Open Access Article
Open Access ArticleUltrasonic-promoted enzymatic preparation, identification and multi-active studies of nature-identical phenolic acid glycerol derivatives†
Teng Sun‡
a,
Haiping Zhang‡a,
Zhe Donga,
Zengshe Liub and
Mingming Zheng *a
*a
aOil Crops Research Institute, Chinese Academy of Agricultural Sciences, Key Laboratory of Oilseeds Processing, Ministry of Agriculture, Oil Crops and Lipids Process Technology National & Local Joint Engineering Laboratory, Hubei Key Laboratory of Lipid Chemistry and Nutrition, Wuhan 430062, China. E-mail: zhengmingming@caas.cn
bBio-Oils Research Unit, United States Department of Agriculture, Agricultural Research Service, National Center for Agricultural Utilization Research, 1815 N. University St., Peoria, Illinois 61604, USA
First published on 16th March 2020
Abstract
Phenolic acid glycerols (PAGs) are a group of rare phytochemicals found from potato periderm, which show great potential in the food, cosmetic and pharmaceutical industries. In this study, seven PAGs were enzymatically synthesized via transesterification of ethyl phenates (EPs) with glycerol by ultrasonic promotion. The conversions of 88.1–98.5% could be obtained in 1–9 h. Compared with the conventional stirring methods, the catalytic efficiency was significantly increased 11.0–44.0 folds by ultrasound assistance. The lipid peroxidation inhibition activity increased 8.1-fold and 14.4-fold compared to the parent phenolic acids (PAs). Furthermore, caffeoyl glycerol and feruloyl glycerol exhibited excellent antimicrobial activity against Escherichia coli compared to the corresponding PAs with minimum inhibitory concentration (MIC) decreasing 4–16-fold. The PAGs can also absorb a much wider and higher amount of the harmful UV-B rays than the corresponding PAs. The present strategy for facile synthesis of multifunctional PAGs paves the way for the development and application of natural phytochemicals and novel ingredients.
Introduction
Phenolic acids (PAs), widely distributed in the plant kingdom such as in fruits, grains, vegetables, olive oil, coffee and herbs, exhibit broad biological and pharmacological activity including inflammatory, antioxidant, anti-carcinogenic and anti-neuroprotective effects. Due to their multiple benefits, PAs and their derivatives have attracted more attention in recent year.1–3 However, poor solubility of these compounds in both water and oil media limits their further application in the food, cosmetic and pharmaceutical industries.4 Therefore, the modification of these compounds is essential to enhance their usefulness and widen their application field. As an example, phenolic acid glycerols (PAGs) such as feruloyl glycerol and caffeoyl glycerol were prepared by phenolic acids/phenolic ethyl phenates with glycerol in transesterification.As early as 2000, several PAGs such as 1-mono feruloyl glycerol were found in potato periderm, which were all-natural phytochemicals with exceptional free radical scavengers and peroxyl lipid oxidation inhibitors.5,6 Moreover, the introduce of glycerol rather than longer alkyl chain alcohols would be more advantageous in the UV filter, which stems from the more hydrophilic character of glycerol to facilitate the penetration of the fractional formulation into the skin.4,7 However, the preparation of such natural phytochemicals on a large scale as well as their structural identification confronts difficulties related to the trace amount of PAGs in nature (<0.1%).4 Until now, only feruloyl glycerol, caffeate glycerol and 4-methoxy cinnamoyl glycerol have been synthesized in the enzymatic method but other kinds of PAGs with different PAs have rarely been reported.3,8,9 Among the reported references, the studies only focused on the synthesis technology of PAGs and much less attention on their structural identification and their functional properties.10–12 Apart from antioxidant and anti-UV, PAs have also been reported to have remarkable antibacterial effects against Gram-positive and Gram-negative bacteria.2,13,14 A recent study demonstrated that the PA-rich extracts from peanut meal exhibited a high antibacterial effect, even comparable to Ampicillin.2,15 However, the antimicrobial performance of the PAGs has not yet been studied.
Suffering from high enzyme loading but poor catalytic performance in the enzymatic synthesis of PAGs,16 a facile and high efficiency strategy to prepare PAGs is still desirable. Recently, an effective enzymatic synthesis strategy with ultrasound pretreatment was developed and has been applied in the preparation of phytosterol esters, flavonoid esters and feruloyl glycerol with superior performance.17,18 Known as an outstanding process pretreatment technique, ultrasound can significantly shorten reaction time and remarkably increase conversion.19–21 Thus, it would be wise to introduce ultrasound pretreatment in PAGs synthesis.
In the present study, seven PAGs were synthesized by enzymatic transesterification of glycerol with seven different ethyl PAs by ultrasound promotion. The structures of seven PAGs were identified precisely by HPLC-MS and nuclear magnetic resonance (NMR). The antioxidant capacity of these PAGs was evaluated by 2,2-diphenyl-1-picrylhydrazyl (DPPH), fluorescence recovery after photobleaching (FRAP) and β-carotene/linoleic acid methods. Furthermore, the antimicrobial and anti-UV-B activities were also evaluated for the first time, which opens new opportunities for developing PAGs as a group of promising multifunctional and nature-identical ingredients.
