Open Access Article
Open Access ArticleComparative investigations of in vitro and in vivo bioactivity of titanium vs. Ti–24Nb–4Zr–8Sn alloy before and after sandblasting and acid etching
Xin Liua,
Yumei Niu†
 *b,
Weili Xie†*a,
Daqing Weic and
Qing Duc
*b,
Weili Xie†*a,
Daqing Weic and
Qing Duc
aDepartment of Prosthodontics, The First Affiliated Hospital of Harbin Medical University, Harbin, Heilongjiang Province, China. E-mail: xwl811@126.com
bDepartment of Endodontics, The First Affiliated Hospital of Harbin Medical University, Harbin, Heilongjiang Province, China. E-mail: niuyumei@hrbmu.edu.cn
cHarbin Institute of Technology School of Materials Science and Engineering, Harbin, Heilongjiang Province, China
First published on 22nd June 2020
Abstract
To avoid the failure of clinical surgery due to “stress shielding” and the loosening of an implant, a new type of alloy, Ti–24Nb–4Zr–8Sn (TNZS), with a low Young's modulus acted as a new implant material in this work. Meanwhile, the surface characteristics, MC3T3-E1 cell behavior and in vivo osseointegration of the titanium and TNZS before and after sandblasting and acid etching were studied comparatively. TNZS and Ti had the same microstructure based on the transmission electron microscopy results. Meanwhile, the TNZS alloy had a lower Young's modulus and surface nanohardness compared with pure titanium. However, the corrosion resistance of Ti was better than that of the TNZS sample in simulated body fluid solution. In addition, the TNZS alloy after sandblasting and acid etching (SLATNZS) had excellent cell adhesion, proliferation, differentiation, ALP activity and in vivo osseointegration ability such as there being almost no soft tissue as compared with other implants. Based on the current results, the new type of Ti–24Nb–4Zr–8Sn alloy showed good potential and promising application prospects in its biochemical aspects.
1. Introduction
Titanium and its alloys show potential in hard tissue repair and replacement due to their good biocompatibility and good corrosion resistance.1,2 Meanwhile, titanium and its alloys possess excellent mechanical properties, while their high Young's modulus could lead to stress shielding after implantation, which could result in the absorption of native bone tissue around the titanium implants.3–5 Thus, researchers have developed a new β-type, Ti–24Nb–4Zr–8Sn (TNZS) for biological applications. It has a lower Young's modulus (about 33 GPa, close to that of human bone) than that of the typical α + β type Ti–6Al–4V alloy (120 GPa) or pure titanium (103.4 GPa), which could avoid “stress shielding” between the native bone and the implant.6–10 Thus, the TNZS alloy has attracted more researchers' attention.As is well known, the sandblasting and acid-etching method (SLA) has been widely used in the surface modification of dental titanium implants, and SLA treated dental titanium implants have been successfully applied clinically. However, these still take place in clinical surgery. Moreover, previous studies had reported that surface characteristics such as surface wettability, roughness, surface topology and surface chemistry played an important role in new bone construction during healing. In detail, the micro-scale and sub-micro-scale topological structures played an important role in bone remodeling at an early stage of healing,11 which could promote the mechanical interlocking between the implant and native bone, thus retaining the stability of the implant. Besides, the sub-micro and micro-scale structures had similar sizes of cell dimensions and absorption pits, leading to the adhesion and proliferation of osteoblasts in vitro and enhancement of bone-to-implant contact in vivo.12 In past decades, some studies reported that a nano-scale surface structure can enhance the osteoblast response behavior and gene expression.12–16 Meanwhile, the suitable wettability and roughness on the implant surface had a critical role in the interaction between the biomaterial and the bioenvironment, especially water and protein, and further influenced cell behavior.17–19 Thus, a hierarchical micro–nano-scale topological structure with suitable surface roughness and wettability could effectively achieve excellent cell biomaterial interaction at an early stage of healing. In this work, the common surface modification method (the SLA method) was applied to change the surface structure of the titanium and TNZS plates. The surface characters of the titanium and TNZS plates before and after SLA treatment, such as wettability, roughness, Young's modulus and hardness, were studied comparatively. Meanwhile, the MC3T3-E1 cell response behavior and in vivo osseointegration of the titanium and TNZS before and after SLA treatment were also studied.
