Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Efficient deoxygenation of waste cooking oil over Co3O4–La2O3-doped activated carbon for the production of diesel-like fuel†
G. Abdulkareem-Alsultan *abe,
N. Asikin-Mijan
*abe,
N. Asikin-Mijan a,
G. Mustafa-Alsultanf,
H. V. Lee
a,
G. Mustafa-Alsultanf,
H. V. Lee d,
Karen Wilson
d,
Karen Wilson e and
Y. H. Taufiq-Yap*ac
e and
Y. H. Taufiq-Yap*ac
aCatalysis Science and Technology Research Centre (PutraCat), Faculty of Science, Universiti Putra Malaysia, 43400 UPM Serdang, Selangor, Malaysia. E-mail: taufiq@upm.edu.my; Fax: +603-89466758; Tel: +603-89466809
bChemical and Environmental Engineering Department, Faculty of Engineering, Universiti Putra Malaysia, 43400 UPM Serdang, Selangor, Malaysia. E-mail: kreem.alsultan@yahoo.com; Tel: +601-82534058
cChancellery Office, Universiti Malaysia Sabah, 88400, Kota Kinabalu, Sabah, Malaysia
dNanotechnology & Catalysis Research Centre (NanoCat), Institute of Postgraduate Studies, University Malaya, 50603 Kuala Lumpur, Malaysia
eSchool of Science, RMIT University, Melbourne, VIC 3001, Australia
fMaintenance of Rotary Machine Equipment, South Refineries Company, Basra, Iraq
First published on 30th January 2020
Abstract
Untreated waste cooking oil (WCO) with significant levels of water and fatty acids (FFAs) was deoxygenated over Co3O4–La2O3/ACnano catalysts under an inert flow of N2 in a micro-batch closed system for the production of green diesel. The primary reaction mechanism was found to be the decarbonylation/decarboxylation (deCOx) pathway in the Co3O4–La2O3/ACnano-catalyzed reaction. The effect of cobalt doping, catalyst loading, different deoxygenation (DO) systems, temperature and time were investigated. The results indicated that among the various cobalt doping levels (between 5 and 25 wt%), the maximum catalytic activity was exhibited with the Co![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) La ratio of 20
La ratio of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 wt/wt% DO under N2 flow, which yielded 58% hydrocarbons with majority diesel-range (n-(C15 + C17)) selectivity (∼63%), using 3 wt% catalyst loading at a temperature of 350 °C within 180 min. Interestingly, 1 wt% of catalyst in the micro-batch closed system yielded 96% hydrocarbons with 93% n-(C15 + C17) selectivity within 60 min at 330 °C, 38.4 wt% FFA and 5% water content. An examination of the WCO under a series of FFA (0–20%) and water contents (0.5–20 wt%) indicated an enhanced yield of green diesel, and increased involvement of the deCOx mechanism. A high water content was found to increase the decomposition of triglycerides into FFAs and promote the DO reaction. The present work demonstrates that WCO with significant levels of water and FFAs generated by the food industry can provide an economical and naturally replenished raw material for the production of diesel.
20 wt/wt% DO under N2 flow, which yielded 58% hydrocarbons with majority diesel-range (n-(C15 + C17)) selectivity (∼63%), using 3 wt% catalyst loading at a temperature of 350 °C within 180 min. Interestingly, 1 wt% of catalyst in the micro-batch closed system yielded 96% hydrocarbons with 93% n-(C15 + C17) selectivity within 60 min at 330 °C, 38.4 wt% FFA and 5% water content. An examination of the WCO under a series of FFA (0–20%) and water contents (0.5–20 wt%) indicated an enhanced yield of green diesel, and increased involvement of the deCOx mechanism. A high water content was found to increase the decomposition of triglycerides into FFAs and promote the DO reaction. The present work demonstrates that WCO with significant levels of water and FFAs generated by the food industry can provide an economical and naturally replenished raw material for the production of diesel.
1. Introduction
A range of geopolitical, economic and environmental issues (e.g. global warming, diminishing petroleum deposits, increasing crude oil prices and demand for energy independence) have promoted recent interest in biofuels. Accordingly, fuels obtained from biomass (biofuels) are considered as a carbon-neutral and renewable substitute for fossil fuels. In particular, much attention has been paid to bio-oils, which can readily be obtained via the pyrolysis (thermal and catalytic cracking) of biomass. Nevertheless, the considerably low selectivity of this approach, which generates a range of unwanted products (primarily oxygenates), requires additional development. On the other hand, the well-established biofuel, which is known as biodiesel or fatty acid methyl esters (FAMEs), is composed of highly oxygenated compounds and suffer from several inevitable drawbacks such as poor storage stability and poor cold-flow behaviour.1,2 Consequently, elimination of oxygen-bonded compounds is necessary to achieve desirable diesel properties. Significantly, the high oxygen content of biodiesel and bio-oils gives rise to most of their disadvantages, including a lower heat content than that of conventional fossil fuels. Thus, the production of (oxygen-free) hydrocarbons from the catalytic deoxygenation (DO) of fatty acids and their derivatives has attracted interest. In principle, DO involves oxygen extraction from fatty acids and their derivatives via decarboxylation (–CO2) and decarbonylation (–CO, H2O) (deCOx) reactions under an H2-free atmosphere.There are several solid acid catalysts containing metal sulfides, noble metals, metal phosphides, metal carbides, metals, and transition metal oxides with supporting materials reported for the DO reaction. Sulfonated acidic catalysts show high affinity toward the production of hydrocarbon fractions, but suffer from sulphur leaching and affect the quality of oil.3,4 Meanwhile, high acidity noble metal catalysts are costly,5 which make them unattractive. Medium acidic catalysts, such as metal phosphates and carbides, and mesoporous catalysts, such as SBA-15, MCM-41, and HMS catalysts, are generally selected for biofuel production since they exhibit low affinity toward the deactivation of the catalyst together with high DO activity.6 However, the complexity of their synthesis makes them unappealing.7 Thus, the drawbacks of the above mentioned catalysts encourage the exploration of non-sulphated, low-cost, and facile catalysts for the production of high-quality renewable fuel.
The use of metal oxides in the DO reaction has been continuously reported and proven to be selective towards the formation of hydrocarbon fractions.8 Common transition metal oxides (TMO) that are used in the DO process include Mn, Ni, Co, W, Mo, Cu, Fe and Zn. Among them, Co exhibits the highest rate of decarboxylation9,10 and formation of rich-paraffin species. The enhancement of decarboxylation by Co is realized through its excellent acidic–basic properties.11 Furthermore, Co also facilitates the formation of olefins (alkenes) via decarbonylation pathways, producing water as a by-product. However, water has a negative effect on Co, which is easily oxidized and deactivated. Recently, the incorporation of a Ca promoter in Co-supported SiO2–Al2O3 led to substantial catalyst stability and suggested the stability of Co relies on the incorporation of a basic metal, where the basic metal promoter leads to the inhibition of water production by lowering the affinity for the decarbonylation reaction.12 For instance, a report on the DO of waste cooking oil (WCO) via deCOx over acid-base catalysts (CaO–La2O3/ACnano and Ag2O3–La2O3/ACnano) recently disclosed that the basic sites in La2O3 play key role in the removal of C–O-bonded species via decarboxylation, and thereby lead to greater enhancement of the DO activity and product selectively toward n-(C15 + C17) fractions.3,12 Furthermore, the basic sites of La2O3 suppress coke colonization. Recently, Benito and co-workers examined the effect of the addition of La2O3 to Ni/Al2O3, and observed that La2O3 promotion led to increased catalyst longevity, probably due to the oxygen donation from La2O2CO3 species to produce CO from carbon deposits.13 Even though a series of binary oxide CaO–La2O3 and Ag2O3–La2O3-supported ACnano have been studied in details,3,12 their DO performance was unsatisfactory and rapidly deactivated by coke deposition. Based on previous findings, the incorporation of Co in La2O3/AC may be promising for the improvement in DO performance and the enhancement of anti-coking character. Furthermore, the use of Co2O3–La3O4 as a metal promoter on AC support has not been reported to date in the literature.
As is known, DO under an inert atmosphere is not beneficial for the formation of desired paraffins products. Green diesel consists of low mixtures of paraffin compounds, which are typically not beneficial for reducing NOx emission and possess a low cetane number. Furthermore, DO under an inert atmosphere prevents the production of a high mass of green diesel since the volatile liquids with non-condensable gases easily escape during the reaction. Thus, since the DO reaction atmosphere plays an important role in enhancing the formation of paraffins, in the present work, WCO was deoxygenized in a semi-batch inert flow reactor and micro-batch reactor over the binary oxide of Co3O4–La2O3 on ACnano catalyst. The micro-batch reactor is a one-dimensional reactor (10 cm length × 0.8 cm diameter), providing rapid heat and high mass transfer rates.14 Although the micro-batch reactor has been successfully utilized for the continuous operation of fast and highly exothermic reactions and transformations involving toxic and explosive chemicals such as hydrogenation, alkylation, oxidation, and polymerization,15–17 this reactor system is rarely adopted in the DO reaction for the production of green diesel. Instead of varying the DO reactor set-up, the stoichiometric study of Co content (5–25 wt%), reaction time, catalyst loading, water and FFA content were explored. This study provides new insight into the interaction of Co3O4–La2O3 on ACnano support and offer an alternative approach to curb coke deposition and improve the longevity of the catalyst during the DO reaction together with the formation of saturated hydrocarbon. The present work also provides new in-depth insight on the DO of WCO in inert and closed reaction systems.