Experimental section
Chemicals and reagents
Ethyl ferulate, ethyl caffeate, ferulic acid, caffeic acid, cinnamic acid, p-hydroxycinnamic acid, m-hydroxycinnamic acid, p-methoxycinnamic acid and 2-methoxycinnamic acid (>99%) were purchased from Aladdin Reagent (Shanghai, China). Ethyl cinnamate, ethyl p-hydroxycinnamate, ethyl m-hydroxycinnamate, ethyl p-methoxycinnamate and ethyl 2-methoxycinnamate (>95%) were obtained from Shanghai Drum Hill Biotechnology (Shanghai, China). Glycerol (99%, dehydrated using an activated 3 Å molecular sieve before use) and ethyl acetate were purchased from Tianjin Guoyao Chemical (Tianjin, China). The catalyst is CALB immoPlus™, immobilized lipase from Candida Antarctica, obtained from Purolite (Wales, UK). The mobile phase of HPLC was made of glacial acetic acid and methanol (HPLC grade), purchased from Merck Chemical Technology (Shanghai, China). All other regents were analytical grade. The Inertsil ODS-SP column (5 μm, 250 mm × 4.6 mm) was purchased from GL Science Inc. (Tokyo, Japan). UV-vis spectrophotometer was DU800 (Beckman Coulter, Brea, CA).Enzymatic synthesis and purification of PAGs
The PAGs were synthesis by enzymatic alcoholysis of phenolic acid ethyl esters with glycerol in solvent free system (Fig. 1). Experiments were carried out in a 25 mL glass bottle with magnetic stirring (200 rpm) (IKA, Staufen, Germany) in the presence or absence of ultrasound assistance irradiated from the microtip probe (diameter of 3 mm) of an ultrasonic homogenizer (Scientz-IID, Zhejiang, China). The ultrasonic-promoted enzymatic reaction device was shown in Fig. S1.† The enzymatic reaction was conducted by combining 8 mmol of ethyl phenates (EPs) and 80 mmol glycerol and lipase (6% of the total weight of the substrates). The ultrasonic parameters were set as following: the ultrasonic parameters were set as following: ultrasonic output (28 W mL−1), ultrasonic intermittent ratio (3 s/9 s, working/waiting) and ultrasonic time (1–9 h). The composition of the crude products was monitored by HPLC (LC-M20A, SHIMADZU, Kyoto, Japan).Immobilized lipase was removed from the mixture after reaction by centrifuge (5000 rpm). The crude product was extracted by solvent mixture (water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 2
ethyl acetate = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) for three times, the excess EPs dissolved in ethyl acetate layer and the excess glycerol dissolved in water layer. Meanwhile, the white PAGs powder precipitated in water layer during each extraction. Then, the purified PAGs (white powder) could be obtain after centrifugal separation from the water layer and freeze-dried. The obtained PAGs were subject to ELSD-HPLC and analysed for purity.
1, v/v) for three times, the excess EPs dissolved in ethyl acetate layer and the excess glycerol dissolved in water layer. Meanwhile, the white PAGs powder precipitated in water layer during each extraction. Then, the purified PAGs (white powder) could be obtain after centrifugal separation from the water layer and freeze-dried. The obtained PAGs were subject to ELSD-HPLC and analysed for purity.
Analysis and structural identification of PAGs
The products were monitored by HPLC with the detector photodiode array (PDA) and evaporative light-scattering detector (ELSD). The detecting condition was set on as following: elution was composed of methanol (A) and 0.5% acetic acid (B) at a flow rate of 1 mL min−1 with 10 μL injection volume. The elution sequence was from 58% B (v/v) to 100% B (v/v) in 20 min consecutively in a linear gradient, followed by 100% B for 2 min at 40 °C. The elusion was analyzed at 280 nm and 325 nm. The ELSD conditions were: gain, 6; gas pressure, 60 psi; drift tube, 40 °C.The identification of PAGs was performed on a Shimadzu MS-8050 mass spectrometer (Tokyo, Japan), with an APCI interface in positive mode. The other MS parameters was selected as following: isospray voltage was set at 4 kV, DL temperature 250 °C, heat block temperature 400 °C, nebulizing gas 2 L min−1 and drying gas 10 L min−1, heating gas 10 L min−1, interface temperature 300 °C; collision energy was set at 35 V. The mass range was from 100 to 1000 m/z. The PAGs were further characterized by 1H NMR spectroscopy (Bruker Avance II HD, Faellanden, Switzerland) with D6-DMSO (0.01% TMS) and CDCl3 (0.01% TMS) as solvent at 400 MHz frequency.