2. Materials and methods
2.1 Preparation and characterization of materials
The potentiodynamic polarization tests of pure Ti and Ti2448 plates before and after MAO treatment were performed on a Gamry 600 (Reference 600, Gamry, USA) electrochemistry workstation. Each of the samples (exposed area of 0.3 cm2) was immersed into a conventional three-electrode cell containing the simulated body fluid (SBF) solution under open conditions to act as the working electrode, a platinum plate as the auxiliary electrode and a saturated calomel electrode (SCE) as the reference. After a 5 min delay under open conditions, the samples reached a stable state. The potentiodynamic polarization curves were measured from +1 V to −1 V according to the open circuit potentials (referenced to the SCE) with a scanning rate of 1 mV S−1.
2.2 In vitro studies
2.3 In vivo animal experiment
The animal experiments used in this study were approved by the Ethics Committee of the First Affiliated Hospital of Harbin Medical University, and were carried out under the control of the University's Guidelines for Animal Experimentation. The in vivo implantation surgery process and study methods were reported in detail in our previous investigations.23 In this study, fifteen New Zealand rabbits with weight of 2.5–3 kg each were studied. Then, the Ti, TNZS, SLATi and SLATNZS implants were placed into four machined holes with size of ϕ 2 × 6 mm3 on the tibias of the rabbits. The interfacial bonding status between the implant and bone was evaluated using the X-ray radiographic technique (DHF-155HII, Hitachi, Japan) and micro-computed tomography (Micro-CT, Siemens Co., America), Materialise's interactive medical image control system 20 (MINICS 20, Materialise Co., Belgium), and VG stained cross-sectional histological analysis.2.4 Statistical analysis
SAS 6.0 software was used to perform the statistical analysis, and the data had statistical significance when p < 0.05. All the in vitro and in vivo experiments and surface characterizations were quantified with at least three independent replicates.3. Results
