Open Access Article
Open Access ArticleElectrochemical detection of C-reactive protein using functionalized iridium nanoparticles/graphene oxide as a tag
Yuchan Ma,
Jiao Yang,
Tong Yang ,
Yan Deng,
Mengqiao Gu,
Min Wang,
Rong Hu
,
Yan Deng,
Mengqiao Gu,
Min Wang,
Rong Hu * and
Yunhui Yang
* and
Yunhui Yang *
*
College of Chemistry and Chemical Engineering, Yunnan Normal University, Kunming, Yunnan, P. R. China. E-mail: yyhui2002@aliyun.com; hudierong_168@163.com
First published on 6th March 2020
Abstract
C-reactive protein (CRP) has become a recognized indicator of inflammation. CRP concentration in serum is an important indicator for monitoring early heart damage, and it is also a newly discovered coronary heart disease-associated inflammatory factor. A conductive nano-hybrid material composed of Au NPs and ionic liquid functionalized molybdenum disulfide (Au NPs/IL-MoS2) was prepared and utilized to immobilize primary CRP antibodies. Subsequently, 1,5-diaminonaphthalene (DN) was adsorbed onto graphene oxide (GO) through π–π stacking, which was used to load iridium nanoparticles (Ir NPs) as a tag to label secondary CRP antibodies. The large surface area of Au NPs/IL-MoS2 and the excellent electrocatalytic properties of Ir NPs/GO-DN toward the reduction of H2O2 resulted in a highly sensitive assay for CRP antigens. This immunosensor exhibited wide linear ranges from 0.01 to 100 ng mL−1 and a lower detection of limit of 3.3 pg mL−1 (S/N = 3). This CRP immunosensor can be applied in real serum sample analysis with satisfactory results, indicating that the immunosensor has potential applications in biomedical detection.
1 Introduction
C-reactive protein (CRP) has become a recognized indicator of inflammation. It has a very low CRP concentration of 1–10 μg mL−1 in a healthy body. When the concentration of this protein is higher than 10 μg mL−1, there may be inflammation in vivo.1 Moreover, CRP concentration in serum is an important indicator for monitoring early heart damage, and it is also a newly discovered coronary heart disease-associated inflammatory factor. Therefore, it plays a key role in the diagnosis of coronary heart disease.2 In recent years, the detection of CRP has not only been widely used to monitor various types of inflammatory states, but also to explore the practical effects of inflammatory treatments.At present, many methods and techniques have been developed to detect CRP, such as fluorescence,3 turbidimetry4 and immunoturbidimetry,5 as well as radiological and enzyme-linked immunosorbent assays.6 Although these methods are highly specific, they all have their own limitations, such as complicated operation, long time consumption, large sample consumption, and expensive equipment. In comparison, electrochemical immunosensors are attracting more and more attention because of some advantages such as being simple, rapid, easily detectable, sensitive and inexpensive. For instance, Zhang's group developed an RNA aptamer-based electrochemical aptasensor for C-reactive protein detection using functionalized silica microspheres as immunoprobes.7 In the same year, they made use of copper nanoparticles and hybridization chain reaction amplifying signal for electrochemical detection of C-reactive protein.8 Furthermore, because of the combination of highly sensitive sensing techniques and specific immune reaction, the electrochemical immunosensor, can be used for real-time monitoring of antigens and have application prospects in clinical diagnosis.9–13
Graphene oxide (GO) is a 2D planar structure with sp2 bonded carbon atoms, which has a special crystal form and high electronic quality. Since its discovery in 2004, GO has had a tremendous impact in many fields of science and technology because of its remarkable physical and chemical properties. It has a high specific surface area (the theoretical specific surface area of single-layer graphene is 2630 m2 g−1), good flexibility and impermeability, excellent thermal conductivity and electrical conductivity, high mechanical strength, strong electron transport capacity and so forth.14,15
As a very precious metal element, iridium has been widely used in fluorescent labeling, molecular sensing,16 and catalysis in recent years. For example, in the field of catalysis, iridium nanoparticles (Ir NPs) have been recognized as the preferred catalyst because of their high activity, stability and stereoselectivity. At the same time, because of their high specific surface area and high density of active sites, their physical and chemical properties are different from many other nanomaterials. In this work, 1,5-diaminonaphthalene (DN) was first adsorbed onto graphene oxide (GO) through π–π stacking.17 Then, it was found that Ir nanoparticles can be loaded onto GO-DN via amino group of DN. Ir NPs/GO-DN were first reported as enzyme mimics showing intrinsic catalase-and peroxidase-like activities, which can be used as a tag to label CRP antibody to construct a sandwich CRP immunosensor.
