Open Access Article
Open Access ArticleDevelopment and validation of a headspace needle-trap method for rapid quantitative estimation of butylated hydroxytoluene from cosmetics by hand-portable GC-MS
Chiranjit Ghosh ,
Varoon Singh
,
Varoon Singh ,
Jonathan Grandy and
Janusz Pawliszyn*
,
Jonathan Grandy and
Janusz Pawliszyn*
Department of Chemistry, University of Waterloo, 200 University Avenue West, Ontario N2L 3G1, Canada. E-mail: janusz@uwaterloo.ca
First published on 12th February 2020
Abstract
Butylated hydroxytoluene (BHT) is widely used as a stable and inexpensive antioxidant in skin care products. BHT is effective at penetrating the epidermis, and prolonged exposure to BHT has been reported to have toxic effects on the lungs, liver, and kidneys. Given that the carcinogenic factor of BHT is closely related to the dose and the length of exposure of the compound on a specific organ, quantitative estimation of BHT in BHT-containing cosmetics is very important in terms of quality control measurements for products used in daily life. In this study, we have developed a new method based on purge-and-trap technology using a headspace needle trap device (NTD) in addition to a comparative SPME methodology which was both coupled to portable gas chromatography (GC) toroidal ion trap mass spectrometer (MS) for rapid determination of BHT in cosmetics. The sample was placed in a glass vial and heated to 60 °C for 30 min to promote partitioning of BHT into the headspace, where it was then pre-concentrated by the Tenax/carboxen NTD. To verify these results, a divinylbenzene/polydimethylsiloxane SPME fiber was also used for headspace extraction. To quantify BHT in a sample, a standard addition calibration method was employed. A six-point calibration curve showed high linearity (R2 = 0.98) within a dynamic concentration range of 1000–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ng mL−1 BHT in the sample matrix. Estimation of BHT by NTD yielded a concentration value of around 5000 ng/0.1 g body lotion, whereas the measurement of BHT using the SPME method yielded a value of around 5500 ng/0.1 g body lotion. The attained results indicate that the amount of BHT estimated by the proposed needle trap method was well-matched with the standard SPME technique. The results obtained from both methods were well matched (∼90%). One of the advantages imparted by this method is that it is based on headspace sampling instead of direct extraction of BHT via direct immersion into the sample matrix; as such, the method does not contaminate the instrument after sampling. Moreover, the use of a portable GC-MS enables on-site quality control measurements of healthcare products, thus circumventing the need for transportation and laborious laboratory sample preparation protocols.
000 ng mL−1 BHT in the sample matrix. Estimation of BHT by NTD yielded a concentration value of around 5000 ng/0.1 g body lotion, whereas the measurement of BHT using the SPME method yielded a value of around 5500 ng/0.1 g body lotion. The attained results indicate that the amount of BHT estimated by the proposed needle trap method was well-matched with the standard SPME technique. The results obtained from both methods were well matched (∼90%). One of the advantages imparted by this method is that it is based on headspace sampling instead of direct extraction of BHT via direct immersion into the sample matrix; as such, the method does not contaminate the instrument after sampling. Moreover, the use of a portable GC-MS enables on-site quality control measurements of healthcare products, thus circumventing the need for transportation and laborious laboratory sample preparation protocols.
