 Open Access Article
Open Access ArticleEffect of tannin addition on chromatic characteristics, sensory qualities and antioxidant activities of red wines
Lingxi Lia,
Zhe Lib,
Zongmin Weic,
Weichao Yud and
Yan Cui *d
*d
aSchool of Functional Food and Wine, Shenyang Pharmaceutical University, 110016, Shenyang, China
bChina Resources Double-Crane Pharmaceutical Co., Ltd., 100102, Beijing, China
cSchool of Traditional Chinese Materia Medica, Shenyang Pharmaceutical University, 110016, Shenyang, China
dSchool of Pharmacy, Shenyang Pharmaceutical University, 110016, Shenyang, China. E-mail: cuiyan_13@126.com
First published on 17th February 2020
Abstract
Tannin addition as an enological practice has been widely used in the winemaking process because of their ability of improving the aroma and sensory characteristics and stabilizing of color of red wine. In this study, hydrolysable, condensed tannins and their mixtures in different ratios were added into two Merlot wines to investigate their effect on the wine overall quality. The contents of 15 phenolic compounds were detected by HPLC-DAD, CIELAB color parameters were measured using a chromatic aberration meter, sensory evaluation was accomplished using the assessment standards established by the American Wine Association, and antioxidant activities were analyzed using DPPH and ABTS radical tests. The results indicated that adding tannins affected phenolic composition, contents and color of wine. The specific effects varied by tannins. Furthermore, tannin addition, especially the mixed tannins, improved the sensory qualities and antioxidant activities greatly. The mixed tannins added with a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 between hydrolyzable and condensed tannins exhibited a better effect on both sensory qualities and antioxidant activities, and it could be recommended as an ideal tannin addition for wine quality improvement.
1 between hydrolyzable and condensed tannins exhibited a better effect on both sensory qualities and antioxidant activities, and it could be recommended as an ideal tannin addition for wine quality improvement.
1. Introduction
Enological tannins are commercial, complex polyphenolic compounds obtained from various botanical sources. The use of enological tannins is officially authorized by the Organisation Internationale de la Vigne et du Vin (OIV) for must and wine clarification in winemaking because of their affinity to bind proteins and to prevent iron haze in wines.1 Enological tannins are divided into hydrolysable and condensed tannins based on their chemical structures. Hydrolysable tannins are glucose esters of gallic (gallotannins) and ellagic acids (ellagitannins). They present in wine extracted from oak wooden barrels where the wine is kept during the aging process, or were added during winemaking.2,3 Condensed tannins, also called proanthocyanidins, are large macromolecules formed by polymerisation of flavan-3-ol subunits (such as (+)-catechin, (−)-epicatechin, and/or (−)-epigallocatechin and epicatechin-3-O-gallate), and the subunits vary from grape skins, seeds, and stems.4 All grape-derived tannins having enological importance are condensed tannins.3 Nowadays, enological tannins have been commonly used in different winemaking scenarios in order to modify aroma and sensory profile, prevent color loss or facilitate the fining of white or rose wines.5–7 Though hydrolysable tannins originating from oak possess higher antioxidant activity than some tannins extracted from grape skin or seed,8 experienced winemakers would prefer to use grape tannins rather than oak tannins.9 It might be due to the ability of grape tannins (that is proanthocyanidins) to improve the wine aroma complexity and that they could react with anthocyanins to form pigmented polymers to significantly stabilize wine color.7,10 Whereas, hydrolysable tannins could act as copigments to protect wine anthocyanins from oxidation, because they may regulate oxidation–reduction phenomena.11 Besides, the addition of both hydrolysable and condensed tannins into wine can differently modulate the sensory perception of wine, and in particular the effect on wine astringency depends on many factors such as tannin typology, timing, dose, and grape variety.12,13Studies have been conducted for the effect of addition of enological tannins on wine sensory quality.4,7,14,15 The effect on the addition of enological tannins on the color and pigment composition of red wines made from Vitis vinifera L. cv Tempranillo grapes was studied by García-Estévez et al.14 The results showed a higher formation of anthocyanin-derived pigments was observed in the red wines containing the exogenous enological tannin. Moreover, these wines showed lower lightness (L*) values and higher chroma 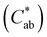 values than control wines, indicating a higher stabilization of color. Neves et al.4 reported that the addition of grape seed tannins had obvious effects of increasing color intensity and antioxidant activity only in the wines poor in polyphenols. Chen et al.7 investigated effects of pre-fermentative addition of enological tannins on wine color, anthocyanins, volatile compounds and sensorial properties. Results indicated that tannin treatments significantly improved wine aroma complexity and sensorial properties. However, the concentration of some stable pigments was negatively affected by tannin addition. Rinaldi et al.15 found that after 1 year aging with enological tannins, there was no increase in the intensity of wine astringency, but an improvement of mouthfeel sensations was achieved with wood-derived tannins. On the other hand, several research studies have indicated that the addition of commercial enological tannins to wines could be less effective to improve the wine sensory quality.5,12,16 For example, with addition of 200 mg L−1 of white grape seed tannins post-fermentation to Cynthiana, no significant increase in total phenolics and little color differences in wine were found.17 The reason for these results is probably due to the low quality or insufficient amount of enological tannins applied. The type of tannins added and the loss of these tannins during the winemaking process could be another possible explanation for the marginal effect of additional tannin on wine quality.4 Furthermore, the amount of tannin added should be carefully considered, because over-adding exogenous enological tannins may result in a dramatic decrease of total phenolic concentrations after alcoholic fermentation and negatively affect mouthfeel and wine structure.13
values than control wines, indicating a higher stabilization of color. Neves et al.4 reported that the addition of grape seed tannins had obvious effects of increasing color intensity and antioxidant activity only in the wines poor in polyphenols. Chen et al.7 investigated effects of pre-fermentative addition of enological tannins on wine color, anthocyanins, volatile compounds and sensorial properties. Results indicated that tannin treatments significantly improved wine aroma complexity and sensorial properties. However, the concentration of some stable pigments was negatively affected by tannin addition. Rinaldi et al.15 found that after 1 year aging with enological tannins, there was no increase in the intensity of wine astringency, but an improvement of mouthfeel sensations was achieved with wood-derived tannins. On the other hand, several research studies have indicated that the addition of commercial enological tannins to wines could be less effective to improve the wine sensory quality.5,12,16 For example, with addition of 200 mg L−1 of white grape seed tannins post-fermentation to Cynthiana, no significant increase in total phenolics and little color differences in wine were found.17 The reason for these results is probably due to the low quality or insufficient amount of enological tannins applied. The type of tannins added and the loss of these tannins during the winemaking process could be another possible explanation for the marginal effect of additional tannin on wine quality.4 Furthermore, the amount of tannin added should be carefully considered, because over-adding exogenous enological tannins may result in a dramatic decrease of total phenolic concentrations after alcoholic fermentation and negatively affect mouthfeel and wine structure.13
Although there are a large number of commercial tannins available on the market and they are reported to might improve some certain characteristics of finished wines, there is little information about their effect on wine overall quality. In this work, considering the characteristic of hydrolysable and condensed tannins, the mixture of both enological tannins, were utilized to study the specific influence of tannin adding on wine overall quality, including wine color, tasting characters, and antioxidant activities for two Merlot wines.
