Open Access Article
Open Access ArticleCembrane-type diterpenoids from the gum resin of Boswellia carterii and their biological activities†
Xiaowei Sunab,
Yanling Genga,
Xiao Wang a,
Dawei Qin*b and
Jinqian Yu
a,
Dawei Qin*b and
Jinqian Yu *a
*a
aQilu University of Technology (Shandong Academy of Sciences), Shandong Analysis and Test Center, Shandong Key Laboratory of TCM Quality Control Technology, Jinan, 250014, P. R. China. E-mail: yujinqian87528@126.com
bSchool of Chemistry and Pharmaceutical Engineering, Qilu University of Technology (Shandong Academy of Sciences), Jinan 250353, Chin. E-mail: aqdw109@163.com
First published on 3rd January 2020
Abstract
Eight new cembrane-type diterpenoids, boscartins AH–AK (1–8), along with two known ones (9-10), were isolated from the gum resin of Boswellia carterii. Compounds 1–3 were characteristic of high oxidation assignable to three epoxy groups, while compounds 4–8 were characteristic of two epoxy groups. Spectroscopic examination was used to elucidate their structures. All isolates were evaluated for antiproliferative activity against HCT-116 human colon cancer cells, anti-inflammatory activity against nitric oxide (NO) production, and hepatoprotective activity in vitro. All of them showed weak antiproliferative activity (IC50 > 100 μM), 8 exhibited potent inhibitory effects on NO production (IC50 of 14.8 μM), with the others showing weak anti-inflammatory activity (IC50 > 30 μM), and 1 exhibited more potent hepatoprotective activity than the positive control, bicyclol, at 10 μM against the damage induced by paracetamol in HepG2 cells.
1. Introduction
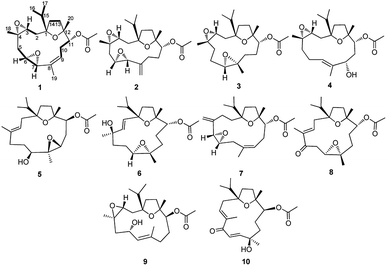
Boswellia carterii Birdw. belonging to the family of Burseraceae is greatly known for its ability to secrete a gum resin by the bark, which was known as olibanum. The gum resin of Boswellia carterii has been used in traditional Chinese medicine (TCM) for blood-activating and pain-relieving drugs to treat inflammatory diseases, blood stasis, traumatic injuries, and so on.1 Previous studies have led to the identification of the active compounds from the gum resin of Boswellia carterii mainly as mono-, sesqui-, di- and tri-terpenoids, which have been discovered to show anti-inflammatory, cytotoxic, hepatoprotective, antibacterial, and antifungal activities.2–10 Among these different kinds of metabolites, diterpenoids and triterpenoids have been identified as the main bioactive ones, which have been mainly classified into the cembrane-type and prenylaromandendrane-type diterpenoids and the oleanane-type and ursane-type triterpenoids. In particular, cembrane-type diterpenoids bearing a 14-membered oxygenated macrocycle have received more and more interest in the laboratory studies of structural modification and natural product research, however, which are mainly contained in soft corals.11–15 Due to the difficulty of obtaining these soft corals, there has been an increasing research into the cembrane-type diterpenoids obtained from terrestrial plants. Nowadays, more and more pharmacological studies have been carried out to research the chemical diversity of cembrane-type diterpenoids reported from the gum resin of Boswellia carterii, the acquisition of which is in great demand from the viewpoints of structural and pharmacological properties.2,3,16,17As part of an ongoing research for cembrane-type diterpenoids with diverse structures and significant activities from the gum resin of Boswellia carterii, a phytochemical investigation of the petroleum ether extract was conducted on the basis of the cytotoxic activity against HCT 116 cell, anti-inflammatory activity against nitric oxide (NO) production, and hepatoprotective activity in vitro. During this study, eight new cembrane-type diterpenoids (1–8) and two known ones (9-10) (Fig. 1) were obtained from the gum resin of Boswellia carterii. What's interesting, all the obtained cembrane-type diterpenoids were characteristic of high oxidation assignable to multiple epoxy groups. Herein, the isolation and structural elucidation of the new compounds are discussed, as well as the antiproliferative activity against HCT-116 human colon cancer cell, anti-inflammatory activity against nitric oxide (NO) production, and protective effect on cytotoxicity induced by paracetamol in HepG2 cells of all the isolated compounds.
2. Results and discussion
The gum resin of B. carterii was extracted with 95% ethanol to offer a crude extract, which was further suspended in 20% aqueous ethanol and partitioned successively with petroleum ether (PE), CH2Cl2, and EtOAc. The partitioned PE part was eluted rapidly by silica gel chromatography with PE and CH2Cl2. The eluted PE part was subjected to multiple column chromatography (CC) to afford eight newly described cembrane-type diterpenes (1–8) and two known cembranes (9-10). The NMR spectra analysis of these isolated compounds also revealed the characteristic structure category of cembrane-type diterpenoids, with elucidated as preliminary common cembranoid featured by one fourteen-member ring incorporated with one isopropyl.14,15Compound 1 was presented as colorless oil, the molecular formula of which was determined as C22H34O5 based on its positive HRESIMS ion peak at m/z 379.2505 [M + H]+ (calcd for 379.2440) with six indices of hydrogen deficiency. The IR spectrum suggested the absorption bands for hydroxy (3476 cm−1) and ester carbonyl (1736 cm−1) groups. Its 1H NMR data (Table 1) displayed signals of one isopropyl at δH 0.91 (3H, d, J = 6.8 Hz, Me-16), 0.96 (3H, d, J = 6.8 Hz, Me-17), 2.17 (1H, m, H-15), three methyl singlets at δH 1.18 (3H, s, Me-20), 1.34 (3H, s, Me-18) and 1.51 (3H, s, Me-19), one olefinic proton at δH 5.43 (1H, dd, J = 6.0, 10.8 Hz, H-9) and four oxymethine protons at δH 4.75 (1H, d, J = 10.8 Hz, H-11) and 2.94 (1H, dt, J = 4.0, 8.8 Hz, H-6). Its further analysis of the 13C NMR and HSQC spectra showed signals of five methyls, five methylenes, six methines including one olefinic methine at δC 125.3 (C-9), four oxymethines at δC 59.2 (C-3), 53.6 (C-6), 56.1 (C-7), 80.9 (C-11), and four quaternary carbons including three oxygenated tertiary carbons at δC 89.0 (C-1), 57.7 (C-4), and 83.8 (C-12), and one quaternary olefinic carbon at δC 137.9 (C-8). Thus, the aforementioned evidence hinted at the presence of the diagnostic cembrane-type diterpenoid with one isopropyl, three methyl singlets, and one double bond in compound 1. What's more, one acetyl group was also revealed in compound 1 deduced by δH 2.08 (3H, s) and δC 170.8, 21.1, in conjuction with IR absorption at 1709 cm−1, assigned to C-11 based on the HMBC correlation of 
