 Open Access Article
Open Access ArticleThe green exfoliation of graphite waste and its suitability for biosensor applications
Tarek H. Taha*a,
Mohamed S. Elnouby *b,
M. A. Abu-Saied
*b,
M. A. Abu-Saied c and
Saad Alamride
c and
Saad Alamride
aEnvironmental Biotechnology Department, Genetic Engineering and Biotechnology Research Institute (GEBRI), City of Scientific Research and Technological Applications (SRTA-City), New Borg El-Arab City, 21934, Alexandria, Egypt. E-mail: t.h.taha@gmail.com
bComposite and Nanostructured Materials Research Department, Advanced Technology and New Materials Research Institute, City of Scientific Research and Technological Applications (SRTA-City), New Borg El-Arab City, 21934, Alexandria, Egypt. E-mail: m_nano2050@yahoo.com
cPolymer Materials Research Department, Advanced Technology and New Materials Research Institute, City of Scientific Research and Technological Applications (SRTA-City), New Borg El-Arab City, 21934, Alexandria, Egypt
dDepartment of Biology, College of Science, King Khalid University, P. O. Box 9004, Abha, Saudi Arabia
eResearch Center for Advanced Materials Science (RCAMS), King Khalid University, P. O. Box 9004, Abha 61413, Saudi Arabia
First published on 4th March 2020
Abstract
This work is concerned with the bio-exfoliation of graphite using a soil bacterium. The isolated bacterium showed a detectable ability to oxidize and change its physical appearance and chemical structure. Multiple characterization procedures were used to study the physical and chemical changes. Raman and FTIR spectroscopy proved that the isolate G3 partially exfoliated the graphite into multi-layer sp2 graphitic layers. Scanning electron microscopy (SEM) proved that there was a change in morphology between untreated graphite waste and that manipulated by bacteria. Cyclic voltammetry results proved that the green exfoliated graphite (GEG) was suitable for use in biosensor applications and showed a noticeable ability to detect methanol, even at lower concentrations. These findings are considered as promising for the biological manipulation of graphite waste for environmental purposes. In addition, it is proved that the bacterial transformation of graphite into other GEG structures occurs without needing the chemically hazardous methods that are currently applied.
Introduction
Recently, nanomaterial-based analytical methods have gained continuous attention for use in different applications, such as: biological research, medicinal and pharmaceutical analysis, food safety and environmental monitoring. Nanomaterials are considered as potential analytical probes, owing to their high surface-to-volume ratio, exceptional physicochemical properties, high adsorption and reactive capacity and other beneficial properties not present in bulk materials.1Various nanomaterials have been employed to serve various functions in the design of biosensors. A biosensor is a compact analytical device which relies on the immobilization of bio-molecules on a transducer surface.2 Among the various types of biosensors available, electrochemical biosensors offer numerous advantages over conventional methods due to their simplicity, high sensitivity, and rapidity.
Graphene is considered to have the highest surface-to-volume ratio with an optical transparency of 97% that results from its existence as a single layer consisting of one-atom thickness.3 Its electrical and thermal conductivities are currently reported to be superior to most conductive elements such as silver, copper and gold.4,5 These powerful, thermal, mechanical, and electrical properties suggest that it is possible to use graphene in various applications such as batteries, hydrogen storage, bioanalytics, photocatalysis, drug carriers, and sensors.6–11 The two predominant methods that include chemical vapor deposition (CVD) and exfoliation are mainly used for the preparation of graphene. However, there are several drawbacks to using the CVD method because it is expensive and difficult to scale up for use industrially.12,13 The exfoliation method is composed of two steps: the first one is the oxidation of graphite into graphene oxide followed by its reduction into reduced graphene oxide or graphene as the second step.14,15 However, the second step involves the use of hazardous substances such as hydrazine which adds undesired nitrogen groups onto the surface and shows elevated energy demands.16,17
There is a wide distribution of ethanol in many applications including alcoholic beverages and liquid cosmetics, and it is not harmful in small quantities. However, methanol can be converted into strongly toxic metabolites which can cause damage to the nervous system and finally lead to death.18 In some countries, commercial fraud is used to blend methanol with alcoholic beverages instead of ethanol to reduce the production costs. The serious problem comes from the fact that both alcohols have the same physical properties and cannot easily be distinguished. Therefore, finding a reliable method to detect small amounts of methanol in ethanol is essential.19
The aim of the current work is concerned with maximizing the benefits of graphite waste and reducing its undesired environmental impacts. It depends on the isolation of a bacterial isolate that can manipulate the graphite powder waste and convert it, using green chemistry, into other precious and eco-friendly forms such as the GEG structure. The work was extended to characterize the carbonic structure obtained by instrumental analysis using Raman, Fourier transform infrared (FTIR) and SEM techniques. The benefits of the structure obtained were investigated by testing its ability to be used as biosensor for the detection of environmental hazardous materials such as methanol especially at very low concentrations.
