A diastereoselective approach to amino alcohols and application for divergent synthesis of dolastatin 10†
Xiao-Di
Nie
a,
Zhuo-Ya
Mao
a,
Wen
Zhou
a,
Chang-Mei
Si
*a,
Bang-Guo
Wei
 *a and
Guo-Qiang
Lin
b
*a and
Guo-Qiang
Lin
b
aInstitutes of Biomedical Sciences and School of Pharmacy, Fudan University, 220 Handan Road, Shanghai, 200433, China. E-mail: sicm@fudan.edu.cn; bgwei1974@fudan.edu.cn
bShanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai 200032, China
First published on 13th November 2019
Abstract
A diastereoselective approach to obtain amino alcohols 10 through SmI2-induced radical addition of chiral imine 8 with 2-(benzyloxymethylsulfonyl)pyridine 9 is described. This approach was easily used for the synthesis of non-natural amino acid 15, a flexible key fragment whose utility was demonstrated in the divergent synthesis of dolastatin 10 (1) and its nine analogues 31a, 31c, 31d, 31e, 31f, 31g, 40a, 40b and 40c were obtained.
Introduction
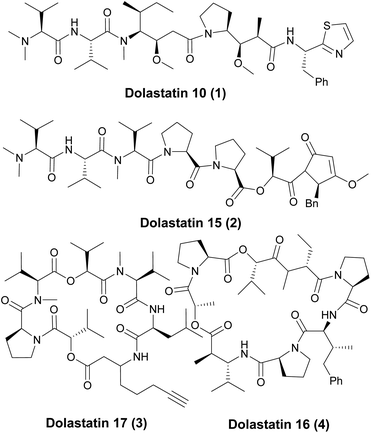
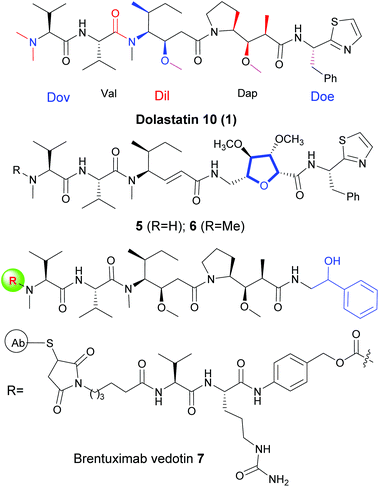
Bioactive small peptides derived from peptidic secondary metabolites are a promising class of compounds for drug discovery,1 due to their interesting biological activities, including antimalarial, antifungal, antimicrobial, cytotoxic and neurotoxic properties.2 Among them, linear peptides displaying a variety of physiological activities and possessing attractive structures have attracted significant attention in recent years.3 Several of these bioactive peptides or cyclodepsipeptides were selected as novel pharmaceuticals and are currently being evaluated in human clinical trials for cancer treatment.4 As a prime instance, dolastatins, a large family of compounds named star molecules, were gradually isolated from the marine mollusk Dolabella auricularia over the last 30 years.5 Among the family, several are of the linear peptide form6 and several are of the cyclodepsipeptide form (Fig. 1).7 Most of them exhibit strong activity against a variety of cancers, and the mechanism of action is through a unique mode of action, which is different from that of paclitaxel, vinblastine and epothilones.8Dolastatin 10 (1) is one of the star molecules of its family compounds, which shows strong antitumor activity in sub-nanomolar concentrations against a wide range of tumor cell lines.9 Structurally, dolastatin 10 (1) led to five subunits (Fig. 2): N,N-dimethyl valine (Dov), L-valine (Val), (3R,4S,5S)-dolaisoleucine (Dil), (2R,3R,4S)-dolaproine (Dap) and protected (S)-dolaphenine (Doe) fragments. Due to the extensive biological activity against a variety of cancers and easily modified chemical structures, the synthetic dolastatin 10 (1) and its analogues have attracted significant attention and several important synthetic methods have been achieved.10 But most of the modifications of dolastatin 10 are focused on the Doe and Dov units. As a result, dolastatin 10 (1) and its analogues have inspired the design of auristatin as the potent ‘warhead’ component of antibody–drug conjugates (ADC) which has been approved as the antineoplastic agent for cancer chemotherapy and was evaluated in clinical trials in recent years.11 There are also a few modifications for Dap fragments.12 However, to the best of our knowledge, structural modifications for the Dil unit are not well studied, and the main reason is that the Dil fragment is difficult to synthesize. Moreover, another tricky reason for the synthesis of dolastatin 10 is the effective condensation between the Dil and Dov-Val fragments.10c
In recent years, we have been focusing on the divergent synthesis of bioactive secondary metabolites,13 and very recently, we have established a diverse approach to obtain dolastatin 10 through a SmI2-induced cross-coupling process to obtain the Dap fragment.13d As a continuation of our interest in developing the divergent synthesis of dolastatins and investigating their structure–activity relationships, herein, we present an asymmetric approach to obtain the Dil fragment and divergent synthesis of more analogues of dolastatin 10 (1).
