Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Base mediated spirocyclization of quinazoline: one-step synthesis of spiro-isoindolinone dihydroquinazolinones†
Rapolu Venkateshwarluab,
V. Narayana Murthya,
Krishnaji Tadiparthic,
Satish P. Nikumbha,
Rajesh Jinkalaa,
Vidavalur Siddaiahb,
M. V. Madhu babua,
Hindupur Rama Mohana and
Akula Raghunadh *a
*a
aTechnology Development Centre, Custom Pharmaceutical Services, Dr. Reddy's Laboratories Ltd., Hyderabad 500049, India
bDepartment of Organic Chemistry and FDW, Andhra University, Visakhapatnam 530045, India. E-mail: raghunadha@drreddys.com
cDepartment of Chemistry, CHRIST (Deemed to be University), Hosur Road, Bengaluru 560029, India
First published on 4th March 2020
Abstract
A novel approach for the spiro-isoindolinone dihydroquinazolinones has been demonstrated from 2-aminobenzamide and 2-cyanomethyl benzoate in the presence of KHMDS as a base to get moderate yields. The reaction has been screened in various bases followed by solvents and a gram scale reaction has also been executed under the given conditions. Based on the controlled experiments a plausible reaction mechanism has been proposed. Further the substrate scope of this reaction has also been studied.
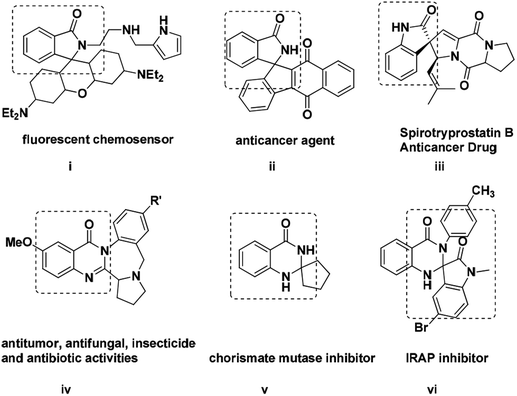
Because of their strong potent biologically activity, heterocyclic compounds have been a constant source of inspiration for the invention of new drugs especially for pharmaceutical and agro chemical industries.1 Indeed, investigation of novel methods for the synthesis of various natural products and heterocycles has always been a challenging task in modern organic chemistry. Amid all, spiro based scaffolds have been found to be very interesting because of their structural diversity. In spite of their intrinsic structures and immense biological activity there is a tremendous demand for the chemistry of spiro-isoindolinone dihydroquinazolinones.2 Indeed, nitrogen containing heterocyclic compounds like spiro-oxindole, spiro-isoindoline, spiro-isoindolinone are playing a significant role in medicinal chemistry and synthetic transformations.3 Moreover these compounds present in many natural products as a core unit like Lennoxamine, Zopiclone, Taliscanine and Pazinaclone (Fig. 1). In addition, many unnatural spiro-isoindolinones show significant biological activities acting as anti HIV-1, antiviral, antileukemic, anesthetic and antihypertensive agents.4,5 Notably, the spiro-isoindolinone dihydroquinazolinone unit has been found to be a combination of two potent pharmacophore units of dihydroquinazolinone and spiro-isoindolinone. Inspite of their remarkable biological activity afore mentioned, various methods have been developed for their synthesis like lithiation approaches, base mediated protocols, Diels–Alder and Wittig reactions, electrophilic and radical cyclization, metal-catalysed reactions and various electrochemical procedures.6
