Open Access Article
Open Access ArticlePEGylated gold nanorods with a broad absorption band in the first near-infrared window for in vivo multifunctional photoacoustic imaging†
Yiping Wanga,
Yiduo Wua,
Qiang Wena,
Pengwei Li *a,
Ying Wangb,
Huabei Jiangc and
Wendong Zhanga
*a,
Ying Wangb,
Huabei Jiangc and
Wendong Zhanga
aMicro-Nano System Research Center, College of Information and Computer, Taiyuan University of Technology, Taiyuan 030024, Shanxi, China. E-mail: lipengwei@tyut.edu.cn
bCollege of Mechanics, Taiyuan University of Technology, Taiyuan 030024, Shanxi, China
cBiomedical Optics Laboratory, Department of Medical Engineering, College of Engineering, University of South Florida, Tampa, FL 33620, USA
First published on 28th January 2020
Abstract
Nanoparticles with absorbances in the near-infrared window (NIR, 700–1300 nm) are ideal contrast agents for in vivo imaging of deep tissue with high signal-to-noise ratios. By using CTAB and L(+)-ascorbic acid (AA) as ligands to effectively balance particle nucleation and growth, PEGylated Au nanorods (NRs) with broad absorption bands (from 650 to 1100 nm) in the first NIR window could be successfully realized. The morphologies, crystal structures, absorption and biotoxicities of the samples were determined by TEM, TGA, UV-vis and MTT assay. The results indicated that the presence of a thin poly(ethylene glycol) (PEG) shell could greatly improve the biocompatibility of the Au NRs (1.7 times that of non-PEGylated Au NRs), making them harmless to living cells. Moreover, the prepared PEGylated Au NRs displayed the highest image contrast and SNR values (1.1–1.5 times that of commercial Au nanospheres and NRs), with excitation lasers of 532, 680 and 828 nm, showing their great potential for use in multicolor photoacoustic imaging in vivo. With the prepared PEGylated Au NRs, a functional image of oxygen saturation was constructed in a single step without changing the contrast agent.
1 Introduction
Near-infrared (NIR) light penetrates deep into the body and is subject to minimal interference from the scattering, absorption and autofluorescence of tissue and blood, which makes it favorable for in vivo photoacoustic imaging (PAI) and photothermal therapy (PTT).1–4 In particular, light within the two transparent biological windows, located at 650–950 nm (NIR-I window) and 1000–1350 nm (NIR-II window), can image deep tissue with high signal-to-noise ratios (SNRs).5–8 Nanoparticles, especially those with strong absorption in the NIR region, are promising labels for exogenous PAI and PTT agents due to their unique optical properties.9–13 However, most of the studied nanoparticles, such as small-molecule dyes,14 semiconductors,15–17 upconversion nanoparticles,18,19 carbon-based nanotubes and rGO,20–22 have low thermal stabilities or exhibit some degree of biotoxicity, which limits their in vivo applications.Au nanoparticles, which have excellent chemical stabilities, low biotoxicities and tunable absorbance bands in the NIR region, have been reported as promising contrast agents for NIR PAI and PTT.23–28 Au nanoparticles designed with a rod-in-shell structure and tailored to be responsive in the first and second NIR windows have been prepared and applied for hyperthermia-based therapy.29 Likewise, gold nanostars have also been successfully synthesized, with greater than 320-fold fluorescence enhancement in the first NIR-I window and 50-fold enhancement in the NIR-II region.30 In addition, gold nanorods (with an aspect ratio of approximately 3.5) emitting at approximately 700 nm have been prepared and used as contrast agents in a combined PA/Raman imaging system for the detection of ovarian cancer.31 Biodegradable gold nanovesicles with an ultrastrong plasmonic coupling effect have also been successfully synthesized and applied to PAI and PTT.32 However, it is still a challenge to tune the bandwidth of the emission of Au nanoparticles in the NIR region,33–35 which is necessary for realizing multicolor labeling to synchronously image multiple targets with high SNRs.36 For instance, multifunctional PAI of oxygen saturation requires at least two different wavelength lasers for synchronous image.37
Herein, we report a seed-assistant method to synthesize Au nanorods (NRs) with a broad absorption band ranging from 650 nm to 1100 nm or greater. The synthesized Au NRs were modified with thiolated poly(ethylene glycol) (thiol-PEG) and utilized in PAI. The results reveal that the sample has low cytotoxicity and can achieve excellent imaging efficacy of the blood vessels in mouse brain. The obtained multicolor PA images were analyzed to probe the metabolism in vivo and pixel-by-pixel calculations allowed multifunctional PA images of oxygen saturation to be obtained.
