Ag-Catalyzed cycloisomerization of 1,6-enynamide: an intramolecular type II Alder-ene reaction†
Mei-Hua
Shen
 *,
Yu-Mei
Zhang
,
Chun
Jiang
,
Hua-Dong
Xu
*,
Yu-Mei
Zhang
,
Chun
Jiang
,
Hua-Dong
Xu
 and
Defeng
Xu
*
and
Defeng
Xu
*
School of Pharmaceutical Engineering and Life Science, Changzhou University, Changzhou 213164, China. E-mail: shemmh@cczu.edu.cn; markxu@cczu.edu.cn
First published on 18th November 2019
Abstract
Promoted by a silver catalyst, 1,6-enynamides with a cyclic ene moiety can undergo cycloisomerization to fused N-heterobicycles. This transformation presents an unusual type II Alder-ene reaction and is believed to proceed through a catalytic cycle of formation of a keteneiminium silver intermediate, subsequent 6-endo-trig cyclization and protodemetalation.
The Alder-ene reaction1 is a powerful chemical transformation that efficiently combines two unsaturated components (ene donor and enophile) through C–C bond formation and its intramolecular version, i.e., Alder-ene cycloisomerization,2 is highly useful for ring construction. Alkynes are frequently used as enophiles in the intramolecular ene reaction. Electro-deficient alkynes normally react readily with alkenyl ene donors.3 Although electroneutral alkynes are less reactive enophiles for thermal ene reactions with ordinary alkenes, related cycloisomerization can be realized by introduction of ring strain4 or a metal catalyst.5 On the other hand, electron rich alkynes are rarely reported as enophiles in the Alder-ene reaction. In line with our interest in ene rections6 and in order to answer the question whether electron rich alkynes can participate in Alder-ene reactions as enophiles, we started a program studying the Alder-ene cycloisomerization of ynamides, a typical class of electron-rich alkynes.7
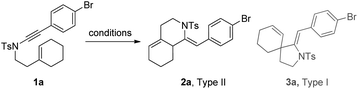
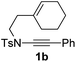
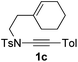
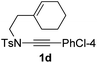
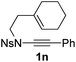
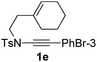
1,6-Enynamide 1a, formed conveniently by coupling of 4-bromophenylethynyl bromide with related alkenyl sulfonamide,8 was used for an initial evaluation (Table 1). Refluxing of 1a in toluene for 10 hours resulted in no reaction (entry 1). Then we turned our attention to metal catalysis. To our delight, a catalytic amount of a rhodium complex RhCl(PPh3)3 can promote the Alder-ene cycloisomerization to give a single product 2a in a modest yield at lower temperature (entry 2, 80 °C). Fused bicycle 2a is a type II intramolecular Alder-ene product,9 and interestingly, the expected type I congener spiro 3a was not detected.10 Copper salts CuCl and CuSO4 can also affect this reaction, giving similar yields (entries 3 and 4). Further research found that Ag2SO4 as a catalyst was superior to the more cationic silver salts AgOTf and AgSbF6 and was able to increase the yield of 2a to 50% under the same conditions (entries 5–7).11 Brief solvent screening study indicated that dichloroethane (DCE) was a better medium than toluene, dichloromethane (DCM) and chloroform for this reaction (entries 7–10).