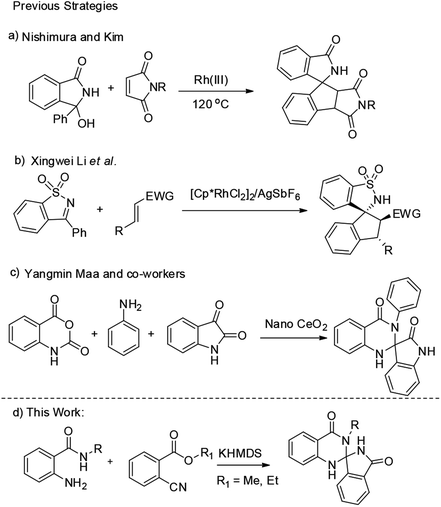
Previously, a number of metal catalyzed reactions have also been reported for the spiroannulations.7 Among all, Nishimura et al. developed an Ir(I) catalyzed [3 + 2] annulation of benzosultam and N-acylketimines with 1,3-dienes via C–H activation for the synthesis of aminocyclopentene derivatives. Further, Xingwei Li et al. developed a Rh(III)-catalysed [3 + 2] annulation of cyclic N-sulfonyl or N-acyl ketimines with activated alkenes for the preparation of various spirocyclic compounds.8 Recently Yangmin Ma et al. developed a one pot nano cerium oxide catalyzed synthesis of spiro-oxindole dihydroquinazolinone derivatives (Scheme 1).5c However, development of these type of novel compounds is always challenging and more attractive. Indeed, to the best of our knowledge there are no reports for the synthesis of spiro-isoindolinone dihydroquinazolinones. This led us to give more attention to study these compounds.
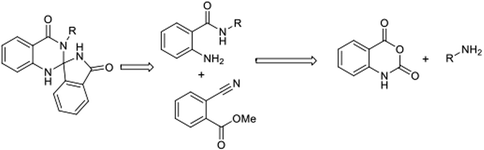
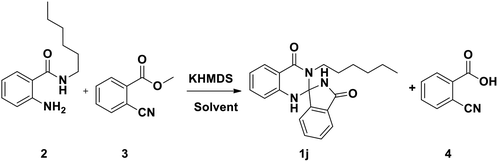
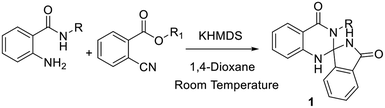
In continuation of our earlier efforts9 for the synthesis of various dihydroquinazolinones, herein we would like to report KHMDS mediated synthesis of novel spiro-isoindolinone dihydroquinazolinones. We envisioned the retro synthetic pathway for these compounds, as depicted in Scheme 2. Accordingly these compounds could be synthesised from 2-aminobenzamide and methyl-2-cyanobenzoate or ethyl-2-cyanobenzoate.
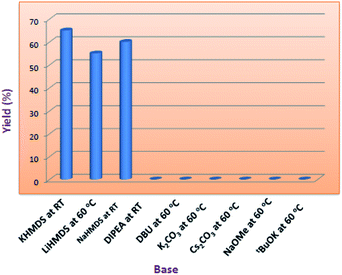
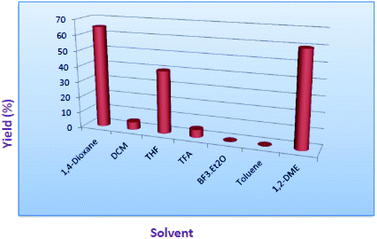
Indeed, in order to understand the reaction conditions, we have commenced the reaction by taking 2-amino-N-hexyl-benzamide (2) and methyl-2-cyanobenzoate (3) as a model substrates. However, in the initial phase of reaction optimisation, we have screened the reaction in different bases (Fig. 2) and to our delight amongst all the bases KHMDS, LiHMDS and NaHMDS were amenable to get moderate yields. However the reaction had not progressed at low temperatures (5 °C) and could improve the yield at room temperature. Moreover the reaction underwent complete conversion with 1.5 equivalents of base. Further, the reaction was also executed with ethyl-2-cyanobenzoate and could replicate the same yield. Incontinuation, the reaction was futile when the reaction was carried out in DIPEA, DBU, K2CO3 and Cs2CO3. Subsequently, the reaction in NaOMe and tBuOK produced exclusively the hydrolysis product (4) of methyl-2-cyanobenzoate (Table 1). To circumvent this problem the reaction has also been screened in various solvents.