1 Introduction
Antioxidants are widely used in skin care products to prevent skin aging, which is typically associated with the appearance of wrinkles, dry skin, dark spots, and loss of skin elasticity and thickness. Antioxidants aid in the maintenance of skin health by terminating the formation of free radicals, which facilitate aging of the skin by damaging DNA, proteins, and lipids in the skin. Free radicals are known to be produced during exposure of skin to sunlight, polluted air, and unhygienic environments.1Owing to its chemical stability and affordability, butylated hydroxytoluene (BHT), a well-known synthetic antioxidant, is extensively used in various cosmetic formulations at concentrations ranging from 0.0002% to 0.5%.2 BHT effectively penetrates the skin, and a portion of the absorbed amount remains within the layers of the skin. The reported effects of BHT on human health remain very controversial. BHT has been approved by the Food and Drug Administration (FDA) and is generally recognized as safe (GRSA) in food additives and preservatives while used in low quantities. According to guidelines from the Food and Agriculture Organization (FAO) of the United States and World Health Organization (WHO), the daily limit of BHT consumption in foods should be restricted to 0–0.125 mg kg−1, whereas the acceptable range of BHT by European Economic Community is 0–0.05 mg kg−1.3 However, previous studies have demonstrated that a single oral dose of BHT within a range of 0.5 to 1.0 g kg−1 may damage renal and hepatic functions in rats.4 Similarly, short-term repeated exposure to BHT was found to cause hepatic toxicity in male and female rats.2,5 Further studies have shown that oral ingestion of BHT can contribute to the development of tumors in the liver, lungs, and gastrointestinal tract of animals.5,6 Apart from potential hepatic and renal effects, long-term skin exposure to BHT has been reported to have a toxic effect on lung tissue.2 Another report7 has asserted that BHT itself is not genotoxic; rather, BHT alters the genotoxicity of other agents. In clinical tests, exposure to pure BHT was found to cause moderate irritation.8 Skin sensitivity tests on small groups of participants determined that application of 1–2% BHT did not show negative effects on patients.9 The Cosmetics Ingredient Review (CIR) Expert Panel recommends using BHT at a maximum concentration of 0.5% in cosmetics. Although the potential negative effects of BHT on human health have been widely discussed in numerous reports and generated much controversy, it is widely agreed that the carcinogenic factor of BHT is related to its dose and the length of exposure of the compound to a specific organ. Therefore, quantitative estimations of BHT in cosmetics regularly used in daily life are very important, as periodic use of these products can lead to bioaccumulation of BHT in the body, and may cause adverse effect on human health.
Antioxidants in foods, cosmetics, and pharmaceuticals are determined mainly by micellar electrokinetic capillary chromatography, supercritical fluid extraction, cloud point extraction, flow injection analysis followed by high-performance liquid chromatography (HPLC), and spectrophotometric methods.10–15 To date, very few studies have utilized gas chromatography (GC) methods to estimate BHT in target samples. The few methods reported have consisted of cumbersome steps such as solvent extraction and solvent evaporation, rendering their application complex and tedious.16 To overcome these limitations, solid-phase microextraction (SPME) has been proposed as an alternative sample preparation method for analysis of BHT in target sample matrices by GC-MS or GC-MS/MS.16–19
Within the last few years, portable gas chromatography-mass spectroscopy (GC-MS) has emerged as a viable tool for on-site analysis of organic analytes present in gaseous samples. The capabilities of field-portable GC-MS have been well established for environmental and forensic analysis.20,21 However, to the best of our knowledge, no study to date has reported the application of portable GC-MS instrumentation for the determination of BHT in samples. In addition, most reports to date on BHT have entailed determination of this compound in foods stuffs as matrices of choice. Therefore, there is a pressing need for the development of a simple and robust method for the rapid determination of BHT in daily life products, such as cosmetics. Herein, a commercial body lotion listing BHT as its main antioxidant ingredient has been chosen as a representative cosmetics complex matrix. The determination of BHT in cosmetics was chosen for study owing to the fact that human skin is exposed to cosmetics for long time periods following their application on the skin.
Needle trap (NT) is a well-established sample preparation method for exhaustive extraction of volatile organic compounds (VOCs) as well as semivolatile organic compounds (SVOCs).19,22,23 The main advantage imparted by the needle trap method is that the needle trap devices (NTDs) are able to trap both free and particle-bound analytes present in media. Further, the sample analysis is easily achieved by thermal desorption of the needle trap device (NTD) in a gas chromatography system. In this regard, specialized needle trap devices have already been successfully used for the on-site quantitation of formaldehyde from car exhaust on portable GC-MS instrumentation.24 However, most NTD applications reported to date have entailed direct extraction from gaseous samples. Recently, NTD was successfully coupled with the thin-film membrane (TF-SPME) for transferring analytes from TF-SPME to NTD and applied for headspace sampling from water samples,25 demonstrating the potential of this method as an effective analytical tool for fast and accurate determination of analytes from liquid sample media.