| Entry | Catalyst | Solvent | Temp. (°C) | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: 1a (0.2 mmol), catalyst (0.04 mmol, 20 mol%), solvent (2 mL), 60, 80 or 120 °C oil bath, N2, 10 h in a sealed tube. b Isolated yield. | ||||
| 1 | — | PhMe | 120 | — |
| 2 | RhCl(PPh3)3 | PhMe | 80 | 34 |
| 3 | CuCl | PhMe | 80 | 36 |
| 4 | CuSO4 | PhMe | 80 | 42 |
| 5 | AgOTf | PhMe | 80 | 40 |
| 6 | AgSbF6 | PhMe | 80 | 28 |
| 7 | Ag2SO4 | PhMe | 80 | 50 |
| 8 | Ag2SO4 | DCM | 60 | 45 |
| 9 | Ag2SO4 | CHCl3 | 80 | 20 |
| 10 | Ag 2 SO 4 | DCE | 80 | 55 |
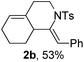
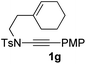
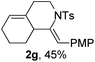
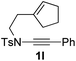
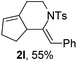
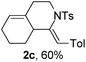
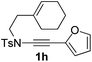
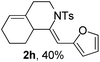
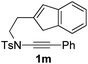
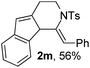
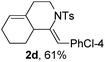
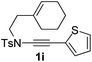
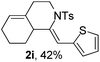
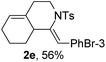
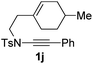
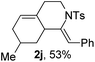
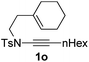
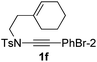
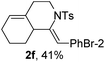
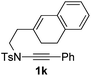
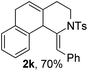
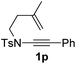
Next, using DCE as the solvent and Ag2SO4 as the catalyst, more N-Ts ynamides 1 were subjected to this reaction to investigate the substrate scope (Table 2). 1b–g, substrates with variously substituted phenylethynyl groups were all effectively suitable for this reaction, giving the corresponding octahydroisoquinolines 2b–g efficiently with yields ranging from 41% to 61%. Furanyl and thiophenyl enynamides 1h and 1i were successfully transformed into the corresponding N-heterobicycles 2h and 2i in good yields. Ynamide 1j with a methyl group on the cyclohexenyl moiety reacted smoothly as well. Benzene-fused cyclohexene in 1k can serve as a nice ene donor and tricycle 2k was obtained in 70% yield. Cyclopentenyl substrate 1l and its benzene-fused homologue 2m were subjected to the same reaction conditions to give the corresponding bicyclic products 2l and 2m with comparable yields. Switching the N-tosyl group to the N-nosyl group delivered similar outcomes. A complex reaction mixture was observed for alkyl ynamide 1o under the same conditions, suggesting that the aryl ynamide is required for this reaction. Analysis of the NMR spectra of this reaction mixture indicates that it might be composed of the desired product mixed with by-products derived from hydrolysis of both the substrate and product and isomerization of the eynamide moiety (such as the 1,3-H-shift). The cyclic alkene is also critical for the success of this cycloisomerization process evidenced by a complex reaction mixture obtained for 1p, which carries a noncyclic ene donor.
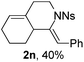
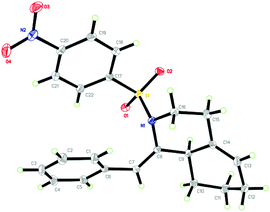
The structure of 2n was determined unambiguously by the single crystal X-ray analysis. The ORTEP picture shows clearly that there is an octahydroisoquinoline core and an exocyclic alkene group with Z-geometry (Fig. 1). The structure of other products was assigned accordingly by analogy of NMR data.
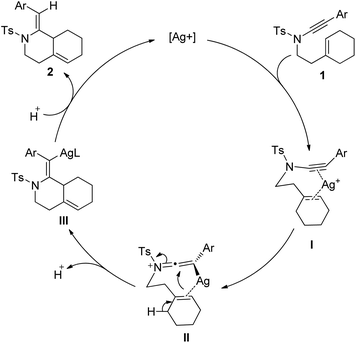
According to the above findings, a reaction mechanism was proposed (Scheme 1). Coordination of the silver cation with enynamide 1 followed by ynamide activation gives a keteneiminium silver intermediate II; then, a 6-endo-trig attack of the cycloalkene on the keteneiminium moiety led to a vinyl silver intermediate III which underwent protodemetalation affording the fused bicyclic product. This mechanism can explain both the exclusive formation of the type II ene product over the spiro type I ene product and the Z-configuration of the exocyclic alkene.