2. Materials and methods
2.1 Materials
For the present study, the feedstock (palm oil-based WCO supplied by a restaurant at Serdang, Selangor, Malaysia) was used without purification. Table 1 summarises the characteristics of the crude WCO, including free fatty acid content and water content. The level of free fatty acids (18.4% by weight) is reflected by the total acid number (TAN) of 36.8 mg KOH g−1. The fatty acid content of the WCO included the saturated palmitic (45.68%), stearic (4.25%) and myristic acid (1.3%), together with the unsaturated oleic (40.19%) and linoleic acid (7.90%). Anhydrous silver nitrate (CoNO3, 99.99% pure) was supplied by Sigma Aldrich (UK), lanthanum nitrate hexahydrate (La (NO3)3·6H2O, 99.0% pure) was supplied Merck (Germany), phosphoric acid (H3PO4, 85.0–87.0% pure) was supplied by J. T. Baker (USA), and Walnut shells (Juglans sp.) were obtained from a market in Basra (Iraq). Liquid alkane and alkene standards (n = C8–C20) for gas chromatographic analysis were supplied by Sigma Aldrich and used without additional purification. The internal standard was 1-bromohexane. GC-grade n-hexane, purity >98%, supplied by Merck (Germany), was used for dilution. N2 gas with 99% purity was supplied by Linde Malaysia Sdn Bhd.| Properties | Value |
|---|---|
| Density (g cm−3) | 0.87 |
| Viscosity (mm2 s−1) | 4.85 |
| Moisture content (% wt) | 1–5 |
| Acid value (mg KOH g−1) | 36.81 |
| FFA content (%) | 18.40 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|
| Fatty acid composition | |
| Myristic acid, C14:0 | 1.93 |
| Palmitic acid, C16:0 | 45.68 |
| Stearic acid, C18:0 | 4.25 |
| Oleic acid, C18:1 | 40.19 |
| Linoleic acid, C18:2 | 7.95 |
2.2 Catalyst preparation
A two-step process and additional treatment with H3PO4 were chosen for the preparation of activated carbons with a prolate shape, surface functionality and high purity. Initially, 50 g of dried walnut shell powder with a particle size in the range of 1–200 mesh was subjected to pyrolysis in an N2 atmosphere at a heating rate of 5 °C min−1 up to 700 °C for 5 h. The sample was heated in a tube furnace using a ceramic vertical reactor. The carbonaceous product was activated by concentrated phosphoric acid at 158 °C for 12 h, then washed with hot water to pH = 7 and further dried in an oven overnight to form activated carbon (AC). The AC was then infused with 20 wt% La(NO3)·6H2O and 5–30 wt% Co(NO3) under continuous stirring for 6 h. The impregnation of the metal on the support was optimized via the vacuum impregnating procedure, and this was facilitated with a vacuum machine (Edwards RV12) at 6.3 × 10−6 mbar. Subsequently, a Stuart RE300DB rotary evaporator was employed for drying, under heating and reduced pressure for 3 h at 55 °C and −60 kPa, respectively. Subsequently, the AC-doped metals underwent calcination at 700 °C, which lasted for a 4 h period under an N2 flow. The metal-doped AC was denoted as Co2O3(x)–La2O3(y)/ACnano, where x = 5, 10, 15, 20, 25, and 30 wt%.2.3 Material characterization
For the purpose of identifying the chemical composition and the state of dispersion of the metal-doped AC catalysts before and after the chemical reaction, X-ray diffraction (XRD) analysis was applied (Shimadzu, model XRD-6000). The Brunauer–Emmett–Teller (BET) technique was employed using an N2 adsorption/desorption analyser (Thermo-Finnigan Sorpmatic 1990 series) to obtain information on the surface area, and pore size and volume distribution of the catalysts. Typically, the catalyst sample was degassed at a temperature of 150 °C overnight to eliminate foreign gases and moisture from the surface of the catalysts. The analysis was performed at −196 °C, and the desorption and adsorption of nitrogen (N2) on the surface of the catalyst was performed in a vacuum chamber. Temperature-programmed desorption (TPD) was employed to examine the acidity and basicity of the catalysts. Here, two probe molecules, NH3 and CO2, were employed (TPD–CO2 and TPD–NH3, respectively). A Thermo Finnigan TPD/R/O 1100 instrument equipped with a thermal conductivity detector (TCD) was employed to conduct the examination. Typically, about 0.05 g of catalyst was pre-treated with an N2 gas flow, which lasted for 30 min at a temperature of 250 °C. This process was followed by exposing the catalyst to CO2 gas for adsorption within 1 h and flushing with N2 gas to remove excess CO2. Subsequently, CO2 desorption from the basic sites of the catalyst was identified by using the TCD under a flow of helium gas (30 mL min−1) in the temperature range of 50–900 °C. The temperature was kept constant for 30 min. NH3 adsorption/desorption was performed using similar steps as the TDP-CO2 method. An electron microscope (LEO 1455 VP) was employed to record field-emission scanning electron microscopy (FESEM) images, which was equipped with energy dispersive X-ray (EDX) (facilitated by a Rayny EDX-720 spectrometer) to determine the elemental composition of the catalyst samples (P, Co, La, O and C). X-ray photoelectron spectroscopy (XPS) was used to study the chemical states on the surface of the Co2O3–La2O3/ACnano sample, and measurements were performed with a Microprobe PHI Quantera II under ultrahigh vacuum (UHV) conditions (base vacuum of ∼10−8 Pa) at room temperature. For the X-ray source, radiation of Mg Kα (hν = 1253.6 eV) was employed. Furthermore, thermal analysis using a thermogravimetry analyzer (TGA) (TGA 1000i, Instrument Specialists Inc, United States) was applied to identify the level of deposition of coke and carbon on the spent catalyst. Both the catalyst and spent catalyst were heated in the temperature range of 25–900 °C range (30 °C min−1) with an airflow of 40 mL min−1.2.4 Catalytic deoxygenation of WCO
The DO of WCO was carried out in a 250 mL mechanically stirred semi-batch reactor. WCO (∼10 g) was placed in the reactor together with 0.5 wt% of catalyst, and prior to the initiation of the experiment, inert N2 gas was flushed into the reactor accompanied by continuous stirring of the mixture. The purpose of this process was to ensure that the O2 and moisture from the air was eliminated before heat was applied. Afterwards, the mixture was continuously treated with a constant flow of N2 (flow rate of 20 mL min−1) at atmospheric pressure to maintain the inert conditions for the DO reaction. The DO reaction was performed at 300 °C for 1 h. A cooler was used to facilitate the condensation of the deoxygenated products, where the condensates were collected in a vessel of the batch reactor. After completing each experiment, the mixture was cooled to room temperature. For comparison, a 10 cm length and 0.8 cm diameter stainless steel tube was used as a micro batch reactor closed system, where initially, the catalyst and WCO were mixed and placed in the micro-reactor and then the system was flushed using nitrogen gas for 10 min with a flow of 3 mL min−1 and then the valve was closed and the reactor heated at a heating rate of 50 °C min−1 to the desired temperature, and after the reaction was finished, the system was cooled using ice-cold water. The mixture of catalyst, char and product were separated using a centrifuge (3500 rpm for 10 min). The final deoxygenated liquid products were further analysed by using gas chromatography with a flame ionization detector (GC-FID), gas chromatography-mass spectrometry (GC-MS) and Fourier-transform infrared spectroscopy.2.5 Water and FFA content effect
