 Open Access Article
Open Access ArticleA preparation of β-glucans and anthocyanins (LoGiCarb™) lowers the in vitro digestibility and in vivo glycemic index of white rice
Jaslyn Jie Lin Leea,
Barnabas Chan*b,
Cui Chunc,
Kalpana Bhaskarand and
Wei Ning Chen *a
*a
aSchool of Chemical and Biomedical Engineering, Nanyang Technological University, 62 Nanyang Drive, Singapore 63745. E-mail: wnchen@ntu.edu.sg
bBountifood Pte. Ltd, 52 International Road, #02-02, Singapore 619626. E-mail: barnabas.chan@bountifood.com
cCollege of Light Industry and Food Sciences, South China University of Technology, No. 381, Wushan Road, Tianhe District, Guangzhou, China
dCentre for Applied Nutrition Services, Glycemic Index Research Unit, School of Applied Science, Temasek Polytechnic, 21 Tampines Ave 1, Singapore 529757
First published on 31st January 2020
Abstract
The effect of a proprietary blend of β-glucan, anthocyanins and resistant dextrin (LoGICarb™) on the (1) in vitro digestibility and (2) in vivo glycemic response of humans to white rice, were carried out. The amounts of glucose released, rapidly digestible starch, and predicted glycemic index of white rice were significantly reduced, with addition of LoGICarb™. The mean glycemic index (GI) value of white rice, were also reduced from 72 to 55.0 ± 4.52, in 14 test subjects. These effects were due to the combination of anthocyanins and β-glucans in one sachet of LoGICarb™. The anthocyanins could bind α-amylase, reducing the amount of available enzymes for starch digestion, thus slowing down starch digestion in white rice. In addition, β-glucans helped increase the viscosity of meal bolus. This is the first study that demonstrated addition of plant-based extracts could significantly decrease the digestibility and GI value of cooked white rice.
1. Introduction
Rice is an important daily staple for a large proportion of the global population. White rice has a high glycemic index (GI) of 72,1 and contributes significantly to dietary glycemic load. While white rice is known to have a high GI, it is still the most commonly-consumed form of rice due to its soft and fluffy texture.There have been well-conducted studies on the efficacy of certain plant extracts that can lower the digestibility of starches.2–5 Anthocyanins have been shown to have an effect on multiple pathways of starch digestion, including inhibition of amylase and glucosidase,6,7 increasing the proportions of slowly digestible starch (SDS) and resistant starch (RS) and decreasing the proportion of rapidly digestible starch2,3,5 and altering the starch matrix.
β-Glucan have been demonstrated to decrease the digestibility of starches by increasing the viscosity of bolus, thus increasing the transit time of the meal in the gastric and intestine. The increased viscosity also slows down the access of enzymes to the food.8
LoGiCarb (Bountifood Pte Ltd) contains a proprietary blend of β-glucan, anthocyanins and resistant dextrin, developed to improve the glycemic response of the human body to white rice. A small amount of resistant dextrin was included in the recipe to further increase dietary fibre content, and improve the flowing capability of the sachet. The anthocyanins in this proprietary product have a unique proportion of cyanidin, pelagornis and delphinidin, which are mostly presented in the form of 3-glucoside. This study was performed to determine the effects of this proprietary blend of anthocyanins, β-glucans and resistant dextrin on (1) the in vitro digestibility and (2) the in vivo glycemic response to white rice.