Experimental work
Graphite waste
The graphite waste (G0) was obtained as unused powder that originated by cutting a big graphite bar into small electrodes prepared for electrochemical applications at City of Scientific Research and Technological Applications.Sample preparation
Soil samples were collected from different locations at the New Borg El-Arab City. Each sample was serially diluted from 10−1 to 10−7 using sterile normal saline.Isolation of graphite manipulating bacteria
The isolation of graphite manipulating bacteria was dependent on the ability of bacterial isolates to grow on Luria–Bertani agar plates (LB) supplemented with graphite powder. In the beginning, LB agar medium was prepared and supplemented with 0.25 g L−1 graphite powder. The prepared LB-graphite bottle was autoclaved at 115 °C and 6.9 kPa for 20 min. After sterilization, the agar medium was poured into sterile Petri plates and transferred into 4 °C for solidification. Soil dilutions were spread over the plates with a sterile glass spreader using 50 μL of each dilution. The plates were incubated at 30 °C for three days. The formed bacterial colonies were picked up and purified on LB agar plates and considered as presumptive graphite manipulating bacteria.Liquid broth manipulation process
The purified isolated graphite manipulating bacteria were re-cultivated in a graphite containing broth medium for the examination of their real ability to bio-convert graphite into other carbon structures. The process was dependent on the inoculation of 1 mL of microbes grown overnight in 10 mL of sterile minimal broth containing (g L−1): NaCl, 0.5; K2HPO4, 0.3; KH2PO4, 0.4; yeast extract, 0.1; distilled H2O, 1000 mL, and with addition of 0.25 g L−1 of graphite. The flasks were incubated at 30 °C and 150 rpm for 72 h.Lysis of bacterial cells
After incubation, the bacterial cells were lysed and washed out leaving the transformed carbon structure in a pure form for subsequent characterization. The lysis procedure started with centrifugation of the culture containing bacterial cells at 6000 rpm for 5 min, followed by removal of the supernatant. The carbon-bacterial pellets formed were dispersed in 2 mL of 10% sodium dodecyl sulfate solution (SDS) for 30 min at 50 °C, followed by centrifugation at 6000 rpm for 5 min. The supernatant was discarded and the pellets were washed twice with 1 mL of absolute ethanol and once with 1 mL of distilled water with centrifugation at 6000 rpm after each washing step. The carbonic pellets were finally air dried and were submitted for the instrumental characterization.Microbial identification
Characterizations
The electrochemical experiments were conducted on a potentiostat (Autolab 87070, Metrohm) in a three electrode configuration system. All electrochemical experiments were carried out in a cell containing 100 mL of phosphate buffered saline solution (PBS, pH 7.0) or 100 mL of ethanol. Each electrolyte was amended with different doses of methanol (0–1.0 mL/100 mL). A graphite rod was used as the auxiliary electrode, an Ag/AgCl wire as the reference electrode, together with the previously prepared working electrode.
Results and discussion
Isolation of graphite manipulating bacteria
After the cultivation and incubation of serially diluted soil on the graphite containing plate, the grown bacterial isolates were collected up and purified. As shown in Fig. 1, only one isolate was able to grow and formed a single white big colony. This bacterial isolate was considered as the presumptive graphite manipulating isolate and was transferred to graphite containing broth to confirm its ability to manipulate the graphite.Raman spectroscopy
Raman spectroscopy is considered to be a fast and non-destructive method, which provides a direct understanding of the electron–phonon interactions, which indicate a high sensitivity to electronic and crystallographic structures.20 Fig. 2 compares the Raman spectra of samples G0 and GEG measured at 532 nm excitation. The three most intense peaks were observed at ∼1340 cm−1 (D band), ∼1570 cm−1 (G band) and ∼2670 cm−1 (2D band).The D band is recognized as the disorder, or defect band, whereas, the G band is the results of an in-plane vibrational mode of the sp2 hybridized carbon atoms that comprise graphene sheets.20 Raman spectra could be used to characterize the level of disorder in carbon nanostructures using the peak ratio of ID/IG. Herein, this ratio decreased from 0.195896 to 0.124136 which may be due to the increasing defect density.