2 Experimental section
2.1 Synthesis of Au nanorods
Au NRs were synthesized by a modified seed-mediated synthetic method.38,39 In a typical procedure (Scheme 1), 10.3 mL of 0.025 M HAuCl4 (Sinopharm Chemical Reagent Co., Ltd., ≥99.9%) and 1.823 g of cetyl trimethyl ammonium bromide (CTAB) surfactant (Tianjin Guangfu Fine Chemical Research Institute, ≥99.0%) were first added to a beaker. Then, deionized water (18 MΩ) was added to bring the total concentration of HAuCl4 to be 2.5 × 10−3 M, and CTAB of 0.05 M. Four flasks tagged as A, B, C and D, were separately transferred into 10 mL, 4.5 mL, 4.5 mL and 45 mL of the above-mentioned solution in proper order. Then, 350 μL of 0.01 M ice-cold NaBH4 (Sinopharm Chemical Reagent Co., Ltd, ≥98.0%) was added to flask A, and stirred for 6.5 min. 0.4 mL of solution in flask A and 25 μL of 0.1 M L(+)-ascorbic acid (AA) (Tianjin Shentai Chemical Industry Co., Ltd., ≥99.7%) were transferred into flask B, and the solution was stirred for 3 min. And then, 0.4 mL of solution in flask B and 25 μL of 0.1 M AA were added to flask C, stirred for 3 min again. Finally, 4 mL solution of flask C and 250 μL of 0.1 M AA were added to flask D, stirred for 5 s and left undisturbed in a water bath at 28 °C for 12 h, until the reaction completed. The top layer of the solution was carefully removed, and 10 mL of deionized water was added to disperse the precipitate; the solution was centrifuged, and washed several times with distilled water to fully remove excess CTAB.2.2 Surface modification of the Au nanorods
First, 10 mg of thiol-PEG (Nanjing Pengsheng Biological Technology Co. Ltd) was dissolved in 1 mL of deionized water and sonicated for 10 min. Then, the solution was treated with 50 mL of a 0.1 M NaBH4 solution under sonication for another 15 min to prevent the possible dimerization of thiol-PEG (PEG-S-S-PEG). Second, the washed Au nanorods were dispersed in 10 mL of deionized water, mixed with the above thiol-PEG solution (10 mL) via stirring for 5 min and then left undisturbed for 5 h. Finally, the sample was centrifuged several times for 10 min at 3500 rpm and dispersed in 4 mL of deionized water for further application.2.3 Characterization
The morphologies and structures of the as-synthesized Au nanorods were identified by transmission electron microscopy (TEM, JEOL 2100F, 200 kV). UV-vis absorbances of the various Au nanorods were identified by spectrophotometry (Shimadzu, 3100 UV-vis-NIR). TGA analysis was performed on NETZSCH (STA-409-pc) using a heating rate of 20 °C min−1 up to 700 °C under N2 condition. The PA signals were recorded and normalized by a unit rotation scanning PA detection system (Fig. S1†), which contained a laser (Surelite I-20, Continuum), an optical parametric oscillator (OPO, Surelite OPO Plus), a nonfocused ultrasonic transducer (PMUT, V310-SU, Olympus, 5 MHz), a stepper motor rotating table and motor control box (MC, M600, Beijing Zhuo Li Han Guang Instrument Co., Ltd.), preamplifier (5077 PR, Olympus), and a PCI4732 data acquisition (DAQ) card. A 532 nm laser beam was directly emitted from the laser, and further laser wavelengths were obtained via the OPO.2.4 Cell viability experiments