2. Materials and methods
2.1. Materials
Oat bran extract (77.5% β-glucans), black rice extract (10.05% anthocyanidins, 25% anthocyanidins), and resistant dextrin (82% dietary fibre) were obtained from Bountifood Pte Ltd (Singapore). Kangaroo brand Australia rice was purchased from FairPrice supermarket (Singapore). Pepsin from porcine gastric mucosa (≥250 units per mg solid), α-amylase from porcine pancreas (Type VI-B, ≥10 units per mg solid), pancreatin from porcine pancreas (8 × USP specification), porcine bile extract, sodium acetate, sodium potassium tartrate tetrahydrate, and 3,5-dinitrosalicylic acid were obtained from Sigma-Aldrich (St Louis, Mo, USA). Amyloglucosidase from Aspergillus niger (3260 units per mL), MES monohydrate, Tris buffer salt, and invertase (2000 units per mL), and available carbohydrates/dietary fibre assay kit (K-ACHDF) were purchased from Megazyme (Bray, Co. Wicklow, Ireland).2.2. Sample preparation
The LoGICarb™ sachet (1.3 g) is a mixture of oat bran extract, black rice extract and resistant dextrin. The white rice (58.51 g) were washed twice, and mixed with 58.51 g water, followed by cooking in a rice cooker. The LoGICarb™ sachet (1.3 g) were added to the cooked white rice and mixed for 3 min.For in vitro digestion, the sample was transferred to a food blender, and blended for 10 min to produce the samples for in vitro digestion. A standard white bread sample was used as a control.
For in vivo glycemic index study, white rice was portioned into serving size of 58.51 g to provide 50 g available carbohydrate. The rice was mixed with 1 LoGICarb sachet for 3 min, and served to the subjects with 250 mL of water.
2.3. In vitro digestion
The in vitro digestibility study was performed according to a standardized static in vitro digestion method.9 The simulated digestion fluids, including simulated salivary fluid (SSF), simulated gastric fluid (SGF) and simulated intestinal fluid (SIF) were prepared according to the standardized composition as reported by ref. 9.All buffers and food samples were pre-conditioned to 37 °C before in vitro digestion. The minced-cooked rice samples with and without plant extracts (5 g each) were added into a test tube containing 5 mL SSF buffer containing α-amylase. This was vortexed for 20 s at 2500 rpm to initiate formation of bolus, and the oral digestion was further continued in a 37 °C water bath for 100 s. The total oral digestion process was 2 min. The gastric digestion phase was then continued by the addition of 10 mL SGF buffer (pH 2.0) to the oral phase sample. The sample was incubated in a shaking water bath (160 rpm, 37 °C) for 2 h. Next, the pancreatic digestion was initiated by addition of 20 mL SIF buffer (pH 7.0) and was returned to the shaking water bath. Aliquots (0.5 mL) were withdrawn from the test tubes at timed intervals of 0, 10, 20, 30, 45, 60, 90, 120, 150, and 180 min. The aliquots were quenched by immediate boiling for 10 min in 100 °C water bath, followed by storage in a refrigerator (4 °C) until analysis.
2.4. Reducing sugar analysis
The procedure of reducing sugar analysis was modified from4 DNS reagent was prepared, the composition of the reagent consists of 150 g sodium potassium tartrate, 5.475 g DNS acid, 100 mL 2 M NaOH and 400 mL deionized water. The aliquots samples from free glucose and in vitro digestion were diluted with deionized water (5–25 times). After dilution, 0.5 mL of DNS reagent was mixed with 0.5 mL of diluted samples by vortex for 15 s. The tubes were then placed in boiling water for 10 min and cooled in an ice-water bath for 5 min. The samples were then transferred to a 96-well plate, and the absorbance was read at 540 nm using a Bio-tek Synergy™ plate reader (Vermont, U.S.). The absorbance values were converted to glucose equivalents using a glucose standard curve.2.5. Rapidly digestible starch (RDS), slowly digestible starch (SDS) and predicted glycemic index (pGI) determination
| SDS = (G120 − G20) × 0.9 |
| HI = AUC (test food)/AUC (white bread) × 100 |
The pGI was obtained according to the equation pGI = 0.549 HI + 39.71, developed by ref. 13. The pGI value of the control white bread was 100. To convert the pGI to glucose as a standard reference, the pGI was multiplied by 0.7 to obtain pGIglucose.
2.6. In vivo glycemic index study
Fifteen healthy volunteers between age 19–60 years were recruited. Informed consent was obtained from all volunteers. The inclusion criteria were: no known food allergy and tolerance, not-pregnant, and not under medications known to affect glucose tolerance. Subjects that were diabetic, using anti-hyperglycemic drugs or insulin, had undergone major medical/surgical event requiring hospitalization during last 3 months, having a disease or drug influencing digestion and nutrients absorption, or using steroids, protease inhibitors or antipsychotics were excluded from the study.