However, the 2D band is at almost double the frequency of the D band and originates from the second order Raman scattering process. This band is used to determine the graphene layer thickness, and the degree of defects could be confirmed by using the ratio I2D/IG. In the present study this ratio was 0.384777 and 0.32377 for the graphite and GEG samples, respectively. Al-Sherbini et al., prepared graphene sheets with a high number of defects and a large number of graphene layers were exfoliated from graphite with an I2D/IG of 0.46.20
Herein, these peaks hardly shifted. The full width at half maximum (FWHM) together with the intensity of the peaks could express more about the structure. Table 1 summarizes the positions, intensity and FWHM for the main three peaks for both samples. Sample GEG has a much broader and upshifted 2D band with respect to the G0 sample, which indicated that GEG has multilayered graphene formed form graphite (G0).21 Moreover, the 2D band of graphene was more uniform compared to that of the graphite sample as shown in Fig. 3.22 Roscher et al., reported that, the R2 value of graphene was higher than that of graphite.23
| Band | G0 | GEG | |
|---|---|---|---|
| D | Peak | 1342 | 1343.5 |
| ID | 306.9264 | 239.5548 | |
| FWHM | 51.561 | 68.374 | |
| G | Peak | 1570 | 1567 |
| IG | 1566.78 | 1929.771 | |
| FWHM | 30.649 | 32.476 | |
| ID/IG | 0.195896 | 0.124136 | |
| 2D | Peak | 2685 | 2688 |
| I2D | 602.861 | 624.8059 | |
| I2D/IG | 0.384777 | 0.32377 | |
| FWHM | 77.695 | 84.986 | |
| R2 | 0.936 | 0.9368 |
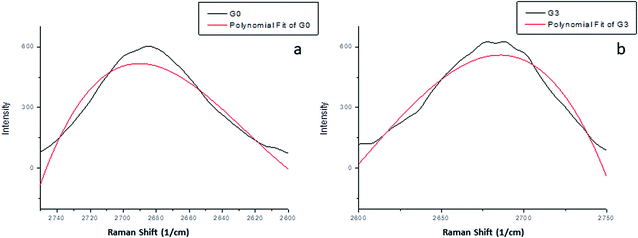 | ||
| Fig. 3 Raman 2D bands of (a) graphite (G0) and (b) GEG (G3); both plots show the measured data profile fit. | ||
FTIR analysis
The FTIR spectra of graphite (Fig. 4a) indicate the chemical inertness of bulk graphite.24 Whereas, the FTIR spectra of GEG showed various functional groups (Fig. 4b). A highly intense band at 3428 cm−1 was due to the O–H stretching vibration which indicated the presence of OH and/or COOH functional groups within the structure. Very weak bands at 2857 cm−1 and 2924 cm−1 were due to the symmetric and asymmetric stretching vibration of C–H bond, respectively. Carbonyl C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration (1739 cm−1), C
O stretching vibration (1739 cm−1), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibration of the unoxidized graphitic domain (1641 cm−1), O–H deformation (1462 cm−1), C–O stretching of epoxy groups (1237 cm−1) were seen in the FTIR spectra of GEG.25,26 Thus, the results obtained from FTIR confirmed the presence of various oxygen containing functional groups such as hydroxyl, epoxy, carboxyl, carbonyl, within the GEG structure. The results of the FTIR analyses performed by Song et al.,27 Marcano et al.,15 Galpaya et al.,24 Bao et al.,28 and Chowdhury et al.,29 were in agreement with the results obtained in the current research.
C stretching vibration of the unoxidized graphitic domain (1641 cm−1), O–H deformation (1462 cm−1), C–O stretching of epoxy groups (1237 cm−1) were seen in the FTIR spectra of GEG.25,26 Thus, the results obtained from FTIR confirmed the presence of various oxygen containing functional groups such as hydroxyl, epoxy, carboxyl, carbonyl, within the GEG structure. The results of the FTIR analyses performed by Song et al.,27 Marcano et al.,15 Galpaya et al.,24 Bao et al.,28 and Chowdhury et al.,29 were in agreement with the results obtained in the current research.