Molybdenum disulfide (MoS2) is a typical metal sulfide material, which structure is similar to that of GO. Furthermore, MoS2 is cheap and has a good ability to absorb visible light. Its excellent physical and chemical properties make it widely used in photocatalytic degradation of organic matter, lithium ion batteries, biosensors and other fields.18 However, it is hard to control both the size and coverage density of nanoparticles on MoS2. To solve this problem, Liu et al. reported an ionic liquid functionalized reduced graphene oxide loaded with gold nanoparticle nanocomposite (IL-rGO-Au). The cations of the amine-terminated ionic liquid (IL-NH2) can be introduced into the graphene oxide (rGO), contributing to stabilization of ionic liquid functionalized reduced graphene oxide (IL-rGO) dispersions via electrostatic repulsion. Besides, the surfaces of IL-rGO are evenly covered with uniform AuNPs, and the AuNPs deposited on IL-rGO are noticeably dense.14 Inspired by their work, we try to use ionic liquid to functionalize molybdenum disulfide so that gold nanoparticle can be loaded to obtain a nanocomposite (Au NPs/IL-MoS2) which can control size and coverage density of nanoparticles on MoS2 just like IL-rGO. With the assistance of IL-NH2, Au NPs were uniformly and densely absorbed on the surfaces of the IL-MoS2. Then, this nanocomposite was modified on the surface of a glassy carbon electrode to immobilize CRP antibody.
Meanwhile, Ir NPs/GO-DN with the properties of peroxidase mimic enzyme was used as a label to construct a sandwich-type immunosensor for the determination of C-reactive protein. The quantitative detection of CRP antigen was achieved by detecting the catalytic current of the label (Ir NPs/GO-DN) to the reduction of hydrogen peroxide. This is the first time to demonstrate the use of Ir NPs/GO-DN and Au NPs/IL-MoS2 for constructing electrochemical immunosensor.
2 Material and methods
2.1 Reagents and apparatus
CRP, C-reactive protein antibody were obtained from Shanghai Linc-Bio Co., Ltd (Shanghai, China); chitosan (chit), bovine serum albumin (BSA), phosphate buffer saline (PBS), L-cysteine were purchased from Sigma-Aldrich (Shanghai, China); graphene oxide (GO) was purchased from Shanghai Yuanye Biological Co., Ltd (Shanghai, China); sodium molybdate (Na2MoO4·2H2O) was obtained from Aladdin Industrial Corporation Co., Ltd (Shanghai, China); 1,5-diaminonaphthalene (DN) was bought from Beijing Biotechnology Co., Ltd (Beijing, China); 1-aminopropyl-3-methylimidazolium chloride (IL-NH2) was purchased from Shanghai Chengjie Chemical Co., Ltd (Shanghai, China); HAuCl4·xH2O, H2IrCl6·6H2O was purchased from J&K Scientific Co., Ltd (Beijing, China); glutaraldehyde was bought from Boao Extension Technology Co., Ltd (Beijing, China). All reagents used in this experiment were of analytical grade. Ultrapure water was used throughout whole experiment.Cyclic voltammetry (CV), electrochemical impedance spectroscopy (EIS), and differential pulse voltammetry (DPV) were carried out with a CHI 660D electrochemistry workstation (CH Instruments Co., Ltd Shanghai, China). The transmission electronic microscopy (TEM) images were carried out with JEM-2100 (JEOL Co., Ltd Japan). The scanning electronic microscopy (SEM) analysis was performed using S-3000N (Hitachi science systems Co., Ltd Japan). The centrifuge was performed with TGL-20M (Xiangyi Laboratory Instrument Development Co., Ltd Hunan, China). The experiment used a three-electrode system comprised of the glassy carbon electrode as the working electrode, the saturated calomel electrode as the reference electrode, and the platinum electrode as the counter electrode.
