Open Access Article
Open Access ArticleNovel ultra-small Pd NPs on SOS spheres: a new catalyst for domino intramolecular Heck and intermolecular Sonogashira couplings†
Bhairi Lakshminarayana,
T. Vinodkumar ,
G. Satyanarayana
,
G. Satyanarayana * and
Ch. Subrahmanyam
* and
Ch. Subrahmanyam *
*
Department of Chemistry, Indian Institute of Technology Hyderabad, Kandi, Sangareddy, 502285, Telangana, India. E-mail: csubbu@iith.ac.in; Tel: +91 40 2301 6054 Tel: +91 40 2301 6050
First published on 28th January 2020
Abstract
We report a novel catalyst Pd/SOS that catalyzes the dual C–C bond forming coupling of an iodoarene moiety with an internal alkene and an external alkyne via an intramolecular Heck reaction, followed by an intermolecular Sonogashira reaction, respectively. The catalyst was characterized using XRD, IR, XPS, SEM and TEM analyses. Notably, for the first time, cheap and readily available new silica [nanosilica on microsilica (SOS)] material-supported ultra-small Pd nanoparticles (2.20 nm) are employed for the efficient synthesis of dihydrobenzofuran and oxindole derivatives in a domino one-pot reaction. Significantly, a sub-molar quantity of Pd (0.3 mol%) was found to be sufficient to furnish the products in very good to near quantitative yields. Gratifyingly, the catalyst could be recycled up to five cycles with a marginal loss (∼no loss) of the product.
Introduction
Nowadays, nanomaterials are an integral part of almost all technological developments. Although the field of the synthesis of nanomaterials has well expanded in the last two decades, the innovation of unique nanostructures with scalable and sustainable synthesis remains a critical challenge. In the recent past, supported ultra-small Pd nanoparticles have been the area of intense research.1 The unsupported Pd nanoparticles tend to aggregate and are difficult to handle in catalytic reactions.1 To overcome this aggregation, various supporting materials have been developed including microporous and mesoporous metal oxides,2 carbon,3,4 and polymer materials.5 The recent development of silica-supported metal nanoparticles has further boosted the fundamental research and industrial potential of heterogeneous catalysts because these are inexpensive and eco-friendly materials with reasonable catalytic performance and stability.6High-surface-area silica has broad applications in many fields such as catalysis, medical imaging and drug delivery.7 Vitally, the natural properties of silica can be tuned by fluctuating various parameters, for example, the size, shape and morphology.7 The interest in silica nanospheres with different sizes, measurements and morphology is persistently increasing since modern industries have a huge preference for such materials.8 The effectiveness of these materials is mostly due to their micro and mesostructures, which enable dynamic atoms to scatter on the extensive inner surface and enhance the activity.9 The availability of active sites inside the nano-silica particles is vital as the poor accessibility of active sites will restrict their applications when significant mass transport is essential.7 Hence, high-surface-area nano-silica with the better availability of active sites is required. Moreover, silica has tunable pore structures with a non-toxic nature. Therefore, due to these reasons, silica is the best solid support for organic transformations. Based on the advantages of silica, we have synthesized novel silica material-supported ultra-fine Pd particles and showed their efficacy for the domino preparation of dihydrobenzofuran and oxindole derivatives.
Particularly, dihydrobenzofurans and oxindoles are ubiquitous scaffolds with interesting biological properties.10,11 Specifically, 3,3′-disubstituted dihydrobenzofurans and oxindoles constitute the main core structures of natural products and pharmaceutical compounds.12,13 Therefore, these core structures (dihydrobenzofurans and oxindoles) have drawn considerable attention from the synthetic community. As a result, notable domino protocols were established for their synthesis via homogeneous catalysis.12,13 We have also established the efficient synthesis of benzofurans, oxindoles, and indolines under homogenous catalysis.14,15 Domino reactions have been proven to be incredible synthetic organic reactions in chemistry as they permit the formation of at least two or more bonds in a single operation. To the best of our knowledge, there are no reports on domino reactions via heterogeneous catalysis using sub-molar quantities of the catalyst. Therefore, the development of novel heterogeneous catalysts has attracted interest from the scientific community. Moreover, heterogeneous catalysis is a powerful tool for environmentally benign processes. For this reason, when compared to traditional methods, heterogeneous catalysis is the new and promising alternative for the formation of 3,3′-disubstituted dihydrobenzofurans and oxindole derivatives.