Scanning electron microscopy (SEM)
Fig. 5 shows the SEM micrographs of both of graphite and GEG. The GEG represented have a lamellar structure with two characteristics figures, (i) aggregated layers in a cluster form, and (ii) needle-like growth. These two different shapes indicate that the microbial isolate has significantly effects on the graphite powder morphology and structure, and a new morphological structure was formed. These results were matched with the Raman and FTIR results which proved the structural change between the original graphite powder and the newly formed GEG structure. | ||
| Fig. 5 SEM micrographs: (a and b) low and high magnification images of graphite powder, and (c and d) low and high magnification images of GEG. | ||
Suggested BIO mechanism for the formation of GEG structure
The exfoliation of graphite into graphitic layers is practically associated by two primary methods that were applied separately. The first method was mainly dependent on an oxidation process that involves the chemical incorporation of an atom between the sheet flakes of graphite. Liu et al., proposed that the bacterial cells probably produced metabolites that served as surfactants and promoted the contact of the bacterial cells to the graphitic material.11 This contact can result in extracellular electron transfer pathways at the cell/graphitic material interface that finally resulted in the formation of graphite oxide or graphene oxide.30 The other method was dependent on an incorporation of a big molecule (other than oxygen) that helped in the exfoliation of the stacked sheets of graphite, which was accompanied by chemical modifications. The current exfoliation mechanism is assumingly attributed to the first method. The large number of oxygen atoms detected by the characterization process outbalanced the ability of the isolated microbe to oxidize the graphite waste and exfoliate its flakes into other oxygenic graphitic structures.Molecular identification of the G3 isolate
The PCR amplification of 16S rRNA from the extracted genomic DNA of isolate G3 showed a 1500 bp amplified band when compared with a 1 kb DNA ladder. The amplified gene obtained was collected from the agarose gel, extracted, purified and submitted to sequencing (Sigma, Germany). The sequence obtained was compared with the 16S rRNA sequences deposited in the GenBank database and revealed that the sequence was 100% similar to that of Bacillus toyonensis strains. The sequence was deposited in the GenBank database under the accession number MN606105. The relatedness of the current isolate and other similar strains are represented in the phylogenetic tree shown in Fig. 6.Electrochemical measurements
 | ||
| Fig. 7 Cyclic voltammograms of prepared electrodes of (a) G0, and (b) GEG in PBS buffer solution (pH 7.0) with different concentrations of methanol (0–1.0 mL/100 mL buffer). | ||
However, the blank graphite electrode (G0) had no sensing behavior towards methanol in PBS buffer solution as shown in Fig. 7a.
The previously described measurements suggest that the sensing mechanism was as follows: firstly, methanol was adsorbed on the electrode surface, which was followed by its dissociation through various steps.31 The organic intermediate species of methanol oxidation were dissociated on the electrode surface by interacting with the surface OH− species.32 Finally, the adsorbed species effected the electrochemical behavior of the electrode.
As shown in Fig. 9, the biosensor response of the GEG electrode towards methanol was tested in 100 mL ethanol in the presence of different concentrations of methanol (0.0–0.01 volume ratios). It was noticeable that, there was a detectable response behavior of the GEG electrode even at relatively low concentrations of methanol. Calibration curves of the two ranges of potential (+0.3 and −0.5 V) are presented in Fig. 10.
 | ||
| Fig. 9 Cyclic voltammograms of the prepared electrode of GEG in ethanol with different concentrations of methanol (0–1.0 mL/100 mL ethanol). | ||
The sensing efficiency of the GEG electrode towards methanol in PBS buffer was higher than the detected efficiency in ethanol solution as shown in Fig. 8 and 10. The two regions of potential (+0.3 and −0.5 V) were used in both electrochemical tests of buffer and ethanol media for comparison.