Antioxidant evaluation
1,1-Diphenyl-2-picrylhydrazyl (DPPH) radical scavenging assay: 0.5 mL of PAGs sample (1.0 mM in methanol) was mixed with 2.5 mL of 0.1 mM DPPH in methanol. The reaction mixture was incubated in the dark at room temperature for 30 min. The mixture was put into the quartz cell for ultraviolet detection radical at 517 nm to calculate the scavenging activity.22β-Carotene/linoleic acid assay: β-carotene was dissolved in chloroform solution with the concentration 0.1 mg mL−1. 40 mg linoleic acid was added in 4 mL of above liquid, followed by 400 mg Tween 40, then chloroform was distilled off under reduced pressure. 100 mL distilled water was added to the above residue and dissolved as reaction solution. 0.2 mL PAGs sample (1.0 mM in methanol) was added into 4 mL reaction solution, then the mixture solution was measured at 450 nm against pure methanol (blank) using a UV-vis spectrophotometer in a 1 cm quartz cell for 0 min and 180 min. The antioxidant activity (AA) was calculated using the following equation:
| % AA = [1 − (Asample 0 − Asample 180)/(Acontrol 0 − Acontrol 180)] × 100. |
Ferric reducing antioxidant power (FRAP) assay: the FRAP solvent was comprised of 5 mL of a 10 mmol L−1 2,4,6-tris(2-pyridyl)-s-triazine (TPTZ) solution (156 mg TPTZ dissolve in 50 mL 40 mmol L−1 HCl), 5 mL of 20 mmol L−1 FeCl3·6H2O and 50 mL of 0.1 mol L−1 acetate buffer (pH 3.6). The FRAP solvent was incubated at 37 °C for 10 min. Then, 2 mL of the above liquid were taken out into a 10 mL colorimetric tube, followed by 0.5 mL of 1 mmol L−1 PAGs sample and distilled water to filled in the whole 10 mL. The solutions were kept at room temperature for 20 min and then were analysed on UV-vis at 593 nm.22 The result was shown by mmol TE/L using Trolox as the reference.
Activated bacteria and preparation
Escherichia coli DH5α, Bacillus subtilis 168, Staphylococcus aureus SI-27, and Saccharomyces cerevisiae were purchased from Wuhan University, China Center for Type Culture Collection (Wuhan, China). Bacteria: the bacteria required for the experiment on the LB agar medium is yeast extract 5 g, NaCl 5 g, peptone 10 g, distilled water volume to 1000 mL. Agar addition amount is 1.8%, incubated at 37 °C, in a constant temperature incubator for 24 h. Saccharomyces cerevisiae: the experiment required bacteria inoculated into YPD Agar medium (yeast extract 10 g, peptone 20 g, glucose 20 g, distilled water volume to 1000 mL). Agar addition amount is 1.8%, incubated at 28 °C for 48 h. All experiments were aseptic and stored at 4 °C for use. Bacteria was inoculated into Luria broth (LB) at 37 °C, 220 rpm for 12 h and made with sterile saline at a certain concentration (E. coli, Staphylococcus aureus 108 cfu mL−1, Bacillus subtilis 106 cfu mL−1). Saccharomyces cerevisiae was inoculated into YPD at 28 °C, 180 rpm for 12 h and made with sterile saline at 106 cfu mL−1.Determination of the minimum inhibitory concentration (MIC)
The 48-well plate was sterilized for use. Before use, the 48-well plate was irradiated with UV light for at least 20 min. To each well, 0.5 mL medium containing sample was added at a concentration of 10, 5, 2.5, 1.25, 0.63 and 0.32 mg mL−1. Then 10 μL Escherichia coli, Staphylococcus aureus, Bacillus subtilis and yeast were added to the 48-well plates. Bacteria was cultured at 37 °C for 24 h (yeast at 28 °C for 48 h), and the turbidity of the bacterial liquid was observed. The MIC of the control group (10% DMSO in distilled water) was determined by the degree of clarification.UV absorption test
PAs and PAGs of 1 mmol L−1 were dissolved in methanol, and the absorption spectrum in the range of 200–800 nm was measured against a blank (methanol).Result and discussion
Effect of ultrasound irradiation on the conversion of PAGs
Under the optimized conditions, a comparable study was performed on the transesterification of glycerol with different EPs under stirring with ultrasound assistance. The reaction time and conversion under ultrasonic pretreatment were 4.0–14.0 times shorter and 1.4–7.5 times higher than that of mechanical stirring, respectively (Table 1). In this study, the turnover number (TON) defined as the molar amount of converted substrate per gram per hour effective enzyme loading was applied to evaluate the catalytic efficiency. The TON of ultrasound assistance was 12.0–45.0 times higher than under mechanical stirring, which was the best performance in the enzymatic synthesis of PAGs. However, by extending the reaction time during stirring, the conversion of EPs decreased. This phenomenon may be ascribed to the excess ethanol accumulated during the reaction reverses the transesterification reaction. On the contrary, the transient high temperature generated by ultrasound could promote the volatilization of ethanol, which pushed the reaction forward.19 Benefiting from the cavitation effect of ultrasound, the contact probabilities of substrate and enzyme could be increased, thereby further accelerating the reaction.18,23| EPs | Ultrasound assistance | Mechanical stirring | TONu/TONs | ||||