The current study introduces a new method based on headspace needle trap sampling coupled to hand-portable gas chromatography toroidal ion trap mass spectrometry (GC-TMS) for the rapid determination of BHT in cosmetics. By incorporating the use of a hand-portable analyzer and other portable experimental components, the presented method thus facilitates determinations outside of the laboratory environment, extending the applicability of the method beyond typical laboratory analysis. In addition to carrying out statistical validation of the method, experimental results attained via NT were also compared with those attained via headspace (HS) SPME, showing good agreement between the two techniques. Succinctly, the newly developed HS-NTD method exploiting GC-TMS for estimation of BHT in cosmetics was demonstrated to be not only rapid, robust and cost-effective, but also reproducible, accurate, and reliable.
2 Experimental
2.1 Chemicals and supplies
The butylated hydroxytoluene (BHT) standard was purchased from Millipore Sigma (Bellefonte, PA, USA). BHT-containing and non-BHT containing cosmetics (Vaseline intensive care) were purchased from the local market. HPLC grade acetone was obtained from Caledon Laboratories Ltd. (Georgetown, ON, Canada). Ultrapure water was obtained using a Barnstead/Thermodyne NANO-pure ultrapure water system (Dubuque, IA). The 19 gauge Tenax/Carboxen (CAR) Custodian needle trap device, 65 μm divinylbenzene/polydimethylsiloxane (DVB/PDMS) SPME fibers were supplied by Torion Technologies of PerkinElmer Inc. (American Fork, UT). The 20 mL crimp vial was purchased from Millipore Sigma (Bellefonte, PA, USA). The helium gas cylinder was supplied by Praxair. The ingredients of the BHT-containing commercial body lotion used as a representative matrix are described in its label as follows: water, mineral oil, glycerin, cetostearyl, alcohol, petrolatum, avenasativa straw extract, sodium cetostearylsulphate, glyceryl stearate, paraffin, dimethicone, microcrystalline wax, sodium carboxyl methylcellulose, methylparaben, DMDM hydantoin, propylparaben, simethicone, disodium EDTA, sotanean oil, BHT, perfume, alpha-isomethyl ionone, amyl cinnamal, butylphenylmethylpropional, citronellol, geraniol, hexyl cinnamal, limonene, linalol.2.2 Portable GC-MS analysis
A Torion Tridion-9 GC-toroidal ion trap MS (Torion Technologies Inc.) was utilized for analysis of BHT in the selected matrix. This GC-MS instrument weighs 14.5 kg (32 lb), has small dimensions of 38.1 × 39.4 × 22.9 cm, and operates on battery power. The GC column consists of a low thermal mass (LTM) MXT-5 (5 m × 0.1 mm × 0.4 μm) Siltek-treated stainless-steel column (Restek Co. Bellefonte, PA). The injector temperature was maintained at 280 °C, the maximum recommended for Tenax sorbents, as to ensure the complete desorption of the target analyte from the NTD device. The oven temperature was programmed as described: the temperature was initially held at 37 °C for 2 s, ramped at a rate of 2 °C s−1 to 220 °C, then held for 50 s. Desorption of the NTD device was performed at 280 °C for 1 s in splitless mode, followed by a 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 split for 10 s, and finally a 50
1 split for 10 s, and finally a 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 split for the 30 s. This desorption protocol was chosen to give the best balance between the higher sensitivity given by splitless injections while providing high enough injector flow rates via split-flow to desorb the NTD quickly enough as to not effect the chromatographic peak shape of early eluters during the chromatographic run. The temperature of the ion-trap heater was 155 °C, whereas the transfer-line temperature was set at 250 °C during analysis. Helium was used as carrier gas at a fixed flow rate of approximately 0.3 mL min−1. Performance validation of the instrument was achieved by quantitation of various compounds present in the Calion-13 standard mixture.
1 split for the 30 s. This desorption protocol was chosen to give the best balance between the higher sensitivity given by splitless injections while providing high enough injector flow rates via split-flow to desorb the NTD quickly enough as to not effect the chromatographic peak shape of early eluters during the chromatographic run. The temperature of the ion-trap heater was 155 °C, whereas the transfer-line temperature was set at 250 °C during analysis. Helium was used as carrier gas at a fixed flow rate of approximately 0.3 mL min−1. Performance validation of the instrument was achieved by quantitation of various compounds present in the Calion-13 standard mixture.