All bio-experimental procedures were approved by IACUC committee at Taiyuan University of Technology. And the experiments were carried out in accordance with the approved guidelines. HeLa cells were cultured in the standard cell medium recommended by the American Type Culture Collection (ATCC) at 37 °C under a 5% CO2 atmosphere. Cells were seeded into 96-well plates and incubated with different concentrations of Au nanoparticles (Au NRs, Au NR@PEG, and commercial Au nanospheres and Au NRs) for 24 h. The relative cell viabilities were determined by a standard methyl thiazolyl tetrazolium (MTT) assay and imaged via an optical microscope.2.5 In vitro and in vivo photoacoustic imaging
The Au NR@PEG (1 nM) solutions were irradiated under 828 nm excitation at a power density of 15 mJ cm−2 for 30 min (laser on), then by natural cooling at room temperature without laser irradiation (laser off). This cycle was repeated five times and the UV-vis spectra of the samples were obtained to characterize the photostability of their absorption properties. Agar powder (2 g, Gene Company Ltd.) was dissolved in a beaker in 100 mL of deionized water and mixed well with a glass rod. The turbid liquid was heated to boiling in a microwave oven (Midea Group Co., Ltd.). Then, the liquid was removed and stirred in a water bath for 20 min at 60 °C until the liquid thickened. Then, the viscous material was poured into a 4.5 cm diameter cylindrical mold, cooled and solidified. Finally, the solid agar was used as a biological tissue phantom due to its absorbance of NIR excitation approximating that of biological systems.A 0.9 mm diameter glass capillary, which could be filled with fresh ox blood or blood mixed with various concentrations of Au NR@PEG in specific experiments, was implanted into the surface of the phantom to simulate blood vessels. The phantom was placed under water and irradiated with a 680 nm or 828 nm laser at a power density of 15 mJ cm−2 (Fig. S2†).
The mouse was transiently narcotized with isoflurane, then 0.04 mL/10 g of 10 wt% chloral hydrate was intraperitoneally injected to ensure the mouse was thoroughly anesthetized. The mouse head was gently shaved of hair and smoothly coated with an ultrasonic coupling agent (Boline Healthcare Ltd). The wavelength of the laser was adjusted to 828 nm, and the mouse was placed under water. Then, the cerebral blood vessels of the mouse were imaged before and after intravenously (IV) injecting the contrast agents (Au NR@PEG, commercial Au nanospheres and Au nanorods; 1 nM solutions, 0.1 mL/10 g body weight). The laser excitation was changed to 680 and 532 nm, and the above experiment was repeated. Notably, when the contrast agent was changed, subsequent IV injection should occur at least 24 h later to allow the residual contrast agent to be completely metabolized.