Conclusions
The current work describes the use of green method to substitute the hazardous chemical materials previously used for graphite exfoliation process. The formation of GEG was characterized using Raman and FTIR spectroscopy combined with SEM. The GEG obtained presented high sensitivity toward low concentrations of methanol in both of phosphate buffered saline solution and ethanol. This applied green method is considered to be ecofriendly and a cost-effective method for the bioremediation of graphite waste into valuable graphitic material. It is recommended that more effort is made with this research for the complete exfoliation of graphite into carbon nanostructures.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to express their gratitude to the Research Center of Advanced Materials – King Khalid University, Saudi Arabia for support with grant number: RCAMS/KKU/002-19.References
- G. Maduraiveeran and W. Jin, Nanomaterials based electrochemical sensor and biosensor platforms for environmental applications, Trends Environ. Anal. Chem., 2017, 13, 10–23 CrossRef CAS.
- R. Batool, A. Rhouati, M. H. Nawaz, A. Hayat and J. L. Marty, A Review of the Construction of Nano-Hybrids for Electrochemical Biosensing of Glucose, Biosensors, 2019, 9, 46 CrossRef CAS PubMed.
- R. Kumar, B. R. Mehta, M. Bhatnagar, S. Ravi, S. Mahapatra, S. Salkalachen and P. Jhawar, Graphene as a transparent conducting and surface field layer in planar Si solar cells, Nanoscale Res. Lett., 2014, 9, 349 CrossRef PubMed.
- A. A. Balandin, S. Ghosh, W. Bao, I. Calizo, D. Teweldebrhan, F. Miao and C. N. Lau, Superior thermal conductivity of single-layer graphene, Nano Lett., 2008, 8, 902–907 CrossRef CAS PubMed.
- B. Marinho, M. Ghislandi, E. Tkalya, C. E. Koning and G. de With, Electrical conductivity of compacts of graphene, multi-wall carbon nanotubes, carbon black, and graphite powder, Powder Technol., 2012, 221, 351–358 CrossRef CAS.
- X. Zhu, J. Li, H. He, M. Huang, X. Zhang and S. Wang, Application of nanomaterials in the bioanalytical detection of disease-related genes, Biosens. Bioelectron., 2015, 74, 113–133 CrossRef CAS PubMed.
- S. Shi, W. Jiang, T. Zhao, K. E. Aifantis, H. Wang, L. Lin, Y. Fan, Q. Feng, F. z. Cui and X. Li, The application of nanomaterials in controlled drug delivery for bone regeneration, J. Biomed. Mater. Res., Part A, 2015, 103, 3978–3992 CrossRef CAS PubMed.
- V. Palermo, I. A. Kinloch, S. Ligi and N. M. Pugno, Nanoscale mechanics of graphene and graphene oxide in composites: a scientific and technological perspective, Adv. Mater., 2016, 28, 6232–6238 CrossRef CAS PubMed.
- Y. Liu, H. Cai, F. Wang, J. Wang, Q. Huang, Z. Fu and Y. Lu, Graphene on {116} faceted monocrystalline anatase nanosheet array for ultraviolet detection, Nanoscale, 2018, 10, 3606–3612 RSC.
- B. A. Lehner, V. A. Janssen, E. M. Spiesz, D. Benz, S. J. Brouns, A. S. Meyer and H. S. van der Zant, Creation of Conductive Graphene Materials by Bacterial Reduction Using Shewanella oneidensis, ChemistryOpen, 2019, 8, 888–895 CrossRef CAS PubMed.
- L. Liu, C. Zhu, M. Fan, C. Chen, Y. Huang, Q. Hao, J. Yang, H. Wang and D. Sun, Oxidation and degradation of graphitic materials by naphthalene-degrading bacteria, Nanoscale, 2015, 7, 13619–13628 RSC.
- B. Chen, H. Huang, X. Ma, L. Huang, Z. Zhang and L.-M. Peng, How good can CVD-grown monolayer graphene be?, Nanoscale, 2014, 6, 15255–15261 RSC.
- Y. Zhang, L. Zhang and C. Zhou, Review of chemical vapor deposition of graphene and related applications, Acc. Chem. Res., 2013, 46, 2329–2339 CrossRef CAS PubMed.
- S. Abdolhosseinzadeh, H. Asgharzadeh and H. S. Kim, Fast and fully-scalable synthesis of reduced graphene oxide, Sci. Rep., 2015, 5, 10160 CrossRef CAS PubMed.
- D. C. Marcano, D. V. Kosynkin, J. M. Berlin, A. Sinitskii, Z. Sun, A. Slesarev, L. B. Alemany, W. Lu and J. M. Tour, Improved synthesis of graphene oxide, ACS Nano, 2010, 4, 4806–4814 CrossRef CAS PubMed.