Ionization on the Tridion-9 mass analyzer was performed using a 70 eV in-trap electron gun source. The ion trap was operated in full scan mode using an m/z range of 43–500 and a scan time of 50 ms.
2.3 Determination of BHT in body lotion by the purge-and-trap method
A standard addition calibration approach was used for the determination of BHT in body lotion. Briefly, the calibration method consisted of the addition of known quantities of the target analyte (BHT) into a sample matrix having an unknown quantity of the analyte, thus accounting for possible sample matrix effects. In this study, 0.1 g body lotion was weighed and put into a 20 mL crimp-cap vial. A series of BHT standards in concentrations of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ng mL−1, 7000 ng mL−1, 5000 ng mL−1, 3000 ng mL−1, and 1000 ng mL−1 were prepared by dilution of a 1000 μg mL−1 BHT standard stock solution with acetone. Briefly, 10 μL of BHT standard was added into the matrix, followed by 4 mL of water in each vial. The sample matrix was vortexed for 5 min, then placed on a rotating apparatus for stirring at 1200 rpm for 5 min. Thereafter, each vial was heated at 60 °C for 30 min so as to enable enrichment of BHT in the headspace of the vial and its extraction by the Tenax/CAR needle trap device. In this study, 10 mL of headspace gas was driven through the NTD for extraction. Headspace SPME (HS-SPME) analysis was carried out with the use of a 65 μm DVB/PDMS SPME fiber. Following extraction, both the fiber and the NTD were desorbed at 280 °C in the portable GC-MS injector. Each sample was analyzed at least in triplicate. A plot of the peak area responses was constructed for the range of 1000–10
000 ng mL−1, 7000 ng mL−1, 5000 ng mL−1, 3000 ng mL−1, and 1000 ng mL−1 were prepared by dilution of a 1000 μg mL−1 BHT standard stock solution with acetone. Briefly, 10 μL of BHT standard was added into the matrix, followed by 4 mL of water in each vial. The sample matrix was vortexed for 5 min, then placed on a rotating apparatus for stirring at 1200 rpm for 5 min. Thereafter, each vial was heated at 60 °C for 30 min so as to enable enrichment of BHT in the headspace of the vial and its extraction by the Tenax/CAR needle trap device. In this study, 10 mL of headspace gas was driven through the NTD for extraction. Headspace SPME (HS-SPME) analysis was carried out with the use of a 65 μm DVB/PDMS SPME fiber. Following extraction, both the fiber and the NTD were desorbed at 280 °C in the portable GC-MS injector. Each sample was analyzed at least in triplicate. A plot of the peak area responses was constructed for the range of 1000–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ng mL−1 BHT using the aforementioned external BHT standards. The unknown concentration of BHT in the target sample was determined by extrapolating the plot of at y = 0.
000 ng mL−1 BHT using the aforementioned external BHT standards. The unknown concentration of BHT in the target sample was determined by extrapolating the plot of at y = 0.