2. Materials and methods
2.1. Chemicals and samples
Castalin, vescalagin, castalagin were obtained from Shanghai ZZBIO Co., Ltd (Shanghai, China). Gallic acid, ellagic acid, vanillic acid, caffeic acid, polydatin (+)-catechin, (−)-epicatechin were purchased from Chengdu Must Bio Technology Co., Ltd. (Chengdu, China). 2,2-Diphenyl-1-picrylhydrazyl (DPPH) and 2,20-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS) were purchased from Sigma-Aldrich (St. Louis, MO, USA).Proanthocyanidin dimer B1, B2 and B2-3′-O-gallate (B2-3′-O-G), trimer C1 and anthocyanin malvidin-3-O-glucoside were extracted and purified (with purity > 95%) as our previous methods.18–20
Chemicals used for wine total polyphenols, flavones, proanthocyanidins and anthocyanins analyses, and all organic solvents used for sample preparation (analytical grade) and HPLC analysis (chromatographic grade) were purchased from Chemical Branch of Shandong Yuwang industrial Co., Ltd. (Shandong, China).
One hydrolysable tannin sample was provided from Proenol Industria Biotecnologica Lda., another condensed tannin sample was provided from Biocrático Lda. (Portugal). Two Central Valley red wines from Chile (RWA, 2015; RWB, 2016) were selected for this study.
2.2. Determination of total polyphenols, flavonoids, proanthocyanidins and anthocyanins in wines
The total polyphenols, flavonoids, anthocyanins and proanthocyanidins of wines were determinated before tannins adding. The total polyphenol content was estimated with gallic acid as the equivalent using Folin–Ciocalteu colorimetric method with a few modifications.21 The total polyphenol content was calculated as the followed regression equation of the standard curve of gallic acid:| Y = 77.97X − 0.3605, r2 = 0.9992 |
Aluminum chloride colorimetric method was used to determine the total flavonoid content of the wine samples with little modifications.22 Rutin was used as the standard for a calibration curve. The total flavonoid content was calculated using the following linear equation based on the calibration curve:
| Y = 0.8918X + 0.0512, r2 = 0.9995 |
Total proanthocyanidin content was equivalent calculated by (+)-catechin and the regression equation obtained from the standard curve as follows:
| Y = 1.7107X + 0.0389, r2 = 0.9990 |
Total anthocyanins were determined using the pH differential method.23 Cyanidin-3-O-glucoside (c3g) with a molar extinction coefficient of 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 and molecular weight of 449.2 was used as the standard and the results expressed as milligrams of c3g equivalents per liter.
900 and molecular weight of 449.2 was used as the standard and the results expressed as milligrams of c3g equivalents per liter.
2.3. Preparation of red wines with the addition of tannins
Two young red wines (1 year old and 2 years old, respectively) made from Merlot were used as subjects of this study due to their low amounts in polyphenols and no exogenous tannin addition. Two high quality commercial tannins (one hydrolysable and one condensed tannin) were selected based on our previous work (to be published), and their mixtures were used for addition into red wines. Three tannin mixtures were obtained according to different ratios (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; 3
1; 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) and the dosage of these tannins (0.6 g L−1) applied to red wine corresponded to the dose recommended by the company instructions. Referring to the tannin contents of each sample, the actual additions were given as Table 1.
3) and the dosage of these tannins (0.6 g L−1) applied to red wine corresponded to the dose recommended by the company instructions. Referring to the tannin contents of each sample, the actual additions were given as Table 1.
| Samples | Addition of TAN HT (g L−1) | Addition of TAN CT (g L−1) |
|---|---|---|
| a HT, hydrolysable tannin; CT, condensed tannin. | ||
| X-Control | 0 | 0 |
| X-MIX1 | 0.27 | 0.30 |
| X-MIX2 | 0.41 | 0.15 |
| X-MIX3 | 0.14 | 0.45 |
| X-TAN HT | 0.55 | 0 |
| X-TAN CT | 0 | 0.60 |
2.4. Determination of phenolic compounds after tannin addition
15 phenolic compounds were selected as index component. Castalin, gallic acid, vescalagin, castalagin, proanthocyanidin B1, (+)-catechin, vanillic acid, caffeic acid, proanthocyanidin B2, (−)-epicatechin, proanthocyanidin B2-3′-O-G, C1, malvidin-3-O-glucoside, polydatin and ellagic acid were prepared in methanol at the concentrations of 32.64, 65.00, 24.12, 35.28, 200.00, 66.25, 20.00, 16.32, 200.00, 51.50, 27.65, 16.00, 195.00, 8.80 and 43.75 mg L−1, respectively. Wine samples were filtered through a 0.22 μm filter membrane prior to HPLC-DAD analysis. The HPLC-DAD apparatus used in this work was a Waters e2695 system, equipped with an autosample system, and a photodiode array detector. The detect wavelength were performed at 280, 320 and 525 nm. The column was an Innoval C18 (250 × 4.6 mm, 5 μm). The column temperature was 30 °C. The flow rate was fixed at 0.7 mL min−1. Two elution solvents, A (water/formic acid; 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v) and B (acetonitrile/water/formic acid; 58
2, v/v) and B (acetonitrile/water/formic acid; 58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 42
42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v/v), were used with the following elution program: 0 min, 2% B; 15 min, 5% B; 20 min, 8% B; 40 min 10% B; 75 min 15% B; 80 min, 20% B; 85 min, 30% B; 87 min, 31% B; 87.1–100 min 100% B.
2, v/v/v), were used with the following elution program: 0 min, 2% B; 15 min, 5% B; 20 min, 8% B; 40 min 10% B; 75 min 15% B; 80 min, 20% B; 85 min, 30% B; 87 min, 31% B; 87.1–100 min 100% B.