In this study, for the first time, we synthesized a novel silica material, i.e., nano-silica uniformly distributed on the surface of bulk silica (SOS). Moreover, it was identified that ultra-small particles were dispersed on the surface of the SOS material. As an application in organic transformation, the products were obtained in very good to near quantitative yields in a domino fashion and by using sub-molar quantities of the catalyst. Also, we successfully recycled the novel catalyst without a considerable loss of the product.
Results and discussion
Synthesis of SOS
Polyvinylpyrrolidone (PVP, 10k MW, 1.262 g) and cetyltrimethylammonium bromide (CTAB, 0.0625 g) were dissolved in distilled water (20 mL). To this solution, methanol (40 mL) was added with stirring; then, ammonium hydroxide (2.8%, 10 mL) was added and the solution was stirred for 15 min. Finally, to this stirred solution, 3-mercaptopropyl trimethoxysilane (MPTMS, 2 mL) was added. The resultant solution was stirred overnight at room temperature. The formed SOS spheres were collected by centrifugation and dried. Furthermore, these SOS spheres were calcined at 600 °C for 5 h. The synthetic process of SOS is shown in Scheme 1.Synthesis of Pd/SOS catalyst
To a round bottom flask, we added 55 mg (0.31 mmol) of PdCl2, 30 mg of PVP (10k MW) and water (25 mL) at room temperature. The resultant mixture was stirred at 80 °C for 30 min. To the reaction mixture cooled to room temperature, we added 0.126 M of aq. NaBH4 solution and 500 mg of SOS and stirred at 80 °C for 3 h. The resultant Pd/SOS was washed with water (2 × 10 mL) and EtOH (2 × 10 mL) and dried at 110 °C for 12 h under vacuum. The resultant black coloured material was grained and used as such for further analysis and its catalytic applications. The synthetic process of Pd/SOS is shown in Scheme 2.Characterization
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in polyvinylpyrrolidone (PVP), which is decorated on the surface of SOS.23
O in polyvinylpyrrolidone (PVP), which is decorated on the surface of SOS.23
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C/C–C, C–N, C–O, and O–C
C/C–C, C–N, C–O, and O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, respectively.31 The core-level spectrum of N 1s shows a peak at 399.1 eV, which originates from the pyridinic nitrogen (N) in PVP.32
O, respectively.31 The core-level spectrum of N 1s shows a peak at 399.1 eV, which originates from the pyridinic nitrogen (N) in PVP.32
| Element | Atomic conc. [%] | Error [%] | Mass conc. [%] | Error [%] |
|---|---|---|---|---|
| Pd | 0.10 | 0.05 | 0.74 | 0.34 |
| C | 62.75 | 0.50 | 50.95 | 0.62 |
| Si | 0.96 | 0.41 | 18.90 | 0.70 |
| O | 27.19 | 0.39 | 29.41 | 0.44 |
Catalytic performance
Optimization studies
To begin with, the optimization for the domino intramolecular Heck cyclization followed by intermolecular Sonogashira coupling (for the synthesis of dihydrobenzofuran derivatives) was planned. In this regard, 1-iodo-2-((2-methylallyl)oxy)benzene 1a and phenylacetylene 2a were chosen as the starting materials. Thus, 1a (0.25 mmol) and 2a (0.5 mmol) were treated in the presence of the Pd/SOS (0.3 mol%) catalyst and K2CO3 (0.5 mmol) base in DMF (0.5 mL) at 100 °C for 1 h. To our delight, the expected dihydrobenzofuran 3aa was isolated in a near quantitative yield (Table 2, entry 1). In contrast, the reaction was found to be inferior with other bases [Cs2CO3 (53%), diazabicycloundecene (DBU) (82%) and NEt3 (44%), (Table 2, entries 