3 Results and discussion
The typical morphologies and structures of the three different Au nanostructures were initially determined by scanning electron microscopy (SEM) and UV-vis (Fig. 1). Fig. 1a shows the typical morphology of the synthesized Au NRs, which had diameters of approximately 22 nm and aspect ratios ranging from 6 to 18. The commercial Au nanospheres had a diameter of 25 ± 5 nm and are shown in Fig. 1b. Fig. 1c presents the typical shape of the commercial Au nanorods, which had a diameter of approximately 10 nm and lengths of 33 ± 7 nm. The absorption spectra of the samples were determined by UV-vis (Fig. 1d). Compared with the commercial Au nanospheres (absorption peak at approximately 520 nm) and nanorods (absorption peaks at 520 and 800 nm), the synthesized Au NRs exhibit a broad absorption band (ranging from 650 to 1100 nm) over the first NIR window (650–950 nm),7 indicating their potential widespread use in NIR imaging in the medical field. It is suspected that this broadening of the longitudinal surface plasmon resonance (SPR) band is dominated by differences in the aspect ratios of different Au nanorods.40,41The prepared Au NRs can be conveniently transferred to the aqueous phase for PEGylation, as shown schematically in Fig. 2. Fig. 2a is a brightfield TEM image, which shows the typical rod-like characteristics of the sample. Fig. 2b displays the UV-vis absorption spectra of the synthesized Au NRs before and after being PEGylated. Though they have similar absorption features, the PEGylated Au NRs exhibit a 16.5 nm redshift of the absorption peak. This redshift may be ascribed to the thin PEG shell coating the Au NRs (Fig. 2c).TGA curve for Au NR@PEG evaluated by mass loss was recorded as a function of temperature (Fig. S3†). The total mass loss for Au NR@PEG was 35.3%, which indicated successful coating of PEG on the surface of Au NR. The selective area electron diffraction (SAED) pattern ([022] zone axis direction) in Fig. 2d reveals the cubic structure of the sample and demonstrates its good crystallinity. Fig. 2e shows the high-resolution TEM (HRTEM) image of the top surface of a single rod in area ‘1’ highlighted by the red rectangle in Fig. 2c. The interplanar spacing was measured as 0.235 nm, corresponding to the Au {111} facet in the cubic phase.
The UV-vis spectra of Au NR@PEG showed no obvious decrease in the absorbance at 828 nm after five cycles of laser on/off (Fig. S4†). The result indicated that Au NR@PEG showed excellent photostability after a long period of laser irradiation. High absorption enhancement dominated the relatively weak background solvent spectrum in the NIR region for PAI.6,10 Simulated blood vessels (Fig. 3a) were imaged separately at 532, 680 and 828 nm (Fig. 3b). From the PA images obtained under 532 nm excitation (Fig. 3b: b1, b4, b7 and b10), Au NR@PEG had a SNR almost equal to that of the commercial Au nanoparticles; when the laser wavelength was changed to 680 nm (Fig. 3b: b2, b5, b8 and b11), Au NR@PEG showed the highest image contrast of all four samples, and under 828 nm excitation (Fig. 3b: b3, b6, b9 and b12), Au NR@PEG was still one of the best contrast agents and provided the highest SNR. In summary, the synthesized Au NR@PEG exhibited promising multicolor PAI with high SNRs. The UV-vis absorption spectra of fresh ox blood and blood mixed with different concentrations of the PA contrast agents are shown in Fig. 3c, the results of which also reflect the enhancement of the PA effect by Au NR@PEG at different wavelengths, including approximately 532 nm and the broad NIR band from 650 to 1100 nm.
Additional in vitro PA properties of the PEGylated Au NRs are shown in Fig. 4, where Au NR@PEG was employed as a PAI contrast agent, mixed with fresh ox blood at different concentrations and illuminated with an 828 nm wavelength laser (Fig. 4a). The amplitudes of the PA signals demonstrate an obvious increase after Au NR@PEG had been added. The signal increase was nearly linear with respect to the concentration of Au NR@PEG from 0.25 to 1.0 nM (Fig. 4b). When the concentration reached 1 nM, the amplitude of the generated PA signal of the mixture was approximately 18.3 times higher than that of blood alone. The PA spectra and optical spectra of the three different Au nanocrystals (Au NR@PEG and commercial Au nanospheres and NRs) are shown in Fig. 4c–e. The multiwavelength PA spectra were obtained by collecting the intensities of the PA signals at different wavelengths (from 680 to 900 nm) when a 1 nM aqueous solution was added to the glass capillary tubes. Clearly, the PA spectra agree well with the absorption spectra of the samples, indicating that Au NR@PEG could provide excellent enhancement of the PA signal over a broad NIR region from 680 nm to 900 nm.