2.2 Preparation of materials
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) for above 2 h. Then, the resulting solution was centrifuged at 6000 rpm, followed by washing and dispersing in ultrapure water (1 mg mL−1).
1) for above 2 h. Then, the resulting solution was centrifuged at 6000 rpm, followed by washing and dispersing in ultrapure water (1 mg mL−1).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and kept stirring at 25 °C for 48 h. The above solution was centrifuged and washed with ethanol and water for three times, respectively. The obtained black powder was dried at 60 °C for 24 h under vacuum condition.17
1), and kept stirring at 25 °C for 48 h. The above solution was centrifuged and washed with ethanol and water for three times, respectively. The obtained black powder was dried at 60 °C for 24 h under vacuum condition.17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and after ultrasonication for 30 min, 4 mL of a 1% H2IrCl6·6H2O solution was added. Then, 3 mL of 0.1 mol L−1 NaBH4 solution was added dropwise, and the mixture was uniformly stirred for 4 h. The obtained product was centrifuged, followed by washing with ethanol and ultrapure water for three times, and dried in vacuum drying oven at 60 °C for 24 h.
1), and after ultrasonication for 30 min, 4 mL of a 1% H2IrCl6·6H2O solution was added. Then, 3 mL of 0.1 mol L−1 NaBH4 solution was added dropwise, and the mixture was uniformly stirred for 4 h. The obtained product was centrifuged, followed by washing with ethanol and ultrapure water for three times, and dried in vacuum drying oven at 60 °C for 24 h.2.3 Preparation of C-reactive protein immunosensor
The glassy carbon electrodes were polished with emery paper and alumina slurry of different particle size. Finally, the glassy carbon electrodes were washed with nitric acid aqueous solution (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) anhydrous ethanol and ultrapure water for 5 minutes in sequence and dried in air.
1) anhydrous ethanol and ultrapure water for 5 minutes in sequence and dried in air.
The appropriate amount of Au NPs/IL-MoS2 (1 mg mL−1) and chitosan (5 mg mL−1) were mixed (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
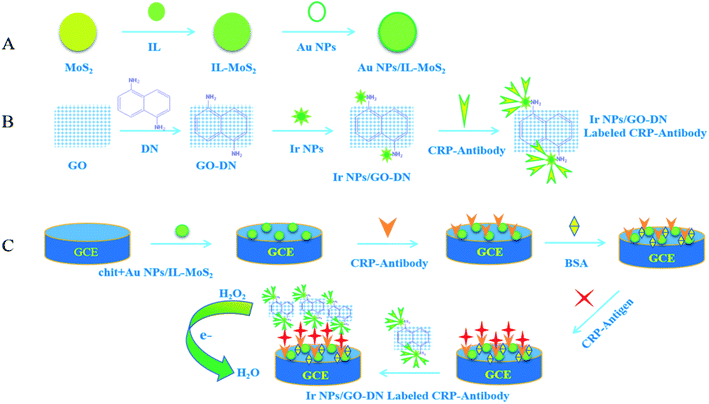
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Then, 10 μL of mixed liquid was pipetted onto the surface of electrode. It was washed with PBS buffer solution after dried. 10 μL of 10 μg mL−1 CRP antibody was dropped on the electrode surface for overnight at 4 °C. Next, the modified glassy carbon electrodes were washed for three times with PBS solution. 10 μL of 1% BSA solution was added to block non-specific sites at 37 °C for 1 h. Finally, the electrode was washed with PBS solution for three times and dried naturally. The immunosensor can be used to incubate different concentrations of CRP, the preparation process of CRP immunosensor was shown in Fig. 1.
1). Then, 10 μL of mixed liquid was pipetted onto the surface of electrode. It was washed with PBS buffer solution after dried. 10 μL of 10 μg mL−1 CRP antibody was dropped on the electrode surface for overnight at 4 °C. Next, the modified glassy carbon electrodes were washed for three times with PBS solution. 10 μL of 1% BSA solution was added to block non-specific sites at 37 °C for 1 h. Finally, the electrode was washed with PBS solution for three times and dried naturally. The immunosensor can be used to incubate different concentrations of CRP, the preparation process of CRP immunosensor was shown in Fig. 1.