2 to 3)]. When water was used as the solvent, 3aa was obtained in poor to moderate yields (Table 2, entries 5 to 8). The addition of 0.25 mmol of phase-transfer catalysts [tetrabutylammonium iodide (TBAI), tetrabutylammonium bromide (TBAB) and benzyl triethylammonium chloride (BTEAC)] to the reaction in water and in the presence of the base K2CO3 improved the yields of 3aa (Table 2, entries 9 to 11). The reaction with 0.25 mmol of BTEAC with the base DBU afforded 3aa in 75% yield (Table 2, entry 12). On the other hand, the product 3aa was obtained in 78% and 90% yields in the polar solvents DMA and DMSO, respectively (Table 2, entries 13 and 14). Only starting materials were recovered back without the base or the catalyst or at room temperature (Table 2, entries 15 to 17). Only trace amounts of the product 3aa were noted when the reaction was performed at 50 °C (Table 2, entry 18). A poor yield of 3aa was obtained when the reaction was conducted under neat conditions (Table 2, entry 19). From the above-mentioned results, the best optimal conditions are those given in Table 1 entry 1 for the formation of the dihydrobenzofuran 3aa.| Entry | Base | Additive (PTC) | Solvent | Yield 3aa (%) |
|---|---|---|---|---|
| a Reaction conditions: 1-iodo-2-((2-methylallyl)oxy)benzene 1a (68 mg, 0.25 mmol), phenylacetylene 2a (51 mg, 0.5 mmol), catalyst (0.3 mol% of Pd), base (0.5 mmol), additive (0.25 mmol), solvent (0.5 mL).b Isolated product yield.c With no catalyst.d At room temperature.e At 50 °C. | ||||
| 1 | K2CO3 | — | DMF | 99 |
| 2 | Cs2CO3 | — | DMF | 53 |
| 3 | DBU | — | DMF | 82 |
| 4 | NEt3 | — | DMF | 44 |
| 5 | K2CO3 | — | Water | 42 |
| 6 | Cs2CO3 | — | Water | 30 |
| 7 | DBU | — | Water | 40 |
| 8 | NEt3 | — | Water | 15 |
| 9 | K2CO3 | TBAI | Water | 60 |
| 10 | K2CO3 | TBAB | Water | 65 |
| 11 | K2CO3 | BTEAC | Water | 77 |
| 12 | DBU | BTEAC | Water | 75 |
| 13 | K2CO3 | — | DMA | 78 |
| 14 | K2CO3 | — | DMSO | 90 |
| 15 | — | — | DMF | — |
| 16c | K2CO3 | — | DMF | — |
| 17d | K2CO3 | — | DMF | — |
| 18e | K2CO3 | — | DMF | Trace |
| 19 | K2CO3 | — | Neat | 35 |
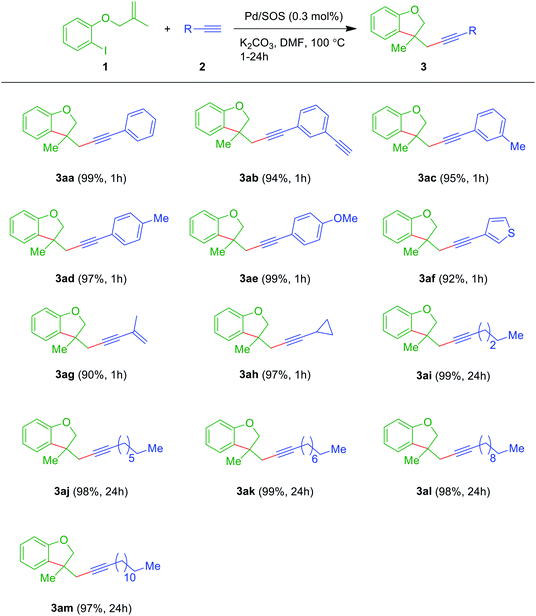
With the optimized reaction conditions in hand (Table 2, entry 1), to check the generality of this domino process, the reaction was explored with other ortho-iodoarylallyl ethers 1 and terminal alkynes 2. To our delight, the reaction was feasible and furnished the products 3aa–3am in excellent to near quantitative yields under the standard reaction conditions (Table 3). As noted above (Fig. 8), the reaction was faster with arylacetylenes 2a–2f than the aliphatic ones 2g–2m. It is worth mentioning that the reaction mixture was exclusively transformed into a single product 3ab containing a free terminal acetylenic C–H, wherein only one acetylene moiety was utilized in the reaction out of two. No other product (i.e., the product in which both alkynes of 2b reacted with two molecules of ether 1a) was formed other than 3ab even by altering the ratios of 1a and 2b. This free terminal acetylenic group would allow further derivatization under traditional coupling reactions. Also, the method was smooth with heteroaryl alkyne 2f. Furthermore, the reaction was found to exhibit excellent chemo-selectivity with enyne 2g, in which an alkyne moiety was involved in Sonogashira coupling over Heck coupling with a terminal ene functional group. Apart from alkyl terminal alkynes, cyclopropyl alkyne 2h was found to be suitable for this domino process.