|---|---|---|---|---|---|---|---|
| Time (h) | Conversion (%) | TONu (mmol g−1 h−1) | Time (h) | Conversion (%) | TONs (mmol g−1 h−1) | ||
a TON: turnover number was evaluated on the basis of the molar amount of converted substrate per gram per hour effective enzyme loading. Reaction condition: ethyl phenate/glycerol 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (mol mol−1), reaction temperature 65 °C, enzyme loading 6%, ultrasonic power 280 W, ultrasonic intermittent ratio: 3 s/9 s (working/waiting). 10 (mol mol−1), reaction temperature 65 °C, enzyme loading 6%, ultrasonic power 280 W, ultrasonic intermittent ratio: 3 s/9 s (working/waiting). |
|||||||
| Ethyl cinnamate | 1 | 93.9 | 14.3 | 24 | 61.3 | 0.4 | 35.8 |
| Ethyl p-hydroxycinna-mate | 3 | 89.6 | 4.5 | 24 | 17.5 | 0.1 | 45.0 |
| Ethyl m-hydroxycinna-mate | 6 | 89.4 | 2.1 | 24 | 17.3 | 0.1 | 21.0 |
| Ethyl p-methoxycinna-mate | 6 | 88.1 | 2.0 | 24 | 12.7 | 0.1 | 20.0 |
| Ethyl 2-methoxycinna-mate | 6 | 88.6 | 2.2 | 24 | 17.1 | 0.1 | 22.0 |
| Ethyl caffeate | 9 | 90.1 | 1.5 | 24 | 12.1 | 0.1 | 15.0 |
| Ethyl ferulate | 3 | 98.5 | 4.8 | 24 | 71.7 | 0.4 | 12.0 |
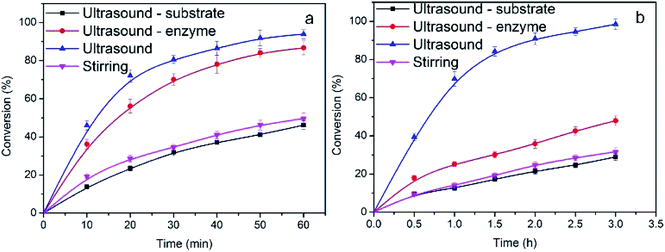
In order to evaluate the effect of ultrasound irradiation on enzyme activity and substrate solubility, ultrasound pretreatment was applied to the lipase and substrate individually. Two different ultrasound pretreatment patterns were designed: one was continuous ultrasound irradiation throughout the reaction and the other was only pretreatment at the beginning of reaction for a certain time.24,25 The controlled trial was performed by ultrasound pre-irradiation (1 h) of only lipase and in the absence of lipase after which the lipase was added in. As shown in Fig. 2, there was no significant difference in conversion among stirring and ultrasonic pretreatment of only the substrate (without the lipase). In contrast, in ultrasound pretreatment of lipase for 1 h, the conversion increased sharply. This result may stem from an alteration in the statement of the lipase ‘lid’ from closed to open after being activated by ultrasound pretreatment, which contributed to the transesterification process.20,26 Continuous ultrasound irradiation showed the highest conversion among the different ultrasound modes. Considering the conversion and reaction efficiency, ultrasound irradiation throughout the reaction was selected in this study.
Structure characterization of PAGs
The enzymatic synthesis of PAGs was analysed by HPLC-ELSD. The chromatograms of raw materials including glycerol, EPs and corresponding PAGs obtained after purification are shown in Fig. 3. The result from HPLC chromatogram indicated that the purification of PAGs was successful with a highly purity (>95%) and no residual ethyl PAs or glyceride were detected by HPLC.The synthesized PAGs were identified by HPLC/APCI-MS. The mass spectrograms of PAGs are shown in Fig. 4. The MS of peak #1 (Fig. 4a) showed an molecular ion of 223.1 m/z [M + H]+, daughter ion of 161.3 m/z [M + H–C2H6O2]+ ascribe to methyl cinnamate. The cinnamoyl daughter ion was at 131.1 m/z [M + H–C3H8O3]+. The MS of peak #2 (Fig. 4b) revealed an abundant addition of 261.1 m/z [M + Na]+ and an molecular ion of 239.2 m/z [M + H]+, daughter ion of 179.1 m/z [M + H–C2H6O2]+, which was the daughter for methyl hydroxycinnamate. The other daughter ion at 147.1 m/z [M + H–C3H8O3]+ belonged to hydroxycinnamoyl. The MS of peak #3 and #4 displayed in Fig. 4c and d, respectively, showing that higher intensity of m/z 275.2 [M + Na]+ and the daughter ion at m/z 193.2 [M + H–C2H6O2]+ corresponded to methyl methoxycinnamate, and daughter ion of m/z 161.1 [M + H–C3H8O3]+ was for methoxy cinnamoyl. The MS of peak #5 (Fig. 4e) produced the abundant addition of m/z 277.2 [M + Na]+ and daughter ions at m/z 195.1 [M + H–C2H6O2]+ ascribe to methyl methoxy caffeate, and daughter ion of m/z 161.1 [M + H–C3H8O3]+ ascribe to caffeoyl. The MS of peak #6 (Fig. 4f) displayed an daughter ion of m/z 291.1 [M + Na]+ and an addition ion of m/z 269.1 [M + H]+, daughter ion at m/z 209.1 [M + H–C2H6O2]+ ascribe to methyl ferulate, and daughter ion of m/z 177.1 [M + H–C3H8O3]+ ascribe to ferulyl. It is worth mentioning that the monoglycerol with m-hydroxycinnamic acid and 2-methoxy cinnamic acid were synthesized and identified for the first time.