The water and FFA contents have a direct effect on the DO reaction. Fatty acids are known to be the primary composition during the DO reaction of triglycerides. Therefore, oleic acid was chosen as a model reactant. The water content in WCO will affect hydrolysis process of TG, where the TG molecules split into free fatty acids and propane. To vary the FFA content, the WCO used in this study consisted of average triglycerides of 92 wt%, free fatty acids of 6.6 wt%, and unknown impurities of 1.4 wt%. The feedstock was free of water. Additional water or free fatty acid (oleic acid) was mixed with the WCO to adjust the initial parameters for the reaction tests. The fatty acid composition of the WCO was evaluated according to the standard AOCS method (1997), F9a-44 (Table 1). The WCO consisted of C14–C20 fatty acids with the majority being unsaturated C18:1 (oleic acid) and saturated C16:0 (palmitic acid).2.6 Product analysis
The reaction was analysed using an Agilent 7890A gas chromatograph (GC) fitted with a flame-ionization detector and an HP-5 capillary column (30 m × 0.25 mm × 0.33 mm). The sample (1 mL) was injected with a split ratio of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The carrier gas was nitrogen with a flow rate of 11 mL min−1. The injector temperature was 280 °C and the detector temperature was 300 °C. The oven was programmed to maintain a temperature of 40 °C for 4 min before ramping up to 280 °C at a rate of 10 °C min−1, maintaining this final temperature for 5 min. The products of the reaction were identified by comparing the retention times on the gas chromatograph with known standards and by examining the fragmentation patterns on an Agilent 5970 mass spectrometric (MS) detector. Calibration curves were used to provide quantitative analysis for each compound of interest. The determination of hydrocarbon yield (X) on the catalyst performance was evaluated by GC-FID using eqn (1) as follows:18
1. The carrier gas was nitrogen with a flow rate of 11 mL min−1. The injector temperature was 280 °C and the detector temperature was 300 °C. The oven was programmed to maintain a temperature of 40 °C for 4 min before ramping up to 280 °C at a rate of 10 °C min−1, maintaining this final temperature for 5 min. The products of the reaction were identified by comparing the retention times on the gas chromatograph with known standards and by examining the fragmentation patterns on an Agilent 5970 mass spectrometric (MS) detector. Calibration curves were used to provide quantitative analysis for each compound of interest. The determination of hydrocarbon yield (X) on the catalyst performance was evaluated by GC-FID using eqn (1) as follows:18
 | (1) |
 | (2) |
3. Results and discussion
3.1 Characterization of Co3O4(x)–La2O3(y)/ACnano catalysts
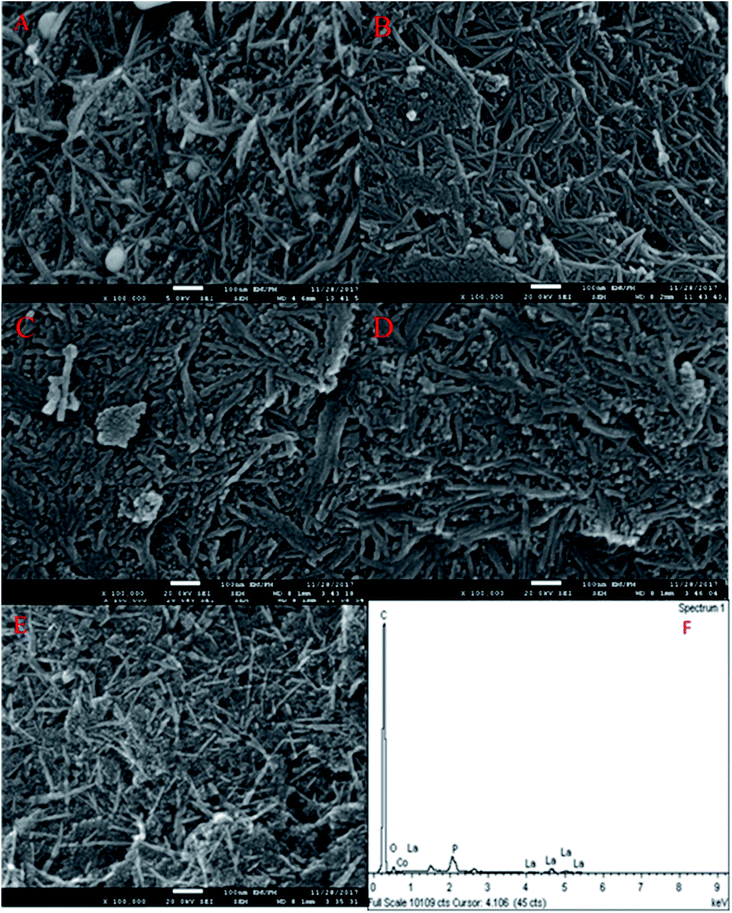
The FESEM images of the nanosized walnut shell-derived AC catalyst were presented in our recent report.3 The result indicated the presence of nanocrystalline fibrous particles with a thickness in the range of 15–20 nm on the AC catalyst support. Interestingly, the FESEM images of the Co3O4(x)–La2O3(y)/ACnano catalysts, where x = 5, 10, 15, 20, 25 wt% and y = 20 wt%, showed well-dispersed, needle-like particles with a long and thin slab of nano-structures (Fig. 1A–E). The particle length and diameter were within the range of 190–290 nm and 11–23 nm, respectively. A further increment in Co species led to the formation of compact micro-sized agglomerates. Based on the EDX analysis, the white spherical particles are attributed to Co species (identified by EDX analysis), which decreased in size and disappeared with an increase in the content of Co species contents. The change in morphology at different Co loading was due to the dopant effect, and thus resulted in aggregation.19 The needle-like structures were deformed as the Co aggregates grew excessively on the catalyst surface. Fig. 1F and Table 2 show the EDX analysis of the Co3O4(x)–La2O3(y)/ACnano catalysts, which indicate the presence of C, O, P, La, and Co elements. Thus, the treatment of AC with H3PO4 resulted the presence of P,20,21 while the successful surface impregnation of La and Co was reflected by the presence of the corresponding elements in the EDX analysis. According to Table 2, the atomic ratio of Co![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) La in the catalysts was consistent with the expected (x
La in the catalysts was consistent with the expected (x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) y) values of 5
y) values of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, 10
20, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, 15
20, 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, 20
20, 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 and 25
20 and 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20. Overall, each of the Co3O4(x)–La2O3(y)/ACnano catalysts had a maximum carbon content of >39%, which is expected to provide outstanding mechanical properties for both stability and thermal catalytic activity during DO reactions.
20. Overall, each of the Co3O4(x)–La2O3(y)/ACnano catalysts had a maximum carbon content of >39%, which is expected to provide outstanding mechanical properties for both stability and thermal catalytic activity during DO reactions.
| Catalysta | Element compositionb (%) | ||||
|---|---|---|---|---|---|
| C | O | P | La | Co | |
| a Theoretical Co/La atomic ratio of catalyst.b Experimental Co/La atomic ratio in the synthesized catalyst determined by EDX. | |||||
| Co3O4(5)–La2O3(20%)/ACnano | 52.44 | 11.74 | 12.71 | 18.73 | 4.38 |
| Co3O4(10)–La2O3(20%)/ACnano | 49.37 | 10.46 | 9.27 | 19.60 | 11.30 |
| Co3O4(15)–La2O3(20%)/ACnano | 47.63 | 9.40 | 9.24 | 18.39 | 15.34 |
| Co3O4(20)–La2O3(20%)/ACnano | 43.16 | 8.43 | 10.21 | 19.23 | 18.97 |
| Co3O4(25)–La2O3(20%)/ACnano | 38.98 | 5.31 | 9.47 | 19.50 | 26.74 |
Fig. 2A presents the XRD analysis of AC and Co3O4(x)–La2O3(y)/ACnano catalysts. AC displayed a peak centred at 2θ = 25.0°, which indicates the presence of amorphous carbonaceous materials.22 In the case of the Co3O4(x)–La2O3(y)/ACnano catalysts, the amorphous AC was altered to a highly crystalline state after the incorporation of the Co–La metals. Here, the XRD analysis revealed peaks at 2θ = 9.1°, 17.1°, 31.5°, 36.7°, 43.1°, 54.5°, 59.3° and 62.7°, which correspond to the Co3O4 phase (JCPDS File no. 00-001-1152).23 The XRD peaks at 2θ = 14.1°, 18.1°, 25.3°, 26.6°, 29.6°, 39.2°, 46.6°, 54.6°, and 77.6° are attributed to the La2O3 phase (JCPDS File no. 00-037-1497).24,25 The highly crystalline nature of the Co3O4(x)–La2O3(y)/ACnano catalyst is due to the intercalation of La and Co ions in the AC matrix.26,27 Upon increasing the Co dosage (from 5 to 25 wt%), the occurrence of bimetallic lanthanum cobalt oxide phases (CoLaO3) at 2θ = 23.3°, 40.2° and 69.9° (JCPDS File no. 00-006-0491)28,29 (Fig. 2B and C) evidenced the effective integration of the bimetallic (Co–La) structure into the ACnano catalyst. Moreover, the bimetallic CoLaO3 peaks corresponding to the (110)1 plane gradually shifted to a lower angle with an increase in the Co content. This phenomenon indicates that more Co atoms were located deep in the La2O3 lattice with an increase in the Co content, indicating that the higher the Co content, the higher the amount of Co–LaO3 solid solution is formed.2 The homogeneous substitution of Co with La easily occurred because the radius of La3+ (1.16 Å) is larger than that of Co2+ (0.65 Å). Also the incorporation of Co-rich species in the Co3O4(x)–La2O3(y)/ACnano catalyst promoted the dispersion of CoLaO3 and reduced the crystallinity of CoLaO3.3,4 The present finding is consistent with the EDX results, where the oxygen content was reduced by 54% after the incorporation of 25 wt% of Co species (Table 2). Although the atomic radius of oxygen (1.67 Å) is larger than that of Co (0.65 Å), excess replacement by Co increased the size of the unit cell for CoLaO3.11 The increment in the average crystallite size for the Co3O4(x)–La2O3(y)/ACnano catalyst with an increase in Co dosage is depicted in Table 3.