After the successful accomplishment of the synthesis of dihydrobenzofurans, we turned our attention to the synthesis of oxindole derivatives. Thus, the reaction was performed with 4 and terminal alkynes 2 under standard reaction conditions. Notably, as anticipated, the reaction was found to be quite successful and furnished the desired oxindoles 5aa–5bo in excellent to near quantitative yields. Also, it was observed that in the case of oxindole synthesis, the required reaction times, particularly for aromatic alkynes, were slightly longer (Table 4).
Recyclability test
To check the sustainability of the catalyst and its retainable activity, a recyclability study was also carried out; the results are shown in Fig. 9. The catalyst was recovered by using centrifugation and washings with ethanol followed by water; it was further dried in a vacuum oven at 60 °C overnight. The recovered Pd/SOS catalyst was then subjected to the next catalytic cycles. The recovered catalyst was found to be active without an appreciable reduction in the product's (3aa) yield under the established conditions. Thus, based on the above-mentioned results, it was confirmed that the Pd/SOS catalyst has enough stability and can be reused.Mechanism
Based on previous explorations via homogenous catalysis, a conceivable reaction path can be detailed, as depicted in Scheme 3. The Pd(II) intermediate P could be formed upon activating the iodoarene bond of ortho-iodoarylallyl ether 1 by the Pd(0) heterogeneous catalyst. The subsequent intramolecular Heck step would form a bicyclic species Q. The coupling of Q with terminal acetylene 2 would generate R. Lastly, dihydrobenzofuran 3 can be formed via reductive elimination from R, regenerating the Pd(0) nanocatalyst.Conclusion
In this study, we have successfully achieved the synthesis of a novel heterogeneous catalyst. The catalyst was composed of ultra-small Pd nanoparticles on SOS (nano-silica uniformly distributed on the surface of bulk silica). Also, it was characterized by various physicochemical techniques. In addition, the efficiency and applicability of the catalyst have been demonstrated by the domino synthesis of dihydrobenzofurans and oxindoles using a sub-molar quantity of the catalyst (0.3 mol%). Moreover, this reaction showed functional group tolerance. The catalyst was robust and recyclable.Conflicts of interest
There are no conflicts to declare.References
- F. Jiang, R. Li, J. Cai, W. Xu, A. Cao, D. Chen, X. Zhang, C. Wang and C. Shu, J. Mater. Chem. A, 2015, 3, 19433 RSC.
- B. Lakshminarayana, J. Chakraborty, G. Satyanarayana and Ch. Subrahmanyam, RSC Adv., 2018, 8, 21030 RSC.
- B. Lakshminarayana, L. Mahendar, J. Chakraborty, G. Satyanarayana and Ch. Subrahmanyam, J. Chem. Sci., 2018, 130, 47 CrossRef.
- B. Lakshminarayana, L. Mahendar, P. Ghosal, G. Satyanarayana and Ch. Subrahmanyam, ChemistrySelect, 2017, 2, 2700 CrossRef CAS.
- S. Wu, J. Dzubiella, J. Kaiser, M. Drechsler, X. Guo, M. Ballauff and Y. Lu, Angew. Chem., Int. Ed., 2012, 51, 2229 CrossRef CAS PubMed.
- M. Dhiman, B. Chalke and V. Polshettiwar, ACS Sustainable Chem. Eng., 2015, 3, 3224 CrossRef CAS.
- N. Bayal, B. Singh, R. Singh and V. Polshettiwar, Sci. Rep., 2016, 6, 24888 CrossRef CAS PubMed.
- V. Polshettiwar, D. Cha, X. Zhang and J. M. Basset, Angew. Chem., Int. Ed., 2010, 49, 9652 CrossRef CAS PubMed.