To examine the biotoxicity of the Au NRs for active targeting, HeLa cells were incubated with and without Au nanoparticles (Au NRs, Au NR@PEG and commercial Au nanospheres and NRs) at concentrations of 0.25–1.0 nM. A standard MTT assay was carried out to determine the viability of the cells (Fig. 5a). A plot and photographs of the dose-dependent cytotoxicity (Fig. 5a and S5†) show that most of the cells survived when cultured in the presence of Au NR@PEG, even at high concentrations of up to 1 nM (77.3%), compared with the survival of the other groups, after 24 h. This suggests that Au NR@PEG possess low cell cytotoxicity and good biocompatibility42,43 and may be a promising PA contrast agent. Hematoxylin and eosin stained histological images (Fig. 5b) of four organs (heart, liver, kidney, and spleen) from mice treated with Au NR@PEG at 24 h postinjection show no appreciable abnormalities and show extremely low levels of residual Au nanoparticles in the body, which agrees well with the results above and indicates the relatively minimal effects of PEGylated Au NRs on biological organisms.
Appropriately applying contrast agents can increase the imaging depth and spatial resolution of in vivo PAI compared to those of traditional optical imaging methods.6,44 Au NR@PEG, which provides a suitable absorption band in the NIR region and possesses low toxicity, was IV injected into a mouse (1 nM, 0.1 mL/10 g body weight) to obtain PA images in a living body (Fig. 6). The cerebral blood vessels of the mouse (Fig. 6a) were imaged with a PA system at several time points, ranging from 0 min to 1 day (Fig. 6b and c), under 828 nm laser irradiation. It is obvious that the PA signals were very weak and the blood vessels could barely be imaged at low contrast prior to Au NR@PEG being IV injected and metabolized over 2 h postinjection. Strong PA signals appeared after Au NR@PEG had been IV injected and reach a maximum at 25 min postinjection, indicating the efficient accumulation of the nanorods, likely owing to the enhanced permeability and retention (EPR) effect of living organisms.19 The PA signal intensity returned to the initial value after 120 min, suggesting that Au NR@PEG was rapidly metabolized.
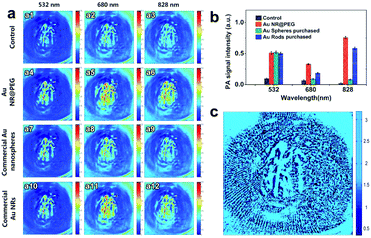
To verify the potential of Au NR@PEG for multicolor PAI, mouse was IV injected with the different Au nanocrystals (1 nM, 0.1 mL/10 g body weight) and imaged at different excitation wavelengths (532, 680 and 828 nm) with a power density of 15 mJ cm−2 (Fig. 7a).34 Under 532 nm excitation, Au NR@PEG (Fig. 7: a4) exhibited contrast improvement equivalent to that of the commercial Au nanomaterials (Fig. 7: a7 and a10). Under 680 nm excitation, Au NR@PEG (Fig. 7: a5) and the commercial Au nanorods (Fig. 7: a11) showed the highest image contrast. When the laser wavelength was adjusted to 828 nm, Au NR@PEG (Fig. 7: a6) maintained the best image quality among the different contrast agents. In short, the prepared Au NR@PEG provided the best image enhancement at all the excitation wavelengths. These results are more obvious in the histogram of the relative PA signals (Fig. 7b). Image contrast (calculated from ten randomly selected points, which were at the same position for each PA image) and the SNR were also analyzed systemically to evaluate the image quality (Table 1). Compared with that of the control group, the image contrast of Au NR@PEG improved by 61.2% under 680 nm excitation and 80.0% under 828 nm excitation, which are largest enhancements among all of the samples. Likewise, the SNRs of the images of the Au NR@PEG group also greatly improved by 34.8%, 53.2% and 24.2% under 532 nm, 680 nm at 828 nm excitation, respectively.