2.5. Chromatic characteristics and sensory evaluation of wine with tannin addition
Chromatic characteristics of the red wines with tannin addition were determined according to the CIELab universal color appreciation system, using a portable colorimeter. The results were expressed by the cylindrical coordinates L* (psychometric lightness), c* (psychometric chroma), and h* (hue angle) values and the axes of a three-dimensional color space a*(measure of redness) and b* (measure of yellowness). ΔE*(color difference) was used for a comprehensive measure of color and could be calculated as ΔE = [(ΔL)2 + (Δa)2 + (Δb)2]/2.Sensory evaluation of the red wines with different tannin addition was implemented by 15 professionals who had acquired WSET (Wine & Spirit Education Trust) Level 3 certification. Sample wines were prepared in duplicate and were completed blind tasting in two rounds within 30 minutes. Wine evaluation chart (Fig. 1) of the American Wine Association (AWS) was used as a guideline for the tasters, which was scored from five factors including wine appearance, aroma/bouquet, taste/texture, aftertaste and overall impression.24 The final results were given based on the average scores.
2.6. Antioxidant activities of wine with tannin addition
The antioxidant activities of wine with tannin addition were analyzed using DPPH and ABTS assays. They were conducted by measuring free-radical scavenging capacities and the specific implementations referred to our previous work.25 The antioxidant activity of each sample was expressed as the clearance rate.2.7. Statistical analysis
All experimental results were performed in triplicate and results are expressed as mean ± standard deviation (SD). Statistical analysis was performed using SPSS (Version 22.0, Chicago, IL, USA) and carried out by one-way ANOVA.3. Results and discussion
3.1. Total polyphenols, flavonoids, anthocyanins and proanthocyanidins in wines
The contents of phenolic compounds in wine vary greatly with grape varieties, season, climate, maturity and aging.26 In this study, young Merlot wines were selected as sample wines for tannin addition due to their varietal characteristic brought about lower tannin contents. Their total polyphenols, flavonoids, anthocyanins and proanthocyanidins were assayed before adding tannins. The results were presented in Table 2 and it indicated that the wines exerted low contents of polyphenols and were suitable as the experiment wines for tannin addition.| Samples | TP (mg L−1) | TFO (mg L−1) | TA (mg L−1) | TAC (mg L−1) |
|---|---|---|---|---|
| a TP, total polyphenols; TFO, total flavonoids; TA, total proanthocyanidins; TAC, total anthocyanins. | ||||
| RWA | 1281 | 2416 | 1567 | 81.54 |
| RWB | 1196 | 2498 | 1736 | 72.73 |
3.2. Determination of phenolic compounds in wines after tannin addition
The contents of 15 phenolic compounds in wine before and after tannin addition were showed in Table 3. Tannins added not only had effect on phenolic composition but also contents. Except for increasing in exogenous components, the contents of gallic acid, (+)-catechin, (−)-epicatechin, procyanidin B1, and B2-3′-O-G obviously increased with the addition of any tested tannin. The contents of vanillic acid, caffeic acid, procyanidin B2 decreased with the addition of tannin when hydrolysable tannins accounted for a large proportion. After tannin addition, the content of malvidin-3-O-glucoside slightly reduced probably due to their reaction trend with proanthocyanidins and formed polymeric pigments.25 Tannin addition affected the varieties and contents of phenolic compounds in wine, that might be further influence the wine color, mouthfeel and even quality.14,15 Tannins added in different proportions exerted different effects on phenolic contents in wine.| Compound | Castalin | Gallic acid | Vescalagin | Castalagin | B1 | Catechin | Vanillic acid | Caffeic acid | B2 | Epicatechin | B2-3′-O-G | C1 | Mv-3-O-glu | Polydatin | Ellagic acid | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a B1, procyanidin B1; B2, procyanidin B2, B2-3′-O-G, procyanidin B2-3′-O-G; C1, procyanidin C1; Mv-3-glu, malvidin-3-O-glucoside. Statistical analysis was conducted on RWA and RWB respectively. Different letters in a column indicated significant differences at P < 0.05; statistically, a, b, c, d, e, and f followed the values indicating significant differences among these values. | ||||||||||||||||
| RWA (mg L−1) | Control | — | 40.19 ± 0.15f | — | — | 63.99 ± 0.04f | 23.00 ± 0.03f | 7.17 ± 0.05c | 11.28 ± 0.01c | 55.57 ± 0.14d | 13.57 ± 0.02f | 5.49 ± 0.04f | — | −50.03 ± 0.04a | 2.90 ± 0.06c | 7.59 ± 0.03de |