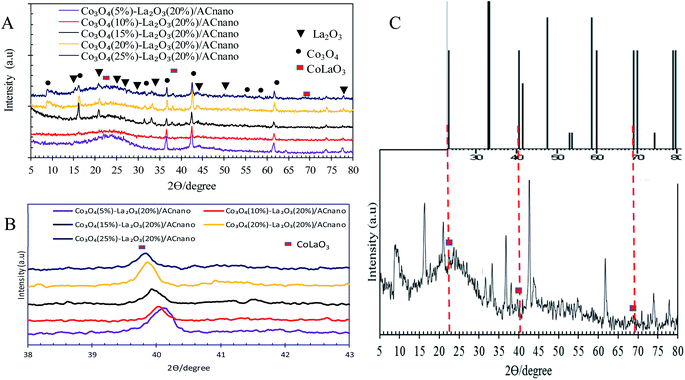 | ||
| Fig. 2 (A) XRD patterns of Co3O4(x)–La2O3(20%)/ACnano catalysts, (B) bimetallic phase shifting and (C) bimetallic phase of CoLa2O3 of Co3O4(25)–La2O3(20%)/ACnano catalyst. | ||
| Catalyst | XRDa | BETb | TPD-NH3c | TPD-CO2c | |||
|---|---|---|---|---|---|---|---|
| Crystallite sizea (nm) | Surface areab (m2 g−1) | Pore volumeb (cm3 g−1) | NH3 desorption temperaturec (°C) | Amount of NH3 adsorbedc (μmol g−1) | CO2 desorption temperaturec (°C) | Amount of CO2 adsorbedc (μmol g−1) | |
| a The crystallite size was determined from the highest intensity line broadening of the XRD peak at 2θ = 43.1°.b BET surface area.c NH3/CO2 desorption peak for all the catalysts. | |||||||
| Co3O4(5%)–La2O3(20%)/ACnano | 38.40 | 668.53 | 0.64 | 127/730/806 | 148.74/2584.76/19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 266.00 266.00 |
635 | 3289.72 |
| Co3O4(10%)–La2O3(20%)/ACnano | 44.49 | 634.76 | 0.61 | 130/634/800 | 214.39/1019.17/16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 247.95 247.95 |
546 | 3510.07 |
| Co3O4(15%)–La2O3(20%)/ACnano | 46.23 | 514.36 | 0.57 | 138/819/842 | 249.84/1191.16/18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 843.94 843.94 |
906 | 3865.96 |
| Co3O4(20%)–La2O3(20%)/ACnano | 48.41 | 501.79 | 0.52 | 135/609/831 | 254.21/4042.96/26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 165.70 165.70 |
939 | 5292.06 |
| Co3O4(25%)–La2O3(20%)/ACnano | 53.18 | 486.13 | 0.41 | 140/713/832 | 251.25/713.16/4400.43 | 901 | 3272.65 |
Table 3 lists the BET surface area for the Co3O4(x)–La2O3(20)/ACnano catalysts with different Co![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) La ratios. The results indicate a decrease in the BET surface area from 668 to 486 m2 g−1 with an increase in Co dosage from 5 to 25 wt%. The reduction in surface area with an increment in the Co content is due to the destruction of the needle-like structure together with the formation of excessive small aggregates (see FESEM image), which covered the active sites of the catalyst surface.30–32 Moreover, the pore volume of the Co3O4(x)–La2O3(20)/ACnano catalyst decreased from 0.64 to 0.41 nm with an increase in Co content. These observations are attributed to the incorporation of excess active metals (Co and La) in the pores of the AC support, which slightly blocked the porous structure.24
La ratios. The results indicate a decrease in the BET surface area from 668 to 486 m2 g−1 with an increase in Co dosage from 5 to 25 wt%. The reduction in surface area with an increment in the Co content is due to the destruction of the needle-like structure together with the formation of excessive small aggregates (see FESEM image), which covered the active sites of the catalyst surface.30–32 Moreover, the pore volume of the Co3O4(x)–La2O3(20)/ACnano catalyst decreased from 0.64 to 0.41 nm with an increase in Co content. These observations are attributed to the incorporation of excess active metals (Co and La) in the pores of the AC support, which slightly blocked the porous structure.24
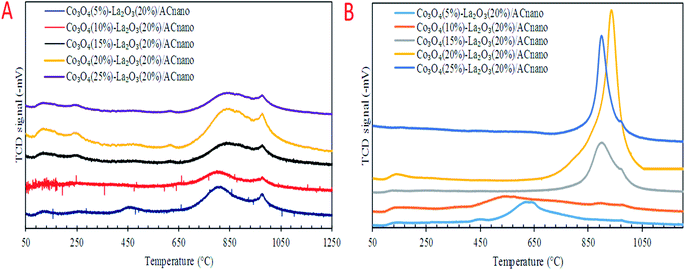
Previous work reported that acidic sites are necessary for enhancing the C–O and C–C bond cleavage via decarboxylation/decarbonylation and cracking pathways.33 Besides acidic sites, basic sites play an important role in promoting decarboxylation and suppressing the formation of coke by reducing the deactivation rate of acidic catalysts.34,35 Hence, the perfect and ideal DO catalyst can be achieved via the co-existence of basic and acidic sites. Thus, the acidity and basicity profiles for the Co3O4(x)–La2O3(y)/ACnano catalyst were quantified by temperature-programmed desorption NH3 (TPD-NH3) and CO2 desorption (TPD-CO2) analyses, and the results are displayed in Fig. 3A and B and Table 3. The TPD–NH3 profiles in Fig. 3A display two distinct NH3 desorption peaks at temperatures of <150 °C and >500 °C, which are associated with the weak and strong acidic sites, respectively.36 An examination of Table 3 reveals that the increment of Co doping from 5 to 20 wt% induced the generation of strong acidic sites, which suggests that the presence of Co-rich species enhances the strength of the acidic sites. However, a dramatic decrease in the concentration of strong acid sites was observed with a further increment in Co content to 25 wt%. This can be attributed to the structural changes caused by the high Co content, which led to damage of the mixed oxide structure (Fig. 1E). The highest acidity distribution (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 208.66 μmol g−1) was observed at a Co content of 20 wt% with the maximum increase of approximately 28% over the Co3O4(5%)–La2O3(20%)/ACnano catalyst. The weak acidity indeed showed negligible changes (148.74–254.21 μmol g−1) for all the Co3O4(x)–La2O3(y)/ACnano catalysts. The basicity (TPD–CO2) profiles of the various Co3O4(x)–La2O3(y)/ACnano catalysts are presented in Fig. 3B and Table 3. Each catalyst displayed the main desorption peak at a temperature of >500 °C, which is attributed to the interaction of CO2 with the strong basic sites of the catalyst. Similar to the trend on the acidity profile, the strong basic site distribution was found to increase with an increase in Co dopant up to 20 wt%. It is generally acknowledged that the basicity content will improve with an increase in Co loading.37–39 This is due to the synergistic effect between Co and La on the activated carbon surface.40–43 However, excess Co dopant covers the active sites of La, and thus provides lower basicity. Consistent with this, a reduction in the distribution of basic sites (5292.06 μmol g−1) was found on the Co3O4(25)–La2O3(20)/ACnano catalyst. In summary, the Co3O4(20%)–La2O3(20%)/ACnano catalyst exhibited the highest CO2 desorption peaks at Tmax = 939 °C with the maximum basic site distribution (5292.06 μmol g−1). Considering the acidity and basicity, an excess of Co dopant (25 wt%) results in high saturation of the Co-active sites, consequently hindering the active sites of La. Since, the capacity to instigate C–O cleavage is directly related to a high distribution of acid and basic active sites, it is speculated that elevated Co dosages of >20% will result in lower DO activity. Based on the result obtained, it is expected that the most effective ratio for converting WCO to hydrocarbon-like structures via DO is the CoO(20%)–La2O3(20%)/ACnano catalyst.
208.66 μmol g−1) was observed at a Co content of 20 wt% with the maximum increase of approximately 28% over the Co3O4(5%)–La2O3(20%)/ACnano catalyst. The weak acidity indeed showed negligible changes (148.74–254.21 μmol g−1) for all the Co3O4(x)–La2O3(y)/ACnano catalysts. The basicity (TPD–CO2) profiles of the various Co3O4(x)–La2O3(y)/ACnano catalysts are presented in Fig. 3B and Table 3. Each catalyst displayed the main desorption peak at a temperature of >500 °C, which is attributed to the interaction of CO2 with the strong basic sites of the catalyst. Similar to the trend on the acidity profile, the strong basic site distribution was found to increase with an increase in Co dopant up to 20 wt%. It is generally acknowledged that the basicity content will improve with an increase in Co loading.37–39 This is due to the synergistic effect between Co and La on the activated carbon surface.40–43 However, excess Co dopant covers the active sites of La, and thus provides lower basicity. Consistent with this, a reduction in the distribution of basic sites (5292.06 μmol g−1) was found on the Co3O4(25)–La2O3(20)/ACnano catalyst. In summary, the Co3O4(20%)–La2O3(20%)/ACnano catalyst exhibited the highest CO2 desorption peaks at Tmax = 939 °C with the maximum basic site distribution (5292.06 μmol g−1). Considering the acidity and basicity, an excess of Co dopant (25 wt%) results in high saturation of the Co-active sites, consequently hindering the active sites of La. Since, the capacity to instigate C–O cleavage is directly related to a high distribution of acid and basic active sites, it is speculated that elevated Co dosages of >20% will result in lower DO activity. Based on the result obtained, it is expected that the most effective ratio for converting WCO to hydrocarbon-like structures via DO is the CoO(20%)–La2O3(20%)/ACnano catalyst.
Since the Co3O4(20%)–La2O3(20%)/ACnano catalyst showed superior acid-base active sites, a detailed study on its surface was performed via XPS analysis. Fig. S1A–E† reveal the deconvolution peaks at a binding energy (BE) of 284.7–289.8 eV (C), 531.8–533.1 eV (O) and ∼133.7–135.7 eV (P) together with the characteristic peaks for La 3d5 and Co 2p3. The O 1s spectra for the Co3O4(20%)–La2O3(20%)/ACnano catalyst present two peaks with the BE values of 529.8 eV, 531.8 eV and 533.1 eV (Fig. S1B†). The XPS peak at 529.8 eV is attributed to the lattice O2− species, O22/O, whereas the peak at 531.6 eV originated from the hydroxyl species, OH−. The highest BE level peak at 533.10 eV is assigned to molecular water adsorbed on the surface of the catalyst.44,45 This implies that the lanthanum and cobalt are in the form of oxides on the catalyst surface. It noteworthy to mention that the O 1s region showed the lowest BE peak compared to other elemental species, which are known to be less electron-rich oxygen species.44 This is in agreement with surface atomic ratio data shown in Table 4, where only 6% O was detected on the Co3O4(20%)–La2O3(20%)/ACnano catalyst. This result is also in accordance with the FESEM-EDX result, where only ∼5% O was detected (Table 2). Thus, this finding confirms that the Co3O4(20)–La2O3(20)/ACnano catalyst is comprised mainly of bimetallic Co–La alloy supported on ACnano. The carbon in the Co3O4(20)–La2O3(20)/ACnano catalyst was detected in the C 1s spectra (Fig. S1C†). Four distinct peaks were obtained at BE of 284.7, 285.8, 286.9 and 289.8 eV. These peaks are attributed to C–C, C–O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C(O)O, respectively, demonstrating that three types of functional groups were incorporated during the synthesis of the catalyst. The value for C 1s belonging C–C was the highest (56%), implying that the ACnano support is mainly composed of carbon-based material, which was connected by C–C bonds. The total C content was relatively high (45%), and thus enhanced the thermally stability of the catalyst at high reaction temperatures.46 Referring to Fig. S1D,† the BE for the deconvolution curves of Co 2p were observed in the range of 781.9 eV (Co 2p3/2) to 797.8 eV (Co 2pl/2). This is evidence of the existence of cobalt metal on the catalyst surface. The significant deconvolution curves of Co 2p suggest that the cobalt metal was well distributed on the external AC surface, and it is speculated that the improvement in DO catalytic activity is due to the greater cobalt dispersion on the AC.47 Fig. S1E† indicates deconvolution of the high-resolution P 2p spectra into a single peak, with binding energies of 133.7 eV for P 2p3/3 and 135.3 eV for P 2p1/2. These observations are linked to the presence of PO43−, which was derived from the phosphate precursor of H3PO4 used in the chemical activation of the walnut shell.25 Fig. S1F† indicates the presence of two regions displayed by La 3d5 in lanthanum oxide (838.3 eV and 835.2 eV), which are associated with La3+ species on the catalyst surface.48 Notably, the XPS results revealed a significant bonding interaction between Co2+ and La3+ in terms of a shift in the binding energies of Co 2p3/2 (from 779.2 (Co3O4, standard) to 781.7), and a shift in the binding energy of La 3d5 (from 834.5 eV (La2O3, standard) to 835.6 + 0.1 eV).44,49 The shift in bond energy is also due to the formation of the bimetallic (La–Co) phase. The metallic bonding of La and Co was substantiated by the XRD results (refer to XRD).