| Conditions | 532 nm | 680 nm | 828 nm | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Fig. | Contrast | SNR | Fig. | Contrast | SNR | Fig. | Contrast | SNR | |
| Control | a1 | 3.182 | 0.9847 | a2 | 2.475 | 0.4004 | a3 | 1.912 | 0.2450 |
| Au NR@PEG commercial | a4 | 4.763 | 1.3276 | a5 | 3.989 | 0.6136 | a6 | 3.442 | 0.3042 |
| Au nanospheres | a7 | 4.692 | 1.1368 | a8 | 2.594 | 0.4129 | a9 | 1.952 | 0.2506 |
| Commercial Au NRs | a10 | 4.755 | 1.3354 | a11 | 3.127 | 0.5527 | a12 | 2.131 | 0.2724 |
Since the oxygenated hemoglobin and deoxygenated hemoglobin in biological tissue have different absorptivities to different laser wavelengths, the distribution of the blood oxygen concentration in the mouse cerebral blood vessels could be calculated and reconstructed according to the above results (Fig. 7c). We obtained the cerebral deoxyhemoglobin (Hb), oxyhemoglobin (HbO2) and oxygen saturation (sO2) values of the mouse cerebral blood vessels at two different excitation wavelengths (680 nm and 828 nm). The sO2 changes were calculated pixel-by-pixel via the following equations, and the pixels were chosen according to the calculated total hemoglobin (HbT) concentration (CHbT = CHbO2 + CHb):37
 | (1) |
 | (2) |
 , φ680 and φ828 are the PA signal amplitudes acquired at the wavelengths of 680 nm and 828 nm, and k is the proportionality coefficient, which can be determined from the acoustic parameters and the wavelength-dependent local light fluence. The difference in oxygen saturation before and after correction was small,45 so it was set to 1 here. Notably, the calculated CHbO2 and CHb values are relative due to the unknown coefficient k, while the oxygen saturation (sO2) value calculated from eqn (1) is absolute.
, φ680 and φ828 are the PA signal amplitudes acquired at the wavelengths of 680 nm and 828 nm, and k is the proportionality coefficient, which can be determined from the acoustic parameters and the wavelength-dependent local light fluence. The difference in oxygen saturation before and after correction was small,45 so it was set to 1 here. Notably, the calculated CHbO2 and CHb values are relative due to the unknown coefficient k, while the oxygen saturation (sO2) value calculated from eqn (1) is absolute.
4 Conclusions
Au NRs with a broad absorption band (650–1100 nm) over the first NIR window were obtained via a seed-assistant method in three steps. The prepared Au NRs can be conveniently transferred to the aqueous phase for PEGylation, showing good photostability and low biotoxicity. Owing to the broad absorption band in the NIR region, Au NR@PEG could significantly improve the efficiency of in vitro and in vivo PAI under multiple excitation wavelengths (532, 680 and 828 nm). Based on this, the synthesized Au NR@PEG could provide a feasible, convenient and efficient means of multifunctional PAI deep in the body, such as for measuring the distribution of oxygen saturation.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 11602159, 51205276, and 61474079), the Special Talents in Shanxi Province (Grant No. 201605D211020), the Scientific & Technological Innovation Programs of Higher Education Institutions in Shanxi (Grant No. 2016136) and the 2018 Study Abroad Program for the University-Sponsored Young Teachers.Notes and references
- Y. Jiang and K. Pu, Small, 2017, 13, 1700710 CrossRef PubMed
.
- D. Cui, C. Xie, Y. Lyu, X. Zhen and K. Pu, J. Mater. Chem. B, 2017, 5, 4406–4409 RSC
.
- X. Wang, Y. Ma, X. Sheng, Y. Wang and H. Xu, Nano Lett., 2018, 18, 2217–2225 CrossRef CAS PubMed
.
- Z. Xu, X. Chen, Y. Jiang, X. Ai, B. Xing and K. Pu, ACS Nano, 2018, 18, 1498–1505 Search PubMed
.
- J. T. Robinson, G. Hong, Y. Liang, B. Zhang, O. K. Yaghi and H. Dai, J. Am. Chem. Soc., 2012, 134, 10664–10669 CrossRef CAS PubMed
.
- C. W. Ng, J. Li and K. Pu, Adv. Funct. Mater., 2018, 28, 1804688 CrossRef
.