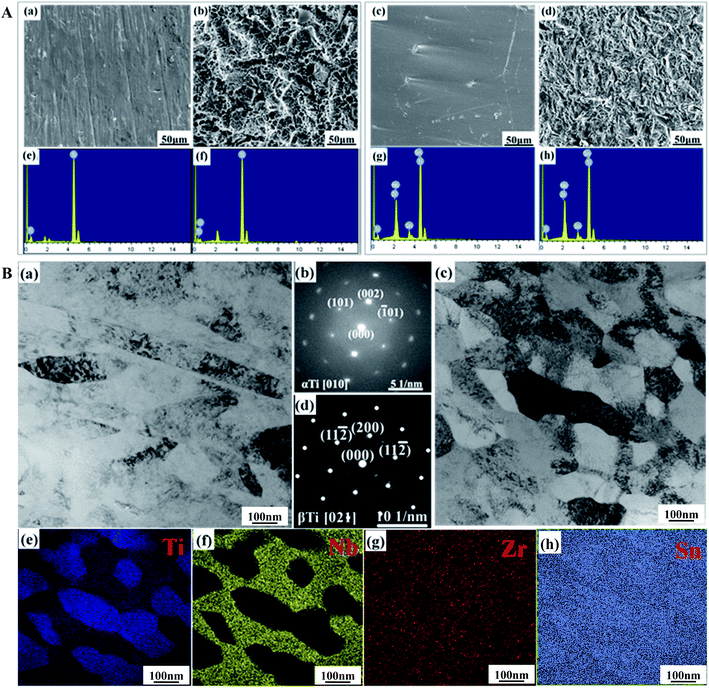
3.1 Characterization of materials
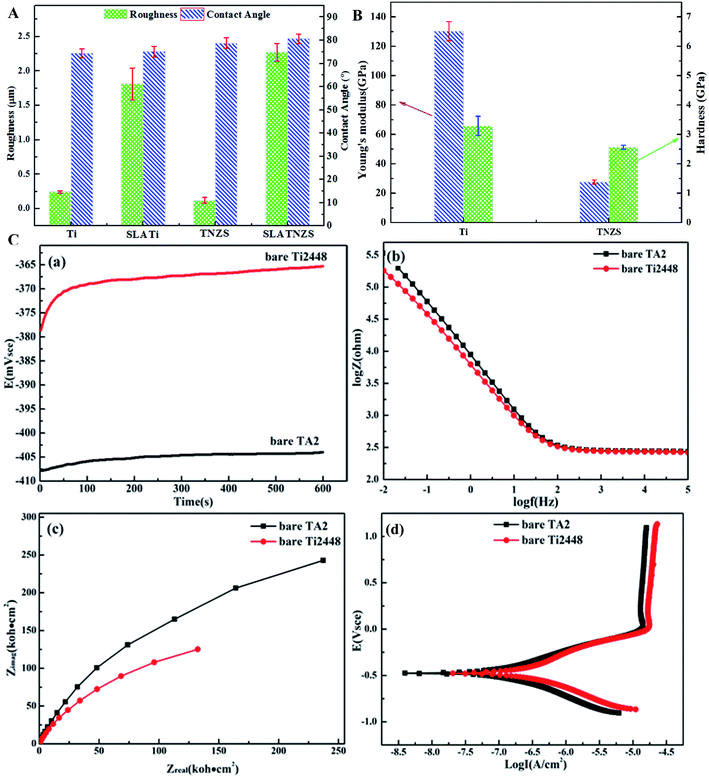
Fig. 2C illustrates the open circuit voltage, impedance modulus, Nyquist plot and potentiodynamic curves of the Ti and TNZS samples in SBF solution. In Fig. 2C(a), it was clearly found that the open circuit voltage of the TNZS sample was greater than that of the Ti sample. In Fig. 2C(b), it can clearly be seen that the Z modulus of TNZS sample is lower than that of the Ti sample in the range of 0.01–100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Hz. In Fig. 2C(c), the Nyquist plots showed that the radius of curvature for pure Ti2448 (150 kΩ cm2) was larger than that for pure Ti (250 kΩ cm2), revealing that the corrosion resistance for pure Ti was better than that for pure Ti2448. The potentiodynamic polarization curves for pure Ti and Ti2448 are shown in Fig. 2C(d). It was clearly found that the pure TNZS samples had the same corrosion potentials as the Ti sample (−0.466 V and −0.466 V), while its corrosion current density was higher than that of the Ti sample (1.90 × 10−8 A cm−2 and 3.64 × 10−9 A cm−2). As a result, the Nb, Zr and Sn elements in the TNZS sample were not effective in improving the corrosion resistance.
000 Hz. In Fig. 2C(c), the Nyquist plots showed that the radius of curvature for pure Ti2448 (150 kΩ cm2) was larger than that for pure Ti (250 kΩ cm2), revealing that the corrosion resistance for pure Ti was better than that for pure Ti2448. The potentiodynamic polarization curves for pure Ti and Ti2448 are shown in Fig. 2C(d). It was clearly found that the pure TNZS samples had the same corrosion potentials as the Ti sample (−0.466 V and −0.466 V), while its corrosion current density was higher than that of the Ti sample (1.90 × 10−8 A cm−2 and 3.64 × 10−9 A cm−2). As a result, the Nb, Zr and Sn elements in the TNZS sample were not effective in improving the corrosion resistance.