| No. | 1 | 2 | 3 | 4 | ||||
|---|---|---|---|---|---|---|---|---|
| δHa | δCa | δH | δC | δH | δC | δH | δC | |
| a 1H and 13C NMR spectra were obtained in CDCl3. | ||||||||
| 1 | 89.0 | 88.2 | 88.9 | 88.9 | ||||
| 2a | 2.01 m | 36.4 | 1.84 d(15.2) | 35.1 | 1.95 d(15.2) | 36.4 | 1.83 dd(3.2, 15.2) | 35.3 |
| 2b | 1.64 overlapped | 1.60 dd(10.4, 15.2) | 1.60 overlapped | 1.58 dd(5.2, 15.2) | ||||
| 3 | 3.06 dd(2.4, 4.0) | 59.2 | 3.08 d(10.4) | 57.8 | 3.14 t(3.2) | 60.1 | 2.92 dd(3.2, 5.2) | 59.4 |
| 4 | 57.7 | 57.2 | 58.8 | 59.2 | ||||
| 5a | 2.79 dd(4.0, 13.2) | 42.9 | 2.61 dd(2.4, 13.8) | 41.4 | 1.65 overlapped | 36.0 | 2.11 m | 37.2 |
| 5b | 0.78 dd(13.2, 10.4) | 1.08 dd(10.4, 13.8) | 1.42 m | 1.45 overlapped | ||||
| 6a | 2.94 dt(8.8, 4.0) | 53.6 | 2.73 d(10.4) | 60.1 | 1.81 m | 25.2 | 2.25 m(2H) | 23.4 |
| 6b | 1.67 m | |||||||
| 7 | 3.56 s | 56.1 | 3.03 s | 57.8 | 3.03 dd(3.6, 6.4) | 58.8 | 5.59 t(6.8) | 128.8 |
| 8 | 137.9 | 143.2 | 59.9 | 134.5 | ||||
| 9a | 5.43 dd(6.0, 10.8) | 125.3 | 2.38 m | 32.3 | 1.61 overlapped | 30.6 | 4.10 d(10.8) | 87.2 |
| 9b | 2.20 m | 1.58 overlapped | ||||||
| 10a | 2.86 td(0.8, 10.8) | 27.6 | 2.15 m | 26.5 | 1.72 m | 23.8 | 2.07 d(12.8) | 31.9 |
| 10b | 2.14 td(10.8, 6.0) | 1.90 m | 1.49 m | 1.82 dt(12.8, 10.8) | ||||
| 11 | 4.75 d(10.8) | 80.9 | 4.82 dd(1.6, 11.6) | 76.3 | 4.84 d(11.2) | 79.4 | 4.78 d(10.8) | 76.1 |
| 12 | 83.8 | 84.1 | 84.1 | 83.0 | ||||
| 13a | 1.96 overlapped | 35.7 | 1.97 overlapped | 31.5 | 1.93 overlapped | 35.6 | 1.88 overlapped | 35.4 |
| 13b | 1.64 overlapped | 1.58 overlapped | 1.65 overlapped | 1.65 overlapped | ||||
| 14a | 1.95 overlapped | 30.0 | 1.97 overlapped | 35.4 | 1.94 overlapped | 29.9 | 1.89 overlapped | 29.8 |
| 14b | 1.64 overlapped | 1.66 m | 1.43 m | 1.46 overlapped | ||||
| 15 | 2.17 m | 32.7 | 1.99 overlapped | 34.5 | 1.80 overlapped | 32.9 | 2.09 overlapped | 33.0 |
| 16 | 0.91 d(6.8) | 18.7 | 0.91 d(6.8) | 17.9 | 0.93 d(6.8) | 18.8 | 0.90 overlapped | 17.1 |
| 17 | 0.96 d(6.8) | 16.8 | 0.94 d(6.8) | 17.6 | 0.95 d(6.8) | 16.9 | 0.90 overlapped | 17.1 |
| 18 | 1.34 s | 17.9 | 1.34 s | 18.3 | 1.27 s | 19.2 | 1.23 s | 17.1 |
| 19 | 1.51 s | 17.6 | 4.92 s, 4.74 s | 110.8 | 1.27 s | 17.0 | 1.73 s | 14.5 |
| 20 | 1.18 s | 21.5 | 1.20 s | 22.4 | 1.10 s | 21.5 | 1.16 s | 21.5 |
 |
2.08 s | 21.1 | 2.10 s | 21.1 | 2.09 s | 21.2 | 2.11 s | 21.3 |
 |
170.8 | 170.6 | 171.2 | 171.3 | ||||
| OH | 7.93 s (OH-9) | |||||||
The cembrane-type planar structure of 1 was unambiguously elucidated by the integrated evidence provided by the 2D NMR experiments. Five different substructural fragments a (C-2–C-3), b (C-5–C-6–C-7), c (C-9–C-10–C-11), d (C-13–C-14), e (C-15–C-16 and C-15–C-17) were readily identified by the correlations from the COSY spectrum, the connectivities of which were achieved by the HMBC correlations (Fig. 2). Pivotal correlations of H2-2, H-3, H-15, Me-16 and Me-17 to C-1; H2-2, H-3, H2-5, H-6, and Me-18 to C-4; H-6, H-7, H-9, H2-10, and Me-19 to C-8; H2-10, H-11, H2-14, and Me-20 to C-12, H-15 to C-1, C-2, C-12 and C-14 from the HMBC data, confirmed the cyclization of a 14-membered macrocycle but also the linkage of the isopropyl to this macrocycle. Additionally, 3 of 6 degrees of unsaturation were accounted for a double bond, an acetyl carbonyl, and a macrocycle, which allowed the remain three degrees of unsaturation for three additional epoxy rings of 1:12-epoxide, 3:4-epoxide, and 6:7-epoxide, in conjuction with the molecular formula and 13C NMR data. The 8, 9 double bond was located according to the HMBC correlations of H-9/C-7/C-8/C-10/C-11, Me-19/C-7/C-8/C-9, and H-7/C-5/C-8/C-9/Me-19. Thus, the planar structure of 1 was assigned as 1:12,3:4,6:7-triepoxy-11-acetoxy-8-cembranene, which represented the first cembrane-type diterpenoid with three epoxy bridges at 1:12, 3:4, and 6:7.
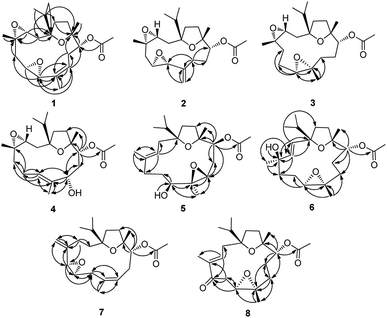
The configurations and structure of 1 were further established by analysis of its NOESY correlations and coupling constants. The NOE correlations of H-3/H-5b, H-7; H-6/H-7, Me-18, Me-19; H-7/H-3, H-5b; H-10a/Me-20, H-3, H-7, H-11; H-10b/H-9; and H-11/H-3, H-10a, Me-20, showed that H-3, H-6, H-7, H-10a, H-11, Me-18 and Me-20 were all in β, thus, rendering the 3,4-oxirane, 6,7-oxirane and the acetyl group at C-11 to be α orientations (Fig. 3). Additionally, the large coupling constant of JH-9,H-10a = 10.8 Hz, and the small coupling constant of JH-9,H-10b = 6.0 Hz, as well as the large coupling constant of JH-11,H-10b = 10.8 Hz, and the small coupling constant of JH-11,H-10a = 0.8 Hz, indicated not only a less than 90° torsional angle between the intersecting H(9)C(9)C(8) and H(10b)C(10)C(9) flats, but also a approximately 180° torsional angle between the intersecting H(10b)C(10)C(11) and H(11)C(11)C(10) flats (Fig. 3), ascertaining the α orientation of the acetyl group at C-11. Similarly, the large coupling constant of JH-5b,H-6 = 10.4 Hz, and the small coupling constant of JH-5a,H-6 = 4.0 Hz, as well as the large coupling constant of JH-5a,H-5b = 13.2 Hz, and the singlet of H-7, indicated not only a approximately 180° torsional angle between the intersecting H(6)C(6)C(5) and H(5b)C(5)C(6) flats, but also the α orientation of the 6,7-oxirane group (Fig. 3). In addition, the olefinic geometry of C-8/C-9 was ascertained as Z form by the NOE correlations of H-9/Me-19. Hence, the structure of 1 was defined as (1S,3R,4S,6R,7R,11R,12R,8Z)-1:12,3:4,6:7-triepoxy-11-acetoxy-8-cembrene (boscartins AH) with the aid of a computer-modeled 3D structure (Fig. 3) generated by MM2 force field calculations for energy minimization using the molecular modeling program Chem 3D Ultra 14.0.