- Y. Jiang, J. Li, X. Zhen, C. Xie and K. Pu, J. Am. Chem. Soc., 2019, 141, 4073–4079 CrossRef PubMed
.
- J. Li, J. Huang, J. Huang, Y. Jia, C. Xie and K. Pu, ACS Nano, 2018, 12, 2643–2651 CrossRef PubMed
.
- J. Lemaster and J. Jokerst, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2017, 9, e1404 CrossRef
.
- Y. Jiang and K. Pu, Acc. Chem. Res., 2018, 51, 1840–1849 CrossRef CAS PubMed
.
- T. Bao, W. Yin, X. Zheng, X. Zhang, J. Yu, X. Dong, Y. Yong, F. Gao, L. Yan, Z. Gu and Y. Zhao, Biomaterials, 2016, 76, 11–24 CrossRef CAS PubMed
.
- Y. Yong, L. Zhou, S. Zhang, L. Yan, Z. Gu, G. Zhang and Y. Zhao, NPG Asia Mater., 2016, 8, e273 CrossRef CAS
.
- X. Zhen, X. Feng, C. Xie, Y. Zheng and K. Pu, Biomaterials, 2017, 127, 97–106 CrossRef CAS PubMed
.
- Q. Chen, C. Wang, C. Liang and Z. Liu, J. Control. Release, 2014, 35, 8206–8214 CAS
.
- X. Qian, S. Shen, T. Liu, L. Cheng and Z. Liu, Nanoscale, 2015, 7, 6380–6387 RSC
.
- H. Xie, Z. Li, Z. Sun, J. Shao, X.-F. Yu, Z. Guo, J. Wang, Q. Xiao, H. Wang and Q.-Q. Wang, Small, 2016, 12, 4136–4145 CrossRef CAS PubMed
.
- Z. Zhou, Y. Sun, J. Shen, J. Wei, C. Yu, B. Kong, W. Liu, H. Yang, S. Yang and W. Wang, Biomaterials, 2014, 35, 7470–7478 CrossRef CAS PubMed
.
- X. Zhu, W. Feng, J. Chang, Y.-W. Tan, J. Li, M. Chen, Y. Sun and F. Li, Nat. Commun., 2016, 7, 10437 CrossRef CAS PubMed
.
- S. K. Maji, S. Sreejith, J. Joseph, M. Lin, T. He, Y. Tong, H. Sun, S. W.-K. Yu and Y. Zhao, Adv. Mater., 2014, 26, 5633–5638 CrossRef CAS PubMed
.
- C. Wang, L. Xu, C. Liang, J. Xiang, R. Peng and Z. Liu, Adv. Mater., 2014, 26, 8154–8162 CrossRef CAS PubMed
.
- M. A. Patel, H. Yang, P. L. Chiu, D. D. T. Mastrogiovanni, C. R. Flach, K. Savaram, L. Gomez, A. Hemnarine, R. Mendelsohn and E. Garfunkel, ACS Nano, 2013, 7, 8147–8157 CrossRef CAS PubMed
.
- S. Gao, L. Zhang, G. Wang, K. Yang, M. Chen, R. Tian, Q. Ma and L. Zhu, Biomaterials, 2016, 79, 36–45 CrossRef CAS PubMed
.
- W. Li and X. Chen, Nanomedicine, 2015, 10, 299–320 CrossRef CAS PubMed
.
- H. Ju, R. A. Roy and T. W. Murray, Biomed. Opt. Express, 2013, 4, 66–76 CrossRef CAS PubMed
.
- E. Locatelli, I. Monaco and M. Comes Franchini, RSC Adv., 2015, 5, 21681–21699 RSC
.
- Z. Li, H. Huang, S. Tang, Y. Li, X.-F. Yu, H. Wang, P. Li, Z. Sun, H. Zhang, C. Liu and P. K. Chu, Biomaterials, 2016, 74, 144–154 CrossRef CAS PubMed
.
- P. Wu, D. Deng, J. Gao and C. Cai, ACS Appl. Mater. Interfaces, 2016, 8, 10243–10252 CrossRef CAS PubMed
.