2.4 Detection method
Different concentrations of CRP were added to the immunosensor and incubated at 37 °C for 60 min. The electrodes were washed for three times with PBS buffer solution and dried after incubation. Then, Ir NPs/GO-DN labeled CRP antibodies were dropped on the electrode surface and incubated at 37 °C for 45 min followed by washing with PBS buffer solution. As the working electrode, CRP immunosensor was put into the 10 mL of 0.1 mol L−1 PBS buffer solution (pH 7.4). Amperometric responses of the immunosensor were recorded using chronoamperometry at −0.2 V constant potential when 100 μL of 3% H2O2 was added at room temperature due to the catalytic reduction of the labeled Ir NPs/GO-DN toward H2O2. This reduction current was in proportion to the concentration of CRP.3 Results and discussion
3.1 Morphology of materials
Au NPs/MoS2 and Au NPs/IL-MoS2 were dispersed in water and their microstructure were observed through TEM. It was found in Fig. 2(A) that only few Au NPs were dispersed on MoS2 without IL. Contrast with it, there were many Au NPs distributed on IL-MoS2 uniformly (Fig. 2(B)), and the size of Au NPs was about 12 nm.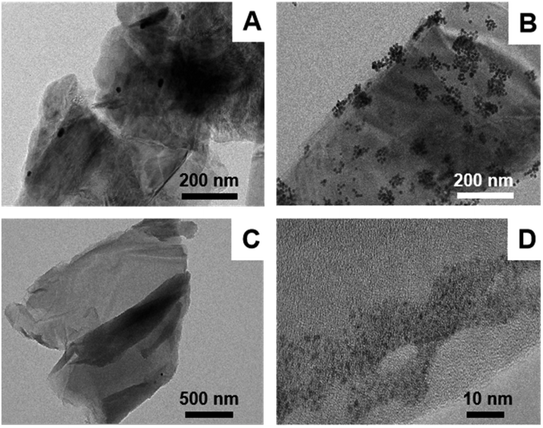 | ||
| Fig. 2 TEM characterizations of different materials: (A) Au NPs/MoS2; (B) Au NPs/IL-MoS2; (C) GO-DN; (D) Ir NPs/GO-DN. | ||
GO-DN was dispersed in water and characterized by TEM. The micromorphology of GO-DN can be seen from Fig. 2(C) which indicated that the GO-DN is distributed in layers. Fig. 2(D) shows TEM images of Ir NPs/GO-DN. It can be seen that Ir NPs were dispersed uniformly with 1 nm of the particle diameter.
3.2 Comparison of the catalytic effect of GO-DN and Ir NPs/GO-DN
The same amount of GO-DN and Ir NPs/GO-DN (1 mg mL−1) were modified on the surface of glassy carbon electrode with certain amount of chitosan. Amperometric responses of the different modified electrodes were recorded using chronoamperometry at −0.2 V constant potential when 100 μL of 3% H2O2 was added to 10 mL of PBS. It can be seen from the Fig. 3 that the catalytic current value of Ir NPs/GO-DN (curve (b)) to the reduction of H2O2 is 3 times larger than that of GO-DN (curve (a)), which indicates that Ir NPs and GO-DN have a good synergistic effect on catalysis to the reduction H2O2, and can obtain higher response current signal. In summary, the highly effective peroxide-mimetic enzyme catalytic activity of Ir NPs/GO-DN composite nanomaterials mainly comes from the reduction of hydrogen peroxide to H2O. Simple mechanism could be inferred: hydrogen peroxide has peroxide bond, O in –O–O– is not the lowest oxidation state, so it is unstable and easy to disconnect. Under normal temperature, the decomposition of hydrogen peroxide can be accelerated by heating; light irradiation with a wavelength of 320–380 nm; or using catalyst such as Au, Pt,21 or Ir.22| H2O2 + Ir = H2IrO2 |
| H2IrO2 + H2O2 = Ir + O2 + 2H2O |
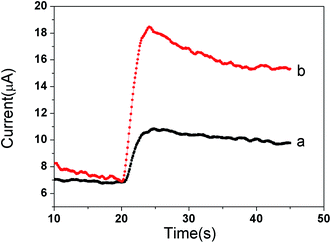 | ||
| Fig. 3 Comparison of the catalytic effect of (a) GO-DN and (b) Ir NPs/GO-DN to the reduction of 3% H2O2. | ||