The structures of PAG were further determined by 1H NMR (Table S1†). Chemical shifts were given on a δ (ppm) scale and the corresponding carbon number was shown in Fig. 4.
Cinnamoyl glycerol (CDCl3, 400 MHz): δ (ppm) 7.74 (1H, d, J = 16.0), 7.54 (2H, m), 7.41 (3H, m), 6.48 (1H, d, J = 16.0), 4.33 (1H, dd, J = 11.7, 5.4), 4.02 (1H, dd, J = 4.8, 4.3), 3.93 (1H, d, J = 4.7), 3.73 (1H, dd, J = 3.5, 11.6).
Coumaroyl glycerol (DMSO, 400 MHz): δ (ppm) 10.03 (1H, s), 7.57 (2H, d, J = 8.5), 6.79 (2H, m), 6.39 (1H, dd, J = 15.9, 6.0), 4.01 (1H, dd, J = 11.2, 6.5), 3.71 (1H, tq, J = 10.0, 5.6, 4.8), 3.53 (1H, dd, J = 11.2, 5.4), 3.35 (1H, dd, J = 11.2, 5.6).
m-Hydroxycinnamoyl glycerol (DMSO, 400 MHz): δ (ppm) 9.65 (1H, d, J = 3.3), 7.57 (1H, dd, J = 16.0, 10.2), 7.15 (1H, m), 6.97 (1H, m), 6.84 (1H, dd, J = 8.2, 2.2), 6.51 (1H, dd, J = 16.0, 2.4), 4.97 (1H, d, J = 5.3), 4.03 (1H, dd, J = 11.2, 6.5), 3.71 (1H, m), 3.35 (1H, m).
p-Methoxy cinnamoyl glycerol (CDCl3, 400 MHz): δ (ppm) 7.69 (1H, m), 7.49 (2H, m), 6.92 (2H, m), 6.33 (1H, m), 4.32 (2H, qd, J = 11.7, 5.3), 4.02 (1H, d, J = 6.6), 3.75 (1H, dd, J = 11.6, 4.0), 3.67 (1H, dd, J = 11.6, 5.6).
2-Methoxy cinnamoyl glycerol (CDCl3, 400 MHz): δ (ppm) 7.95 (1H, d, J = 17.4), 7.77 (1H, s), 7.49 (1H, s), 7.17 (1H, s), 7.06 (1H, s), 6.69 (1H, d, J = 16.2), 4.25 (1H, d, J = 11.0), 3.90 (1H, s), 3.73 (3H, h, J = 4.6), 3.67 (1H, s).
Caffeoyl glycerol (DMSO, 400 MHz): δ (ppm) 8.32 (1H, s), 7.22 (1H, q, J = 4.5, 3.8), 7.02 (1H, m), 6.68 (1H, s), 5.32 (2H, t, J = 4.9), 4.41 (1H, s), 3.50 (1H, d, J = 1.7), 3.43 (1H, m), 3.28 (1H, dd, J = 10.8, 5.6).
Feruloyl glycerol (DMSO, 400 MHz): δ (ppm) 9.63 (1H, s), 7.56 (1H, d, J = 15.9), 7.33 (1H, d, J = 2.0), 7.12 (1H, dd, J = 8.2, 2.0), 6.79 (1H, d, J = 8.1), 4.15 (2H, dd, J = 11.2, 4.1), 4.01 (1H, dd, J = 11.2, 6.5), 3.82 (3H, s), 3.70 (2H, m).