Results and discussion
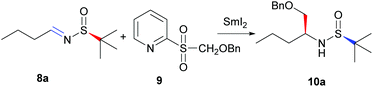
As shown in Table 1, our investigation started with an effective method to obtain the Dil subunit of dolastatin 10. Commercialized chiral N-tert-butanesulfinamide has been undoubtedly one of the most efficient classes of auxiliaries in the past decade,14 and we decided to investigate an asymmetric process through the SmI2-induced radical addition of chiral imine 8a with 2-(benzyloxymethylsulfonyl)pyridine 9. The imine 8a was easily prepared through oxidation and following condensation with N-tert-butanesulfinamide,15 and 2-(benzyloxymethylsulfonyl)pyridine 9 was synthesized through a known procedure.16 When a mixture of 8a (0.5 mmol) and 2-(benzyloxymethylsulfonyl)pyridine 9 (0.75 mmol) was treated with newly prepared SmI2 (0.1 M in THF), the desired product 10a was not produced (Table 1, entry 1). Even when the amount of 9 (1.0 mmol) or SmI2 was increased, the results were still fruitless (Table 1, entries 2 and 3). When a solution of 2-(benzyloxymethylsulfonyl)pyridine 9 in THF was dropped into the mixture of imine 8a with SmI2 and stirred for 30 min, the desired product was obtained in 42% yield with high diastereoselectivity (Table 1, entry 4). Different amounts of 2-(benzyloxymethylsulfonyl)pyridine 9 and SmI2 were screened, and the results are summarized in Table 1 (Table 1, entries 5–8). Although the best yield is 67%, the diastereoselectivity is excellent (Table 1, entry 6).| Entrya | SmI2 (equiv.) | 9 (equiv.) | Yieldb (%) | drc |
|---|---|---|---|---|
| a 2-(Benzyloxymethylsulfonyl)pyridine 9 (in THF) was dropped into the mixture of imine 8a (0.5 mmol) with SmI2, and then stirred for 30 min at room temperature. b Isolated yield. c dr values were determined by 1H NMR of crude products. d The new prepared SmI2 (0.1 M in THF) was dropped into a mixture of 8a with 2-(benzyloxymethylsulfonyl)pyridine 9. | ||||
| 1d | 4.0 | 1.5 | Trace | — |
| 2d | 4.0 | 3.0 | Trace | — |
| 3d | 6.0 | 3.0 | Trace | — |
| 4 | 4.0 | 1.5 | 42 | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 5 | 4.0 | 3.0 | 47 | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 6 | 6.0 | 3.0 | 67 | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 7 | 6.0 | 2.0 | 45 | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 8 | 6.0 | 2.5 | 56 | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
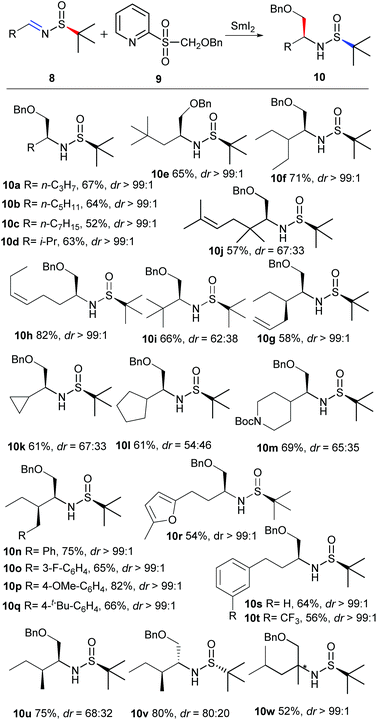
Next, we turned to investigate the scope and limitation of this asymmetric radical addition of imines 8b–8w with 2-(benzyloxymethylsulfonyl)pyridine 9. Different substituted linear alkyl imines 8a–8h were surveyed under the optimal conditions, as summarized in Scheme 1. In general, the reactions with various substituted linear alkyl imines 8a–8h proceeded smoothly in moderate yields with excellent diastereoselectivities (dr > 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). When tert-butyl type imines 8i and 8j were investigated, the results showed that the desired 10i–10j could be produced in moderate yields with low diastereoselectivities. Cyclic alkyl substituted imines 8k–8m were also studied, and it was found that the diastereoselectivities of 10k–10m are still low. Several aryl