3.3 Electrochemical impedance behaviors of different modified electrode interfaces
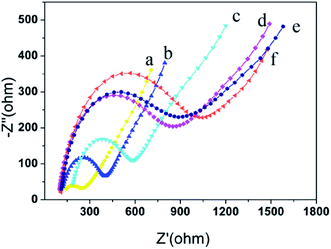
Fig. 4 shows the electrochemical impedance spectroscopy for each interface. The curve (a) is obtained at a bare glassy carbon electrode. It is approximates the straight line, indicating that the bare glassy carbon electrode has good conductivity and hardly affects the electron transfer. The curve (b) is obtained at Au NPs/IL-MoS2/chitosan modified glassy carbon electrode. The impedance value has increased (Ret = 450 Ω) due to the impediment effect on electron transfer of chitosan. The curve (c) is obtained at CRP antibody/Au NPs/IL-MoS2/chitosan modified glassy carbon electrode, and its impedance value increases compared with that of curve (b) (Ret = 600 Ω). As a protein, the CRP antibody is non-conductive, which obstructed the electron transfer and resulted in the increases of impedance value. After the immunosensor blocked with BSA (curve (d)), the impedance value increased (Ret = 750 Ω) compared with that of curve (c). After the immunosensor incubated with CRP antigen, the semicircular diameter increased (curve (e), Ret = 800 Ω) compared with that of the curve (d) because the specific binding of the antibody and antigen obstructed the electron transfer. The curve (f) was obtained after CRP antigen/BSA/anti-CRP/Au NPs/IL-MoS2/chitosan modified electrode was incubated with Ir NPs/GO-DN labeled CRP antibody with the maximum value of the impedance (Ret = 1100 Ω). Thus, it is explained that the electrode is successfully modified layer by layer.3.4 Optimization of experimental variables
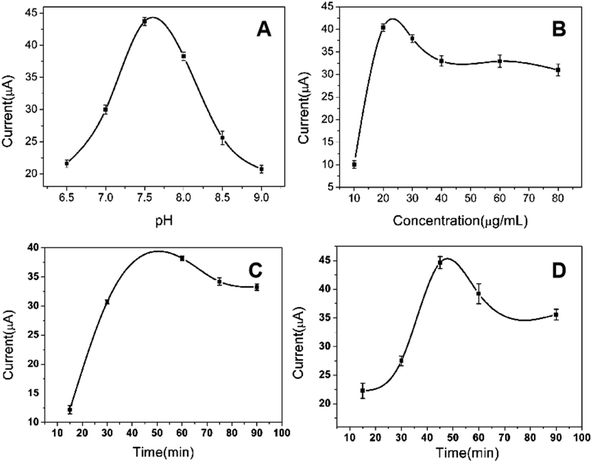
3.5 Immunosensor response characteristics
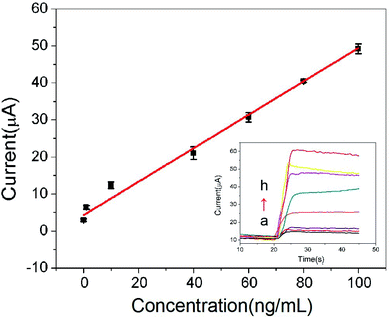
Under the optimal conditions, the calibration curves of the proposed immunosensor was illustrated in Fig. 6. Response current of CRP immunosensor is in proportional to CRP antigen concentration. The linear regression equation is I (μA) = 0.452c (ng mL−1) + 4.31, the linear range is 0.01–100 ng mL−1 with the correlation coefficient of 0.9931. The method demonstrates a good sensitivity with detection limit of 3.3 pg mL−1 (S/N = 3) for CRP. A comparison of different immunoassay for the determination of CRP was shown in Table 1.23–26 The results indicated that the linear range of this immunosensor was better than that of the previous reports.| Method | Linear range | Detection limit | Reference |
|---|---|---|---|
| Label-free immunoassay | 0.2–80 ng mL−1 | 0.04 ng mL−1 | 23 |
| Molecularly imprinted | 0.0625–1 mg mL−1 | 0.0625 mg mL−1 | 24 |
| Fluorescent aptasensor | 0.05–100 ng mL−1 | 0.01 ng mL−1 | 25 |
| Surface plasmon resonance-based immunoassay | 1.2–80 ng mL−1 | 1.2 ng mL−1 | 26 |
| Electrochemical immunosensor | 0.01–100 ng mL−1 | 3.3 pg mL−1 | This work |
3.6 The selectivity of immunosensor
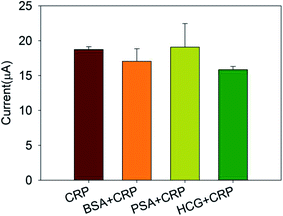