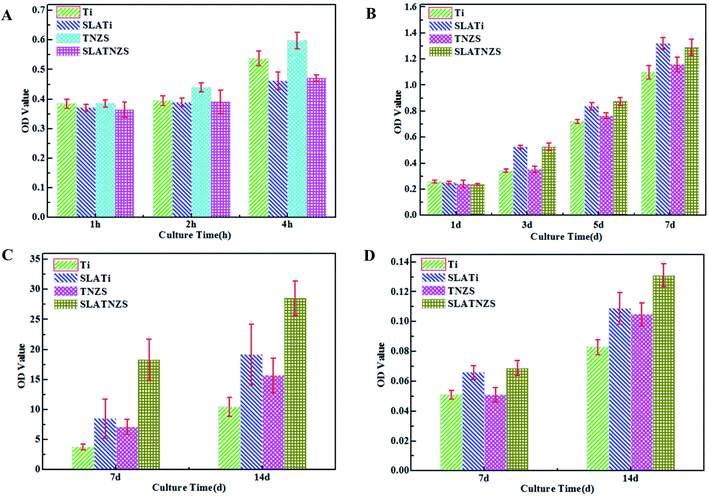
3.2 Results of in vitro studies
3.3 Results of in vivo studies
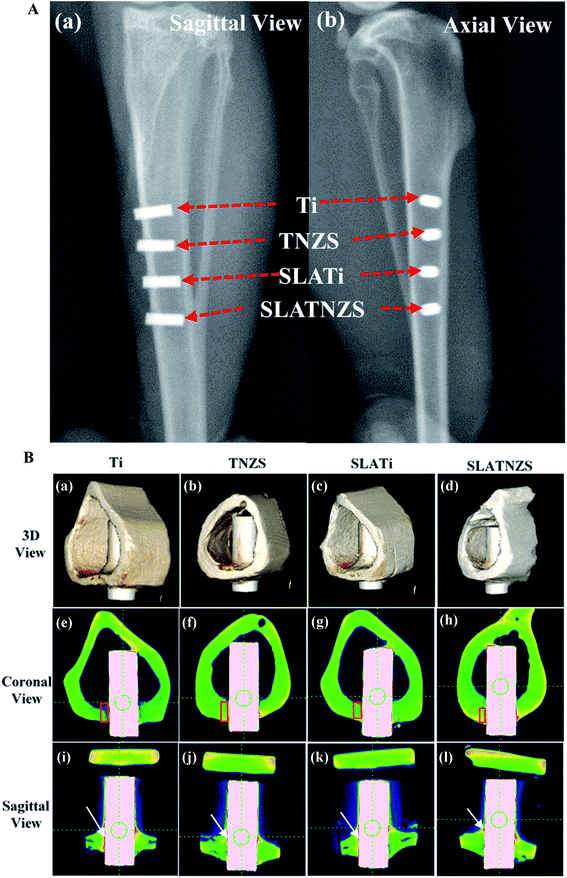
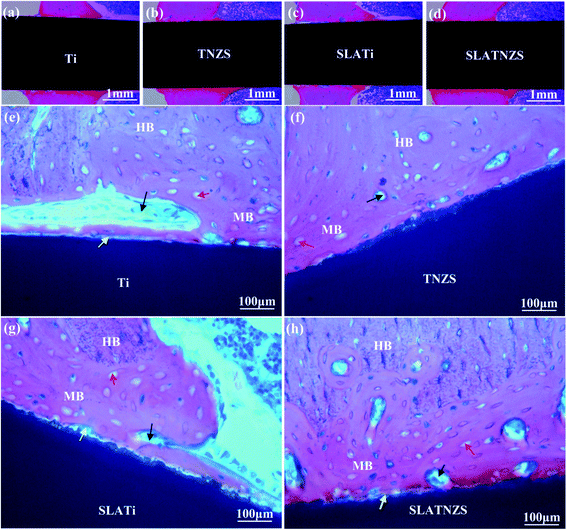
It was notable that the biological tissue had coverage shapes that fit well with the implant surface. Some soft tissue marked by white arrows was formed at the Ti implant–bone interface (Fig. 4B(e) and (i)). However, after sand blasting and acid etching, the amount of soft tissue on the SLATi implant surface was decreased (Fig. 4B(g) and (k)). From the coronal view direction, the bone tissue around the Ti and SLATi implant surfaces was relatively loose. Compared with the Ti implant, a small amount of soft tissue (marked by white arrows) was found at the TNZS and SLATNZS implants and bone interface (Fig. 4B(f), (j) and (h) and (l)). Moreover, as for the TNZS and SLATNZS implant, at the bone–implant interface, the new bone tissue (marked by the red regions in Fig. 4B(e)–(h)) exhibited as relatively dense. The reason for this was attributed to the low modulus and hardness, thus avoiding the absorption of new bone tissue. Meanwhile, there were no cracks observed at the implant–bone interface for the Ti and TNZS implants before and after sandblasting and acid etching.