2.7. Statistical analysis
At least two repeated results within each analysis were obtained. The results were reported in the format of mean ± standard deviation (SD). One-way analysis of variance (ANOVA; P < 0.05) and Duncan's multiple range test were employed to analyze the differences between samples using SPSS Statistics 20 software (IBM, Chicago, IL, U.S.). For GI results, the presence of outliers was determined by an individual result that is variated 2 standard deviations away from the mean group. The outlier was discarded from dataset.3. Results and discussion
3.1. In vitro digestion
The glucose released from white rice sample, white bread and white rice with LoGICarb™ during 180 min of in vitro digestion was showed in Fig. 1.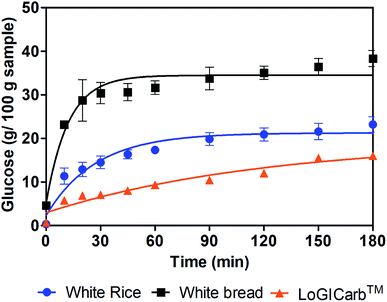 | ||
| Fig. 1 Glucose released from white rice, white bread and white rice with addition of LoGICarb™ during 180 min in vitro digestion. | ||
The RDS, SDS and pGI values of each sample were shown in Table 1. The addition of LoGICarb™ reduced the amount of RDS and SDS from white rice.
| Sample | ACH (g/100 g) | RDS (g/100 g) | SDS (g/100 g) | pGIwhite breadb | pGIglucoseb |
|---|---|---|---|---|---|
| a Within each column, sample with different lower-case letters indicated statistically significant difference among the samples (P < 0.05).b The pGIwhite bread were calculated from Goñi et al. (1997)13 equation, which used white bread as a reference in the hydrolysis index calculation. pGIglucose were calculated by multiplying pGIwhite bread by 0.7. | |||||
| White bread | 54.5 ± 0.7a | 22.8 ± 4.3a | 6.0 ± 2.8a | 94.6 ± 3.2a | 71.0 ± 2.4a |
| White rice | 49.1 ± 2.7a | 11.4 ± 1.5b | 7.2 ± 2.0a | 75 ± 1.5b | 56.2 ± 1.2b |
| LoGICarb™ + white rice | 49.1 ± 2.7a | 6.0 ± 0.1c | 4.6 ± 0.3a | 60.5 ± 0.2c | 45.4 ± 0.2c |
Furthermore, the predicted glycemic index (pGIglucose) was also reduced to about 19% by LoGICarb™, which could possibly reduce the GI of white rice for a low GI (GI ≤ 55) classification.
3.2. In vivo glycemic index
Fifteen healthy human volunteers participated in the in vivo glycemic index study. The general profile of the subjects was shown in Table 2. The average age of 5 females and 10 males, was 39.5 years, with an average BMI of 21.8 kg m−2.| Subject | Age | Gender | Weight (kg) | Height (m) | BMI (kg m−2) |
|---|---|---|---|---|---|
| 1 | 50 | F | 50.6 | 1.53 | 21.6 |
| 2 | 57 | F | 55.6 | 1.6 | 21.9 |
| 3 | 60 | F | 60.3 | 1.61 | 23.3 |
| 4 | 48 | M | 71.8 | 1.7 | 24.8 |
| 5 | 43 | M | 53.5 | 1.66 | 19.4 |
| 6 | 49 | F | 58.2 | 1.61 | 22.5 |
| 7 | 24 | M | 58 | 1.71 | 19.8 |
| 8 | 38 | F | 50.4 | 1.53 | 21.5 |
| 9 | 57 | M | 74 | 1.75 | 24.2 |
| 10 | 23 | M | 73 | 1.72 | 24.7 |
| 11 | 24 | M | 63.8 | 1.84 | 18.8 |
| 12 | 52 | M | 67.1 | 1.7 | 23.2 |
| 13 | 22 | M | 50.2 | 1.61 | 19.4 |
| 14 | 23 | M | 63.7 | 1.84 | 18.8 |
| 15 | 23 | M | 81 | 1.88 | 22.9 |
The average glycemic response curves of the test food and reference food were showed in Fig. 2. The GI was calculated from the IAUC of LoGICarb™ with white rice versus that of glucose. The individual subjects' GI value of LoGICarb™ with white rice were shown to range from 24 to 77 (one outlier at which GI = 104). Thus, the mean GI value of LoGICarb™ with white rice was 55.0 ± 4.52. This enabled white rice to be classified as a low GI (GI ≤ 55) food.