 | ||
Fig. 3 Key NOE ( ) correlations observed for 1–8 (3D structures of 1–8 were generated by MM2 force field calculations for energy minimization using the molecular modeling program Chem 3D Ultra 14.0). ) correlations observed for 1–8 (3D structures of 1–8 were generated by MM2 force field calculations for energy minimization using the molecular modeling program Chem 3D Ultra 14.0). | ||
Compound 2 was presented as colorless oil with the molecular formula of C22H34O5 as determined to be the same with 1 by its positive HRESIMS experiment (m/z 379.2521 [M + H]+, calcd for 379.2440), with six indices of hydrogen deficiency. The IR spectrum suggested the absorption bands for hydroxy (3395 cm−1) and ester carbonyl (1733 cm−1) groups. The 1H and 13C NMR data of 2 were detailedly compared with those of 1, evidencing that 2 was an isomer of 1, which was further confirmed by the integrated evidence provided by the 1D and 2D NMR experiments (Table 1). The 1H NMR data of 2 established not only the replacement of an olefinic methine in 1 by an exo methylene at δH 2.38 and 2.20 (H2-9), but also the replacement of an allylic methyl in 1 by a olefinic exomethylene at δH 4.92 and 4.74 (H2-19). The 13C NMR data of 2 established the replacement of trisubstituted double bond at C8–C9 in 1 by a gem-disubstituted double bond at C8–C19 [C-8 (δC 143.2) and C-19 (δC 110.8)]. Additionally, the HMBC correlations of H2-9 to C-7 (δC 57.8), C-8, C-10 (δC 26.5), C-11 (δC 76.3), C-19, and H2-19 to C-7, C-8, C-9, confirmed the location of the double bond at C8–C19. Thus, the planar structure of 2 was assigned as 1:12,3:4,6:7-triepoxy-11-acetoxy-8,19-cembranene.
The configurations and structure of 2 were further established by analysis of its NOESY correlations and coupling constants. The NOE correlations of H-3/H-2a, H-5b, H-6, H-11, Me-16; H-2a/Me-18; H-5b/H-3, H-7, Me-16, Me-17; H-6/H-9b, H-11, H-19, Me-18; H-7/H-5b, H-11; and H-11/H-3, H-6, Me-18, Me-20, showed that H-3, H-2a, H-5b, H-6, H-7, H-11, Me-18 and Me-20 were all in β, thus, rendering the 3,4-oxirane, 6,7-oxirane and the acetyl group at C-11 to be α orientations (Fig. 3). Additionally, the large coupling constant of JH-3,H-2b = 10.4 Hz, JH-5a,H-5b = 13.8 Hz, J H-6,H-5b = 10.4 Hz, JH-11,H-10b = 11.6 Hz, and the small coupling constant of JH-5a,H-6 = 2.4 Hz, JH-6,H-7 = 0 Hz, JH-11,H-10a = 1.6 Hz, as well as the biogenetic consideration, also indicated the above assigned orientations for H-3, H-6, H-7, H-11. Hence, the structure of 2 was defined as (1S,3R,4S,6R,7R,11R,12R)-1:12,3:4,6:7-triepoxy-11-acetoxy-8,19-cembrene (boscartins AI) with the aid of a computer-modeled 3D structure (Fig. 3) generated by MM2 force field calculations for energy minimization using the molecular modeling program Chem 3D Ultra 14.0.
Compound 3 was presented as colorless oil with the molecular formula of C22H36O5 as determined by its positive HRESIMS experiment (m/z 381.2653 [M + H]+, calcd for 381.2596), with five indices of hydrogen deficiency. The IR spectrum suggested the absorption bands for hydroxy (3455 cm−1) and ester carbonyl (1734 cm−1) groups. The 1H and 13C NMR data of 3 were carefully compared with those of 1, clearly evidencing the consistent 1:12,3:4-diepoxy-11-acetoxy-containing cembrane type backbone between them, with the difference ascribed to signals at C-6 (δC 25.2), C-7 (δC 58.8), C-8 (δC 59.9), and C-9 (δC 30.6). The 1H NMR data of 3 established not only the replacement of an oxymethine in 1 by an exo methylene at δH 1.81 and 1.67 (H2-6), but also the replacement of an olefinic methine in 1 by an exo methylene at δH 1.61 and 1.58 (H2-9). The 13C NMR data of 3 established the epoxy ring of 6:7-epoxide in 1 was migrated to C-7 and C-8 in 3, and the trisubstituted double bond at C8–C9 in 1 was hydrogenised in 3. In-depth 2D NMR scrutiny established the above deduction. Obviously, the notable HMBC correlations of H-7 (δH 3.03) to C-5 (δC 36.0), C-6, C-8, C-9, Me-19 (δC 17.0) and Me-19 (δH 1.27) to C-7, C-8, C-9 indicated an oxygen bridge between C-7 and C-8, constructing a 1:12,3:4,7:8-triepoxy-11-acetoxy-cembrane motif for 3.
The configurations and structure of 3 were further established by analysis of its NOESY correlations and coupling constants. The NOE correlations of H-3/H-15, Me-16, Me-17; H-7/H-11, Me-16, Me-17, Me-18, Me-19; H-11/H-7, H-10a, Me-18, Me-19, Me-20, showed that H-3, H-7, H-11, Me-18, Me-19 and Me-20 were all in β, thus, rendering the 3,4-oxirane, 7,8-oxirane and the acetyl group at C-11 to be α orientations (Fig. 3). Additionally, the large coupling constant of JH-11,H-10b = 11.2 Hz also indicated the above assigned orientation for H-11. Hence, the structure of 3 was defined as (1S,3R,4S,7R,8S,11R,12R)-1:12,3:4,7:8-triepoxy-11-acetoxy-cembrane (boscartins AJ) with the aid of a computer-modeled 3D structure (Fig. 3) generated by MM2 force field calculations for energy minimization using the molecular modeling program Chem 3D Ultra 14.0, as well as the biogenetic consideration.
Compound 4 was presented as colorless oil with the molecular formula of C22H36O5 as determined by its positive HRESIMS experiment (m/z 379.2357 [M − H]−, calcd for 381.2596), with five indices of hydrogen deficiency. The IR spectrum suggested the absorption bands for hydroxy (3454 cm−1) and ester carbonyl (1737 cm−1) groups. The 1H and 13C NMR data of 4 were carefully compared with those of 9 (boscartins Q), clearly evidencing the resembled 1:12,3:4-diepoxy-11-acetoxy-7-cembranene type backbone between them. The only difference between them was ascribed to the migration of a hydroxy group from C-6 in 9 to C-9 in 4, which was further supported by the well-resolved HMBC correlations of H-6 (δH 2.25)/C-4 (δC 59.2), C-5 (δC 37.2), C-7 (δC 128.8), C-8 (δC 134.5); H-7 (δH 5.59)/C-5, C-6 (δC 23.4), C-9 (δC 87.2), Me-19 (δC 14.5); H-9 (δH 4.10)/C-7, C-8, C-10 (δC 31.9), C-11 (δC 76.1), Me-19; H-11 (δH 4.78)/C-9, C-10, C-12 (δC 83.0), C-13 (δC 35.4), Me-20 (δC 21.5),  (δC 171.3); Me-19 (δH 1.73)/C-7, C-8, C-9, thus, constructing a 1:12,3:4-diepoxy-11-acetoxy-7-cembranene-9-ol motif for 4.
(δC 171.3); Me-19 (δH 1.73)/C-7, C-8, C-9, thus, constructing a 1:12,3:4-diepoxy-11-acetoxy-7-cembranene-9-ol motif for 4.