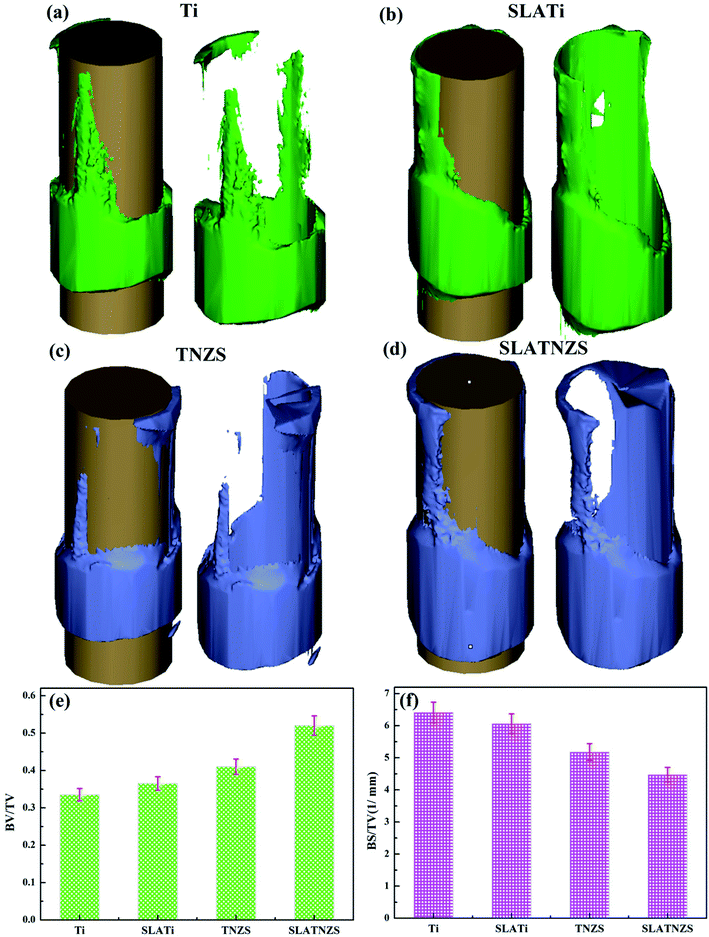
The 3D reconstruction results of the bone and soft tissue surrounding the implants after implantation in the tibias for 16 weeks are illustrated in Fig. 5. All the biological tissues had good connection with the implants and completely covered the implants. After healing for 16 weeks, the results of BV/TV and BS/TV revealed that the bone tissue surrounding the SLATNZS implant was the densest, showing excellent osseointegration ability compared to the other implants. Meanwhile, the results also indicated that the low Young's modulus TNZS implant with a porous surface showed excellent osseointegration ability.
Moreover, the magnified images exhibited that the Ti implant showed poor connection with the surrounding bone tissue because soft tissue was formed at the interface. Almost no soft tissue was found at the TNZS implant–bone interface, showing a direct contact connection with the bone tissue. After sandblasting and acid etching, it was found that some bone tissue was formed at the cavity on the SLATi and SLATNZS implants, suggesting that the rough surface was beneficial to the growth of bone tissue. Meanwhile, the amount of new bone around the SLATNZS implant was more than that around the SLATi implants.
4. Discussion
As is well known, osseointegration is an important evaluation standard for new bone formation around an implant. During healing, there is some biological interaction between the implant and biological tissue, which involve host bone–implant interaction, and cell–implant interaction. The excellent biological interaction between the implant and biological tissue plays an important role in improving the osseointegration ability of orthopedic implants.24–26 Recently, some clinical surgeries have revealed that poor osseointegration easily results in a loosening of the implant and subsequent surgical failure.27,28 To achieve excellent osseointegration ability, many surface modified technologies such as sandblasting and acid etching have been developed. It has been proved that sandblasting is an effective approach to enhancing osseointegration through constructing a rough surface topography. However, after sandblasting, the residual Al2O3 can easily affect normal bone mineralization.29 To avoid this phenomenon, acid etching is effectively applied to remove the residual Al2O3 from sandblasting.30,31 This indicates that the surface modifications could enhance osteoblast response behavior and the formation of new bone tissue.32,33 In this work, the cell response behavior and in vivo osseointegration of the titanium and TNZS before and after sandblasting and acid etching were studied comparatively. The current results suggested that the low Young's modulus porous SLATNZS sample with the micro/nano double scale could synergistically enhance cell proliferation, adhesion and ALP activity in vitro and improve osseointegration in vivo.After implantation, the cell–implant interaction took place first in the biological fluid. The interaction ability depended on the surface characters of the implant. The previous study had demonstrated that the cell adhesion mechanism was that the positively charged protein as the intermediate medium enhanced the cell adhesion via electrostatic attraction. Generally, cell adhesion behavior often relies on the adhesion and exchange of water molecules and protein. On the hydrophobic and negatively charged surface, in the early stage, the water molecules were firstly absorbed on the implant surface, and were randomly distributed, and then the protein with positive charges was absorbed on the modified titanium surface by releasing the water molecules. Finally, the osteoblast was adhered to the coating surface via integration with the membrane protein. However, the superhydrophilic surface could induce the formation of a hydration cation layer via hydrogen bonds on the coating surface, which inhibited the absorption of protein, further suppressing the cell response behavior. Thus, the superhydrophilic and superhydrophobic surfaces were both not beneficial to the protein absorption, and then affected the cell behavior response. Although the superhydrophobic surface could greatly affect the absorbed the protein, it could damage the natural conformation of the protein, and further affect cell adhesion. According to the above-mentioned discussion, it was concluded that a biomaterial with a suitable hydrophilic/hydrophobic feature and surface roughness had excellent cell-material interaction with the surrounding biological fluid.