To investigate the specificity of this immunosensor, 20 ng mL−1 CRP antigens were mixed with 20 ng mL−1 bovine serum albumin (BSA), prostate specific antigen (PSA), and human chorionic gonadotropin (HCG), respectively. Then, the response current was measured by the chronoamperometry and compared with the response current generated by CRP antigen alone. The results were shown in Fig. 7. It can be seen that interferents only caused a slight variety in the response current, indicating strong anti-interference ability and good selectivity of this immunosensor.3.7 The stability of immunosensor
After the CRP immunosensor was storied at 4 °C for 5 days, the response current was measured, which retained about 95.93% of its original response. After 30 days of storage, the CRP immunosensor kept 83.28% of the initial response, indicating that the immunosensor has good stability.3.8 CRP analysis in real serum samples
In this work, the serum samples were obtained from the Hospital of Yunnan Normal University (Kunming, China). Briefly, blood sample were centrifuged with 1000–1200g (relative centrifugal force, RCF) for 5 minutes to get serum sample. After being diluted to 1000 fold with the buffer solution, three serum samples were mixed with CRP antigens standard solution to get final CRP concentration of 5, 10 and 30 ng mL−1, respectively. Then, the CRP concentration of three serum samples was measured using proposed immunosensor. Under the optimal conditions, the average recovery was 101.2%. The results were shown in Table 2, indicating that the immunosensor developed in this experiment has practicality and can be used to detect CRP in serum.| Sample | Added (ng mL−1) | Found (ng mL−1) | Recovery (%) | Average recovery (%) |
|---|---|---|---|---|
| 1 | 5.00 | 4.95 | 99.0 | |
| 2 | 10.00 | 10.23 | 102.3 | 101.2 |
| 3 | 30.00 | 30.73 | 102.4 |
4 Conclusions
Au NPs/IL-MoS2, a conductive nano-hybrid material, has large specific surface area, which can be used to immobilize protein. Meanwhile, Ir NPs@GO-DN was first reported as enzyme mimics showing intrinsic catalase and peroxidase-like activities, which was synthesized and used as a tag to label CRP antibody to construct a sandwich CRP immunosensor. The quantitative detection of CRP antigen was achieved by detecting the catalytic current of Ir NPs/GO-DN to the reduction of hydrogen peroxide. This sensor had the characteristics of low cost, high sensitivity, strong anti-interference ability and low detection limit. It can be used for the determination of CRP in real sample.Conflicts of interest
The authors declare that they have no conflicts of interest concerning.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grants No. 21465026, 21765026).References
- S. Black, I. Kushner and D. Samols, J. Biol. Chem., 2004, 279, 48487–48490 CrossRef CAS PubMed.
- A. May and T. J. Wang, Expert Rev. Mol. Diagn., 2007, 7, 793–804 CrossRef CAS PubMed.
- R. J. Cui, H. C. Pan, J. J. Zhu and H. Y. Chen, Anal. Chem., 2007, 79, 8494–8501 CrossRef CAS PubMed.
- W. L. Roberts, R. Sedrick, L. Moulton and A. Spencer, Clin. Chem., 2000, 46, 461–468 CrossRef CAS.