Conclusions
In summary, a silver catalyzed Alder-ene cycloisomerization of 1,6-enynamide has been achieved for the first time to give unusual type II Alder-ene products. The reaction provides a facile method for the efficient construction of fused N-heterocyclic scaffolds bearing an endo and a Z-exocyclic C–C double bonds selectively, which is valuable in both organic synthesis and medicinal chemistry.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
We thank the National Natural Science Foundation of China (No. 21672027) for financial support. This work is also supported by the High-Level Entrepreneurial Talent Team of Jiangsu Province (2017-37) and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).Notes and references
- (a) H. M. R. Hoffmann, Angew. Chem., Int. Ed. Engl., 1969, 8, 556 CrossRef CAS; (b) T. J. J. Mueller, in Comprehensive Organic Synthesis II, ed. P. Knochel, Elsevier B.V., 2014, vol. 5, p. 1 Search PubMed.
- D. F. Taber, in Intramolecular Diels–Alder and Alder-Ene Reactions, ed. D. F. Taber, Springer-Verlag, Berlin, 1984, p. 61 Search PubMed.
- (a) K. Mikami, K. Takahashi, T. Nakai and T. Uchimaru, J. Am. Chem. Soc., 1994, 116, 10948 CrossRef CAS; (b) E. M. Groen-Piotrowska and M. B. Groen, Recl. Trav. Chim. Pays-Bas, 1993, 112, 627 CrossRef CAS; (c) K. Mikami, K. Takahashi and T. Nakai, J. Am. Chem. Soc., 1990, 112, 4035 CrossRef CAS.
- (a) D. Niu and T. R. Hoye, Nat. Chem., 2013, 6, 34 CrossRef; (b) D. A. Candito, D. Dobrovolsky and M. Lautens, J. Am. Chem. Soc., 2012, 134, 15572 CrossRef CAS; (c) D. A. Candito, J. Panteleev and M. Lautens, J. Am. Chem. Soc., 2011, 133, 14200 CrossRef CAS; (d) R. Karmakar, P. Mamidipalli, S. Y. Yun and D. Lee, Org. Lett., 2013, 15, 1938 CrossRef CAS; (e) I. Tabushi, H. Yamada, Z. Yoshida and R. Oda, Bull. Chem. Soc. Jpn., 1977, 50, 285 CrossRef CAS; (f) M. N. Protopopova and A. Galminas, Izv. Akad. Nauk SSSR, Ser. Khim., 1991, 1229 CAS.
- (a) B. M. Trost, Acc. Chem. Res., 1990, 23, 34 CrossRef CAS; (b) M. P. Munoz, J. Adrio, J. C. Carretero and A. M. Echavarren, Organometallics, 2005, 24, 1293 CrossRef CAS; (c) T. Shibata, M. Yamasaki, S. Kadowaki and K. Takagi, Synlett, 2004, 2812 CrossRef CAS; (d) S. J. Sturla, N. M. Kablaoui and S. L. Buchwald, J. Am. Chem. Soc., 1999, 121, 1976 CrossRef CAS; (e) B. M. Trost, G. J. Tanoury, M. Lautens, C. Chan and D. T. MacPherson, J. Am. Chem. Soc., 1994, 116, 4255 CrossRef CAS; (f) B. M. Trost, D. L. Romero and F. Rise, J. Am. Chem. Soc., 1994, 116, 4268 CrossRef CAS; (g) B. M. Trost and B. A. Czeskis, Tetrahedron Lett., 1994, 35, 211 CrossRef CAS; (h) B. M. Trost and Y. Shi, J. Am. Chem. Soc., 1993, 115, 9421 CrossRef CAS; (i) B. M. Trost and Y. Shi, J. Am. Chem. Soc., 1991, 113, 701 CrossRef CAS; (j) A. Knierzinger, A. Grieder and P. Schoenholzer, Helv. Chim. Acta, 1991, 74, 517 CrossRef CAS.
- T.-S. Liu, H. Zhou, P. Chen, X.-R. Huang, L.-Q. Bao, C.-L. Zhuang, Q.-S. Xu, M.-H. Shen and H.-D. Xu, Org. Lett., 2018, 20, 3156 CrossRef CAS.