O and C(O)O, respectively, demonstrating that three types of functional groups were incorporated during the synthesis of the catalyst. The value for C 1s belonging C–C was the highest (56%), implying that the ACnano support is mainly composed of carbon-based material, which was connected by C–C bonds. The total C content was relatively high (45%), and thus enhanced the thermally stability of the catalyst at high reaction temperatures.46 Referring to Fig. S1D,† the BE for the deconvolution curves of Co 2p were observed in the range of 781.9 eV (Co 2p3/2) to 797.8 eV (Co 2pl/2). This is evidence of the existence of cobalt metal on the catalyst surface. The significant deconvolution curves of Co 2p suggest that the cobalt metal was well distributed on the external AC surface, and it is speculated that the improvement in DO catalytic activity is due to the greater cobalt dispersion on the AC.47 Fig. S1E† indicates deconvolution of the high-resolution P 2p spectra into a single peak, with binding energies of 133.7 eV for P 2p3/3 and 135.3 eV for P 2p1/2. These observations are linked to the presence of PO43−, which was derived from the phosphate precursor of H3PO4 used in the chemical activation of the walnut shell.25 Fig. S1F† indicates the presence of two regions displayed by La 3d5 in lanthanum oxide (838.3 eV and 835.2 eV), which are associated with La3+ species on the catalyst surface.48 Notably, the XPS results revealed a significant bonding interaction between Co2+ and La3+ in terms of a shift in the binding energies of Co 2p3/2 (from 779.2 (Co3O4, standard) to 781.7), and a shift in the binding energy of La 3d5 (from 834.5 eV (La2O3, standard) to 835.6 + 0.1 eV).44,49 The shift in bond energy is also due to the formation of the bimetallic (La–Co) phase. The metallic bonding of La and Co was substantiated by the XRD results (refer to XRD).
| State of element | O 1s | C 1s | Co 2p | La 3d5 | P 2p | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Atomic percent (at%) | 6.1 | 45.2 | 18.8 | 19.9 | 9.9 | |||||
| O 1s. cf1 | O 1s. cf2 | O 1s. cf3 | C–O | C–C | C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
C(O)O | Co 2p1/2 | P 2p3/2 | ||
| Peak position (eV) | 529.81 | 531.83 | 533.10 | 285.81 | 284.70 | 286.90 | 289.81 | 797.80 | 835.60 | 133.72 |
| FWHM | 1.42 | 1.80 | 196 | 1.42 | 1.26 | 1.57 | 1.55 | 2.96 | — | 2.12 |
| Concentration (%) | 16.35 | 56.55 | 27.09 | 27.98 | 55.69 | 10.87 | 5.47 | — | — | — |
3.2 DO of WCO over Co3O4(x)–La2O3(y)/ACnano catalysts and effect of reaction atmosphere
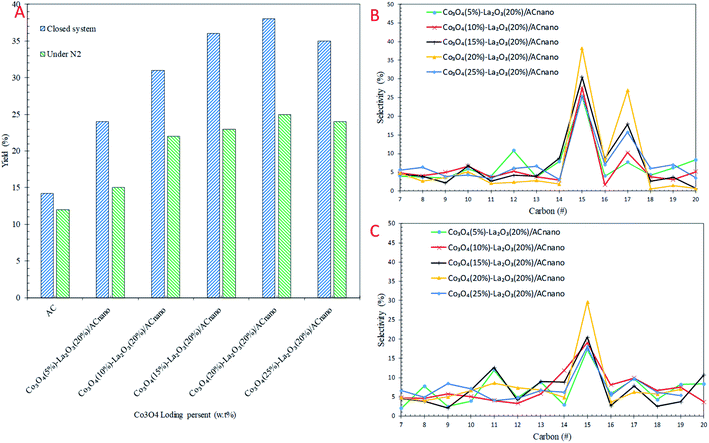
The catalytic DO of WCO over the Co3O4(x)–La2O3(y)/ACnano catalysts was conducted at a constant temperature (300 °C), for 1 h, with 0.5 wt% catalyst loading under two different conditions: (i) N2 flow and (ii) closed system. The detailed GC-FID results are shown in Fig. 4. For comparison, a blank experiment was carried out over the ACnano support. The results showed that the deoxygenized liquid products produced from both reaction systems were composed of saturated and unsaturated hydrocarbon fractions in the range of C8–C20 (Fig. 4A). Notably, ACnano had lower activity for the DO of WCO than the Co3O4(x)–La2O3(20%)/ACnano catalysts. The DO profile showed that a low hydrocarbon yield (12–14%) was achieved from ACnano catalysing DO in both reaction systems. However, when the Co3O4(20%)–La2O3(20%)/ACnano catalyst was applied, high activity was observed with a hydrocarbon yield in range of 25–38%. The yields of liquid hydrocarbon were significantly increased in the order of Co3O4(20%)–La2O3(20%)/ACnano > Co3O4(15%)–La2O3(20%)/ACnano > Co3O4(25%)–La2O3(20%)/ACnano > Co3O4(10%)–La2O3(20%)/ACnano > Co3O4(5%)–La2O3(20%)/ACnano > ACnano. The hydrocarbons yield increased from 33–65% to 27–35% as the Co dopant increased from 5 to 20 wt% and reduced for 25 wt% Co concentration.Theoretically, the WCO was composed majority of ∼46% C16 and ∼52% C18 fatty acids, which were further deoxygenized via decarboxylation/decarbonylation (deCOx) pathways to produce hydrocarbon fractions mainly of n-C15 and n-C17. Fig. 4B and C shows the carbon distribution of the deoxygenated liquid product obtained from both DO systems. Considering that n-(C15 + C17) was significantly found among the hydrocarbon products in all cases, it is conceivable that the n-(C15 + C17) obtained resulted from the decarbonylation (deCOx) synthesis pathway. As the Co content increased, the deCOx rate increased and resulted in formation of n-(C15 + C17) fractions.50 In addition, the total selectivity of n-(C15 + C17) was reduced (∼29–41%) when 25 wt% of Co catalysed the reaction. Although some studies acknowledged that high deCOx activity is dominated by catalysts with rich weak + medium acidic sites (TPD-NH3 desorption temperature <500 °C),25,51,52 it was difficult to correlate this finding since the weak acid obtained from all the catalysts was close in value (Table 3). The high hydrocarbon yield (25–38%) and n-(C15 + C17) fractions (36–65%) over the Co3O4(20%)–La2O3(20%)/ACnano catalyst suggest that the DO activity via deCOx pathways was strongly affected by the surface density of strong acid and basic sites. This indicates that a higher degree of DO occurs over highly acidic and basic catalysts. The pronounced effect of acid-base characteristics in promoting the DO reaction is consistent with our previous investigations. The DO of WCO on Ag2O–La2O3/ACnano and CaO–La2O3/AC catalysts suggested that deCOx is favoured by the existence of a large amount of acid-based sites.3,12
As expected there was a great difference in the hydrocarbon fraction distribution between the DO reaction using the micro-batch closed system and reaction under N2 flow. The DO of WCO under an N2 flow gave a low hydrocarbon yield (25%) and also poor deCOx activity with only 36% n-(C15 + C17) selectivity. Meanwhile, the n-(C15 + C17) selectivity was found to increase significantly in the micro-batch closed system, with 38% hydrocarbon yield and 68% n-(C15 + C17) selectivity. This indicates that the deCOx reaction was promoted by the catalytic reaction under the high pressure built-up in the micro-batch closed system. In addition, this suggests competitive activation of the active sites by the high pressure DO system, which led to an enhancement in the deCOx reaction. The low selectivity toward n-(C15 + C17) was prominent for DO catalysed by Co3O4(x)–La2O3(y)/ACnano under an N2 flow, which is due to the formation of rich oxygen-containing compounds (oxygenates and CO2/CO gases) during the ineffective DO reaction. These oxygenates can act as poisons by strongly adsorbing on the catalyst surface and deactivate the active sites of the catalyst.