| MIX1 | 7.78 ± 0.11c | 47.53 ± 0.01c | 10.56 ± 0.08c | 20.53 ± 0.15c | 70.98 ± 0.12c | 37.41 ± 0.21c | 6.96 ± 0.04d | 11.20 ± 0.02d | 59.79 ± 0.21c | 27.06 ± 0.12c | 18.98 ± 0.06a | 5.56 ± 0.08c | 48.70 ± 0.05d | 3.07 ± 0.11c | 7.93 ± 0.11c | |
| MIX2 | 10.74 ± 0.07b | 50.18 ± 0.13b | 15.65 ± 0.12b | 25.65 ± 0.01b | 67.44 ± 0.07d | 29.90 ± 0.05d | 5.69 ± 0.01f | 10.99 ± 0.01e | 48.06 ± 0.14e | 20.06 ± 0.08d | 11.98 ± 0.04c | 6.39 ± 0.11a | 47.92 ± 0.01f | 2.89 ± 0.03c | 8.01 ± 0.03b | |
| MIX3 | 4.18 ± 0.04d | 44.84 ± 0.05d | 8.47 ± 0.02d | 7.68 ± 0.01d | 75.28 ± 0.05b | 50.47 ± 0.14b | 7.38 ± 0.03b | 11.87 ± 0.03b | 72.03 ± 0.31b | 37.16 ± 0.07b | 8.77 ± 0.03d | 4.73 ± 0.05d | 49.52 ± 0.02b | 3.25 ± 0.01b | 8.38 ± 0.02a | |
| TAN CT | — | 43.99 ± 0.07e | — | — | 79.45 ± 0.05a | 63.97 ± 0.35a | 7.44 ± 0.04a | 12.75 ± 0.03a | 86.3 ± 0.44a | 48.97 ± 0.12a | 12.75 ± 0.11b | 6.074 ± 0.05b | 48.41 ± 0.02e | 3.34 ± 0.02a | 7.55 ± 0.08e | |
| TAN HT | 13.79 ± 0.14a | 57.68 ± 0.22a | 23.01 ± 0.08a | 33.75 ± 0.07a | 64.97 ± 0.05e | 23.80 ± 0.09e | 6.84 ± 0.04e | 10.79 ± 0.04f | 43.18 ± 0.09f | 13.72 ± 0.02e | 5.64 ± 0.06e | — | 49.38 ± 0.09c | 2.86 ± 0.05c | 7.60 ± 0.06d | |
| RWB (mg L−1) | Control | — | 37.39 ± 0.15f | — | — | 37.44 ± 0.02f | 19.24 ± 0.04f | 6.28 ± 0.01c | 12.85 ± 0.03c | 32.67 ± 0.09d | 8.53 ± 0.09f | — | — | 40.95 ± 0.18a | 3.13 ± 0.03d | 7.15 ± 0.04c |
| MIX1 | 8.17 ± 0.06c | 44.35 ± 0.01c | 11.80 ± 0.03c | 20.62 ± 0.18c | 53.37 ± 0.18b | 34.35 ± 0.08c | 5.92 ± 0.02d | 12.03 ± 0.11e | 43.33 ± 0.33a | 22.56 ± 0.07b | 5.86 ± 0.11e | 3.91 ± 0.01d | 40.46 ± 0.11c | 3.78 ± 0.11c | 6.69 ± 0.08d | |
| MIX2 | 11.39 ± 0.03b | 50.77 ± 0.11b | 15.09 ± 0.21b | 25.99 ± 0.23b | 43.28 ± 0.08d | 30.06 ± 0.14d | 5.05 ± 0.03f | 12.25 ± 0.01d | 36.35 ± 0.25b | 17.91 ± 0.06c | 8.16 ± 0.01d | 5.67 ± 0.07b | 40.69 ± 0.04b | 4.81 ± 0.06b | 6.79 ± 0.17d | |
| MIX3 | 6.29 ± 0.11d | 42.54 ± 0.09e | 6.36 ± 0.01d | 7.98 ± 0.01d | 46.70 ± 0.08c | 53.08 ± 0.21b | 6.85 ± 0.04a | 15.38 ± 0.06a | 34.00 ± 0.11c | 13.65 ± 0.11d | 9.23 ± 0.04b | 4.94 ± 0.05c | 41.10 ± 0.11a | 3.75 ± 0.08c | 7.77 ± 0.07a | |
| TAN CT | — | 43.81 ± 0.01d | — | — | 55.99 ± 0.33a | 60.59 ± 0.45a | 6.35 ± 0.05b | 14.31 ± 0.01b | 34.00 ± 0.04c | 38.30 ± 0.05a | 10.87 ± 0.06a | 6.44 ± 0.11a | 40.69 ± 0.09b | 4.81 ± 0.02b | 6.46 ± 0.03e | |
| TAN HT | 13.26 ± 0.01a | 54.66 ± 0.09a | 23.95 ± 0.11a | 29.08 ± 0.13a | 40.95 ± 0.04e | 20.72 ± 0.21e | 5.49 ± 0.03e | 11.66 ± 0.02f | 27.77 ± 0.17e | 10.67 ± 0.07e | 8.26 ± 0.01c | — | 40.72 ± 0.03b | 4.91 ± 0.01a | 7.20 ± 0.01b | |
3.3. Effect of tannin addition on the chromatic characteristics
Based on CIElab color system, the corresponding coordinate point of any color could be found in the space. The L* axis represented wine lightness, and the descriptive terms assigned might include the words “light”, “dark”, etc.27 The L* scale ranged from 0 to 100, when L* = 0, meant black, while L* = 100, meant white. The a* and b* coordinates may be conceptually related to an opponent color theory, which was based on the proposition that the retina of the eye contains opponent color channels that distinguish colors according to their red-versus-green and yellow-versus-blue attributes.28 The higher the value of a* is, the more it tends to red, and the higher the value of b* is, the more it tends to yellow. The parameter c* represented psychometric chroma. The higher the value is, the higher the saturation will be. The value of hue angle (h*) ranged from 0° to 360°, which red wine generally between 0° and 90°, and the lower value of h* lead to purple or ruby red, while higher value lead to brick red or reddish brown.Tables 4 and 5 presented the effect of tannin addition on chromatic characteristics of red wines. From Table 4 it can be seen clearly that there was no significant difference between hydrolysable and condensed tannins added individually into RWA on color parameters. However, compared with control, after tannin addition, hydrolysable or condensed tannin had significant effect on lightness (L*) and color difference (ΔE*). Tannin mixture 2 and 3 showed significant difference and increasing on parameters a* and h* compared to control, which meant the redness shifted up and tended to be brick red or reddish brown. Hydrolysable tannin adding led to the decrease of value b*, which brought to the reduce of yellowness of wine. As to the value of parameter c*, there was a significant difference between tannin mixture 3 and others, so that RWA with the addition of tannin mixture 3 presented a stronger color saturation.