| Entry | Base | Solvent | 1jb (%) | Cyano benzoic acid (4)(%) |
|---|---|---|---|---|
| a Reaction conditions: KHMDS (1 M, 1.5 mmol), 2-amino-benzamide (1 mmol) and methyl-2-cyanobenzoate (1.5 mmol) in 1,4-dioxane (10 mL).b Isolated yield. | ||||
| 1 | KHMDS | 1,4-Dioxane | 60 | 4 |
| 2 | NaHMDS | 1,4-Dioxane | 58 | 3 |
| 3 | NaOMe | 1,4-Dioxane | — | 60 |
| 4 | tBuOK | 1,4-Dioxane | — | 60 |
| 5 | KHMDS | THF | 40 | 5 |
| 6 | KHMDS | 1,2-DME | 60 | 5 |
| 7 | LiHMDS | 1,4-Dioxane | 55 | 5 |
Gratifyingly, among all the solvents 1,4-dioxane, THF and 1,2-dimethoxyethane were found to get good to moderate yields. Whereas, other solvents like DCM ended up with non-polar spots where as in toluene unknown polar impurity was observed. However, there is no reaction progress observed in the presence of trifluoroacetic acid as well as in BF3·Et2O as a solvent (Fig. 3).
With the optimized conditions in hand, we have explored the applicability of our reaction with various substrates by taking various groups like alkyl, cyclopropyl, cyclohexyl, cycloheptyl, benzyl, naphthyl, furan and to our delight all the substrates were well tolerated under the aforementioned optimal conditions (Table 2). The only limitation of this reaction is substituted cyanobenzoate.
| a Reaction conditions: KHMDS (1 M, 1.5 mmol), 2-amino-benzamide (1 mmol) and methyl/ethyl-2-cyanobenzoate (1.5 mmol) in 1,4-dioxane (10 mL). |
|---|
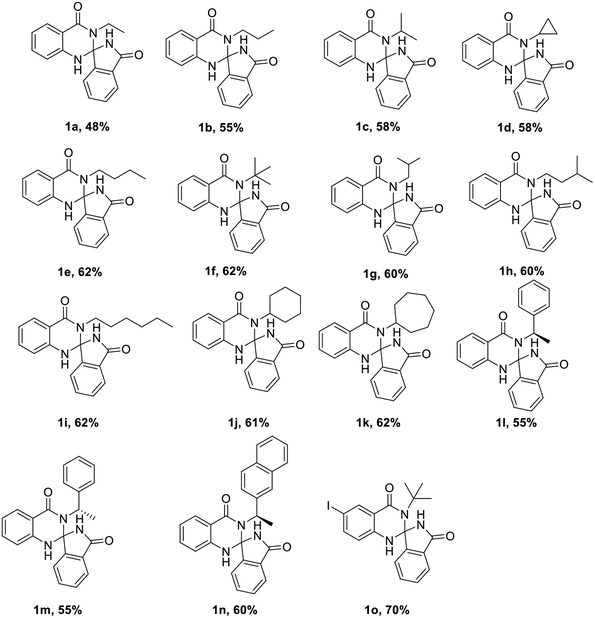 |
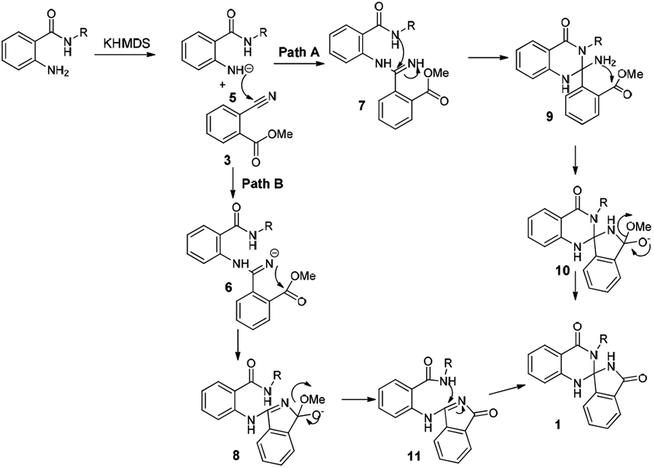
Based on the aforementioned studies and the literature reports, a plausible mechanism for this reaction has been predicted (Scheme 3). Indeed, to gain insight into the mechanism a series of control experiments have been executed under the similar reaction conditions. Initially the reaction has been carried out without base and both the starting materials were intact. Further the reaction without 2-aminobenzamide resulted hydrolysis product. To explore further, the reaction has also been executed on a 10 gram-scale for the synthesis of 1j and has successfully been demonstrated under the aforementioned optimized conditions.