2.4 Headspace needle trap device (HS-NTD) sampling
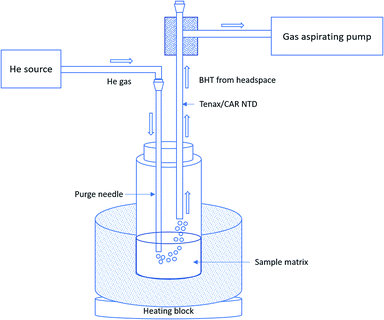
An approximate volume of 4 mL sample solution was introduced into a 20 mL crimp vial. The vial was capped with a PTFE/silicone septum and a screw cap. A Torion gas aspirating metering pump was used to draw BHT from the headspace of the vial into the needle trap device. A tee adapter with one end fitted to the NTD and the other (second part) to the aspirator was used during the sampling procedure. The end of the adaptor was directly inserted into the vial through the septum for headspace extraction. The fittings at the joints of the adapter were properly wrapped with Teflon tape to ensure an air-tight seal between the adaptor and aspirator. A purging needle was placed within the sample solution and guided to a helium gas reservoir (Fig. 1). The aspirator was used during sampling to exert enough driving force so as to pass the headspace gas through the NTD at a pre-fixed sampling rate, facilitating trapping of the analyte within the Tenax/CAR sorbent bed of the needle. Here, we used a fixed sampling volume of 10 mL at a sampling rate of 10 mL min−1. Sampling and purging were performed simultaneously, using the same abovementioned sampling rate. A flow rate of 10 mL min−1 was selected in accordance with the results of a preliminary breakthrough study so as to avoid the breakthrough of the NTD. After extraction, the NTD was injected into the Tridion-9 portable GC-MS for analysis of BHT in the sample.2.5 Needle trap breakthrough test
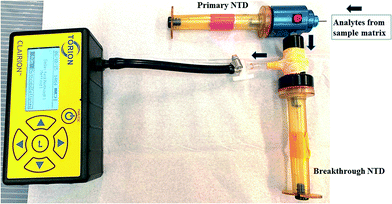
The breakthrough test was designed according to the method described by Grandy et al.,25 as depicted in Fig. 2. In brief, two NTDs were connected in series as shown in the diagram. Samplings at different rates were carried out to assess whether breakthrough occurred. Under a “no breakthrough” scenario, analytes from the sample matrix would be trapped solely by the 1st NTD (primary NTD); however, if breakthrough occurred, then the 2nd NTD (breakthrough NTD) would trap any remaining analyte before the analyte entered the aspirator. This investigation was carried out to determine the optimum sampling rate under which a single NTD was able to trap all analytes during headspace extraction.The breakthrough test was performed by extracting BHT from the sample matrix. The matrix was spiked with 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ng of BHT standard solution.
000 ng of BHT standard solution.
2.6 Sampling by SPME fiber
To validate the results obtained by the HS-NTD method, a similar headspace study was carried out under the same experimental conditions using a 65 μm DVB/PDMS SPME fiber. The sample matrix was prepared in the same manner as detailed for the HS-NTD method. Briefly, a vial was heated for 30 min at 60 °C so as to allow BHT to pre-concentrate from the headspace into the SPME fiber. The fiber was directly inserted into the crimp vial through the septum and exposed to the headspace of the vial for an extraction period of 10 min. Results attained via HS-SPME were then compared with those of the HS-NTD method.2.7 Estimation of amounts of BHT extracted by NTD and SPME
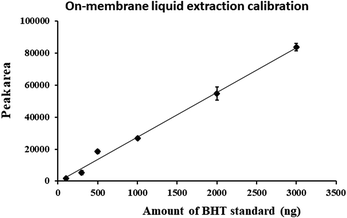
A thin film (TF) liquid injection method described by Grandy, Singh et al.26 was used to assess the amounts of BHT extracted by NTD and SPME. The details of the method are described elsewhere. Briefly, the method is based on an on-membrane liquid injection, where pre-determined quantities of a standard solution are injected onto a membrane using a microsyringe so as to allow the analyte to be wicked onto it. The loaded TF-SPME membrane is then placed into the SPS-3 (PerkinElmer American Fork Utah) thermal desorption unit (TDU), which transfers analytes from the membrane, inserted into a 3.5′′ sorbent tube to the NTD. After desorption at 250 °C for 5 min, analytes trapped into the NTD are then immediately injected into the portable GC-MS for instrumental analysis. This methodology prevents the need for performing liquid injection (LI) to calibrate nanograms injected on the instrument. This lack of traditional LI is important as the injector and column capacity of the Tridion-9 cannot accommodate large solvent volumes. In this work, the response of the instrument was recorded for a series of different BHT standard solutions. A calibration curve was constructed based on peak areas and amounts of analyte injected into the membrane. The results were transformed into a 1/x2 weighing factor so as to avoid possible discrimination of any data corresponding to low concentrations of the standard.3 Results and discussion
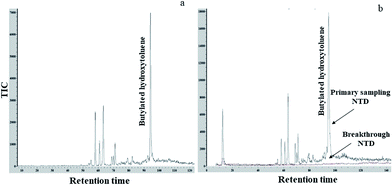
A new method encompassing needle trap sampling coupled to portable GC-MS was developed for the determination of BHT in cosmetics. Attained chromatograms (Fig. 3) displayed no major interfering peaks originating from the sample matrix at a retention time of 95.4 s for BHT. As seen by the chromatogram of the breakthrough test (Fig. 3b), no breakthrough occurred during our sampling process.To investigate the amounts of BHT extracted by NTD and SPME, instrumental responses associated with the quantities of BHT injected into the portable GC-MS following on-membrane liquid injection by NTD and SPME were calculated. Using the calibration curve (Fig. 4), the amounts of BHT extracted from the headspace of the BHT-containing vials were then calculated from the instrumental peak response.