| Addition | L* | a* | b* | c* | h* | ΔE* |
|---|---|---|---|---|---|---|
| a Different letters in a column indicated significant differences at P < 0.05; statistically, a, b, and c followed the values indicating significant differences among these values. | ||||||
| Control | 19.10 ± 0.09b | 5.29 ± 0.43c | 5.41 ± 0.12ab | 7.58 ± 0.38bc | 57.52 ± 0.25b | 8.27 ± 0.12a |
| MIX1 | 19.11 ± 0.11b | 5.20 ± 0.10c | 5.46 ± 0.04ab | 7.54 ± 0.06bc | 57.42 ± 0.08b | 8.24 ± 0.12a |
| MIX2 | 19.07 ± 0.10b | 6.11 ± 1.16ab | 5.50 ± 0.10ab | 8.26 ± 0.93ab | 58.00 ± 0.72a | 8.57 ± 0.33a |
| MIX3 | 19.19 ± 0.03b | 6.59 ± 0.42a | 5.69 ± 0.02a | 8.72 ± 0.32a | 58.24 ± 0.27a | 8.56 ± 0.12a |
| TAN HT | 20.18 ± 0.41a | 4.77 ± 0.56c | 5.02 ± 0.21c | 6.92 ± 0.32c | 57.36 ± 0.08ab | 7.14 ± 0.37b |
| TAN CT | 20.17 ± 1.23a | 5.55 ± 0.87bc | 5.23 ± 0.51bc | 7.64 ± 0.92bc | 57.81 ± 0.18ab | 7.29 ± 1.16b |
| Addition | L* | a* | b* | c* | h* | ΔE* |
|---|---|---|---|---|---|---|
| a Different letters in a column indicated significant differences at P < 0.05; statistically, a, b, c, and d followed the values indicating significant differences among these values. | ||||||
| Control | 18.07 ± 0.03b | 4.89 ± 0.77a | 5.23 ± 0.18a | 7.13 ± 0.55a | 57.37 ± 0.56a | 9.24 ± 0.12a |
| MIX1 | 18.26 ± 0.20b | 3.62 ± 0.28bc | 5.16 ± 0.05a | 6.31 ± 0.17bc | 56.47 ± 0.15c | 8.93 ± 0.20ab |
| MIX2 | 18.08 ± 0.10b | 3.79 ± 0.08bc | 5.25 ± 0.03a | 6.48 ± 0.06bc | 56.47 ± 0.06c | 9.11 ± 0.10a |
| MIX3 | 17.82 ± 0.20b | 4.26 ± 0.40ab | 4.26 ± 0.09a | 6.65 ± 0.19ab | 56.93 ± 0.38b | 9.40 ± 0.23a |
| TAN HT | 19.41 ± 1.13a | 3.86 ± 0.25bc | 4.44 ± 0.47b | 5.38 ± 0.52d | 56.33 ± 0.15c | 7.86 ± 1.12c |
| TAN CT | 19.01 ± 1.23a | 3.41 ± 0.87c | 4.75 ± 0.51b | 5.86 ± 0.92cd | 56.68 ± 0.18bc | 8.27 ± 1.16bc |
As in the case of RWA, it can be seen from Table 5, that there was no significant difference between hydrolysable and condensed tannins added individually into RWB on color parameters. However, compared to control, after tannin addition, hydrolysable and condensed tannins both showed significant difference on each color parameter, so that the addition of hydrolysable or condensed tannin had significant effect on the color of wine. Although hue angle decreased after tannin mixture 1, 2 and 3 addition, there was no significant effect on lightness (L*), yellowness (b*), and color difference (ΔE*). Only tannin mixture 1 and 2 had certain effect on redness (a*) and chroma (c*).
After tannin added, correlation analysis between color attributes and phenolic compound contents in red wine was listed in Table 6. There was significant positive correlation between L* and procyanidin B1, malvidin-3-O-glucoside, respectively. Color parameter a* and c* was significantly positive correlated with procyanidin B1, B2, malvidin-3-O-glucoside, and ellagic acid, respectively, and significantly negative correlated with polydatin. Color parameter b* was significantly positive correlated with procyanidin B2, malvidin-3-O-glucoside and ellagic acid, respectively, and significantly negative correlated with polydatin. Color parameter h* was significantly positive correlated with procyanidin B1, B2, vanillic acid, malvidin-3-O-glucoside, and ellagic acid, respectively, and significantly negative correlated with polydatin. There was no significant correlation between color difference (ΔE*) and the contents of the 15 determined phenolic compounds. The results showed that procyanidin B1, B2, malvidin-3-O-glucoside, polydatin and ellagic acid were the main substances which impacted the color of wine. Procyanidins and anthocyanin exerted effect on the wine color could be related to the widely reported facts that flavanol–anthocyanin combination could effectively improve wine color stability.25,29 Except for procyanidins, phenolic acids and ellagic tannins were also involved in the copigmentation of red wine,30 that might be the reason why the components of hydrolysable tannin had impacts on the color parameter of wine. Polydatin belongs to stilbenes and is the precursor of resveratrol.31 Although, there are rare studies about the effect of polydatin on wine color, stilbenes and resveratrol were reported being related to wine color.32,33 A significant negative correlation between the resveratrol content and b* value was found by Zou et al.,32 and the result was similar to that between polydatin and b* in this study. According to our knowledge, it is the first time that a correlation was studied between polydatin and wine color parameter. The specific effect remains to be further studied. In sum, tannin addition had effect on wine color, and their influence was the result of the interaction of various compounds. However, no certain variation tendency was observed in this study.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | L* | a* | b* | c* | h* | ΔE* | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a **Correlation is signicant at the 0.01 level; *correlation is significant at the 0.05 level. 1 = castalin; 2 = gallic acid; 3 = vescalagin; 4 = castalagin; 5 = procyanidin B1; 6 = catechin; 7 = vanillic acid; 8 = caffeic acid; 9 = procyanidin B2; 10 = epicatechin; 11 = procyanidin dimers B2-3′-O-G; 12 = procyanidin C1; 13 = malvidin-3-O-glucoside; 14 = polydatin; 15 = ellagic acid. | |||||||||||||||||||||
| 1 | 1 | ||||||||||||||||||||