- C. K. Chua and M. Pumera, Chemical reduction of graphene oxide: a synthetic chemistry viewpoint, Chem. Soc. Rev., 2014, 43, 291–312 RSC.
- H. C. Schniepp, J.-L. Li, M. J. McAllister, H. Sai, M. Herrera-Alonso, D. H. Adamson, R. K. Prud'homme, R. Car, D. A. Saville and I. A. Aksay, Functionalized single graphene sheets derived from splitting graphite oxide, J. Phys. Chem. B, 2006, 110, 8535–8539 CrossRef CAS PubMed.
- R. Paasma, K. E. Hovda and D. Jacobsen, Methanol poisoning and long term sequelae–a six years follow-up after a large methanol outbreak, BMC Clin. Pharmacol., 2009, 9, 5 CrossRef PubMed.
- X.-Y. Zou, R. Xie, X.-J. Ju, W. Wang, Z. Liu, X.-Y. Li and L.-Y. Chu, Visual detection of methanol in alcoholic beverages using alcohol-responsive poly(N-isopropylacrylamide-co-N,N-dimethylacrylamide) copolymers as indicators, RSC Adv., 2014, 4, 61711–61721 RSC.
- A.-S. Al-Sherbini, M. Bakr, I. Ghoneim and M. Saad, Exfoliation of graphene sheets via high energy wet milling of graphite in 2-ethylhexanol and kerosene, J. Adv. Res., 2017, 8, 209–215 CrossRef CAS PubMed.
- A. C. Ferrari, Raman spectroscopy of graphene and graphite: disorder, electron–phonon coupling, doping and nonadiabatic effects, Solid State Commun., 2007, 143, 47–57 CrossRef CAS.
- C. Wongchoosuk and Y. Seekaew, 2D Materials, 2019 Search PubMed.
- S. Roscher, R. Hoffmann and O. Ambacher, Determination of the graphene–graphite ratio of graphene powder by Raman 2D band symmetry analysis, Anal. Methods, 2019, 11, 1224–1228 RSC.
- D. Galpaya, M. Wang, G. George, N. Motta, E. Waclawik and C. Yan, Preparation of graphene oxide/epoxy nanocomposites with significantly improved mechanical properties, J. Appl. Phys., 2014, 116, 053518 CrossRef.
- N. A. Kumar, S. Gambarelli, F. Duclairoir, G. Bidan and L. Dubois, Synthesis of high quality reduced graphene oxide nanosheets free of paramagnetic metallic impurities, J. Mater. Chem. A, 2013, 1, 2789–2794 RSC.
- Y. Huang, M. Zeng, J. Ren, J. Wang, L. Fan and Q. Xu, Preparation and swelling properties of graphene oxide/poly (acrylic acid-co-acrylamide) super-absorbent hydrogel nanocomposites, Colloids Surf., A, 2012, 401, 97–106 CrossRef CAS.
- J. Song, X. Wang and C.-T. Chang, Preparation and characterization of graphene oxide, J. Nanomater., 2014, 2014, 276143 Search PubMed.
- C. Bao, L. Song, W. Xing, B. Yuan, C. A. Wilkie, J. Huang, Y. Guo and Y. Hu, Preparation of graphene by pressurized oxidation and multiplex reduction and its polymer nanocomposites by masterbatch-based melt blending, J. Mater. Chem., 2012, 22, 6088–6096 RSC.
- D. R. Chowdhury, C. Singh and A. Paul, Role of graphite precursor and sodium nitrate in graphite oxide synthesis, RSC Adv., 2014, 4, 15138–15145 RSC.
- G. Wang, F. Qian, C. W. Saltikov, Y. Jiao and Y. Li, Microbial reduction of graphene oxide by Shewanella, Nano Res., 2011, 4, 563–570 CrossRef CAS.
- D.-S. Park, M.-S. Won, R. N. Goyal and Y.-B. Shim, The electrochemical sensor for methanol detection using silicon epoxy coated platinum nanoparticles, Sens. Actuators, B, 2012, 174, 45–50 CrossRef CAS.
- B. Tao, J. Zhang, S. Hui, X. Chen and L. Wan, An electrochemical methanol sensor based on a Pd–Ni/SiNWs catalytic electrode, Electrochim. Acta, 2010, 55, 5019–5023 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2020 |