However, in this work, the contact angles on the Ti, SLATi, TNZS and SLATNZS surfaces were similar. The roughness on the SLATi and SLATNZS samples increased compared with the Ti and TNZS samples. Thus, the value of the roughness ranged from 1.7 to 2.2 μm, which was beneficial to cell proliferation, ALP activity and differentiation. Moreover, the previous studies had reported that the matrix stiffness could modify the morphology and focal adhesion characteristics of the cells.34–36 The surface hardness on TNZS was decreased compared with Ti, and the cell behavior for the TNZS sample was better than that for the Ti sample. The current results were in agreement with the previous studies.
As reported in the previous research, the bone reconstruction and bone resorption around the implant took place simultaneously during healing. Many investigations have indicated that host bone resorption often occurs at the implant–bone interface, which could decrease the stability at the interface and result in surgical failure.37,38 Besides, the previous studies indicated that a mismatch in the Young's modulus between the implants and host bone tissue could be generated, leading to bone resorption and implant loosening due to stress shielding.39 Therefore, in this work, to avoid bone resorption and implant loosening at the implant–bone interface, the Ti–24Nb–4Zr–8Sn (TNZS) alloy with a low elastic modulus which was capable of providing a better balance in the Young's modulus between the implant and the bone was studied.40 The X-ray images showed that all implants remained at the initial implanted sites in the tibias, suggesting that the Ti and TNZS based implants had no loosening or displacement. Based on the BV/TV and BS/TV analysis, the intensity of the bone around the SLATNZS implant was high compared with that around the SLATi implant. These results revealed that the low Young's modulus SLATNZS implant could reduce the “stress shielding” after implantation. Meanwhile, there was little soft tissue around the SLATNZS implant surface, and new bone tissue was also found in the micro-pore region based on the histological analysis and the results of our previous study. However, the resolution of the optical microscopy was very low, and could not be used to judge the size of the pore structure on the alloy surface in Fig. 6. In a future study, we would adopt an ultrathin slice of hard histological tissue to study the interface status, and further reveal whether new bone tissue could grow into the micro-pores.
Based on the current results, Ti–24Nb–4Zr–8Sn (TNZS) is a promising candidate in the field of replacement and repair of hard tissue.
5. Conclusion
In this work, sandblasting and acid etching were successfully applied to change the surface characteristics of Ti and TNZS samples. After sandblasting and acid etching, the surfaces of the Ti and TNZS samples exhibited rough and porous characteristics. Compared with pure Ti, the TNZS alloy had a lower Young's modulus and surface hardness. In vitro tests indicated that the SLATNZS sample had better cell adhesion, proliferation, differentiation and ALP activity as compared with pure Ti. Meanwhile, histological analysis indicated that the newly formed bone grew into the micro-pores and almost no soft tissue was found at the implant–bone interface, showing good osseointegration. Therefore, the TNZS alloy as a new implant material is a promising potential candidate in the biochemical field.Conflicts of interest
The authors declare that they have no Conflicts of interest in this work.Acknowledgements
This work was financially supported by Heilongjiang Provincial Health Department Project (ZY18C01), Harbin Science and Technology Bureau, the National Natural Science Foundation of China (Grant No. 81771106), National Natural Science Foundation, Innovation Research Group Natural Fund (Grant No. 51621091), Heilongjiang Provincial Youth Science Foundation (QC2013C043), and National Basic Science Research Program (2012CB933900).References
- R. I. Asri, W. S. Harun, M. A. Hassan, S. A. Ghani and Z. Buyong, J. Mech. Behav. Biomed. Mater., 2016, 57, 95–108 CrossRef CAS PubMed.