The configurations and structure of 4 were further established by analysis of its NOESY correlations and coupling constants, as well as comparison those with 9. The NOE correlations of H-3/H-7, H-11, H-15, Me-16, Me-17, Me-18; H-9/H-7, H-10a, H-11; H-11/H-3, H-9, H-10a, Me-20; Me-18/H-3 showed that H-3, H-9, H-11, Me-18 and Me-20 were all in β, thus, rendering the 3,4-oxirane, the hydroxy group at C-9 and the acetyl group at C-11 to be α orientations (Fig. 3). The NOE correlations of H-11 and Me-18 were opposite to those observed in 9, further ascertaining the above orientations for H-3, H-9, H-11, and Me-18. Additionally, the large coupling constants of JH-9,H-10b = 10.8 Hz, JH-11,H-10b = 10.8 Hz, and JH-10a,H-10b = 12.8 Hz, as well as the little coupling of H-9/H-10a and H-11/H-10a, indicated an approximately 180° torsional angle not only between the intersecting H(9)C(9)C(10) and H(10b)C(10)C(9) flats, but also between the intersecting H(10b)C(10)C(11) and H(11)C(11)C(10) flats (Fig. 3), ascertaining the α orientations for the hydroxy group at C-9 and the acetyl group at C-11. Based on the NOE correlations of H-7/H-3 and Me-19/H-9, the olefinic geometry of C-7/C-8 was ascertained as E form. Hence, the structure of 4 was defined as (1S,3R,4S,9S,11R,12R,8E)-1:12,3:4-diepoxy-11-acetoxy-7-cembranene-9-ol (boscartins AK) with the aid of a computer-modeled 3D structure (Fig. 3) generated by MM2 force field calculations for energy minimization using the molecular modeling program Chem 3D Ultra 14.0, as well as the biogenetic consideration.
Compound 5 was presented as colorless oil with the molecular formula of C22H36O5 as determined to be the same with 4 by its positive HRESIMS experiment (m/z 381.2635 [M + H]+, calcd for 381.2596), with five indices of hydrogen deficiency. The IR spectrum suggested the absorption bands for hydroxy (3425 cm−1) and ester carbonyl (1731 cm−1) groups. The 1H and 13C NMR data of 5 were detailedly compared with those of 4, evidencing that 5 was an isomer of 4, which was further confirmed by the integrated evidence provided by the 1D and 2D NMR experiments (Table 1). The 1H NMR data of 5 established not only the replacement of an oxymethine in 4 by an olefinic methine at δH 5.12 (H-3), but also the replacement of an olefinic methine in 4 by an oxymethine at δH 3.66 (H-7). The 13C NMR data of 5 established the replacement of an epoxy ring of 3:4-epoxide in 4 by a trisubstituted double bond at C3–C4 in 5 [C-3 (δC 118.9) and C-4 (δC 135.4)], the replacement of a trisubstituted double bond at C7–C8 in 4 by an oxymethine at C-7 (δC 69.7) and a nonprotonated oxygenated tertiary carbon at C-8 (δC 64.8). Additionally, the HMBC correlations of H-3 to C-1 (δC 89.2), C-2 (δC 28.8), C-5 (δC 32.3), Me-18 (δC 18.7); H-7 to C-6 (δC 31.2), C-8, Me-19 (δC 16.4); OH-7 (δH 4.25) to C-7; H-9 (δH 2.71) to C-8, C-10 (δC 27.7), Me-19; H-11 to C-9 (δC 63.8), C-10, C-12 (δC 82.8), C-13 (δC 35.3), Me-20 (δC 22.3),  (δC 170.9); Me-18 (δH 1.64) to C-3, C-4, C-5; Me-19 (δH 1.27) to C-7, C-8, C-9, confirmed the location of the double bond at C3–C4, the hydroxy group at C-7, and the epoxy ring at C8–C9, in association with the five degrees of unsaturation. Thus, the planar structure of 5 was assigned as 1:12,8:9-diepoxy-11-acetoxy-3-cembranene-6-ol.
(δC 170.9); Me-18 (δH 1.64) to C-3, C-4, C-5; Me-19 (δH 1.27) to C-7, C-8, C-9, confirmed the location of the double bond at C3–C4, the hydroxy group at C-7, and the epoxy ring at C8–C9, in association with the five degrees of unsaturation. Thus, the planar structure of 5 was assigned as 1:12,8:9-diepoxy-11-acetoxy-3-cembranene-6-ol.
The configurations and structure of 5 were further established by analysis of its NOESY correlations and coupling constants, as well as comparison those with 4. The NOE correlations of H-7/H-10b, Me-19; H-9/H-11, Me-19; H-11/H-9, H-10b; H-10a/Me-20, in association with the absence of H-11/Me-20, and H-7/Me-20, showed that H-10a and Me-20 were both in β, and H-7, H-9, H-11, and Me-19 were all in α, thus, rendering the hydroxy group at C-7, the 8,9-oxirane, and the acetyl group at C-11 to be β orientations (Fig. 3). The NOE correlations of H-11 were opposite to those observed in 4, further ascertaining the above orientations for H-7, H-9, H-11, and Me-19. Additionally, the large coupling constants of JH-7,H-6b = 10.0 Hz, JH-9,H-10a = 10.8 Hz, and JH-11,H-10a = 11.6 Hz, as well as small coupling constant of JH-9,H-10b = 3.2 Hz and the little coupling of H-7/H-6a and H-11/H-10b, indicated an approximately 180° torsional angle between the intersecting H(6b)C(6)C(7) and H(7)C(7)C(6) flats, between the intersecting H(9)C(9)C(10) and H(10a)C(10)C(9) flats, between the intersecting H(10a)C(10)C(11) and H(11)C(11)C(10) flats (Fig. 3), ascertaining the β orientations for the hydroxy group at C-9, the epoxy ring at C8–C9, and the acetyl group at C-11. Based on the NOE correlations of H-3/H-10b and Me-18/H-2a, the olefinic geometry of C-3/C-4 was ascertained as E form. Hence, the structure of 5 was defined as (1S,7S,8S,9R,11S,12R,3E)-1:12,8:9-diepoxy-11-acetoxy-3-cembranene-7-ol (boscartins AL) with the aid of a computer-modeled 3D structure (Fig. 3) generated by MM2 force field calculations for energy minimization using the molecular modeling program Chem 3D Ultra 14.0, as well as the biogenetic consideration.
Compound 6 was presented as colorless oil with the molecular formula of C22H36O5 as determined to be the same with 4 and 5 by its positive HRESIMS experiment (m/z 403.2455 [M + Na]+, calcd for 403.2460), with five indices of hydrogen deficiency. The IR spectrum suggested the absorption bands for hydroxy (3427 cm−1) and ester carbonyl (1733 cm−1) groups. The 1H and 13C NMR data of 6 were detailedly compared with those of 4 and 5, evidencing that 6 was an isomer of 4 and 5, which was further confirmed by the integrated evidence provided by the 1D and 2D NMR experiments (Table 2). The 1H NMR data of 6 established not only the replacement of an exo methylene in 5 by an olefinic methine at δH 5.69 (H-2), but also the replacement of an oxymethine in 5 by an exo methylene at δH 1.99, 0.89 (H2-9). The 13C NMR data of 6 established the migration of a double bond from C3–C4 in 5 to C2–C3 [C-2 (δC 135.9), C-3 (δC 131.2)], and the replacement of a trisubstituted olefinic carbon at C4 in 5 by a nonprotonated oxygenated tertiary carbon at C-4 (δC 81.3), as well as the migration of an epoxy ring from C8–C9 in 5 to C7–C8 [C-7 (δC 64.4), C-8 (δC 61.4) and C-9 (δC 34.3)]. Additionally, the HMBC correlations of H-2 to C-1 (δC 88.9), C-3, C-4, C-14 (δC 34.4); H-3 (δH 5.63) to C-1, C-2, C-4, C-5 (δC 35.0), Me-18 (δC 23.4); OH-4 (δH 10.83) to C-4; H-7 (δH 2.91) to C-5, C-6 (δC 21.9), C-8; H-11 (δH 4.91) to C-9 (δC 34.3), C-10 (δC 25.8), C-12 (δC 83.6), Me-20 (δC 21.2),  (δC 171.2); Me-18 (δH 1.30) to C-3, C-4, C-5; Me-19 (δH 1.30) to C-7, C-8, C-9, confirmed the location of the double bond at C2–C3, the hydroxy group at C-4, and the epoxy ring at C7–C8, in association with the five degrees of unsaturation. Thus, the planar structure of 6 was assigned as 1:12,7:8-diepoxy-11-acetoxy-2-cembranene-4-ol.