The Scheme 3 describes a plausible mechanism for the preparation of compound 1. Initially, KHMDS will abstract N–H proton of amide and nucleophilic nitrogen will attack the cyanobenzoate to get imine intermediate 6 and 7, which on subsequent cyclization lead to the formation of 8 and 9. Finally, the compounds 8 and 9 underwent cyclization to get the spiro-isoindolinone dihydroquinazolinone 1.
Conclusion
In summary we have successfully synthesized atom economic, scalable and novel spiro-isoindolinone dihydroquinazolinones under mild conditions. The substrate scope of the reaction has been studied to get moderate yields and a possible mechanism for this reaction is proposed. Further investigation of these types of model substrates is underway in our laboratory.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to thank Dr. Reddy's Laboratory, Hyderabad for providing facilities to carry out this work.References
- (a) A. Dondoni, A. Massi, S. Sabbatini and V. Bertolasi, J. Org. Chem., 2002, 67, 6979 CrossRef CAS PubMed; (b) V. A. Chebanov, E. A. Muravyova, S. M. Desenko, V. I. Musatov, I. V. Knyazeva, S. V. Shishkina, O. V. Shishkin and C. O. Kappe, J. Comb. Chem., 2006, 8, 427 CrossRef CAS PubMed.
- (a) K. Ding, Y. Lu, Z. Nikolovska-Coleska, G. Wang, S. Qiu, S. Shangary, W. Gao, D. Qin, J. Stuckey, K. Krajewski, P. P. Roller and S. Wang, J. Med. Chem., 2006, 49, 3432 CrossRef CAS PubMed; (b) M. Kitajima, T. Nakamura, N. Kogure, M. Ogawa, Y. Mitsuno, K. Ono, S. Yano, N. Aimi and H. Takayama, J. Nat. Prod., 2006, 69, 715 CrossRef CAS PubMed; (c) N. Bacher, M. Tiefenthaler, S. Sturm, H. Stuppner, M. J. Ausserlechner, R. Kofler and G. Konwalinka, Br. J. Haematol., 2006, 132, 615 CrossRef CAS PubMed; (d) T. Mugishima, M. Tsuda, Y. Kasai, H. Ishiyama, E. Fukushi, J. Kawabata, M. Watanabe, K. Akao and J. Kobayashi, J. Org. Chem., 2005, 70, 9430 CrossRef CAS PubMed; (e) K. Ding, Y. Lu, Z. Nikolovska-Coleska, S. Qiu, Y. Ding, W. Gao, J. Stuckey, K. Krajewski, P. P. Roller, Y. Tomita, D. A. Parrish, J. R. Deschamps and S. Wang, J. Am. Chem. Soc., 2005, 127, 10130 CrossRef CAS PubMed; (f) R. Raunak, V. Kumar, S. Mukherjee, A. K. Poonam Prasad, C. E. Olsen, S. J. C. Schaeffer, S. K. Sharma, A. C. Watterson, W. Errington and V. S. Parmar, Tetrahedron, 2005, 61, 5687 CrossRef.