- A. S. L. Thankamony, C. Lion, F. Pourpoint, B. Singh, A. J. P. Linde, D. Carnevale, G. Bodenhausen, H. Vezin, O. Lafon and V. Polshettiwar, Angew. Chem., Int. Ed., 2015, 54, 2190 CrossRef CAS PubMed.
- S. Peddibhotla, Curr. Bioact. Compd., 2009, 5, 20 CrossRef CAS.
- Q. B. Li, F. T. Zhou, Z. G. Liu, X. F. Li, W. D. Zhu and J. W. Xie, J. Org. Chem., 2011, 76, 7222 CrossRef CAS PubMed.
- M. Sickert, H. Weinstabl, B. Peters, X. Hou and M. Lautens, Angew. Chem., Int. Ed., 2014, 126, 5247 CrossRef.
- Z. Lu, C. Hu, J. Guo, J. Li, Y. Cui and Y. Jia, Org. Lett., 2009, 12, 480 CrossRef PubMed.
- K. Ramesh, S. Basuli and G. Satyanarayana, Eur. J. Org. Chem., 2018, 2018, 2171 CrossRef CAS.
- K. Ramesh and G. Satyanarayana, Green Chem., 2018, 20, 369 RSC.
- B. Lakshminarayana, L. Mahendar, P. Ghosal, B. Sreedhar, G. Satyanarayana and Ch. Subrahmanyam, New J. Chem., 2018, 42, 1646 RSC.
- D. Eisenberg and W. Kauzman, Science, 1969, 166, 861, DOI:10.1126/science.166.3907.861.
- A. K. Kronenberg and G. H. Wolf, Tectonophysics, 1990, 172, 255 CrossRef.
- J. Fukuda, Water in Rocks and Minerals – Species, Distributions, and Temperature Dependences, 2012, DOI:10.5772/35668.
- Y. Wu, L. Zhang, G. Li, C. Liang, X. Huang, Y. Zhang, G. Song, J. Jia and C. Zhixiang, Mater. Res. Bull., 2001, 36, 253 CrossRef CAS.
- J. Lin, H. Chen, T. Fei and J. Zhang, Colloids Surf., A, 2013, 421, 51 CrossRef CAS.
- D. C. Manatunga, R. M. de Silva and K. N. de Silva, Appl. Surf. Sci., 2016, 360, 777 CrossRef CAS.
- B. Li, Y. Zhang, L. Fu, T. Yu, S. Zhou, L. Zhang and L. Yin, Nat. Commun., 2018, 9, 1076 CrossRef PubMed.
- B. Lakshminarayana, G. Satyanarayana and Ch. Subrahmanyam, ACS Omega, 2018, 3, 13065 CrossRef CAS PubMed.
- E. Meku, C. Du, Y. Sun, L. Du, Y. Wang and G. Yin, J. Electrochem. Soc., 2016, 163, F132 CrossRef CAS.
- J. A. L. López, J. C. López, D. V. Valerdi, G. G. Salgado, T. Díaz-Becerril, A. P. Pedraza and F. F. Gracia, Nanoscale Res. Lett., 2012, 7, 604 CrossRef PubMed.
- F. H. Cincotto, T. C. Canevari, A. M. Campos, R. Landers and S. A. Machado, Analyst, 2014, 139, 4634 RSC.
- X. Zhou and T. Shi, Appl. Surf. Sci., 2012, 259, 566 CrossRef CAS.
- B. Ulgut and S. Suzer, J. Phys. Chem. B, 2003, 107, 2939 CrossRef CAS.
- Y. Zhao, J. Zhao, Z. Su, X. Hao, Y. Li, N. Li and Y. Li, J. Mater. Chem. A, 2013, 1, 8029 RSC.
- H. Y. Wu, K. J. Lin, P. Y. Wang, C. W. Lin, H. W. Yang, C. C. M. Ma and T. R. Jan, Int. J. Nanomed., 2014, 9, 4257 CAS.
- Y. Zhang, X. Xia, X. Cao, B. Zhang, N. H. Tiep, H. He, S. Chen, Y. Huang and H. J. Fan, Adv. Energy Mater., 2017, 7, 1700220 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra09429f |
| This journal is © The Royal Society of Chemistry 2020 |