(δC 171.2); Me-18 (δH 1.30) to C-3, C-4, C-5; Me-19 (δH 1.30) to C-7, C-8, C-9, confirmed the location of the double bond at C2–C3, the hydroxy group at C-4, and the epoxy ring at C7–C8, in association with the five degrees of unsaturation. Thus, the planar structure of 6 was assigned as 1:12,7:8-diepoxy-11-acetoxy-2-cembranene-4-ol.
| No. | 5a | 6b | 7a | 8a | ||||
|---|---|---|---|---|---|---|---|---|
| δH | δC | δH | δC | δH | δC | δH | δC | |
| a 1H and 13C NMR spectra were obtained in CDCl3.b 1H and 13C NMR spectra were obtained in DMSO-d6. | ||||||||
| 1 | 89.2 | 88.9 | 89.9 | 89.8 | ||||
| 2a | 2.32 dd(5.6, 16.0) | 28.8 | 5.69 d(15.6) | 135.9 | 1.89 m | 28.5 | 2.47 dd(6.0, 14.0) | 31.5 |
| 2b | 1.97 dd(5.6, 16.0) | 1.87 m | 2.38 dd(10.0, 14.0) | |||||
| 3a | 5.12 t(5.6) | 118.9 | 5.63 d(15.6) | 131.2 | 2.37 m | 30.8 | 6.54 dd(6.0, 9.2) | 141.1 |
| 3b | 1.99 m | |||||||
| 4 | 135.4 | 81.3 | 146.4 | 141.1 | ||||
| 5a | 2.23 m | 32.3 | 2.13 m | 35.0 | 3.18 s | 58.5 | 198.8 | |
| 5b | 2.17 m | 1.74 overlapped | ||||||
| 6a | 1.85 overlapped | 31.2 | 1.74 overlapped | 21.9 | 2.90 dt(9.6, 2.0) | 62.4 | 2.95 dd(8.8, 13.6) | 40.6 |
| 6b | 1.76 overlapped | 1.55 overlapped | 2.82 d(13.6) | |||||
| 7a | 3.66 d(10.0) | 69.7 | 2.91 t(5.6) | 64.4 | 2.75 d(16.8) | 40.9 | 3.25 d(8.4) | 59.8 |
| 7b | 1.86 overlapped | |||||||
| 8 | 64.8 | 61.4 | 133.7 | 61.7 | ||||
| 9a | 2.71 dd(3.2, 10.8) | 63.8 | 1.99 overlapped | 34.3 | 5.31 dd(5.6, 9.6) | 120.6 | 2.06 m | 34.1 |
| 9b | 0.89 m | 0.96 m | ||||||
| 10a | 2.11 dt(11.6, 2.8) | 27.7 | 1.90 overlapped | 25.8 | 2.21 dd(5.6, 14.4) | 29.3 | 1.93 m | 25.1 |
| 10b | 2.14 t(11.6) | 1.53 overlapped | 2.11 dt(9.6, 14.4) | 1.63 overlapped | ||||
| 11 | 4.90 d(11.6) | 74.4 | 4.91 d(9.6) | 77.1 | 4.93 dd(3.2, 9.6) | 76.4 | 4.70 d(10.8) | 74.2 |
| 12 | 82.8 | 83.6 | 83.5 | 83.2 | ||||
| 13a | 1.76 overlapped | 35.3 | 1.55 overlapped (2H) | 35.6 | 1.74 overlapped | 35.8 | 1.67 overlapped (2H) | 34.9 |
| 13b | 1.71 overlapped | 1.70 overlapped | ||||||
| 14a | 1.80 overlapped | 31.0 | 1.84 overlapped (2H) | 34.4 | 1.73 overlapped(2H) | 30.3 | 1.78 overlapped | 30.7 |
| 14b | 1.76 overlapped | 1.66 overlapped | ||||||
| 15 | 1.76 overlapped | 36.0 | 1.65 m | 38.2 | 1.80 m | 36.3 | 1.83 m | 36.8 |
| 16 | 0.87 d(6.8) | 19.0 | 0.81 t(6.8) (6H) | 18.8 | 0.88 d(6.8) | 19.0 | 0.93 d(6.8) | 19.0 |
| 17 | 0.95 d(6.8) | 16.7 | 0.81 t(6.8) (6H) | 17.7 | 0.97 d(6.8) | 16.7 | 1.01 d(6.8) | 16.8 |
| 18 | 1.64 s | 18.7 | 1.30 s(6H) | 23.4 | 4.92 s, 4.81 s | 109.4 | 1.79 s | 11.8 |
| 19 | 1.27 s | 16.4 | 1.30 s(6H) | 16.9 | 1.64 s | 17.9 | 1.44 s | 17.0 |
| 20 | 1.23 s | 22.3 | 1.05 s | 21.2 | 1.20 s | 22.9 | 1.18 s | 22.0 |
 |
2.10 s | 21.1 | 2.00 s | 21.3 | 2.10 s | 21.2 | 2.00 s | 21.0 |
 |
170.9 | 171.2 | 171.3 | 171.0 | ||||
| OH | 4.79 d(4.8) (OH-7) | 10.78 s(OH-4) | ||||||
The configurations and structure of 6 were further established by analysis of its NOESY correlations and coupling constants, as well as comparison those with 4 and 5. The NOE correlations of OH-4/H-5a, Me-16, Me-19; H-7/H-9b, H-11, Me-19; H-11/H-7, H-9b, H-10a, Me-19, Me-20; H-10a/Me-20, showed that OH-4, H-7, H-11, Me-19 and Me-20 were both in β, and Me-18 was in α, thus, rendering the hydroxy group at C-4, the 7,8-oxirane, and the acetyl group at C-11 to be α orientations (Fig. 3). Additionally, the large coupling constant of JH-11,H-10b = 9.6 Hz, as well as the little coupling of H-11/H-10b, ascertained the β orientation for the hydroxy group at C-4, as well as the α orientations for the epoxy ring at C7–C8 and the acetyl group at C-11. Based on the large coupling constant of JH-2,H-3 = 15.6 Hz, the olefinic geometry of C-2/C-3 was ascertained as E form. Hence, the structure of 6 was defined as (1S,4R,7S,8R,11R,12R,2E)-1:12,7:8-diepoxy-11-acetoxy-2-cembranene-4-ol (boscartins AM) with the aid of a computer-modeled 3D structure (Fig. 3) generated by MM2 force field calculations for energy minimization using the molecular modeling program Chem 3D Ultra 14.0, as well as the biogenetic consideration.