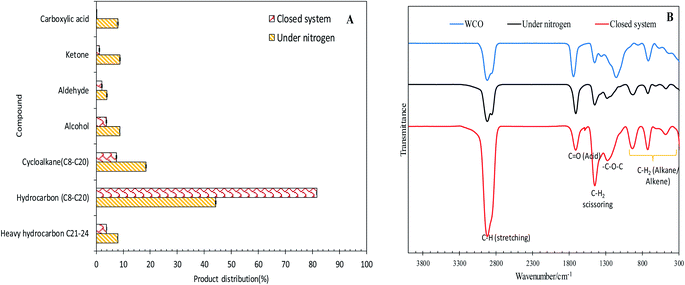
The efficacy of WCO DO under the closed and N2 flow systems over the Co3O4(20%)–La2O3(20%)/ACnano catalysts was further confirmed by GCMS, as shown in Fig. 5A. It was observed that the DO of WCO in the micro-batch closed system produced a hydrocarbon fraction two times larger than that in the N2 flow system. Interestingly, the peaks for carboxylic acids could not be observed in the deoxygenated liquid product from the micro-batch closed system, where in contrast the N2 flow system produced >8% carboxylic acid. Moreover, the amount of other oxygenate species such as ketones, aldehydes and alcohols was remarkably reduced (7.13%) in the micro-batch closed system. Thus, it can be concluded that the Co3O4(20%)–La2O3(20%)/ACnano catalyzed DO in the micro-batch closed system offers successive cracking reactions via C–O cleavage, producing rich hydrocarbon fuel mixtures. The FTIR analysis was performed to study the chemical functional groups of the WCO (feedstock) and deoxygenized liquid products (Fig. 5B). The FTIR spectrum of WCO showed absorption bands at 2915 cm−1 (–CH), 1692 cm−1 (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) stretching, 1447 cm−1 (–CH2) scissoring, 1285 cm1 (–C–O–C) and 726 cm−1 (–(CH)n– bending for alkane).3 The FTIR results for WCO and the liquid deoxygenated product show that all the spectra were normalized by the intensity of the absorption band centered at 2753–3000 cm−1 (CH stretching, aliphatic). It is noteworthy that the liquid deoxygenated products showed a significant intensity reduction for the absorption band at 1692 cm−1, which belongs to C
O) stretching, 1447 cm−1 (–CH2) scissoring, 1285 cm1 (–C–O–C) and 726 cm−1 (–(CH)n– bending for alkane).3 The FTIR results for WCO and the liquid deoxygenated product show that all the spectra were normalized by the intensity of the absorption band centered at 2753–3000 cm−1 (CH stretching, aliphatic). It is noteworthy that the liquid deoxygenated products showed a significant intensity reduction for the absorption band at 1692 cm−1, which belongs to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (fatty acid), and the absence of C–O–C (from carbonyl group in WCO) absorption features at 1285 cm−1. This result indicates the removal of oxygen species via deCOx pathways.53,54 The DO close system exhibited a significantly lower intensity for the C
O (fatty acid), and the absence of C–O–C (from carbonyl group in WCO) absorption features at 1285 cm−1. This result indicates the removal of oxygen species via deCOx pathways.53,54 The DO close system exhibited a significantly lower intensity for the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–O–C peaks compared to DO in the N2 flow system under the same experimental conditions.
O and C–O–C peaks compared to DO in the N2 flow system under the same experimental conditions.
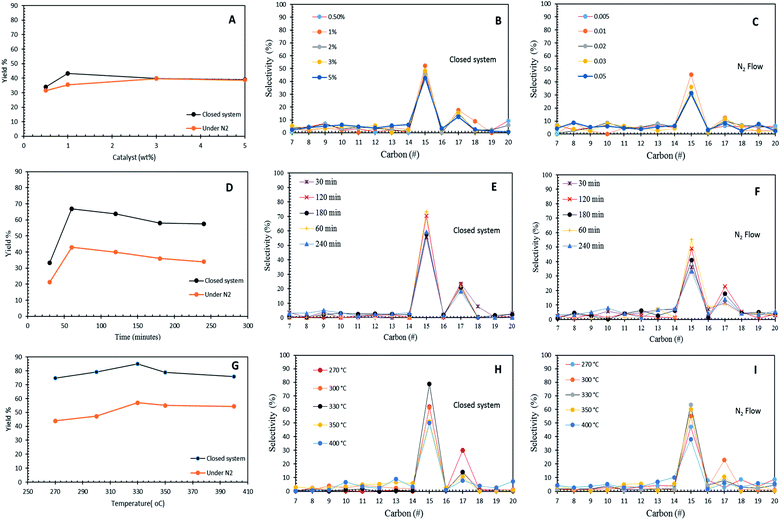
3.3 Optimization studies
The effects of the Co3O4(20%)–La2O3(20%)/ACnano catalyst loading (0.5 wt% to 5 wt%) towards hydrocarbon yield and product selectivity at 300 °C temperature and 60 min reaction time under inert conditions and in the micro-batch closed system with a stirring speed of 400 rpm are shown in Fig. 6A–C. The results show that the DO activity increased with an increment in catalyst loading within the range of 0.5 wt% to 1 wt%, which yielded 43% and 35% of hydrocarbons with 69% and 47% n-C15 + n-C17 selectivity for the reaction using the micro-batch closed system and under N2 flow, respectively. This finding is possibly due to the fact that the increase in the catalyst loading enhanced the exposure of active sites for the accessibility of the feedstock in the DO reaction.55 However, a further increase in the catalyst loading to >1 wt% resulted a reduction in hydrocarbon yield to <40% and n-C15 + n-C17 selectivity to <43% in both DO systems. This is due to the occurrence of parallel or secondary reactions, such as polymerization pathways. The excess active sites due to a high catalyst loading increased the rapid-rate reaction on the catalyst surface, which simultaneously resulted in shortening of the catalyst life span by coke formation caused by polymerization reaction.56 In the present study, 1 wt% catalyst loading was observed to be optimal for the catalytic DO reaction.Fig. 6D–F show the effect of reaction time in the range of 30 min to 240 min with 1 wt% Co3O4(20%)–La2O3(20%)/ACnano catalyst loading at a temperature of 300 °C in the micro-batch closed system and inert N2 flow condition. The results showed that the DO activity depends on the reaction time. As the reaction time increased from 30 min to 60 min, the hydrocarbon yield increased from 33% to 66% and 21% to 39% for DO in the micro-batch closed system and inert condition, respectively. Meanwhile, the closed and inert N2 flow system also showed an increment of n-C15 + n-C17 selectivity from 79% to 94%, and 47% to 58%, respectively. Further prolonging the time to 240 min led to a slight reduction in the hydrocarbon yield from 66% to 57% and 39% to 34%, and the n-C15 + n-C17 selectivity was reduced to >77% and 47%, respectively. The insignificant changes in the hydrocarbon yield and n-C15 + n-C17 selectivity indicate that DO via deCOx reaction was highly unaffected in this time range. The lowest catalytic activity was observed at the longest reaction time (240 min), which is because unfavourable side reactions (i.e., polymerization) occur during a longer reaction time. These side reactions accelerated the deactivation of the catalyst since the polymerization process generates coke on the catalyst. Coke formation reduces the effectiveness of the catalyst and leads to the accumulation of different compounds on the catalyst surface, covering the active sites, and thus suppressing the reaction at the catalytic centres of the catalyst.57
The effect of reaction temperature in the DO of WCO was investigated in the temperature range of 270 °C to 400 °C, with a Co3O4(20%)–La2O3(20%)/ACnano catalyst loading of 1 wt%, and reaction time of 60 min in the micro-batch closed and inert N2 flow system with a stirring speed of 400 rpm (Fig. 6G–I). The DO activity increased with an increase in temperature from 270 °C to 330 °C. Both DO systems showed a similar trend of activity, whereby the highest catalytic activity was found at a temperature of 330 °C. This proves that Co3O4(20%)–La2O3(20%)/ACnano is thermally stable since it is capable to withstand at a high temperature. A further increase in temperature to >330 °C led to the retardation of catalytic activity with a lower hydrocarbon yield and n-C15 + n-C17 selectivity. The high reaction temperature enhanced the occurrence of cracking via C–C scission in the triglycerides and n-C15 + n-C17 fractions into lighter fractions.58 Therefore, the formation of lighter fractions (i.e., gaseous products) and gasoline fraction (i.e., C8–C12) simultaneously reduced the hydrocarbon yield and n-C15 + n-C17 selectivity. In this study, the optimum reaction temperature in the N2 flow system was achieved at 330 °C, 1 wt% catalyst within 60 min, with 52% hydrocarbon yield and 71% n-C15 + n-C17 selectivity. On the other hand, the optimum catalytic DO (hydrocarbon yield = 85%, n-C15 + n-C17 = 93%) was achieved within 60 min and 1 wt% catalyst loading at 330 °C in the micro-batch closed system.
3.4 Effect of water and FFA content in DO of WCO
Other important factors affecting the DO activity are water and FFA content in the feedstock. It was reported in a previous study that the addition of water to the DO feedstock produced three beneficial effects: (1) low acidic and other low-value oxygenated components, (2) prevent the beneficial components that are partially miscible in water to not easily be excluded from DO; and (3) in particularly strong catalysts, water regulates the catalyst site interactions with the feedstock components.59 The investigation of the content of water and FFA on the DO of WCO over the Co3O4(20%)–La2O3(20%)/ACnano catalyst was performed using the optimum parameters of 1 wt%, 60 min at 330 °C in the micro-batch closed system. All the obtained results are displayed in Fig. S2A–D.† Some publications suggest the negative impact of water towards DO activity, where water results in the modification of the external catalyst surface, simultaneously leading to the loss of some active metals species, and reduces the catalytic DO activity. Interestingly, the impact of water on the DO of WCO over the Co3O4(20%)–La2O3(20%)/ACnano catalyst showed a positive effect (Fig. S2A and B†). In the present study, increasing the water content from 1 wt% to 5 wt% resulted in an increase in the hydrocarbon yield from 82% to 88%, while the n-C15 + n-C17 selectivity increased from 90% to 93%. This indicates that the addition of water to WCO facilitated the C–O cleavage of fatty acids via the deCOx reaction. The hydrocarbon yield and selectivity reached ∼88% and 93% n-C15 + n-C17 when the water content was >5 wt%, respectively. This indicates that the cobalt and lanthanum supported on ACnano were chemically and thermally stable at high temperature even with a large amount of water in the feedstock.Further study on the effect of FFAs towards the DO reaction was performed. Since the WCO consisted mainly of C18 of fatty acids, oleic acid (C18) was chosen as a model reactant. The FFA% of the WCO was controlled by further adding commercial oleic acid as a representative natural FFA. The FFA% for the feedstock mixture (WCO + oleic acid) used was in the range of 18% to 38% (Fig. S2C and D†). It was found that all the feedstocks were effectively deoxygenized via the selective deCOx reaction with a higher percentage of hydrocarbon fraction of >93%, and the product was predominantly selective toward n-C15 + n-C17 fractions. The total hydrocarbons was found to increase from 88% to 96% with an increase in FFA content from 18% to 38%. In contrast, the value of n-C15 + n-C17 selectivity was reduced to 84% from 94%. The results suggest that an FFA-rich feed encourages C–C cleavage rather than C–O cleavage. Evidently, n-C9, n-C12 and n-C13 were found to be prominent in the DO of the FFA-rich feedstocks (FFA > 23%). It has been reported that a higher content of FFA in the feedstock will lead to a decrease in DO activity and favour the cracking reaction. Overall, the DO of the FFAs–WCO mixtures was still favorable toward C–O cleavage and produced rich diesel-like fuels. This suggests the Co3O4(20%)–La2O3(20%)/ACnano catalyst is highly adaptable to water–FFA conditions in the DO process, and thus it can be applied for other realistic feedstocks.