| 2 | 0.884** | 1 | |||||||||||||||||||
| 3 | 0.976** | 0.919** | 1 | ||||||||||||||||||
| 4 | 0.983** | 0.896** | 0.967** | 1 | |||||||||||||||||
| 5 | −0.199 | 0.041 | −0.162 | −0.133 | 1 | ||||||||||||||||
| 6 | −0.457 | −0.287 | −0.488 | −0.501 | 0.455 | 1 | |||||||||||||||
| 7 | −0.551 | −0.372 | −0.501 | −0.548 | 0.678* | 0.463 | 1 | ||||||||||||||
| 8 | −0.436 | −0.494 | −0.513 | −0.549 | −0.359 | 0.614* | 0.076 | 1 | |||||||||||||
| 9 | −0.364 | −0.171 | −0.328 | −0.314 | 0.894** | 0.471 | 0.698* | −0.266 | 1 | ||||||||||||
| 10 | −0.435 | −0.161 | −0.406 | −0.395 | 0.700* | 0.842** | 0.448 | 0.16 | 0.727** | 1 | |||||||||||
| 11 | 0.113 | 0.229 | 0.058 | 0.133 | 0.57 | 0.499 | 0.184 | −0.051 | 0.43 | 0.55 | 1 | ||||||||||
| 12 | −0.098 | −0.081 | −0.212 | −0.107 | 0.383 | 0.734** | −0.017 | 0.331 | 0.33 | 0.678* | 0.695* | 1 | |||||||||
| 13 | −0.074 | 0.128 | −0.020 | −0.012 | 0.861** | 0.032 | 0.663* | −0.590* | 0.752** | 0.29 | 0.328 | −0.021 | 1 | ||||||||
| 14 | 0.177 | 0.197 | 0.174 | 0.120 | −0.567 | 0.154 | −0.607* | 0.42 | −0.517 | 0.027 | 0.015 | 0.174 | −0.762** | 1 | |||||||
| 15 | 0.073 | 0.066 | 0.091 | 0.037 | 0.596* | 0.051 | 0.557 | −0.314 | 0.551 | 0.075 | 0.322 | 0.043 | −0.095 | −0.686* | 1 | ||||||
| L* | 0.076 | 0.472 | 0.212 | 0.147 | 0.671* | 0.12 | 0.427 | −0.493 | 0.543 | 0.422 | 0.304 | −0.122 | 0.676* | −0.273 | 0.306 | 1 | |||||
| a* | −0.184 | −0.112 | −0.137 | −0.165 | 0.705* | 0.063 | 0.55 | −0.419 | 0.717** | 0.266 | 0.197 | 0.083 | 0.819** | −0.760** | 0.876** | 0.381 | 1 | ||||
| b* | −0.181 | −0.146 | −0.165 | −0.067 | 0.585* | −0.12 | 0.22 | −0.627* | 0.619* | 0.295 | 0.112 | 0.157 | 0.631* | −0.574 | 0.381 | 0.187 | 0.687* | 1 | |||
| c* | −0.25 | −0.23 | −0.251 | −0.22 | 0.715** | 0.127 | 0.543 | −0.352 | 0.729** | 0.307 | 0.197 | 0.223 | 0.785** | −0.789** | 0.799** | 0.219 | 0.953** | 0.786** | 1 | ||
| h* | −0.316 | −0.194 | −0.274 | −0.286 | 0.751** | 0.178 | 0.644* | −0.311 | 0.730** | 0.354 | 0.182 | 0.131 | 0.826** | −0.803** | 0.808** | 0.411 | 0.969** | 0.669* | 0.951** | 1 | |
| ΔE* | −0.119 | −0.514 | −0.246 | −0.192 | −0.553 | −0.08 | −0.360 | 0.446 | −0.429 | −0.351 | −0.269 | 0.187 | −0.560 | 0.169 | −0.149 | −0.975** | −0.190 | −0.050 | −0.031 | −0.228 | 1 |
3.4. Effect of tannin addition on the sensory qualities of wine
The effect of the addition of tannins on the red wine sensory qualities were illustrated in Table 7. As to RWA, after addition of hydrolysable/condensed/mixture 1/mixture 2 tannins, the total scores of wine sensory evaluation were higher than control which meant a total sensory quality promotion. In contrast, the wine added tannin mixture 3 got a lower sensory evaluation score than control due to the worse wine taste and structure as well as insufficient aftertaste. As regards RWB, the sensory qualities of all the red wines were improved after tannin addition. The wines with mixture tannin addition made better performance on total score than others. The wine added tannin mixture 1 obtained the highest score with a clear shiny body, typical varietal aromas and fresh fruity flavors, balanced and well aftertaste.| Sensory evaluation (score) | Total scores | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Appearance, 3 Max | Aroma and bouquet, 6 Max | Taste and texture, 6 Max | After taste, 3 Max | Overall impression, 2 Max | ||||||||
| RWA | RWB | RWA | RWB | RWA | RWB | RWA | RWB | RWA | RWB | RWA | RWB | |
| Control | 2.0 | 2.2 | 4.9 | 4.9 | 4.3 | 4.1 | 2.2 | 2.3 | 1.4 | 1.1 | 14.8 | 14.6 |
| MIX1 | 2.3 | 2.4 | 5.2 | 5.2 | 4.4 | 5.2 | 2.3 | 2.4 | 1.5 | 1.8 | 15.7 | 17.0 |
| MIX2 | 2.2 | 2.2 | 5.2 | 5.2 | 3.9 | 4.8 | 2.3 | 2.2 | 1.6 | 1.2 | 15.2 | 15.6 |
| MIX3 | 2.2 | 2.3 | 5.0 | 5.2 | 3.7 | 4.6 | 1.6 | 2.1 | 1.4 | 1.4 | 13.9 | 15.6 |
| TAN HT | 2.3 | 2.1 | 5.0 | 5.0 | 4.5 | 4.7 | 1.9 | 2.0 | 1.6 | 1.1 | 15.4 | 14.9 |
| TAN CT | 2.3 | 2.2 | 4.8 | 5.1 | 4.4 | 4.6 | 2.6 | 2.1 | 1.4 | 1.0 | 15.5 | 15.0 |
Overall, the wine added hydrolysable tannin tasted astringent and bitterness which consistent with the sensory properties of ellagitannins.34,35 The wine added condensed tannin exhibited more astringent, which might be due to the high content of proanthocyanidins in the condensed tannins. Cheynier et al.36 reported that proanthocyanidins with 4–6 linkages bound more readily to proteins and possibly are more astringent than the smaller molecular weight flavanols. The tannin added might cause a reaction with polyphenolic compounds in wine. One involves acetaldehyde-mediated condensation between flavanols leading to molecules referred to as ethyl-bridged flavanols.37 Vidal et al.38 verified that ethyl-bridged flavanols, provided they were present in sufficient quantity in wine, could contribute astringency to wine through their flavanic composition as do the proanthocyanidins and additionally could increase bitterness. The wines with tannin mixture 1 adding combined the characteristics of both hydrolysable and condensed tannins, with rich flavor, balanced body, longer finish and well qualities. In summary, tannin added could improve the sensory qualities of wines especially the mixture tannins. Some studies reported the similar results. Parker et al.12 found a slight increase in astringency by addition of grape seed extract and Kovac et al.39 found an increased taster preference for wines with added seeds.