- M. Sun, F. Liu, Y. Zhu, W. Wang, J. Hu, J. Liu, Z. Dai, K. Wang, Y. Wei, J. Bai and W. Gao, Nanoscale, 2016, 8, 4452–4457 RSC
.
- M. F. Tsai, S.-H. G. Chang, F.-Y. Cheng, V. Shanmugam, Y.-S. Cheng, C.-H. Su and C.-S. Yeh, ACS Nano, 2013, 7, 5330–5342 CrossRef CAS PubMed
.
- I. G. Theodorou, Z. A. R. Jawad, Q. Jiang, E. O. Aboagye, A. E. Porter, M. P. Ryan and F. Xie, Chem. Mater., 2017, 29, 6916–6926 CrossRef CAS
.
- J. V. Jokerst, A. J. Cole, D. V. D. Sompel and S. S. Gambhir, ACS Nano, 2012, 6, 10366–10377 CrossRef CAS PubMed
.
- P. Huang, J. Lin, W. Li, P. Rong, Z. Wang, S. Wang, X. Wang, X. Sun, M. Aronova and G. Niu, Angew. Chem., Int. Ed., 2013, 52, 13958–13964 CrossRef CAS PubMed
.
- S. Mallidi, T. Larson, J. Tam, P. P. Joshi, A. Karpiouk, K. Sokolov and S. Emelianov, Nano Lett., 2009, 9, 2825–2831 CrossRef CAS PubMed
.
- Y. Jiang, P. K. Upputuri, C. Xie, Y. Lyu, L. Zhang, Q. Xiong, M. Pramanik and K. Pu, Nano Lett., 2017, 17, 4964–4969 CrossRef CAS PubMed
.
- H. J. Wang, K. H. Yang, S. C. Hsu and H. Y. Huang, Nanoscale, 2016, 8, 965 RSC
.
- Z. L. Zhang, D. W. Pang, Z. Q. Tian, D. L. Zhu, C. N. Zhu and P. Jiang, ACS Appl. Mater. Interfaces, 2013, 5, 1186–1189 CrossRef PubMed
.
- D. Wu, J. Yang, G. Zhang and H. Jiang, Biomed. Opt. Express, 2017, 8, 2276–2286 CrossRef CAS PubMed
.
- N. R. Jana, L. Gearheart and C. J. Murphy, J. Phys. Chem. B, 2001, 105, 4065–4067 CrossRef CAS
.
- H. Y. Wu, H. C. Chu, T. J. Kuo, C. L. Kuo and M. H. Huang, Chem. Mater., 2005, 17, 6447–6451 CrossRef CAS
.
- H. Y. Wu, W. L. Huang and M. H. Huang, Cryst. Growth Des., 2009, 7, 831–835 CrossRef
.
- L. S. Slaughter, W.-S. Chang, P. Swanglap, A. Tcherniak, B. P. Khanal, E. R. Zubarev and S. Link, J. Phys. Chem. C, 2010, 114, 4934–4938 CrossRef CAS
.
- L. Cheng, J. Liu, X. Gu, H. Gong, X. Shi, T. Liu, C. Wang, X. Wang, G. Liu, H. Xing, W. Bu, B. Sun and Z. Liu, Adv. Mater., 2014, 26, 1886–1893 CrossRef CAS PubMed
.
- Z. Li, Y. Hu, M. Chang, K. A. Howard, X. Fan, Y. Sun, F. Besenbacher and M. Yu, Nanoscale, 2016, 8, 16005–16016 RSC
.
- C. Kim, C. Favazza and L. V. Wang, Chem. Rev., 2010, 110, 2756–2782 CrossRef CAS PubMed
.
- M. Sivaramakrishnan, K. Maslov, H. F. Zhang, G. Stoica and L. V. Wang, Phys. Med. Biol., 2007, 52, 1349–1361 CrossRef PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra10442a |
| This journal is © The Royal Society of Chemistry 2020 |