- (a) A. Mekareeya, P. R. Walker, A. Couce-Rios, C. D. Campbell, A. Steven, R. S. Paton and E. A. Anderson, J. Am. Chem. Soc., 2017, 139, 10104 CrossRef CAS; (b) C.-Z. Zhong, P.-T. Tung, T.-H. Chao and M.-C. P. Yeh, J. Org. Chem., 2017, 82, 481 CrossRef CAS; (c) M.-C. P. Yeh, Y.-S. Shiue, H.-H. Lin, T.-Y. Yu, T.-C. Hu and J.-J. Hong, Org. Lett., 2016, 18, 2407 CrossRef CAS PubMed; (d) M.-C. P. Yeh, C.-J. Liang, H.-F. Chen and Y.-T. Weng, Adv. Synth. Catal., 2015, 357, 3242 CrossRef CAS; (e) C. D. Campbell, R. L. Greenaway, O. T. Holton, P. R. Walker, H. A. Chapman, C. A. Russell, G. Carr, A. L. Thomson and E. A. Anderson, Chem. – Eur. J., 2015, 21, 12627 CrossRef CAS; (f) C. D. Campbell, R. L. Greenaway, O. T. Holton, H. A. Chapman and E. A. Anderson, Chem. Commun., 2014, 50, 5187 RSC; (g) P. R. Walker, C. D. Campbell, A. Suleman, G. Carr and E. A. Anderson, Angew. Chem., Int. Ed., 2013, 52, 9139 CrossRef CAS; (h) K. A. DeKorver, X.-N. Wang, M. C. Walton and R. P. Hsung, Org. Lett., 2012, 14, 1768 CrossRef CAS; (i) R. L. Greenaway, C. D. Campbell, O. T. Holton, C. A. Russell and E. A. Anderson, Chem. – Eur. J., 2011, 17, 14366 CrossRef CAS; (j) H. Wakamatsu, M. Sakagami, M. Hanata, M. Takeshita and M. Mori, Macromol. Symp., 2010, 293, 5 CrossRef CAS; (k) S. Couty, C. Meyer and J. Cossy, Angew. Chem., Int. Ed., 2006, 45, 6726 CrossRef CAS; (l) J. R. Dunetz and R. L. Danheiser, J. Am. Chem. Soc., 2005, 127, 5776 CrossRef CAS; (m) N. Saito, Y. Sato and M. Mori, Org. Lett., 2002, 4, 803 CrossRef CAS; (n) J. Huang, H. Xiong, R. P. Hsung, C. Rameshkumar, J. A. Mulder and T. P. Grebe, Org. Lett., 2002, 4, 2417 CrossRef CAS.
- (a) X.-N. Wang, H.-S. Yeom, L.-C. Fang, S. He, Z.-X. Ma, B. L. Kedrowski and R. P. Hsung, Acc. Chem. Res., 2014, 47, 560 CrossRef CAS; (b) C. Jiang, P.-P. Yu, Q. Zhang, H.-D. Xu and M.-H. Shen, Chin. Chem. Lett., 2019, 30, 266 CrossRef CAS; (c) Y. Zhang, R. P. Hsung, M. R. Tracey, K. C. M. Kurtz and E. L. Vera, Org. Lett., 2004, 6, 1151 CrossRef CAS.
- W. Oppolzer and V. Snieckus, Angew. Chem., Int. Ed. Engl., 1978, 17, 476 CrossRef.
- B. M. Trost, M. Lautens, C. Chan, D. J. Jebaratnam and T. Mueller, J. Am. Chem. Soc., 1991, 113, 636 CrossRef CAS.
- For selected recent reviews on silver catalysed reactions, see: (a) G. Fang, X. Cong, G. Zanoni, Q. Liu and X. Bi, Adv. Synth. Catal., 2017, 359, 1422 CrossRef CAS; (b) Y. Wang, R. K. Kumar and X. Bi, Tetrahedron Lett., 2016, 57, 5730 CrossRef CAS; (c) G. Fang and X. Bi, Chem. Soc. Rev., 2015, 44, 8124 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, characterization data and NMR spectra for all new compounds. CCDC 1951227. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9qo01258c |
| This journal is © the Partner Organisations 2020 |