3.5. Effect of tannin addition on the antioxidant activities of wine
For determination of antioxidant activities, DPPH and ABTS assays were adopted and the influence of the addition of tannins on antioxidant activities of the wines was shown in Table 8. The results indicated that tannin added could improve the wine antioxidant activities obviously. RWA with tannin mixture 1 adding exhibited the most powerful DPPH˙ scavenging activity followed by the wine with mixture 3. The DPPH˙ scavenging activities of the wines added other tannins showed no significant difference, but higher than the control. RWBs added tannin mixture 1 and 2 showed the highest DPPH clearance rate followed by the wine with condensed tannin added. RWA added tannin mixture 3 showed the strongest ABTS˙ scavenging activity. The RWAs with other tannin samples added exhibited similar ABTS˙ scavenging activities with no significant difference. RWBs added condensed tannin indicated the most powerful ABTS˙ scavenging capacity followed by the wines added three mixture tannin with no significant difference. RWB with hydrolysable tannin addition showed the least ABTS˙ scavenging activity among tannin added wines. In general, the wines with tannin mixture 1 and 3 added showed the strongest antioxidant activities.| Addition | DPPH clearance rate | ABTS clearance rate | ||
|---|---|---|---|---|
| RWA (%) | RWB (%) | RWA (%) | RWB (%) | |
| a Different letters in a column indicated significant differences at P < 0.05; statistically, a, b, c, and d followed the values indicating significant differences among these values. | ||||
| Control | 37.47 ± 2.85d | 38.68 ± 1.90c | 34.74 ± 1.50c | 33.18 ± 1.69d |
| MIX1 | 46.59 ± 1.67a | 44.63 ± 1.91ab | 38.41 ± 1.47b | 44.38 ± 2.52b |
| MIX2 | 42.16 ± 1.44bc | 46.51 ± 0.74a | 38.38 ± 1.20b | 42.39 ± 2.57b |
| MIX3 | 43.28 ± 1.43b | 40.38 ± 1.40c | 43.94 ± 2.02a | 42.37 ± 1.35b |
| TAN HT | 40.72 ± 1.74c | 39.98 ± 0.91c | 36.70 ± 1.73b | 37.55 ± 1.08c |
| TAN CT | 42.16 ± 0.73bc | 43.30 ± 2.32b | 37.21 ± 2.27b | 49.61 ± 1.45a |
Stepwise multiple regression model was used and the β coefficient of each constituent was analyzed to evaluate the contribution of single phenolic compound to antioxidant capacity. For DPPH and ABTS radical scavenging capacity values, vescalagin, castalagin, procyanidin C1 and polydatin showed positive contributions with the regression β coefficient of 0.514, 0.739, 0.452 and 0.500, respectively. There was no significant correlation between other compounds and the antioxidant activites. Tannins are complex compounds and their antioxidant capacities mainly originate by large amount of phenolic hydroxyl groups and electron-donating groups at benzene ring. Researchers have confirmed that both condensed and hydrolysable tannins possessed strong antioxidant activities and their structure–activity relationship was elucidated.40–43 Structure–activity studies for monomeric and polymeric phenolic compounds showed that 4 moles of radical were scavenged per-substituted diphenol group.44 Zhang and Hou45 inferred that condensed tannin with ECG structure exhibited stronger antioxidative activity than ellagic tannin based on the approach of computational chemistry. Zalacain et al.46 found that the antioxidant activity of ellagic tannin was higher than condensed tannin with (−)-epicatechin structure. Therefore, based on our results, the wine added mixture of both condensed and hydrolysable tannins showed a better antioxidant activity. Furthermore, the chemical synergy of the action of multiple compounds of tannin and phenolic compound might also affect the antioxidant activity of wine with tannin addition.47 Although, it was concluded that tannin addition had obvious effect of increasing antioxidant activity in this study, Neves et al.4 reported that the addition of grape seed tannins had a significant effect on the antioxidant activity only in the wines poor in polyphenols. More different types of wine and tannin should be considerable and applied in further study.
4. Conclusion
The effect of the addition of tannin (hydrolyzable, condensed and mixture tannins) on the wine overall quality including phenolic composition, color, sensory characteristics, and antioxidant activities of red wine was assessed. Tannin addition affected the phenolic composition and contents which might further promote the color of wines, and the influences varied by different tannins. The addition of tannin played a positive role of improving the sensory qualities and increasing antioxidant activities, and the mixture tannins exhibited the obvious effect. After tannin addition, the mixture tannin with a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 between hydrolyzable and condensed tannins obtained the best results on both sensory quality and antioxidant activity, could be recommended as an ideal tannin addition in our study. Furthermore, the type and ratio of tannins chosen to be added should be based on the characteristics of the wine.
1 between hydrolyzable and condensed tannins obtained the best results on both sensory quality and antioxidant activity, could be recommended as an ideal tannin addition in our study. Furthermore, the type and ratio of tannins chosen to be added should be based on the characteristics of the wine.
Conflicts of interest
The authors have declared no conflict of interest.Acknowledgements
This work was supported by Guidance Plan of Natural Science Foundation of Liaoning in China (2019-ZD-0463) and Program for Liaoning Excellent Talents in University (LJQ2015106).References
- OIV, International code of oenological practices, part II, oenological practices and treatments, 3.2 clarification of wine, Paris, France, 2012 Search PubMed.
- I. Garcia-Estevez, M. T. Escribano-Bailon, J. C. Rivas-Gonzalo and C. Alcalde-Eon, J. Agric. Food Chem., 2012, 60, 1373–1379 CrossRef CAS PubMed.
- M. J. Herderich and P. A. Smith, Aust. J. Grape Wine Res., 2008, 11, 205–214 CrossRef.
- A. C. Neves, M. I. Spranger, Y. Q. Zhao, M. C. Leandro and B. S. Sun, J. Agric. Food Chem., 2010, 58, 11775–11782 CrossRef CAS PubMed.
- A. B. Bautista-Ortín, A. Martínez-Cutillas, J. M. Ros-García, J. M. López-Roca and E. Gómez-Plaza, Int. J. Food Sci. Technol., 2005, 40, 867–875 CrossRef.
- E. Obreque-Slíer, A. Peña-Neira, R. López-Solís, C. Ramírez-Escudero and F. Zamora-Marín, Eur. Food Res. Technol., 2009, 229, 859–866 CrossRef.
- K. Chen, C. Escott, I. Loira, J. M. Del Fresno, A. Morata, W. Tesfaye, F. Calderon, S. Benito and J. A. Suarez-Lepe, Molecules, 2016, 21, 1445 CrossRef PubMed.
- M. J. Carvalho, V. Pereira, A. C. Pereira, J. L. Pinto and J. C. Marques, Food Bioprocess Technol., 2015, 8, 2309–2318 CrossRef CAS.
- F. Sonni, M. Cejudo Bastante, F. Chinnici, N. Natali and C. Riponi, J. Sci. Food Agric., 2009, 89, 688–696 CrossRef CAS.
- T. C. Somers, Phytochemistry, 1971, 10, 2175–2186 CrossRef CAS.