Antioxidant evaluation
The antioxidant activity of PAs has been reported in several literatures.11,27 However, the antioxidant activity of PAGs only focused on a single category.6,16,21 Based on the synthesis and identification of seven PAGs mentioned above, the antioxidant activities of PAs and PAGs were evaluated using DPPH radical scavenging, FRAP and β-carotene/linoleic acid assay, respectively.As shown in Fig. 5a, cinnamic acid (2.3%) and cinnamic glycerol (5.9%) showed weak radical scavenging ability. Among the methoxy-containing cinnamic acid derivatives, ferulic acid showed stronger antioxidant activity (93.8%). One reason may be –OCH3 at C-3 had electron-withdrawing ability; the other reason could be the hydrogen bond between –OH and –OCH3 oxygen weakens the O–H bond, facilitating radical formation. Therefore, the hydrogen radical of the hydroxyl group was more active and thus exhibited stronger antioxidant activity than the mono-hydroxycinnamic acid derivatives.28 In addition, the amount of hydroxyl groups may have a dominant influence on their radical scavenging ability. Therefore, as a bis-hydroxycinnamic acid derivative, caffeic acid, showed the highest antioxidant capacity (95.0%).29,30 The radical-scavenging ability of PAGs was similar to the parent PAs, indicating that transesterification modification does not show a negative influence on the antioxidant properties of PAs. The radical-scavenging capacity order of PAGs and usual antioxidants was L-ascorbic acid > L-ascorbyl palmitate > α-tocopherol > caffeoyl glycerol > feruloyl glycerol > TBHQ > BHT > p-hydroxycinnamoyl glycerol > p-methoxy cinnamoyl glycerol > 2-methoxy cinnamoyl glycerol > m-hydroxy cinnamoyl glycerol > cinnamoyl glycerol. Caffeoyl glycerol (94.6%) and feruloyl glycerol (92.6%) exhibited better radical scavenging capability than BHT and were equal to TBHQ.
The total antioxidant capacity was evaluated by FRAP assay. As shown in Fig. 5b, although the antioxidant ability of feruloyl glycerol was 16% less than the ferulic acid, it was still higher than the conventional antioxidants, such as L-ascorbic acid, L-ascorbyl palmitate, α-tocopherol, BHT and TBHQ. Under the experimental conditions (pH 3.6), PAs of 4-OH were observed to obviously reduce iron ability, however, the antioxidant ability of corresponding PAGs decreased obviously. The reason could be that esterification affected the interaction of –OH and –COOH at low pH, and thus decreased the total antioxidant capacity. The antioxidant capacity of ferulic acid was less affected by transesterification than that of caffeic acid, which only retained 63.2% of the initial antioxidant ability. This phenomenon could be explained in that the dihydroxy PAs were more susceptible to esterification modification than monohydroxy PAs. Furthermore, the –OCH3 group in ferulic acid might promote the activity of –OH radicals.
The peroxidation inhibitory ability was measured by the β-carotene/linoleic acid emulsion oxidation method. As shown in Fig. 5c, all PAs showed weak peroxidation inhibitory ability (<20%), which might be ascribed to the poor solubility of PAs in the emulsified system. Compared with the corresponding PAs, the peroxidation inhibitory ability of caffeoyl glycerol (68.2%) and feruloyl glycerol (74.8%) increased 8.1-fold and 14.4-fold, respectively, which showed much better peroxidation inhibitory ability than BHT and L-ascorbyl palmitate. This phenomenon could be explained in that the transesterification of EPs and glycerol increase the amphipathic nature of PAs, which improves the dispersibility of PAGs in the emulsification system, and thus greatly enhanced the lipid peroxidation inhibitory ability of PAs.
Antimicrobial activity
The development of a natural and safe bacteriostatic has been gaining popularity. In recent years, the wide antibacterial activities of PAs have been reported.8,31 However, the antibacterial activity of PAGs has never been reported in any literature. In the present work, Escherichia coli, Staphylococcus aureus, Bacillus subtilis and Saccharomyces cerevisiae, which belong to the Gram-negative bacteria, Gram-positive bacteria and fungus families were used to evaluate the antimicrobial activity of PAGs. The samples concentration was kept in the range of 0.32–10 mg mL−1 for antimicrobial ability evaluation using the MIC method. As shown in Table 2, the prepared PAGs exhibited better antimicrobial ability for Gram-negative bacteria than for Gram-positive bacteria. Compared with p-methoxy cinnamic acid, caffeic acid and ferulic acid, the corresponding PAGs showed much superior anti-E. coli activities, with MIC values decreasing 16.0-fold, 8-fold and 4-fold, respectively. The MIC values of 2-methoxy cinnamoyl glycerol and feruloyl glycerol were 2.5 mg mL−1 and 1.25 mg mL−1, respectively, which decreased 4-fold and 2-fold than their PAs for anti-Bacillus subtilis, respectively. In anti-Staphylococcus aureus, the MIC value of feruloyl glycerol decreased 2-fold compared with ferulic acid. However, it failed to show any significant advantage of PAGs over PAs in antifungal activity. Taking the antibacterial capacity and range into account, p-methoxy cinnamoyl glycerol, 2-methoxy cinnamoyl glycerol, caffeoyl glycerol and feruloyl glycerol exhibited superb application prospects in bacteriostatic. One could deduce that the existence of methoxy cinnamoyl might remarkably increase the antimicrobial activity of PAGs, and the increase in the hydroxyl number might also have a positive effect on the antimicrobial ability.32| Sample | Escherichia coli (mg mL−1) | Bacillus subtilis (mg mL−1) | Staphylococcus aureus (mg mL−1) | Saccharomyces cerevisiae (mg mL−1) |