3.5 Reusability of Co3O4(20%)–La2O3(20%)/ACnano catalyst
The reusability profile of the Co3O4(20%)–La2O3(20%)/ACnano catalyst was investigated via DO reaction in the micro-batch closed system condition under the conditions of 1 wt% catalyst at 330 °C within 60 min (Fig. S3A†). Upon the completion of each cycle, the catalyst was reactivated by simple washing with hexane and reused for the next cycle. The results showed that the Co3O4(20%)–La2O3(20%)/ACnano catalyst performed steadily for eight consecutive runs, maintaining the hydrocarbon yield at >72% with >80% selectivity of C15 and C17 products (Fig. S3A and S4†). This indicates that the Co3O4(20%)–La2O3(20%)/ACnano catalyst possesses mechanical properties and chemical stability. Furthermore, the elemental study indicated a negligible amount of La3+ and Co4+ species leached from the Co3O4(20%)–La2O3(20%)/ACnano catalyst during each reaction. The comparison study show that the leached La3+ and Co4+ in the liquid product of the fresh and 8th-run catalyst gradually increased from 1 ppm to 3 ppm and 1 ppm to 4 ppm, respectively. Hence, the loss in DO activity is due to the dissolution of the active metals during continuous runs. Furthermore, the leaching level is within the maximum range of the EN 12662 Standard Specification for Diesel Fuel Oils contamination content (24 mg L−1),60 which confirms that the Co3O4(20%)–La2O3(20%)/ACnano catalyst shows potential leaching resistance and exhibits good stability.The metal dispersion states and chemical composition study for the Co3O4(20%)–La2O3(20%)/ACnano catalyst (fresh and spent catalysts) were determined by XRD and TGA analysis (Fig. S3B and C†), respectively. The XRD analysis showed that the spent catalysts exhibited similar bimetallic active CoLaO3 phases with higher crystallinity at 2θ = 23.3°, 40.2° and 69.9° (JCPDS File no. 00-006-0491). The crystallite size of the Co3O4(20%)–La2O3(20%)/ACnano particles evaluated at 2θ: 43.1 showed a minor change in the crystallite size of the spent catalyst (Fig. S3B†). The TGA analysis was performed to examine the extent of coke formation during the DO of WCO using the Co3O4(20%)–La2O3(20%)/ACnano catalyst. The results showed a decomposition peak in the temperature range of 290 °C to 550 °C. This is attributed to the combustion of the activated carbon nanorods. The initial decomposition temperature for the fresh and spent catalysts was similar; however, the final decomposition temperature for the spent catalyst was significantly higher >550 °C. Furthermore, the weight loss for the spent catalyst was 3.7 wt% higher than that of the fresh catalyst, which was due to the oxidation of coke in air (Fig. S3C†). The coke is categorized as hard coke, which decomposed completely at a temperature >550 °C.61 The deposited coke will accumulate on the catalyst surface, which covers the active sites on the catalyst and reduces the catalytic DO activity. The negligible loss in DO activity throughout eight reaction runs is an indication that coke formation had an insignificant effect on the active sites of the Co3O4(20%)–La2O3(20%)/ACnano catalyst, which confirms that the La2O3 and Co3O4 supported AC catalyst exhibits high stability. Notably, the use of carbon as a support in the DO reaction has been widely explored due to its excellent thermal stability. For instance, the use of a bimetallic doped carbon catalyst in the DO of non-edible oil including jatropha oil and WCO was recently explored by our group.5,6,11 Ni–Ag/AC, CaO–La2O3/AC, and Ag2O3–La2O3/ACnano were found to be effective in removing the oxygenated compound via deCOx pathways and yielded hydrocarbon in the range of 72–89% with product selectively toward n-C15 + C17 fractions (82–93%) and could be reused four to six times (Table 5). Notably, Co3O4(20%)–La2O3(20%)/ACnano exhibited excellent catalyst activity with hydrocarbon yield and selectivity of 96% and 93%, respectively. Moreover, the Co3O4(20%)–La2O3(20%)/ACnano catalyst showed high catalytic stability with eight times successive reusability with a hydrocarbon yield of >80% and n-C15 + C17 selectivity of >83%. Although the coke formed on the surface of Co3O4(20%)–La2O3(20%)/ACnano was more pronounced than that in previous studies, it still exhibited high stability, reusability and catalytic activity. This suggests by the presence of high acidic active sites with a high surface area and pore volume,12 which are favorable for the DO reaction.
| No. | Catalyst | Support | Reaction | Feed | FFA (%) | Reaction condition | H/C (yield%) | Selectivity (%) | Reusability | Coke (wt%) | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Ni–Ag/ACCFR | Coconut fibre residue | DO | JCO | 15.4 | Catalyst loading: 5 wt% | 80 | 83 (n-C15 + C17) | 5 | 2.5 | 11 |
| Temperature: 350 °C | |||||||||||
| Time: 1 h under N2 flow | |||||||||||
| 2 | CaO–La2O3/AC | Walnut shell | DO | WCO | 18.4 | Catalyst loading: 3 wt% | 72 | 82 (n-C15 + C17) | 6 | 2 | 5 |
| Temperature: 330 °C, | |||||||||||
| Time: 3 h under N2 flow | |||||||||||
| 3 | Ag2O3–La2O3/ACnano | Walnut shell | DO | WCO | 18.4 | Catalyst loading: 1 wt% | 89 | 93 (n-C15 + C17) | 6 | 1.3 | 6 |
| Temperature: 350 °C | |||||||||||
| Time: 2 h under N2 flow | |||||||||||
| 4 | Co3O4(20%)–La2O3(20%)/ACnano | Walnut shell | DO | WCO | 38.4 | Catalyst loading: 1 wt% | 96 | 93 (n-C15 + C17) | 8 | 3.7 | Present study |
| Temperature: 330 °C | |||||||||||
| Time: 1 h and 5% of water content |
4. Conclusion
In the present work, an effective DO reaction was developed by converting triglyceride-based feeds to diesel-like fuel over the Co3O4(x)–La2O3(y)/ACnano catalyst. The improvement in the DO of WCO over the acid–base Co3O4(x)–La2O3(y)/ACnano catalyst was due to the neutralization of the strong acidic sites. DO using a micro-reactor closed system showed better reactivity compared to semi-batch inert flow conditions due to the advantages of high pressure, which is beneficial in maintaining high catalytic activity. The cracking pathway was found to be enhanced with an increase in Co content due to the richness of the high-strength basic and acid sites of the Co3O4(x)–La2O3(y)/ACnano catalyst. Among the Co contents (5–25 wt%), the optimum content of Co for deCOx activity is 20 wt%. The effects of reaction time, catalyst loading, temperature, water and FFA content were further investigated with the optimum DO condition (hydrocarbon fraction = 96%, n-C15 + n-C17 = 93%) achieved using 1 wt% catalyst loading within 60 min at 330 °C, 38.4 wt% FFA and 5% water content in the micro-batch closed system. The efficiency of the Co3O4(20%)–La2O3(20%)/ACnano catalyst was proven to be capable of deoxygenizing low quality feedstocks with a high FFA (38%) and high water content (5 wt%) under the optimum conditions. The Co3O4(20%)–La2O3(20%)/ACnano catalyst showed high stability and reusability up to eight times with the yield and selectivity maintained at >72% and >80% for n-C15 + C17.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the financial support from Ministry of Higher Education Malaysia for Fundamental Research Grant Scheme (FRGS 2014-1), Geran Putra Berimpak (GPB) UPM/800-3/3/1/GPB/2018/9658700, Geran Putra Berimpak GBP/2019/9674500, SATU Joint Research Scheme (ST012-2018), Grand Challenge Grant (GC, Project number: GC001B-14AET) and UM RU grant (RU007C-2017D).References
- S. H. H. Al-jaberi, U. Rashid, F. A. J. Al-doghachi and G. Abdulkareem-alsultan, Energy Convers. Manage., 2017, 139, 166–174 CrossRef CAS.
- A. Alsultan, N. Asikin-mijan and T. Y. Yun Hin, Mater. Sci. Forum, 2016, 840, 348–352 Search PubMed.
- G. Abdulkareem-Alsultan, N. Asikin-Mijan, H. V. Lee, A. S. Albazzaz and Y. H. H. Taufiq-Yap, Energy Convers. Manage., 2017, 151, 311–323 CrossRef.
- P. Mäki-Arvela, M. Snåre, K. Eränen, J. Myllyoja and D. Y. Murzin, Fuel, 2008, 87, 3543–3549 CrossRef.
- H. Dietz, L. Dittmar, D. Ohms, M. Radwan and K. Wiesener, US Pat., 6,285,167, 2001.
- A. E. Barrón C., J. A. Melo-Banda, J. M. Dominguez E., E. Hernández M., R. Silva R., A. I. Reyes T. and M. A. Meraz M., Catal. Today, 2011, 166, 102–110 CrossRef.