- I. Álvarez, J. L. Aleixandre, M. J. García, V. Lizama and J. L. Aleixandre-Tudó, Eur. Food Res. Technol., 2009, 228, 501–510 CrossRef.
- M. Parker, P. A. Smith, M. Birse, I. L. Francis, M. J. Kwiatkowski, K. A. Lattey, B. Liebich and M. J. Herderich, Aust. J. Grape Wine Res., 2007, 13, 30–37 CrossRef CAS.
- J. F. Harbertson, G. P. Parpinello, H. Heymann and M. O. Downey, Food Chem., 2012, 131, 999–1008 CrossRef CAS.
- I. García-Estévez, C. Alcalde-Eon, V. Puente and M. T. Escribano-Bailon, Molecules, 2017, 22, 2046 CrossRef PubMed.
- A. Rinaldi and L. Moio, J. Sens. Stud., 2017, 33, e12325 CrossRef.
- A. B. Bautista-Ortín, J. I. Fernández-Fernández, J. M. López-Roca and E. Gómez-Plaza, J. Food Compos. Anal., 2007, 20, 546–552 CrossRef.
- G. L. Main and J. R. Morris, Am. J. Enol. Vitic., 2007, 58, 365–372 CAS.
- L. X. Li, S. T. Zhang, Y. Cui, Y. Y. Li, L. X. Luo, P. Y. Zhou and B. S. Sun, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2016, 1036–1037, 10–19 CrossRef CAS PubMed.
- S. T. Zhang, Y. Cui, L. X. Li, Y. Y. Li, P. Y. Zhou, L. X. Luo and B. S. Sun, Food Chem., 2015, 188, 422–429 CrossRef CAS PubMed.
- Y. Y. Li, L. X. Li, Y. Cui, S. T. Zhang and B. S. Sun, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2017, 1054, 105–113 CrossRef CAS PubMed.
- V. I. Singleton and J. A. Rossi, Am. J. Enol. Vitic., 1964, 16, 144–158 Search PubMed.
- Z. Jia, M. C. Tang and J. M. Wu, Food Chem., 1999, 64, 555–559 CrossRef.
- M. M. Giusti and R. E. Wrolstad, Characterization and Measurement of Anthocyanins with UV-visible Spectroscopy, John Wiley and Sons, New York, 2001 Search PubMed.
- A. Hartung, J.–Am. Wine Soc., 1999, 31, 116–121 Search PubMed.
- L. X. Li, M. N. Zhang, S. T. Zhang, Y. Cui and B. S. Sun, Molecules, 2018, 23, 1066 CrossRef PubMed.
- L. X. Li and B. S. Sun, Crit. Rev. Food Sci. Nutr., 2017, 59, 563–579 CrossRef PubMed.
- I. L. Weatherall and B. D. Coombs, J. Invest. Dermatol., 1992, 99, 468–473 CrossRef CAS PubMed.
- M. Roderick, Color Physics for Industry, Society of Dyers and Colorists, Bradford, 1987 Search PubMed.
- Y. X. Liu, N. N. Liang, J. Wang, Q. H. Pan and C. Q. Duan, J. Food Sci., 2013, 78, C25–30 CrossRef CAS PubMed.
- J. Heras-Roger, O. Alonso-Alonso, A. Gallo-Montesdeoca, C. Diaz-Romero and J. Darias-Martin, J. Food Sci. Technol., 2016, 53, 2540–2547 CrossRef CAS PubMed.
- S. De Maria, I. Scognamiglio, A. Lombardi, N. Amodio, M. Caraglia, M. Carteni, G. Ravagnan and P. Stiuso, J. Transl. Med., 2013, 11, 264 CrossRef PubMed.
- Y. Zou, Y. Liu, M. Tian and W. Hu, North. Hortic., 2014, 38, 137–139 Search PubMed.
- M. I. Fernandez-Marin, B. Puertas, R. F. Guerrero, M. C. Garcia-Parrilla and E. Cantos-Villar, J. Food Sci., 2014, 79, C310–C317 CrossRef CAS PubMed.
- A. Glabasnia and T. Hofmann, J. Agric. Food Chem., 2006, 54, 3380–3390 CrossRef CAS PubMed.
- J. Robichaud and A. Noble, J. Sci. Food Agric., 1990, 53, 343–353 CrossRef CAS.
- V. Cheynier, C. Prieur, S. Guyot, J. Rigaud and M. Moutounet, in Wine: Nutritional and Therapeutic Benefits, American Chemical Society, 1997 Search PubMed.
- H. Fulcrand, T. Doco, N. E. Es-Safi, V. Cheynier and M. Moutounet, J. Chromatogr. A, 1996, 752, 85–91 CrossRef CAS.
- S. Vidal, I. Francis, A. Noble, M. Kwiatkowski, V. Cheynier and E. Waters, Anal. Chim. Acta, 2004, 513, 57–65 CrossRef CAS.
- V. Kovac, E. Alonso and E. Revilla, Am. J. Enol. Vitic., 1995, 46, 363–367 CAS.
- F. Tian, B. Li, B. Ji, G. Zhang and Y. Luo, LWT--Food Sci. Technol., 2009, 42, 1289–1295 CrossRef CAS.
- H. F. Gu, C. M. Li, Y. J. Xu, W. F. Hu, M. H. Chen and Q. H. Wan, Food Res. Int., 2008, 41, 208–217 CrossRef CAS.
- İ. Gülçin, Z. Huyut, M. Elmastaş and H. Y. Aboul-Enein, Arabian J. Chem., 2010, 3, 43–53 CrossRef.
- H. C. Zhou, Y. M. Lin, S. D. Wei and N. F. Tam, Food Chem., 2011, 129, 1710–1720 CrossRef CAS.
- K. Riedl, S. Carando, H. Alessio, M. McCarthy and A. Hagerman, in Free Radicals in Food, American Chemical Society, 2002 Search PubMed.
- W. H. Zhang and X. Hou, Leather Sci. Eng., 2009, 19, 9–13 CAS.
- A. Zalacain, M. Carmona, C. Lorenzo, I. Blázquez and G. L. Alonso, Antiradical efficiency of different vegetable tannin extracts, 2002 Search PubMed.
- N. P. Seeram, L. S. Adams, S. M. Henning, Y. Niu, Y. Zhang, M. G. Nair and D. Heber, J. Nutr. Biochem., 2005, 16, 360–367 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2020 |