Compound 7 was presented as colorless oil with the molecular formula of C22H34O4 as determined by its positive HRESIMS experiment (m/z 385.2346 [M + Na]+, calcd for 385.2355), with six indices of hydrogen deficiency. The IR spectrum suggested the absorption bands for hydroxy (3442 cm−1) and ester carbonyl (1731 cm−1) groups. The 1H and 13C NMR data of 7 were detailedly compared with those of 1, clearly evidencing the consistent 1:12-triepoxy-11-acetoxy-8-cembranene type backbone between them, with the difference ascribed to signals at C-3 (δC 30.8), C-4 (δC 146.4), C-5 (δC 58.5), C-7 (δC 40.9) and Me-18 (δC 109.4). The 1H NMR data of 7 established the replacement of an oxymethine in 1 by an exo methylene at δH 2.37 and 1.99 (H2-3), the replacement of an exo methylene in 1 by an oxymethine at δH 3.18 (H-5), the replacement of an oxymethine in 1 by an exo methylene at δH 2.75 and 1.86 (H2-7), and the replacement of a methyl in 1 by an olefinic exomethylene at δH 4.92 and 4.81 (H2-18). The 13C NMR data of 7 established the epoxy ring of 3:4-epoxide and the linked methyl at C-18 in 1 were transformed into an exo methylene at C-3 and a gem-disubstituted double bond at C4–C18, and the migration of an epoxy ring from C6–C7 in 1 to C5–C6 [C-5, C-6 (δC 62.4), C-7]. In-depth 2D NMR scrutiny established the above deduction. Obviously, the notable HMBC correlations of H-3 to C-2 (δC 28.5), C-4, C-5, Me-18; H-5 to C-4, C-6, C-7, Me-18; H-6 (δH 2.90) to C-7; H-7a (δH 2.75) to C-6, C-8 (δC 133.7), C-9 (δC 120.6); H-9 (δH 5.31) to C-7, Me-19 (δC 17.9); H-11 (δH 4.93) to C-12 (δC 83.5), C-13 (δC 35.8), Me-20 (δC 22.9),  (δC 171.3); H2-18 to C-3, C-4, C-5; Me-19 (δH 1.64) to C-7, C-8, C-9, indicated a gem-disubstituted double bond at C4–C18, an oxygen bridge between C-5 and C-6, and a trisubstituted double bond at C8–C9, constructing a 1:12,5:6-diepoxy-11-acetoxy-4(18),7-cembrandien motif for 7.
(δC 171.3); H2-18 to C-3, C-4, C-5; Me-19 (δH 1.64) to C-7, C-8, C-9, indicated a gem-disubstituted double bond at C4–C18, an oxygen bridge between C-5 and C-6, and a trisubstituted double bond at C8–C9, constructing a 1:12,5:6-diepoxy-11-acetoxy-4(18),7-cembrandien motif for 7.
The configurations and structure of 7 were further established by analysis of its NOESY correlations and coupling constants, as well as comparison those with 1. The NOE correlations of H-5/H-3a, H-7a, H-11, Me-16, Me-20; H-6/H-3a, H-7a, H-11, Me-17, Me-20; H-11/H-5, H-10a, Me-20, showed that H-5, H-6, H-11, Me-16, Me-17 and Me-20 were both in β, thus, rendering the 5,6-oxirane, and the acetyl group at C-11 to be α orientations (Fig. 3). Additionally, the large coupling constant of JH-6,H-7b = 9.6 Hz, JH-7a,H-7b = 16.8 Hz, JH-9,H-10b = 9.6 Hz, JH-11,H-10b = 9.6 Hz, as well as the little coupling of H-5/H-6 and the small coupling constant of JH-6,H-7a = 2.0 Hz, JH-9,H-10a = 5.6 Hz, JH-11,H-10a = 3.2 Hz, ascertained the α orientations for the epoxy ring at C5–C6 and the acetyl group at C-11. Based on the NOE correlations of H-9/Me-19, the olefinic geometry of C-8/C-9 was ascertained as Z form. Hence, the structure of 7 was defined as (1R,5R,6R,11R,12R,8Z)-1:12,5:6-diepoxy-11-acetoxy-4(18),7-cembrandien (boscartins AN) with the aid of a computer-modeled 3D structure (Fig. 3) generated by MM2 force field calculations for energy minimization using the molecular modeling program Chem 3D Ultra 14.0, as well as the biogenetic consideration.
Compound 8 was presented as colorless oil with the molecular formula of C22H34O5 as determined by its positive HRESIMS experiment (m/z 379.2487 [M + H]+, calcd for 379.2440), with six indices of hydrogen deficiency. The IR spectrum suggested the absorption bands for hydroxy (3448 cm−1), ester carbonyl (1737 cm−1), and conjugated carbonyl (1687 cm−1) groups. The 1H and 13C NMR data of 8 were detailedly compared with those of 10 (boscartins Z), clearly evidencing the consistent 1:12-triepoxy-11-acetoxy-5-oxo-3-cembranene type backbone between them, with the difference ascribed to signals at C-6 (δC 40.6), C-7 (δC 59.8), and C-8 (δC 61.7). The 1H NMR data of 8 established the replacement of an olefinic methine in 10 by an exo methylene at δH 2.95 and 2.82 (H2-6), and the replacement of an olefinic methine in 10 by an oxymethine at δH 3.25 (H-7). The 13C NMR data of 8 established the replacement of a double bond in 10 by an exo methylene and an oxymethine at C-6 and C-7. In-depth 2D NMR scrutiny established the above deduction. Obviously, the notable HMBC correlations of H-3 (δH 6.54) to C-2 (δC 31.5), C-5 (δC 198.8), Me-18 (δC 11.8); H-6 to C-4 (δC 141.1), C-5, C-7; H-7 to C-5, C-6; H-9a (δH 2.06) to C-8, C-10 (δC 25.1), C-11 (δC 74.2), Me-19 (δC 17.0); H-10a (δH 1.93) to C-8, C-9 (δC 34.1); H-11 (δH 4.70) to C-9, C-10, C-12 (δC 83.2), Me-20 (δC 22.0),  (δC 171.0); Me-19 (δH 1.44) to C-7, C-8, C-9, indicated a trisubstituted double bond at C3–C4, a keto at C-5, an oxygen bridge between C-7 and C-8, in association with the six degrees of unsaturation. Thus, the planar structure of 8 was assigned as 1:12,7:8-diepoxy-11-acetoxy-5-oxo-3-cembranene.
(δC 171.0); Me-19 (δH 1.44) to C-7, C-8, C-9, indicated a trisubstituted double bond at C3–C4, a keto at C-5, an oxygen bridge between C-7 and C-8, in association with the six degrees of unsaturation. Thus, the planar structure of 8 was assigned as 1:12,7:8-diepoxy-11-acetoxy-5-oxo-3-cembranene.
The configurations and structure of 8 were further established by analysis of its NOESY correlations and coupling constants, as well as comparison those with 10. The NOE correlations of H-7/H-6b, H-11, Me-17, Me-19, Me-20; H-11/H-3, H-7, H-10a, Me-17, Me-19, Me-20; Me-19/H-9a, H-10a; Me-20/H-10a, showed that H-7, H-11, Me-17, Me-19 and Me-20 were both in β, thus, rendering the 7,8-oxirane, and the acetyl group at C-11 to be α orientations (Fig. 3). Additionally, the large coupling constant of JH-7,H-6a = 8.8 Hz, JH-6a,H-6b = 13.6 Hz, JH-11,H-10b = 10.8 Hz, as well as the little coupling of H-7/H-6b and H-11/H-10b, ascertained the α orientations for the epoxy ring at C7–C8 and the acetyl group at C-11. Based on the NOE correlations of H-3/H-6b, H-11, Me-19, and Me-18/H-2b, the olefinic geometry of C-3/C-4 was ascertained as E form. Hence, the structure of 8 was defined as (1S,7R,8S,11R,12R,8Z)-1:12,7:8-diepoxy-11-acetoxy-5-oxo-3-cembranene (boscartins AO) with the aid of a computer-modeled 3D structure (Fig. 3) generated by MM2 force field calculations for energy minimization using the molecular modeling program Chem 3D Ultra 14.0, as well as the biogenetic consideration.