- (a) A. S. Girgis, Eur. J. Med. Chem., 2009, 44, 91 CrossRef CAS PubMed; (b) G. Periyasami, R. Raghunathan, G. Surendiran and N. Mathivanan, Eur. J. Med. Chem., 2009, 44, 959 CrossRef CAS PubMed; (c) W. Zhang and G. Mei-Lin, Bioorg. Med. Chem., 2009, 17, 2077 CrossRef CAS PubMed; (d) A. Amal Raj, R. Raghunathan, M. R. Sridevi Kumari and N. Raman, Bioorg. Med. Chem., 2003, 11, 407 CrossRef; (e) D.-G. Zhu, V. B. Gandhi, J. Gong, Y. Luo, X. Liu, Y. Shi, R. Guan, S. R. Magnone, V. Klinghofer, E. F. Johnson, J. Bouska, A. Shoemaker, A. Oleksijew, K. Jarvis, C. Park, R. De. Jong, T. Oltersdorf, Q. Li, S. H. Rosenberga and V. L. Giranda, Bioorg. Med. Chem. Lett., 2006, 16, 3424 CrossRef PubMed.
- (a) N. V. Lakshmi, Y. Arun and P. T. Perumal, Tetrahedron Lett., 2011, 52, 3437 CrossRef CAS; (b) T. H. Babu, A. A. Joseph, D. Muralidharan and P. T. Perumal, Tetrahedron Lett., 2010, 51, 994 CrossRef; (c) A. Nandakumar, P. Thirumurugan, P. T. Perumal, P. Vembu, M. N. Ponnuswamy and P. Ramesh, Bioorg. Med. Chem. Lett., 2010, 20, 4252 CrossRef CAS PubMed; (d) G. Shanthi, G. Subbulakshmi and P. T. Perumal, Tetrahedron, 2007, 63, 2057 CrossRef CAS; (e) K. Karthikeyan, R. S. Kumar, D. Muralidharan and P. T. Perumal, Tetrahedron Lett., 2009, 50, 7175 CrossRef CAS; (f) N. V. Lakshmi, P. Thirumurugan and P. T. Perumal, Tetrahedron Lett., 2010, 51, 1064 CrossRef CAS; (g) N. V. Lakshmi, P. Thirumurugan and P. T. Perumal, Synlett, 2010, 6, 955 Search PubMed; (h) K. Karthikeyan, P. M. Sivakumar, M. Doble and P. T. Perumal, Eur. J. Med. Chem., 2010, 45, 3446 CrossRef CAS PubMed; (i) K. Karthikeyan, P. T. Perumal, S. Etti and G. Shanmugam, Tetrahedron, 2007, 63, 10581 CrossRef CAS.
- (a) A. A. Mohammadi, M. Dabiri and H. Qaraat, Tetrahedron, 2009, 65, 3804 CrossRef CAS; (b) K. Engen, J. Savmarker, U. Rosenstrom, J. Wannbery, T. Lundback, A. Jenmalm-Jensen and M. Larhed, Org. Process Res. Dev., 2014, 18, 1582 CrossRef CAS; (c) J. Zhang, J. Zhao, L. P. Wang, J. Liu, D. C. Ren and Y. M. Ma, Tetrahedron, 2016, 72, 936 CrossRef CAS.
- (a) P. Jimonet, A. Boireau, M. Cheve, D. Damour, A. Genevois-Borella, A. Imperato, J. Pratt, J. C. R. Randle, Y. Ribeill, J. M. Stutzmann and S. Mignani, Bioorg. Med. Chem. Lett., 1999, 9, 2921 CrossRef CAS PubMed; (b) T. Ullrich, S. Krich, D. Binder, K. Mereiter, D. J. Anderson, D. Meyer and M. Pyerin, J. Med. Chem., 2002, 45, 4047 CrossRef CAS; (c) D. Stoermer and C. H. Heathcock, J. Org. Chem., 1993, 58, 564 CrossRef CAS; (d) J. Auerbach and S. M. Weinreb, J. Am. Chem. Soc., 1972, 94, 7172 CrossRef CAS PubMed.