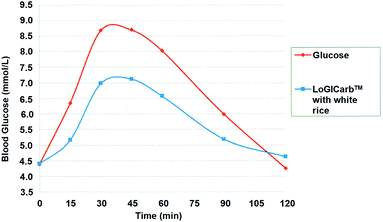 | ||
| Fig. 2 Averaged blood glucose response of 14 subjects after consuming reference (glucose) and test food (LoGICarb™ with white rice). | ||
4. Discussion
This study was specifically designed to evaluate the effects of a blend of β-glucan and anthocyanins on the in vitro digestibility and in vivo glycemic response of humans, to white rice. Both the in vitro and in vivo results were significant and in support of each other, to demonstrate the effectiveness of this blend in lowering the digestibility and predicted GI of cooked white rice. This is the first product, in the scientific literature, that demonstrated that the addition of plant-based extracts leads to a significant decrease in digestibility of cooked white rice, and of its GI value.The mechanisms of the lowering of digestibility of white rice were postulated to be due to the inhibition of α-amylase by anthocyanins.7 The properties of the soluble fibres of β-glucans increased the viscosity of meal bolus and delayed absorption of nutrients in the small intestine,14 as well as modified starches. Cyanidin-3-glucoside, one of the major anthocyanins in black rice extract, was reported to have an IC50 of 0.024 ± 0.003 mM against α-amylase, and the binding of cyanidin-3-glucoside was through GLU233 on α-amylase.7 In bread samples,4 also found that black rice extract could reduce digestion by 6.31% to 17.45%.
Increasing concentration and molecular weight of β-glucans could increase the viscosity of bolus, thus reducing the blood glucose.15 It was also proposed that protein and β-glucans in oat bran extract could form a network to entrap starch, thus further protecting starch from enzyme digestion.16 EFSA panel suggested that 4 g β-glucans per 30 g available carbohydrate should be present in food, for a reduced postprandial glycemic response.14 However, a bread sample containing two times lower the amount of β glucans (3.4 g β-glucans per 50 g carbohydrate) than EFSA's suggestion was found to significantly reduce plasma glucose and serum insulin as compared to plain white bread.17 This was not surprising since the effect of β glucans is both concentration and molecular weight dependent.
Englyst et al. reported that the rapidly available glucose (RAG) is highly correlated with glycemic response, in which RAG (RDS + free glucose) could explain 70% of the variance in glycemic response (P < 0.0001).11 The addition of LoGICarb™ modified the RDS of white rice, which possibly contributed to the reduction of the GI of white rice. However, it was found that the SDS level of white rice also reduced with LoGICarb™ addition, which suggested that part of the starch might have been converted to resistant starch. Similarly, An et al. also observed a reduction in RDS from 47.49% to 32.84%, and an increase of resistant starch from 36.98% to 53.17%, when 20% of black rice extract was incorporated into a wheat gel.2 Further determination of resistant starch content could be performed to confirm the effect of LoGICarb™ on modifying starches in food.
Overall, the results were positively encouraging, that one sachet of LoGICarb™ (1.3 g) could reduce glucose release from white rice and make otherwise high GI white rice1 become a low GI (≤55) food. The effect was due to the combination of black rice extract (anthocyanin) and β-glucans. One sachet of LoGICarb™ contained around 26 mg of anthocyanins (0.054 mmol) to bind to α-amylase, reducing the amount of available enzymes for starch digestion, therefore slowing down the digestion of starch in white rice. Although the level of β-glucans in LoGICarb™ was about 5 times lower than EFSA suggestion, the β-glucans was not subjected to heat and other food processing treatment as in an oat bread, thus could retain molecular integrity and delivery thickening ability better.