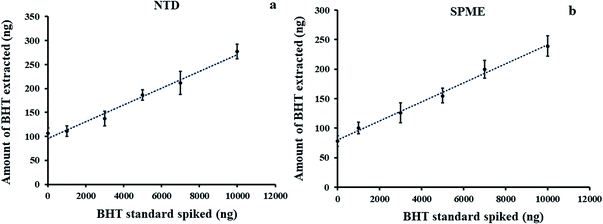
A six-point calibration curve (Fig. 5a) was built with the use of the standard addition method. The curve showed linearity (R2 = 0.98) at a spiking concentration range of 1000–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ng mL−1 BHT standard solution. Three replicates confirmed the data to be reproducible, yielding maximum RSD values of less than 10%. Using this method, the concentration of BHT in body lotion was calculated from the extrapolation of the fitting line to y = 0. As summarized in Table 1, there was a good agreement in the concentrations of BHT present in the body lotion using the NTD and SPME methods with calculated concentrations value of around 5000 ng/0.1 g (Fig. 5a) and 5500 ng/0.1 g (Fig. 5b) respectively.
000 ng mL−1 BHT standard solution. Three replicates confirmed the data to be reproducible, yielding maximum RSD values of less than 10%. Using this method, the concentration of BHT in body lotion was calculated from the extrapolation of the fitting line to y = 0. As summarized in Table 1, there was a good agreement in the concentrations of BHT present in the body lotion using the NTD and SPME methods with calculated concentrations value of around 5000 ng/0.1 g (Fig. 5a) and 5500 ng/0.1 g (Fig. 5b) respectively.
| Sample | BHT (ng) | ||
|---|---|---|---|
| Spiked | Recovery | Accuracy | |
| Non-BHT containing body lotion | 7000 | ∼6500 | ∼93% |
| 5000 | ∼5400 | ∼93% | |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
∼15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
∼99% | |
| BHT containing body lotion | Estimated by standard addition | Estimated by the external calibration method | |
| 5571 | ∼6100 | ∼90% | |
Interestingly, the attained results indicate that the amounts of BHT estimated by the proposed NTD method were similar to the amounts yielded by the standard SPME technique. Although each point in the calibration curve corresponding to the peak area of the concentration of the external BHT standard was different for the NTD and SPME methods, the calculated concentrations of BHT in the unknown sample matrix were found to be well matched. Attained results from both methods depicted that the sampled body lotion contained around 0.005% BHT, which indicates that the concentration of this antioxidant in the sampled body lotion is within the recommended range of 0.0002–0.5% BHT for cosmetic formulations. These results also ascertain that the newly developed NTD method is able to concentrate this semi-volatile compound from the headspace of the investigated cosmetic matrix. One of the advantages of this method is that it extracts from the headspace of the sample, and not directly from the matrix, thus minimizing/circumventing possible instrument contamination. Although the headspace method uses sequential sampling/purging, the dilution effect did not considerably affect the attained results (RSD < 10%) since the positions of the purging needle and sampling needle were affixed prior to each extraction. In this regard, the low purging rate used also contributed to an observed low dilution effect. The headspace volume was kept constant by purging the same amount of gas within the vial. Here, it is important to mention that the SPS-3 thermal desorption unit (TDU) also contains pure-and-trap technology for HS-NTD sampling. However, we did not use the system for our study as it was not possible to couple SPME fiber with the said system. Thus, we would not be able to compare our NTD based results with the traditional SPME method.
It is important to note that due to the relatively high concentrations of BHT found in real-world samples, optimization of LOD and LOQ was not deemed necessary and hence not performed.27 Instead, it was more important to ensure that the calibration methodology was accurate at these high concentrations, and could be used for different cosmetic lotions.