|---|---|---|---|---|
| Cinnamic acid | 2.5 | 5 | 5 | 2.5 |
| Cinnamoyl glycerol | 10 | 10 | 10 | 10 |
| p-Hydroxycinnamic acid | 2.5 | 2.5 | 2.5 | 5 |
| p-Hydroxycinnamoyl glycerol | 10 | 10 | 5 | 5 |
| m-Hydroxycinnamic acid | 2.5 | 2.5 | 2.5 | 10 |
| m-Hydroxycinnamoyl glycerol | 10 | 10 | 5 | >10 |
| p-Methoxy cinnamic acid | 5 | 2.5 | 2.5 | 2.5 |
| p-Methoxy cinnamoyl glycerol | 0.32 | 10 | 5 | 10 |
| 2-Methoxycinnamic acid | 10 | 10 | 5 | 10 |
| 2-Methoxycinnamoyl glycerol | 10 | 2.5 | 5 | 10 |
| Caffeic acid | 2.5 | 1.25 | 2.5 | 2.5 |
| Caffeoyl glycerol | 0.32 | 2.5 | 2.5 | 10 |
| Ferulic acid | 2.5 | 2.5 | 2.5 | 5 |
| Feruloyl glycerol | 0.63 | 1.25 | 1.25 | 10 |
UV absorption evaluation
UV light can be classified into three categories according to the wavelength including UV-A (315–400 nm), UV-B (280–315 nm) and UV-C (100–280 nm). Excessive exposure of UV-A and UV-B could result in free radical, toxic elements into skin cells and even skin cancer. Some natural compounds include typically single or multiple aromatic structures conjugated or unconjugated with carbon–carbon double bonds and thus could absorb light in both UV-A and UV-B wavelength regions. The UV absorption ability of PAs and PAGs are shown in Fig. 6 with the concentration at 1 mmol L−1. The UV spectra of the maximum absorption wavelength (λmax) of PAGs increased by 10–30 nm compared with the parent PAs. The result may be explained that the cinnamoyl structure has a conjugate bond thus maximizing the electron cloud density around the π bond. The reaction, making glycerol combine with the phenolic skeleton instead of H, increased the electron cloud density around the π bond to cause λmax red-shift and the UV absorption bands become wider.33 Although both PAs and PAGs could absorb UV-A and UV-B, the UV absorption values of PAGs were 0.8–26.2% higher and 6.7–55.5% wider than those of PAs. The reason may be the transesterification modification increased the amphiphilic nature of PAs, thus increasing the UV absorption intensity and range.16,34 The study provided the important basic data support for the application of PAGs in sunscreen products. | ||
| Fig. 6 UV absorption evaluation of phenolic acids (PAs) and PAGs in the range of 200–800 nm. The concentration of PAs and PAGs was kept at 1.0 mmol L−1. | ||
Conclusions
In this study, seven PAGs were enzymatically synthesized by ultrasonic assistance with the highest catalytic efficiency among the reported methods. Among the active derivatives, caffeoyl glycerol and feruloyl glycerol exhibited excellent antioxidant activity either in DPPH, FRAP or β-carotene/linoleic acid assay, even comparable or better than the chemical antioxidants such as BHT and TBHQ. Several PAGs such as p-methoxycinnamic acid glycerol, caffeoyl glycerol and feruloyl glycerol, showed much better antimicrobial activities than their parent PAs, with MIC values decreasing 2–16-fold. Compared with PAs, PAGs can absorb a much wider and higher intensity of the harmful UV-A and UV-B rays. Taking advantage of their multifunction including antioxidant, antimicrobial and anti-UV, this study not only paves the way to develop the resultant PA derivatives into the field of multifunctional ingredients, but also opens a new opportunity for advancing the process of phytochemical modification.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was supported by the National Natural Science Foundation of China (31671820, 31972038), the Applied Basic Frontiers Program of Wuhan City (2019020701011474) and the Agricultural Science and Technology Innovation Project of the Chinese Academy of Agricultural Sciences (CAAS-ASTIP-2013-OCRI).Notes and references
- S. A. Heleno, A. Martins, M. J. Queiroz and I. C. Ferreira, Food Chem., 2015, 173, 501–513 CrossRef CAS PubMed.
- O. Ogah, C. S. Watkins, B. E. Ubi and N. C. Oraguzie, J. Agric. Food Chem., 2014, 62, 9369–9386 CrossRef CAS PubMed.
- B. Reis, M. Martins, B. Barreto, N. Milhazes, E. M. Garrido, P. Silva, J. Garrido and F. Borges, J. Agric. Food Chem., 2010, 58, 6986–6993 CrossRef CAS PubMed.
- M. C. Figueroaespinoza and P. Villeneuve, J. Agric. Food Chem., 2005, 53, 2779–2787 CrossRef CAS PubMed.
- J. Graca and H. Pereira, J. Agric. Food Chem., 2000, 48, 5476–5483 CrossRef CAS PubMed.
- D. L. Compton, J. A. Laszlo and K. O. Evans, Ind. Crops Prod., 2012, 36, 217–221 CrossRef CAS.
- A. C. D. Camargo, G. B. Rasera, L. D. P. Silva, V. O. Alvarenga, A. S. Sant'Ana and F. Shahidi, Food Chem., 2017, 237, 538–544 CrossRef PubMed.