5. Conclusion
One sachet of LoGICarb™ (1.3 g) could be applied to white rice, to transform white rice into low GI food. The result was due to a combined effect of black rice anthocyanins and oat β-glucans to inhibit starch digestion enzymes, which increased the viscosity of bolus and modified the starch composition of white rice.Ethics statement
The study was approved by an Independent Ethics Committee, with the reference number of TP-IRB ref: IRB170102. Informed consent was obtained from all volunteers.Conflicts of interest
The authors declared no competing interest.Acknowledgements
The work was supported by Bountifood Pte. Ltd. Authors who are not employed by Bountifood Pte. Ltd. do not receive any financial benefits from any party.References
- F. S. Atkinson, K. Foster-Powell and J. C. Brand-Miller, Diabetes Care, 2008, 31, 2281–2283 CrossRef PubMed.
- J. S. An, I. Y. Bae, S.-I. Han, S.-J. Lee and H. G. Lee, J. Cereal. Sci., 2016, 70, 214–220 CrossRef CAS.
- G. A. Camelo-Méndez, E. Agama-Acevedo, J. Tovar and L. A. Bello-Pérez, J. Cereal. Sci., 2017, 76, 179–185 CrossRef.
- X. Sui, Y. Zhang and W. Zhou, Food Chem., 2016, 196, 910–916 CrossRef CAS PubMed.
- W. Klunklin and G. Savage, Int. J. Food Sci. Technol., 2018, 53, 1962–1971 CrossRef CAS.
- S. Akkarachiyasit, P. Charoenlertkul, S. Yibchok-Anun and S. Adisakwattana, Int. J. Mol. Sci., 2010, 11, 3387–3396 CrossRef CAS PubMed.
- X. Sui, Y. Zhang and W. Zhou, J. Funct. Foods, 2016, 21, 50–57 CrossRef CAS.
- A. Rieder, S. H. Knutsen and S. Ballance, Food Hydrocolloids, 2017, 67, 74–84 CrossRef CAS.
- M. Minekus, M. Alminger, P. Alvito, S. Ballance, T. Bohn, C. Bourlieu, F. Carriere, R. Boutrou, M. Corredig, D. Dupont, C. Dufour, L. Egger, M. Golding, S. Karakaya, B. Kirkhus, S. Le Feunteun, U. Lesmes, A. Macierzanka, A. Mackie, S. Marze, D. J. McClements, O. Menard, I. Recio, C. N. Santos, R. P. Singh, G. E. Vegarud, M. S. Wickham, W. Weitschies and A. Brodkorb, Food Funct., 2014, 5, 1113–1124 RSC.
- H. N. Englyst, S. M. Kingman and J. H. Cummings, Eur. J. Clin. Nutr., 1992, 46(suppl. 2), S33–S50 Search PubMed.
- K. N. Englyst, H. N. Englyst, G. J. Hudson, T. J. Cole and J. H. Cummings, Am. J. Clin. Nutr., 1999, 69, 448–454 CrossRef CAS PubMed.
- A. Wolter, A. S. Hager, E. Zannini and E. K. Arendt, Food Funct., 2014, 5, 564–572 RSC.
- I. Goñi, A. Garcia-Alonso and F. Saura-Calixto, Nutr. Res., 1997, 17, 427–437 CrossRef.
- N. EFSA, Panel on Dietetic Products and Allergies, EFSA J., 2011, 9, 2207 CrossRef.
- P. J. Wood, M. U. Beer and G. Butler, Br. J. Nutr., 2000, 84, 19–23 CrossRef CAS PubMed.
- J. Zhang, K. Luo and G. Zhang, J. Cereal. Sci., 2017, 73, 84–90 CrossRef CAS.
- L. M. N. K. Ekström, E. A. E. Henningsson Bok, M. E. Sjöö and E. M. Östman, J. Funct. Foods, 2017, 32, 106–111 CrossRef.
| This journal is © The Royal Society of Chemistry 2020 |