In order to confirm the robustness of accurately determining BHT concentrations in different body lotions, a non-BHT containing lotion composed of ingredients similar to those of the BHT-containing lotion was submitted to analysis. A non-BHT containing lotion composed of ingredients similar to those of the BHT-containing lotion was submitted to analysis. Known amounts of BHT standards were then spiked in this sample matrix, and instrumental analyses were carried out for external quantitation of BHT.
A calibration curve was then constructed (Fig. 6) from the attained data, yielding accuracy values ranging from 93% to 99% (Table 1).
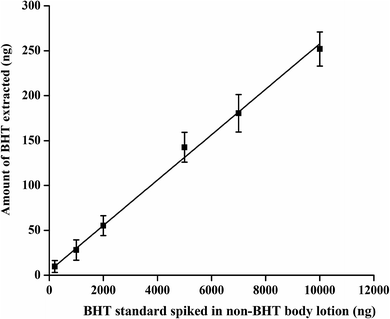 | ||
| Fig. 6 External calibration curve after spiking BHT standard solution into a non-BHT containing body lotion having similar ingredients. The fitting equation is y = 0.02x + 4.8 (R2 = 0.99). | ||
To further confirm the accuracy of our method, similar headspace extractions were carried out by the SPME method on the targeted BHT-containing body lotion. Here, quantification of BHT was achieved through the addition of external standard solutions at different concentrations directly to the target sample matrix, with subsequent monitoring of peak responses of unknown concentrations of BHT in matrix from an established calibration curve. The developed method was shown to exhibit an approximate accuracy of 90% (estimated 6130 ng of our BHT containing lotion) as compared to the standard addition method. The robustness of the calibration methodology was also confirmed via external calibration in the non-BHT containing lotion.
4 Conclusions
The presented work describes the development of a new headspace needle trap analytical method for the rapid determination of BHT in cosmetics by portable GC-MS. The results presented in this study demonstrated the feasibility of utilizing a hand-portable GC-MS for on-site estimation of antioxidants in real-time, thus circumventing the need for laborious laboratory sample preparation procedures and subsequent instrumental analysis in the lab. Utilization of the portable GC-MS, in turn, can largely facilitate rapid decision-making by inspecting agencies, since it can be transported to any place for on-site testing of BHT levels in a variety of healthcare products, thus facilitating quality control measurements. In addition to enabling quantitation, the use of this standard addition calibration method also enabled circumvention of possible matrix effects. However, the standard addition method needs more samples as compared to other conventional methods. The attained results demonstrate good extraction performance and reproducibility for the HS-NTD method. Furthermore, the method was successfully validated through a comparison of results obtained by HS-SPME. Given these results, the developed method can be considered as a sensitive and easy-to-perform method for the estimation of the antioxidant BHT in body lotion. NTD is sometimes considered as a simpler to calibrate technique when compared to SPME since larger quantities of analytes can be extracted by this technique (NTD), leading to enhanced sensitivity.Hence only a nanogram versus response calibration curve is typically required. However, the data attained in this study has demonstrated that the proposed HS-NTD technique is non-exhaustive in nature; as such, the accuracy of the method could be further enhanced by exhaustive extraction by NTD, although such an application would require larger extraction volumes at a higher temperature. It was also encouraging to see that the concentrations determined by the HS-NTD approach yielded comparable results to those attained via traditional HS-SPME. Given that long-term exposure to BHT has been reported to have adverse effects on various organs of the body, efforts should be put into monitoring quantities of BHT in healthcare products that are used regularly as part of daily life, as such products tend to remain in contact with human skin for extended periods of time. Likewise, this tool can also be applied for the analysis of BHT from food samples in real-time; as such, as a future direction, we aim to validate the method for the determination of BHT in foods. As well, the feasibility of the method for analysis of SVOCs in different types of samples is under consideration.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are thankful for the funding provided by the NSERC Industrial Research Chair program to support this research work.References
- I. Bogdan Allemann and L. Baumann, Skin Therapy Lett., 2008, 13, 5–9 CAS.
- R. L. Bronaugh, S. W. Collier, J. E. Storm and R. F. Stewart, J. Toxicol. Cutan. Ocul. Toxicol., 1989, 8, 453–467 CrossRef CAS.