- P. Hongmanorom, A. Luengnaruemitchai, N. Chollacoop and Y. Yoshimura, Energy Fuels, 2017, 31, 8202–8209 CrossRef CAS.
- Y. Rezgui and M. Guemini, Appl. Catal., A, 2005, 282, 45–53 CrossRef CAS.
- H. Zhang, H. Lin and Y. Zheng, Appl. Catal., B, 2014, 160–161, 415–422 CrossRef CAS.
- N. Asikin-Mijan, H. V. Lee, J. C. Juan, A. R. Noorsaadah, H. C. Ong, S. M. Razali and Y. H. Taufiq-Yap, Appl. Catal., A, 2018, 552, 38–48 CrossRef CAS.
- N. Asikin-Mijan, H. V. Lee, J. C. Juan, A. R. Noorsaadah and Y. H. Taufiq-Yap, RSC Adv., 2017, 7, 46445–46460 RSC.
- G. Abdulkareem-Alsultan, N. Asikin-Mijan, N. Mansir, H. V. Lee, Z. Zainal, A. Islam and Y. H. Taufiq-Yap, J. Anal. Appl. Pyrolysis, 2018, 137, 171–184 CrossRef.
- M. Benito, S. Garc, L. Garc and L. Daza, Acc. Chem., 2007, 169, 177–183 CAS.
- M. Kashid, O. Detraz, M. S. Moya, I. Yuranov, P. Prechtl, J. Membrez, A. Renken and L. Kiwi-Minsker, Chem. Eng. J., 2013, 214, 149–156 CrossRef CAS.
- R. Abdallah, B. Fumey, V. Meille and C. de Bellefon, Catal. Today, 2007, 125, 34–39 CrossRef CAS.
- M. T. Janicke, H. Kestenbaum, U. Hagendorf, F. Schüth, M. Fichtner and K. Schubert, J. Catal., 2000, 191, 282–293 CrossRef CAS.
- M. Ueno, H. Hisamoto, T. Kitamori and S. Kobayashi, Chem. Commun., 2003, 3, 936–937 RSC.
- N. Asikin-Mijan, H. V. Lee, G. Abdulkareem-Alsultan, A. Afandi and Y. H. Taufiq-Yap, J. Cleaner Prod., 2016, 167, 1–12 Search PubMed.
- B. Gurunathan and A. Ravi, Bioresour. Technol., 2015, 188, 124–127 CrossRef CAS PubMed.
- J. Sahira and P. P. Bhadra, J. Inst. Eng., 2013, 9, 79–88 Search PubMed.
- S. Yorgun and D. Yıldız, J. Taiwan Inst. Chem. Eng., 2015, 53, 122–131 CrossRef CAS.
- L. N. Silva, I. C. P. Fortes, F. P. De Sousa and V. M. D. Pasa, Fuel, 2016, 164, 329–338 CrossRef CAS.
- V. P. Dinesh, P. Biji, A. Ashok, S. K. Dhara, M. Kamruddin, A. K. Tyagi and B. Raj, RSC Adv., 2014, 4, 58930–58940 RSC.
- B. Balusamy, B. E. Taştan, S. F. Ergen, T. Uyar and T. Tekinay, Environ. Sci.: Processes Impacts., 2015, 17, 1–10 RSC.
- G. Abdulkareem-Alsultan, N. Asikin-Mijan, H. V. V. Lee and Y. H. Taufiq-Yap, Chem. Eng. J., 2016, 304, 61–71 CrossRef CAS.
- J. Wu, Q. Xia, H. Wang and Z. Li, Appl. Catal., B, 2014, 156–157, 265–272 CrossRef CAS.
- E. W. Qian, N. Chen and S. Gong, J. Mol. Catal. A: Chem., 2014, 387, 76–85 CrossRef CAS.
- H. Imai, K. Takami and M. Naito, Mater. Res. Bull., 1984, 19, 1293–1300 CrossRef CAS.
- Z. Pan, R. Wang, M. Li, Y. Chu and J. Chen, J. Energy Chem., 2015, 24, 77–86 CrossRef.
- Ş. Özkara, A. N. Akin, Z. Misirli and A. E. Aksoylu, Turk. J. Chem., 2005, 29, 219–224 Search PubMed.
- I. Khan, K. Saeed and I. Khan, Arabian J. Chem., 2019, 12, 908–931 CrossRef CAS.
- G. Abdulkreem-Alsultan, A. Islam, J. Janaun, M. S. Mastuli and Y. H. Taufiq-Yap, Mater. Lett., 2016, 179, 57–60 CrossRef.
- A. M. Robinson, J. E. Hensley and J. Will Medlin, ACS Catal., 2016, 6, 5026–5043 CrossRef CAS.
- Z. Si, X. Zhang, C. Wang, L. Ma and R. Dong, Catalysts, 2017, 7, 169 CrossRef.
- S. Cheng, L. Wei, X. Zhao and J. Julson, Catalysts, 2016, 6, 195 CrossRef.
- A. Hakim, T. S. Marliza, N. M. Abu Tahari, R. W. N. Wan Isahak, R. M. Yusop, W. M. Mohamed Hisham and A. M. Yarmo, Ind. Eng. Chem. Res., 2016, 55, 7888–7897 CrossRef CAS.
- D. W. Lee, Y. M. Park and K. Y. Lee, Catal. Surv. Asia, 2009, 13, 63–77 CrossRef CAS.
- A. Molaei Dehkordi and M. Ghasemi, Fuel Process. Technol., 2012, 97, 45–51 CrossRef CAS.
- P. A. L. Sheena, A. Sreedevi, C. Viji and V. Thomas, Eur. Phys. J. B, 2019, 92, 1–8 CrossRef CAS.
- E. Laurent and B. Delmon, Appl. Catal., A, 1994, 109, 77–96 CrossRef CAS.
- M. Sajdak, R. Muzyka, J. Hrabak and K. Słowik, J. Anal. Appl. Pyrolysis, 2015, 112, 298–305 CrossRef CAS.
- T. V. Choudhary and C. B. Phillips, Appl. Catal., A, 2011, 397, 1–12 CrossRef CAS.
- S. Lu, L. Wang, T. Qi, D. Qi, X. Xing, Y. Zhang, H. Xiao and S. Zhang, J. Hazard. Mater., 2019, 365, 531–537 CrossRef PubMed.
- N. A. Merino, B. P. Barbero, P. Eloy and L. E. Cadus, Appl. Surf. Sci., 2006, 253, 1489–1493 CrossRef CAS.
- J. L. G. Fierro, Catal. Today, 1990, 8, 153–174 CrossRef CAS.
- M. Haruta, N. Yamada, T. Kobayashi and S. Iijima, J. Catal., 1989, 309, 301–309 CrossRef.
- M. Richard, F. Can, A. G. Fortu, A. Fendler, M. Alves-Fortunato, M. Richard, C. Wang, J. A. Díaz, S. Gil and Y. Guo, Appl. Catal., B, 2016, 180, 29–37 CrossRef.
- S. Yan, S. O. Salley and K. Y. Simon Ng, Appl. Catal., A, 2009, 353, 203–212 CrossRef CAS.
- D. Mukai, S. Tochiya, Y. Murai, M. Imori, T. Hashimoto, Y. Sugiura and Y. Sekine, Appl. Catal., A, 2013, 453, 60–70 CrossRef CAS.
- J. Zhang, Y. Bai, Q. Zhang, X. Wang, T. Zhang, Y. Tan and Y. Han, Fuel, 2014, 132, 211–218 CrossRef CAS.
- Z. Zhong, H. Ang, C. Choong, L. Chen, L. Huang and J. Lin, Phys. Chem. Chem. Phys., 2009, 11, 872–880 RSC.
- H. Dong, L. Zhang, Z. Fang, W. Fu, T. Tang, Y. Feng and T. Tang, RSC Adv., 2017, 7, 22008–22016 RSC.
- J. K. Satyarthi and D. Srinivas, Energy Fuels, 2011, 25, 3318–3322 CrossRef CAS.
- S. Zulkepli, J. Ching, H. Voon, N. Saadah, A. Rahman, J. C. Juan, H. V. Lee, N. S. A. Rahman, P. L. Show and E. P. Ng, Energy Convers. Manag., 2018, 165, 495–508 CrossRef CAS.
- K. C. Kwon, H. May, T. Marolla, B. Nichols, M. Mashburn, H. Mayfield, T. Marolla, B. Nichols and M. Mashburn, Renewable Energy, 2011, 36, 907–915 CrossRef CAS.
- Y. Boyjoo, M. Ang and V. Pareek, Int. J. Photoenergy, 2012, 2012, 1–7 CrossRef.
- M. Arend, T. Nonnen, W. F. Hoelderich, J. Fischer and J. Groos, Appl. Catal., A, 2011, 399, 198–204 CrossRef CAS.
- S. Bezergianni, S. Voutetakis and A. Kalogianni, Ind. Eng. Chem. Res., 2009, 48, 8402–8406 CrossRef CAS.
- Y. Elkasabi, C. A. Mullen, A. L. M. T. M. T. Pighinelli and A. A. Boateng, Fuel Process. Technol., 2014, 123, 11–18 CrossRef CAS.
- J. Bacha, J. Freel, A. Gibbs, L. Gibbs, G. Hemighaus, K. Hoekman, J. Horn, M. Ingham, L. Jossens, D. Kohler, D. Lesnini, J. McGeehan, M. Nikanjam, E. Olsen, R. Organ, B. Scott, M. Sztenderowicz, A. Tiedemann, C. Walker, J. Lind, J. Jones, D. Scott and J. Mills, Chevron Glob. Mark., 2007, pp. 1–117.
- S. K. Sahoo, S. S. Ray and I. D. Singh, Appl. Catal., A, 2004, 278, 83–91 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra09516k |
| This journal is © The Royal Society of Chemistry 2020 |