- W. L. Roberts, L. Moulton, T. C. Law, G. Farrow, M. Cooper-Anderson, J. Savory and N. Rifai, Clin. Chem., 2001, 47, 418–425 CrossRef CAS.
- S. K. Vashist, G. Czilwik, T. Oordt, F. Stetten, R. Zengerle, E. M. Schneider and J. H. T. Luong, Anal. Biochem., 2014, 456, 32–37 CrossRef CAS PubMed.
- J. C. Wang, J. J. Guo, J. J. Zhang, W. J. Zhang and Y. Z. Zhang, Biosens. Bioelectron., 2017, 95, 100–105 CrossRef CAS PubMed.
- J. J. Zhang, W. J. Zhang, J. J. Guo, J. C. Wang and Y. Z. Zhang, Anal. Biochem., 2017, 539, 1–7 CrossRef CAS PubMed.
- A. E. Rachkov, M. I. Rozhko, T. A. Sergeyeva and S. A. Piletsky, Sens. Actuators, B, 1994, 19, 610–613 CrossRef CAS.
- S. Kumar, S. Kumar, M. A. Ali, P. Anand, V. V. Agrawal, R. John, S. Maji and B. D. Malhotra, Biotechnol. J., 2013, 8, 1267–1279 CrossRef CAS PubMed.
- A. Kowalczyk, J. P. Sęk and A. Kasprzak, Biosens. Bioelectron., 2018, 117, 232–239 CrossRef CAS PubMed.
- S. K. Vashist, E. M. Schneider and R. Zengerle, Biosens. Bioelectron., 2015, 66, 159–176 CrossRef PubMed.
- Y. C. Kuo, C. K. Lee and C. T. Lin, Biosens. Bioelectron., 2017, 103, 130–137 CrossRef PubMed.
- N. Liu and Z. Ma, Biosens. Bioelectron., 2014, 51, 184–190 CrossRef CAS PubMed.
- D. Chen, H. B. Feng and J. H. Li, Chem. Rev., 2012, 112, 6027–6053 CrossRef CAS PubMed.
- H. Su, D. D. Liu, M. Zhao, W. L. Hu, S. S. Xue, Q. Cao, X. Y. Le, L. N. Ji and Z. W. Mao, ACS Appl. Mater. Interfaces, 2015, 7, 8233–8242 CrossRef CAS PubMed.
- J. L. Huang, J. N. Tian, Y. C. Zhao and S. L. Zhao, Sens. Actuators, B, 2015, 206, 570–576 CrossRef CAS.
- J. Theerthagiri, R. A. Senthil, B. Senthilkumar, A. R. Poluc, J. Madhavan and M. Ashokkumar, J. Solid State Chem., 2017, 252, 43–71 CrossRef CAS.
- T. Yang, P. Hou, L. L. Zheng, L. Zhan, P. F. Gao, Y. F. Li and C. Z. Huang, Nanoscale, 2017, 9, 17020–17028 RSC.
- K. J. Huang, L. Wang, Y. J. Liu, H. B. Wang and L. L. Wang, Electrochim. Acta, 2013, 109, 587–594 CrossRef CAS.
- M. H. Yang, Y. H. Yang, G. L. Shen and R. Q. Yu, Biosens. Bioelectron., 2006, 21, 1125–1131 CrossRef CAS PubMed.
- J. Y. Chen, Guangzhou Chem., 2013, 38, 1–6 Search PubMed.
- X. Zhang, R. Hu, K. L. Zhang, R. Y. Bai, D. L. Li and Y. H. Yang, Anal. Methods, 2016, 8, 6202–6207 RSC.
- P. Zubiate, C. R. Zamarreño, P. Sánchez, I. R. Matias and F. J. Arregui, Biosens. Bioelectron., 2017, 93, 176–181 CrossRef CAS PubMed.
- Z. Z. Liu, D. Luo, F. L. Ren, F. Y. Ran, W. Chen, B. Q. Zhang, C. M. Wang, H. Chen, J. Wei and Q. H. Chen, RSC Adv., 2019, 9, 11960–11967 RSC.
- S. K. Vashist, E. M. Schneiderc and J. H. T. Luong, Analyst, 2015, 140, 4445–4452 RSC.
| This journal is © The Royal Society of Chemistry 2020 |