- R. S. Lanigan and T. A. Yamarik, Final report on the safety assessment of BHT, Int. J. Toxicol., 2002, 21, 19–94 CrossRef CAS PubMed.
- J. W. Daniel and J. C. Gage, Food Cosmet. Toxicol., 1965, 3, 405–415 CrossRef CAS PubMed.
- A. M. Malkinson, Proc. West. Pharmacol. Soc., 1983, 26, 417–419 CAS.
- R. C. Lindenschmidt, A. F. Tryka, M. E. Goad and H. P. Witschi, Toxicology, 1986, 38, 151–160 CrossRef CAS PubMed.
- E. M. Bomhard, J. N. Bremmer and B. A. Herbold, Mutat. Res., Genet. Toxicol., 1992, 277, 187–200 CrossRef CAS.
- F. S. Mallette and E. Von Haam, AMA Arch. Ind. Hyg. Occup. Med., 1952, 5, 311–317 CAS.
- C. L. Meneghini, F. Rantuccio and M. Lomuto, Dermatology, 1971, 143, 137–147 CrossRef PubMed.
- M. R. Lee, C. Y. Lin, Z. G. Li and T. F. Tsai, J. Chromatogr. A, 2006, 1120, 244–251 CrossRef CAS PubMed.
- M. Borremans, J. Van Loco, P. Roos and L. Goeyens, Chromatographia, 2004, 59, 47–53 CAS.
- G. Burini, J. Chromatogr. A, 1994, 664, 213–219 CrossRef CAS.
- S. H. Kang and H. Kim, J. Pharm. Biomed. Anal., 1997, 15, 1359–1364 CrossRef CAS PubMed.
- A. Abdollahpour, M. Forouhi, M. Shamsipur and Y. Yamini, J. Iran. Chem. Soc., 2010, 7, 516–520 CrossRef CAS.
- H. J. Lin and Y. M. Choong, J. Food Drug Anal., 1999, 7, 291–304 CAS.
- B. Buszewski and M. Szultka, Crit. Rev. Anal. Chem., 2012, 42, 198–213 CrossRef CAS.
- T. R. Fiorentin, M. Fogarty, R. P. Limberger and B. K. Logan, Forensic Sci. Int., 2019, 295, 199–206 CrossRef CAS PubMed.
- F. Elke and P. Wilhelm, Water Res., 2002, 36, 2319–2327 CrossRef.
- A. Wang, F. Fang and J. Pawliszyn, J. Chromatogr. A, 2005, 1072, 127–135 CrossRef CAS PubMed.
- H. Piri-Moghadam, E. Gionfriddo, J. J. Grandy, M. N. Alam and J. Pawliszyn, J. Chromatogr. A, 2018, 1579, 20–30 CrossRef CAS PubMed.
- B. A. Eckenrode, J. Am. Soc. Mass Spectrom., 2001, 12, 683–693 CrossRef CAS PubMed.
- M. Mieth, J. K. Schubert, T. Gröger, B. Sabel, S. Kischkel, P. Fuchs, D. Hein, R. Zimmermann and W. Miekisch, Anal. Chem., 2009, 81, 5851–5857 CrossRef CAS PubMed.
- J. A. Koziel, M. Odziemkowski and J. Pawliszyn, Anal. Chem., 2001, 73, 47–54 CrossRef CAS PubMed.
- J. J. Poole, J. J. Grandy, G. A. Gómez-Ríos, E. Gionfriddo and J. Pawliszyn, Anal. Chem., 2016, 88, 6859–6866 CrossRef CAS PubMed.
- J. J. Grandy, E. Boyaci and J. Pawliszyn, Anal. Chem., 2016, 88, 1760–1767, DOI:10.1021/acs.analchem.5b04008.
- J. J. Grandy, V. Singh, M. Lashgari, M. Gauthier and J. Pawliszyn, Anal. Chem., 2018, 90, 14072–14080 CrossRef CAS PubMed.
- H. J. Suh, M. S. Chung, Y. H. Cho, J. W. Kim, D. H. Kim, K. W. Han and C. J. Kim, Food Addit. Contam., 2005, 22(12), 1176–1188 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2020 |