The two known compounds, boscartins Q (9)3 and boscartins Z (10)3 were identified by comparison of their spectroscopic data (1H and 13C NMR, MS) with the literature values.
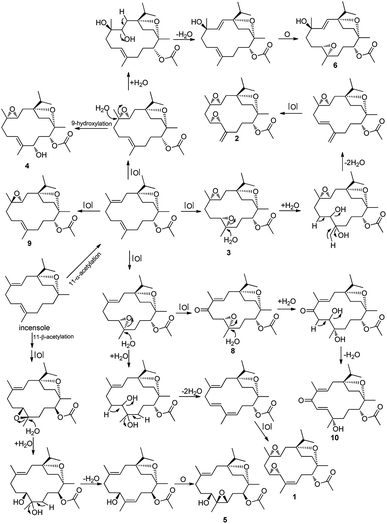
A speculative biogenetic pathway for all the isolated compounds except 2 was shown in Fig. 4. Incensole obtained as the main cembrane-type diterpenoid from the gum resin of B. carterii,14,18–20 is proposed as the precursor for the other cembranes, which gradually acetylized, oxidized, and isomerized to their derivatives. Isolated compounds (1–10) were examined for the antiproliferative activity against HCT-116 human colon cancer cell, anti-inflammatory activity against nitric oxide (NO) production, and hepatoprotective activity, with cisplatin (5.6 μM), dexamethasone (2.2 μM), and bicyclol being used as the positive controls, respectively. All of them showed weak antiproliferative activity (IC50 > 100 μM), and 8 exhibited potent inhibitory effects on NO production (IC50 of 14.8 μM), with the others showing weak anti-inflammatory activity (IC50 > 30 μM), and all compounds except for 7 and 8 exhibited hepatoprotective activity at 10 μM (Table 3), among which 1 exhibited more potent hepatoprotective activity than the positive control, bicyclol, against the damage induced by paracetamol in HepG2 cells (inhibition rate of 45.7%), with 2 exhibiting slightly lower hepatoprotective activity than bicyclol (inhibition rate of 21.0%).
| No. | Cell viability (% of blank) | Inhibition rate (%) |
|---|---|---|
| a Results were expressed as the means ± SD (n = 3 for blank, control, bicyclol and all isolated compounds); bicyclol was used as the positive control (10 μM).b P < 0.01, compared with control group.c P < 0.001, compared with control group.d P < 0.05 compared with control group. | ||
| Blank | 100 | |
| Control | 50.8 | |
| Bicyclol | 67.7b | 27.8 |
| 1 | 75.5c < 0.001 | 45.7 |
| 2 | 68.0b < 0.01 | 21.0 |
| 3 | 57.5 | 6.1 |
| 4 | 63.6c < 0.001 | 13.6 |
| 5 | 51.9 | 2.5 |
| 6 | 61.8c < 0.001 | 9.3 |
| 7 | 49.4 | −2.9 |
| 8 | 44.0 | −4.6 |
| 9 | 62.9d < 0.05 | 12.9 |
| 10 | 64.1c < 0.001 | 14.0 |
3. Experimental section
3.1. General experimental procedures
Optical rotations were obtained using a SEEWE SGW-2 digital polarimeter in MeOH. HRESIMS were determined on a Bruker Impact II mass spectrometer. IR spectra were collected using a Nicolet 5700 spectrometer. NMR spectra were determined using a BURKER 400 NMR in CDCl3 and DMSO-d6. Silica gel (200–300 mesh) and ODS C18 silica gel (YMC Co., Ltd., Japan) were used for column chromatography (CC) separation. Semipreparative CC separations were carried out using a Shimadzu LC-6AD instrument, in association with a SPD-10A detector and a reversed-phase C18 column (YMC-Pack ODS-AU 20 × 250 mm, 10 μm). All solvents used were of analytical grade.3.2. Plant material
Olibanum, the gum resin of Boswellia carterii Birdw., used in this study were the same as those in our previous study,21,22 as well as the medicine origin.3.3. Extraction and isolation
The gum resin of B. carterii (6 kg) was extracted with 95% EtOH, which were portioned subsequently with petroleum ether (PE), CH2Cl2, EtOAc, and n-BuOH as previously described.19,20 The combined PE fraction (2000 g) was further separated by CC over silica gel rapidly eluted with PE, CH2Cl2 and EtOAc. The eluted PE fraction was combined together and further separated by silica gel CC eluted with gradient solvents of PE–EtOAc (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 → 0
0 → 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to yield 4 fractions. Fraction 1 [PE–EtOAc (50
1) to yield 4 fractions. Fraction 1 [PE–EtOAc (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 14.0 g] was applied to silica gel CC eluted with gradient solvents of PE–EtOAc (100
1), 14.0 g] was applied to silica gel CC eluted with gradient solvents of PE–EtOAc (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 → 25
0 → 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to yield 11 fractions (F1-1–F1-11). F1-2 [PE–EtOAc (25
1) to yield 11 fractions (F1-1–F1-11). F1-2 [PE–EtOAc (25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 20 mg] was separated by preparative HPLC system [mobile phase: CH3CN/H2O (90%); flow rate: 3 mL min−1; UV detection at 210] resulting in the isolation of 7 (12 mg). F1-3 [PE–EtOAc (25
1), 20 mg] was separated by preparative HPLC system [mobile phase: CH3CN/H2O (90%); flow rate: 3 mL min−1; UV detection at 210] resulting in the isolation of 7 (12 mg). F1-3 [PE–EtOAc (25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 200 mg] was separated by preparative HPLC system [mobile phase: CH3CN/H2O (90%); flow rate: 5 mL min−1; UV detection at 210] resulting in the isolation of 9 (78 mg). F1-6 [PE–EtOAc (10
1), 200 mg] was separated by preparative HPLC system [mobile phase: CH3CN/H2O (90%); flow rate: 5 mL min−1; UV detection at 210] resulting in the isolation of 9 (78 mg). F1-6 [PE–EtOAc (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 80 mg] was separated by preparative HPLC system [mobile phase: CH3CN/H2O (70%); flow rate: 5 mL min−1; UV detection at 210] resulting in the isolation of 1 (25 mg) and 2 (21 mg). F1-7 [PE–EtOAc (10
1), 80 mg] was separated by preparative HPLC system [mobile phase: CH3CN/H2O (70%); flow rate: 5 mL min−1; UV detection at 210] resulting in the isolation of 1 (25 mg) and 2 (21 mg). F1-7 [PE–EtOAc (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 65 mg] was separated by preparative HPLC system [mobile phase: CH3CN/H2O (70%); flow rate: 5 mL min−1; UV detection at 230] resulting in the isolation of 3 (20 mg) and 8 (5 mg). F1-9 [PE–EtOAc (5
1), 65 mg] was separated by preparative HPLC system [mobile phase: CH3CN/H2O (70%); flow rate: 5 mL min−1; UV detection at 230] resulting in the isolation of 3 (20 mg) and 8 (5 mg). F1-9 [PE–EtOAc (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 80 mg] was separated by preparative HPLC system [mobile phase: CH3CN/H2O (65%); flow rate: 5 mL min−1; UV detection at 210] resulting in the isolation of 4 (13 mg), 5 (6 mg) and 6 (40 mg). Fraction 3 [PE–EtOAc (15
1), 80 mg] was separated by preparative HPLC system [mobile phase: CH3CN/H2O (65%); flow rate: 5 mL min−1; UV detection at 210] resulting in the isolation of 4 (13 mg), 5 (6 mg) and 6 (40 mg). Fraction 3 [PE–EtOAc (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 15.0 g] was applied to silica gel CC eluted with gradient solvents of PE–EtOAc (100
1), 15.0 g] was applied to silica gel CC eluted with gradient solvents of PE–EtOAc (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 → 25
0 → 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to yield 10 fractions (F3-1–F3-10). F3-6 [PE–EtOAc (3
1) to yield 10 fractions (F3-1–F3-10). F3-6 [PE–EtOAc (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 10 mg] was separated by preparative HPLC system [mobile phase: CH3CN/H2O (55%); flow rate: 5 mL min−1; UV detection at 230] resulting in the isolation of 10 (4 mg).