- P. Tengvall and I. Lundström, Clin. Mater., 1992, 9, 115–134 CrossRef CAS PubMed.
- L. J. Xu, Y. Y. Chen, Z. G. Liu and F. T. Kong, J. Alloys Compd., 2008, 453, 320–324 CrossRef CAS.
- Y. Song, D. S. Xu, R. Yang, D. Li, W. T. Wu and Z. X. Guo, Mater. Sci. Eng., A, 1999, 260, 269–274 CrossRef.
- P. Majumdar, S. B. Singh and M. Chakraborty, Mater. Sci. Eng., A, 2008, 489, 419–425 CrossRef.
- Y. L. Hao, S. J. Li, S. Y. Sun, C. Y. Zheng and R. Yang, Acta Biomater., 2007, 3, 277–286 CrossRef CAS PubMed.
- S. J. Li, T. C. Cui, Y. L. Hao and R. Yang, Acta Biomater., 2008, 4, 305–317 CrossRef CAS PubMed.
- S. J. Li, Y. W. Zhang, B. B. Sun, Y. L. Hao and R. Yang, Mater. Sci. Eng., A, 2008, 480, 101–108 CrossRef.
- D. G. Lee, X. Mi, T. K. Eom and Y. Lee, J. Biomater. Tissue Eng., 2016, 6, 798–801 CrossRef.
- F. Liu, F. Wang, T. Shimizu, K. Lgarashi and L. Zhao, Ceram. Int., 2006, 32, 527–531 CrossRef CAS.
- D. L. Cochran, R. K. Schenk, A. Lussi, F. L. Higginbottom and D. Buser, J. Biomed. Mater. Res., 2015, 40, 1–11 Search PubMed.
- A. G. I. Rolando, T. Mclachlan, Y. Cai, S. Berner, R. Tannenbaum, Z. Schwartz, K. H. Sandhage and B. D. Boyan, Biomaterials, 2011, 32, 3395–3403 CrossRef PubMed.
- M. Kulkarni, A. Mazare, E. Gongadze, Š. Perutkova, V. Kralj-Iglič, I. Milošev, P. Schmuki, A. Iglič and M. Mozetič, Nanotechnology, 2015, 26, 062002 CrossRef CAS PubMed.
- M. Kulkarni, A. Flašker, M. Lokar, Š. Perutkova, V. Kralj-Iglič, I. Milošev, P. Schmuki, A. Iglič and M. Mozetič, Int. J. Nanomed., 2015, 10, 1359–1373 CAS.
- D. Kabaso, E. Gongadze, Å. PerutkovÃ, C. Matschegewski, V. Kralj-Iglič, U. Beck, U. van Rienen and A. Iglič, Comput. Methods Biomech. Biomed. Eng., 2011, 14, 469–482 CrossRef CAS PubMed.
- M. Rezazadeh Shirdar, I. Sudin and M. Taheri, Vacuum, 2015, 122, 82–89 CrossRef CAS.
- R. Rasouli, A. Barhoum and H. Uludag, Biomater. Sci., 2018, 6, 1312–1338 RSC.
- T. Sawase, R. Jimbo, K. Baba, Y. Shibata, T. Ikeda and M. Atsuta, Clin. Oral Implants Res., 2010, 19, 491–496 CrossRef PubMed.
- M. Hannig, L. Kriener, W. Hothhannig, C. Becker-Willinger and H. Schmidt, J. Nanosci. Nanotechnol., 2007, 7, 4642–4648 CrossRef CAS PubMed.