- M. Balakrishna, S. S. Kaki, M. S. Karuna, S. Sarada, C. G. Kumar and R. B. Prasad, Food Chem., 2017, 221, 664–672 CrossRef CAS PubMed.
- D. Patil, B. Dev and A. Nag, J. Mol. Catal. B: Enzym., 2011, 73, 5–8 CrossRef CAS.
- C. M. Verdasco-Martín, E. Garcia-Verdugo, R. Porcar, R. Fernandez-Lafuente and C. Otero, Food Chem., 2017, 245, 39–46 CrossRef PubMed.
- J. Wei, X. Huo, Z. Yu, X. Tian, S. Deng, C. Sun, L. Feng, C. Wang, X. Ma and J. Jia, Fitoterapia, 2017, 121, 129–135 CrossRef CAS PubMed.
- Z. Yang, Z. Guo and X. Xu, Food Chem., 2012, 132, 1311–1315 CrossRef CAS PubMed.
- J. A. Laszlo, K. O. Evans, K. E. Vermillion and M. Appell, J. Agric. Food Chem., 2010, 58, 5842–5850 CrossRef CAS PubMed.
- S. Gholivand, O. Lasekan, C. P. Tan, F. Abas and L. S. Wei, Food Chem., 2017, 224, 365–371 CrossRef CAS PubMed.
- S. Sun and B. X. Hu, Food Chem., 2017, 214, 192–198 CrossRef CAS PubMed.
- D. L. Compton, J. A. Laszlo and T. A. Isbell, J. Am. Oil Chem. Soc., 2004, 81, 945–951 CrossRef CAS.
- S. Caillet, J. Côté, J. F. Sylvain and M. Lacroix, Food Control, 2012, 23, 419–428 CrossRef.
- M. A. Polewski, C. G. Krueger, J. D. Reed and G. Leyer, J. Funct. Foods, 2016, 25, 123–134 CrossRef CAS.
- S. R. Bansode and V. K. Rathod, Ultrason. Sonochem., 2017, 38, 503–529 CrossRef CAS PubMed.
- M. M. Zheng, L. Wang, F. H. Huang, L. Dong, P. M. Guo, Q. C. Deng, W. L. Li and C. Zheng, Ultrason. Sonochem., 2012, 19, 1015–1020 CrossRef CAS PubMed.
- C. Xu, H. Zhang, J. Shi, M. Zheng, X. Xiang, F. Huang and J. Xiao, Ultrason. Sonochem., 2018, 41, 120–126 CrossRef CAS PubMed.
- A. Szydłowskaczerniak, I. Bartkowiakbroda, I. Karlović, G. Karlovits and E. Szłyk, Food Chem., 2011, 127, 556–563 CrossRef PubMed.
- M. M. Zheng, Q. Huang, F. H. Huang, P. M. Guo, X. Xiang, Q. C. Deng, W. L. Li, C. Y. Wan and C. Zheng, J. Agric. Food Chem., 2014, 62, 5142–5148 CrossRef CAS PubMed.
- K. S. Jaiswal and V. K. Rathod, Ultrason. Sonochem., 2018, 40, 727 CrossRef CAS PubMed.
- X. Ma, D. Wang, M. Yin, J. Lucente, W. Wang, T. Ding, X. Ye and D. Liu, Ultrason. Sonochem., 2017, 36, 1–10 CrossRef CAS PubMed.
- R. Fernandez-Lafuente, J. Mol. Catal. B: Enzym., 2010, 62, 197–212 CrossRef CAS.
- M. A. Raza and D. Shahwar, J. Chem., 2015, 2015, 1–8 CrossRef.
- H. Kikuzaki, M. Hisamoto, K. Hirose, A. Kayo Akiyama and H. Taniguchi, J. Agric. Food Chem., 2002, 50, 2161–2168 CrossRef CAS PubMed.
- A. Kurekgórecka, A. Rzepeckastojko, M. Górecki, J. Stojko, M. Sosada and G. Świerczekzięba, Molecules, 2013, 19, 78–101 CrossRef PubMed.
- M. Leja, A. Mareczek, G. Wyżgolik, J. Klepacz-Baniak and K. Czekońska, Food Chem., 2007, 100, 237–240 CrossRef CAS.
- L. Y. Chen, C. W. Cheng and J. Y. Liang, Food Chem., 2015, 170, 10–15 CrossRef CAS PubMed.
- A. F. Sánchez-Maldonado, A. Schieber and M. G. Gänzle, J. Appl. Microbiol., 2011, 111, 1176–1184 CrossRef PubMed.
- J. L. Song, L. M. Gong, S. A. Feng, J. H. Zhao, J. F. Zheng and Z. P. Zhu, Spectrochim. Acta, Part A, 2009, 74, 1084–1089 CrossRef PubMed.
- M. Stasiuk and A. Kozubek, Cell. Mol. Life Sci., 2010, 67, 841–860 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra09830e |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2020 |