1), 10 mg] was separated by preparative HPLC system [mobile phase: CH3CN/H2O (55%); flow rate: 5 mL min−1; UV detection at 230] resulting in the isolation of 10 (4 mg).
3.4. Biological assays
| Inhibition rate (%) = [(OD(test) − OD(control))/(OD(blank) − OD(control))] × 100%. |
4. Conclusions
As part of an ongoing research for cembrane-type diterpenoids with diverse structures and significant activities from the gum resin of Boswellia carterii, a phytochemical investigation of the petroleum ether extract was conducted, leading the isolation of eight new cembrane-type diterpenoids, boscartins AH-AK (1–8), along with two known ones (9-10). These eight undescribed cembranoids were characteristic of high oxidation assignable to either three or two epoxy groups connected to the 14-membered acrocycle, as well as a acetyl group at C-11. All isolates were evaluated for antiproliferative activity against HCT-116 human colon cancer cell and anti-inflammatory activity against nitric oxide (NO) production in vitro. All of them showed weak antiproliferative activity (IC50 > 100 μM), and 8 exhibited potent inhibitory effects on NO production (IC50 of 14.8 μM), with the others showing weak anti-inflammatory activity (IC50 > 30 μM), and all compounds except for 7 and 8 exhibited hepatoprotective activity at 10 μM. Results of the inhibitory effects on NO production indicated that a keto functionality in the structure might play an important role for anti-inflammatory activity. As for the hepatoprotective activity, compound 1 exhibited more potent hepatoprotective activity than the positive control, with 2 exhibiting slightly lower hepatoprotective activity than bicyclol, which indicated that three epoxy groups and a double bond in the structure might play an important role for hepatoprotective activity. This study not only provided abundant categories of cembrane-type diterpenoids in gum resin of Boswellia carterii, but also dug out the potential active functionality for further structural modification of these cembranoids.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank associate Professor Fengqin Zhou for the sample authentication. This work was supported by Shandong Provincial Natural Science Foundation, China (ZR2018QH006), Shandong Provincial Key Research and Development Plan (2019GSF108004), and The Priority Research Program of the Shandong Academy of Sciences.References
- Committee of the Chinese Pharmacopia, The Chinese Pharmacopeia, China Medical Science Press, Beijing, 2015, vol. 1, p. 223 Search PubMed.
- J. J. Wang, B. Zheng, J. W. Hu, M. J. Shi, C. J. Wei, X. Wang, H. Sun and T. F. Ji, Phytochemistry, 2019, 163, 126–131 CrossRef CAS.
- Y. G. Wang, J. Ren, J. Ma, J. B. Yang, T. F. Ji and A. G. Wang, Fitoterapia, 2019, 137, 104263 CrossRef CAS.
- H. P. T. Ammon, Phytomedicine, 2019, 63, 153002 CrossRef CAS.
- J. Q. Yu, H. W. Zhao, D. J. Wang, X. Y. Song, L. Zhao and X. Wang, J. Sep. Sci., 2017, 40, 2732–2740 CrossRef CAS.
- S. Bahramzadeh, M. Tabarsa, S. G. You, K. Yelithao, V. Klochkov and R. Ilfat, J. Funct. Foods, 2019, 52, 450–458 CrossRef CAS.
- B. A. Shah, G. N. Qazi and S. C. Taneja, Nat. Prod. Rep., 2009, 26, 72–89 RSC.
- B. Prakash, P. K. Mishra, A. Kedia and N. K. Dubey, LWT–Food Sci. Technol., 2014, 56, 240–247 CrossRef CAS.
- C. L. Woolley, M. M. Suhail, B. L. Smith, K. E. Boren, L. C. Taylor, M. F. Schreuder, J. K. Chai, H. Casabianca, S. Haq, H.-K. Lin, A. A. Al-Shahri, S. Al-Hatmi and D. G. Young, J. Chromatogr. A, 2012, 1261, 158–163 CrossRef CAS.
- S. L. Su, Y. Q. Hua, Y. Y. Wang, W. Gu, W. Zhou, J. A. Duan, H. F. Jiang, T. Chen and Y. P. Tang, J. Ethnopharmacol., 2012, 139(2), 649–656 CrossRef PubMed.
- M. E. F. Hegazy, T. A. Mohamed, A. I. Elshamy, A. R. Hamed, M. A. A. Ibrahim, S. Ohta, A. Umeyama, P. W. Paré and T. Efferth, RSC Adv., 2019, 9, 27183 RSC.
- C. Angulo-Preckler, G. Genta-Jouve, N. Mahajan, M. D. L. Cruz, N. D. Pedro, F. Reyes, K. Iken, C. Avila and O. P. Thomas, J. Nat. Prod., 2016, 79, 1132–1136 CrossRef CAS PubMed.
- N. U. Rehman, H. Hussain, S. Al-Shidhani, S. K. Avula, G. Abbas, M. U. Anwar, M. Górecki, G. Pescitelli and A. Al-Harrasi, RSC Adv., 2017, 7, 42357–42362 RSC.
- A. Al-Harrasi, R. Csuk, A. Khan and J. Hussain, Phytochemistry, 2019, 161, 28–40 CrossRef CAS.
- W. N. Setzer, J. Mol. Model., 2018, 24, 74 CrossRef PubMed.
- F. Pollastro, S. Golin, G. Chianese, M. Y. Putra, A. S. Moriello, L. D. Petrocellis, V. García, E. Munoz, O. Taglialatela-Scafati and G. Appendino, J. Nat. Prod., 2016, 79, 1762–1768 CrossRef CAS PubMed.
- J. Ren, Y. G. Wang, A. G. Wang, L. Q. Wu, H. J. Zhang, W. J. Wang, Y. L. Su and H. L. Qin, J. Nat. Prod., 2015, 78, 2322–2331 CrossRef CAS PubMed.
- S. Al-Shidhani, N. U. Rehman, F. Mabood, M. Al-Broumi, H. Hussain, J. Hussain, R. Csuk and A. Al-Harrasi, Phytochem. Anal., 2018, 29, 300–307 CrossRef CAS PubMed.
- S. K. Avula, H. Hussain, R. Csuk, S. Sommerwerk, P. Liebing, M. Górecki, G. Pescitelli, A. Al-Rawahi, N. U. Rehman, I. R. Green and A. Al-Harrasi, Tetrahedron: Asymmetry, 2016, 27, 829–833 CrossRef CAS.
- S. I. Ali, C. R. Zhang, A. A. Mohamed, F. K. EL-Baz, A. K. Hegazy, M. A. Kord and M. G. Nair, Nat. Prod. Commun., 2013, 8, 1934578X1300801 CrossRef.
- J. Q. Yu, Y. L. Geng, H. W. Zhao and X. Wang, Tetrahedron, 2018, 74, 5858–5866 CrossRef CAS.
- J. Q. Yu, Y. L. Geng, D. J. Wang, H. W. Zhao, L. P. Guo and X. Wang, Phytochem. Lett., 2018, 28, 59–63 CrossRef CAS.
- N. L. Zhu, H. F. Wu, Z. Q. Xu, C. Q. Liu, Y. Tian, M. G. Hu, Z. H. Sun, P. F. Li, G. X. Ma and X. D. Xu, RSC Adv., 2017, 7, 41495–41498 RSC.
- G. J. Balachander, S. Subramanian and K. Ilango, RSC Adv., 2018, 8, 26656–26663 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: NMR spectra for theses new compounds. See DOI: 10.1039/c9ra09776g |
| This journal is © The Royal Society of Chemistry 2020 |