- D. Wei, Q. Du, S. Guo, D. Jia, Y. Wang, B. Li and Y. Zhou, Surf. Coat. Technol., 2018, 340, 93–102 CrossRef CAS.
- R. Zhou, D. Wei, Y. Cao, W. Feng, Q. Du, B. Li, Y. Wang, D. Jia and Y. Zhou, Mater. Sci. Eng., C, 2015, 49, 669–680 CrossRef CAS PubMed.
- Q. Du, D. Wei and S. Wang, Appl. Surf. Sci., 2019, 487, 708–718 CrossRef CAS.
- Q. Du, D. Wei, S. Wang, S. Cheng, Y. Wang, B. Li, D. Jia and Y. Zhou, Chem. Eng. J., 2019, 373, 1091–1110 CrossRef CAS.
- L. Le Guéhennec, A. Soueidan, P. Layrolle and Y. Amouriq, Dent. Mater., 2007, 23, 844–854 CrossRef PubMed.
- R. Agarwal and A. J. García, Adv. Drug Delivery Rev., 2015, 94, 53–62 CrossRef CAS PubMed.
- S. P. Pilipchuk, A. B. Plonka, A. Monje, A. D. Taut, A. Lanis, B. Kang and W. V. Giannobile, Dent. Mater., 2015, 31, 317–338 CrossRef CAS PubMed.
- S. Kotsovilis, I. K. Karoussis and I. Fourmousis, Clin. Oral Implants Res., 2006, 17, 587–599 CrossRef PubMed.
- M. Retzepi, M. P. Lewis and N. Donos, Clin. Oral Implants Res., 2010, 21, 71–79 CrossRef PubMed.
- H. J. Johnson, S. J. Northup, P. A. Seagraves, M. Atallah, P. J. Garvin, L. Lin and T. D. Darby, J. Biomed. Mater. Res., 1985, 19, 489–508 CrossRef CAS PubMed.
- H. Schliephake, A. Aref, D. Scharnweber, S. Bierbaum and A. Sewing, Clin. Oral Implants Res., 2010, 20, 38–44 CrossRef PubMed.
- L. Salou, A. Hoornaert, G. Louarn and P. Layrolle, Acta Biomater., 2015, 11, 494–502 CrossRef CAS PubMed.
- D. Buser, N. Broggini, M. Wieland, R. K. Schenk, A. J. Denzer, D. L. Cochran, B. Hoffmann, A. Lussi and S. G. Steinemann, J. Dent. Res., 2004, 83, 529–533 CrossRef CAS PubMed.
- I. Abrahamsson, T. Berglundh, E. Linder, N. P. Lang and J. Lindhe, Clin. Oral Implants Res., 2010, 15, 381–392 CrossRef PubMed.
- S. Fusco, V. Panzetta, V. Embrione and P. A. Netti, Acta Biomater., 2015, 23, 63–71 CrossRef PubMed.
- Y. Navaro, N. Bleich-Kimelman, L. Hazanov, I. Mironi-Harpaz, Y. Shachaf, S. Garty, Y. Smith, G. Pelled, D. Gazit, D. Seliktar and Z. Gazit, Biomaterials, 2015, 49, 68–76 CrossRef CAS PubMed.
- B. Trappmann, J. E. Gautrot, J. T. Connelly, D. G. Strange, Y. Li, M. L. Oyen, M. A. Cohen Stuart, H. Boehm, B. Li, V. Vogel, J. P. Spatz, F. M. Watt and W. T. Huck, Nat. Mater., 2012, 11, 642–649 CrossRef CAS PubMed.
- H. Kröger, P. Venesmaa, J. Jurvelin, H. Miettinen, O. Suomalainen and E. Alhava, Clin. Orthop. Relat. Res., 1998, 352, 66–74 Search PubMed.
- T. Albrektsson and C. Johansson, Eur. Spine J., 2001, 10, S96–S101 CrossRef PubMed.
- R. Huiskes, H. Weinans and R. B. Van, Clin. Orthop. Relat. Res., 1992, 274, 124–134 Search PubMed.
- Y. L. Hao, S. J. Li, S. Y. Sun, C. Y. Zheng and R. Yang, Acta Biomater., 2007, 3, 277–286 CrossRef CAS PubMed.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2020 |