- (a) L. Dong, C. H. Qu, J. R. Huang, W. Zhang, Q. R. Zhang and J. G. Deng, Chem.–Eur. J., 2013, 19, 16537 CrossRef CAS PubMed; (b) J. R. Huang, Q. Song, Y. Q. Zhu, L. Qin, Z. Y. Qian and L. Dong, Chem.–Eur. J., 2014, 20, 16882 CrossRef CAS PubMed; (c) J.-R. Huang, L. Qin, Y.-Q. Zhu, Q. Song and L. Dong, Chem. Commun., 2015, 51, 2844 RSC; (d) S. S. Li, L. Wu, L. Qin, Y. Q. Zhu, F. Su, Y. J. Xu and L. Dong, Org. Lett., 2016, 18, 4214 CrossRef CAS PubMed; (e) T. Nishimura, Y. Ebe and T. Hayashi, J. Am. Chem. Soc., 2013, 135, 2092 CrossRef CAS PubMed; (f) T. Nishimura, M. Nagamoto, Y. Ebe and T. Hayashi, Chem. Sci., 2013, 4, 4499 RSC; (g) M. B. Zhou, R. Pi, M. Hu, Y. Yang, R. J. Song, Y. Xia and J. H. Li, Angew. Chem., Int. Ed., 2014, 53, 11338 CrossRef CAS PubMed; (h) H. Liu, J. Li, M. Xiong, J. Jiang and J. Wang, J. Org. Chem., 2016, 81, 6093 CrossRef CAS PubMed; (i) S. T. Mei, H. W. Liang, B. Teng, N. J. Wang, L. Shuai, Y. Yuan, Y. C. Chen and Y. Wei, Org. Lett., 2016, 18, 1088 CrossRef CAS PubMed; (j) M. V. Pham and N. Cramer, Chem.–Eur. J., 2016, 22, 2270 CrossRef CAS PubMed.
- (a) M. Nagamoto and T. Nishimura, Chem. Commun., 2014, 50, 6274 RSC; (b) Y. Ebe, M. Hatano and T. Nishimura, Adv. Synth. Catal., 2015, 357, 1425 CrossRef CAS; (c) M. Nagamoto, D. Yamauchi and T. Nishimura, Chem. Commun., 2016, 52, 5876 RSC; (d) L. Bingxian, H. Panjie, Z. Ying, L. Yunyun, B. Dachang and L. Xingwei, Org. Lett., 2017, 19, 5402 CrossRef PubMed.
- (a) V. N. Murthy, N. P. Satish, T. Krishnaji, M. V. Madhubabu, J. Subba Rao, L. V. Rao and A. Raghunadh, RSC Adv., 2018, 8, 22331 RSC; (b) S. P. Kumar, V. N. Murthy, K. R. Ganesh, G. S. Rao, T. Krishnaji and A. Raghunadh, ChemistrySelect, 2018, 3, 6836 CrossRef; (c) C. Jaganmohan, K. P. V. Kumar, G. S. Reddy, S. Mohanty, J. Kumar, B. V. Rao, T. Krishnaji and A. Raghunadh, Synth. Commun., 2018, 48, 168 CrossRef CAS; (d) M. V. Madhubabu, R. Shankar, S. Satish More, M. V. Basaveswara Rao, U. K. Syam Kumar and A. Raghunadh, RSC Adv., 2016, 6, 36599 RSC; (e) R. K. Raghavendra, M. Ramamohan, A. Raghunadh, B. M. Suresh, K. S. Praveen, D. Kalita, E. Laxminarayana, B. Prasad and M. Pal, RSC Adv., 2015, 5, 61575 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra09567e |
| This journal is © The Royal Society of Chemistry 2020 |