including furan substituted linear alkyl imines 8n–8t were also examined, affording the desired products in moderate yields with excellent diastereoselectivities. When 8u, a compound containing chiral α-methyl imine, was investigated, the desired 10u was obtained with low diastereoselectivity. To confirm the effect of different chiral auxiliaries in imine, 8v was also studied and the result suggested that the chiral sulfinamide moiety determined the stereocontrol of this addition process in this example (10v). Imine 8w derived from a ketone was also examined, and the desired product 10w was obtained in moderate yield with high diastereoselectivity. Despite our great efforts to determine the stereochemistry of the newly generated chiral center, the result is fruitless.
1). When tert-butyl type imines 8i and 8j were investigated, the results showed that the desired 10i–10j could be produced in moderate yields with low diastereoselectivities. Cyclic alkyl substituted imines 8k–8m were also studied, and it was found that the diastereoselectivities of 10k–10m are still low. Several aryl including furan substituted linear alkyl imines 8n–8t were also examined, affording the desired products in moderate yields with excellent diastereoselectivities. When 8u, a compound containing chiral α-methyl imine, was investigated, the desired 10u was obtained with low diastereoselectivity. To confirm the effect of different chiral auxiliaries in imine, 8v was also studied and the result suggested that the chiral sulfinamide moiety determined the stereocontrol of this addition process in this example (10v). Imine 8w derived from a ketone was also examined, and the desired product 10w was obtained in moderate yield with high diastereoselectivity. Despite our great efforts to determine the stereochemistry of the newly generated chiral center, the result is fruitless.
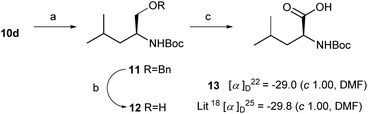
To confirm the stereochemistry of 10a–10v, compound 10d was easily transformed to the known 13. The treatment of 10d with HCl/dioxane in methanol and subsequent protection (Boc2O) afforded ester 11 in 67% overall yield (Scheme 2). The deprotection (Pd/C, H2) of 11 gave alcohol 12 in 81% yield. Dess–Martin oxidation17 and subsequent Pinnick oxidation (NaH2PO4·2H2O, NaClO2)18 gave the known 13 {[α]22D = −29.0 (c, 1.00, DMF), lit.19 [α]25D = −29.8 (c 1.00, DMF)} in 70% overall yield. The spectroscopic and physical data of the synthetic 13 were identical to the reported data.19 Thus the new stereochemistry of product 10d was unambiguously determined as the S-form.
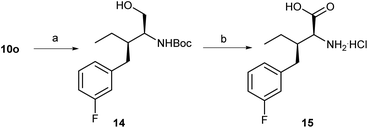
With 10o in hand, another non-natural amino acid 15 was considered as shown in Scheme 3. The removal of the chiral auxiliary (HCl/dioxane) of 10o and protection (Boc2O) gave an ester, which was deprotected with hydrogen (H2) in the presence of Pd/C to obtain alcohol 14 in 88% overall yield (Scheme 3). Dess–Martin oxidation and subsequent Pinnick oxidation (NaH2PO4·2H2O, NaClO2) generated a crude acid without further purification, which was treated with dry hydrogen chloride in methanol to obtain the desired amino acid hydrochloride 15 {[α]25D = −33.6 (c 0.50, MeOH)} in 66% overall yield.
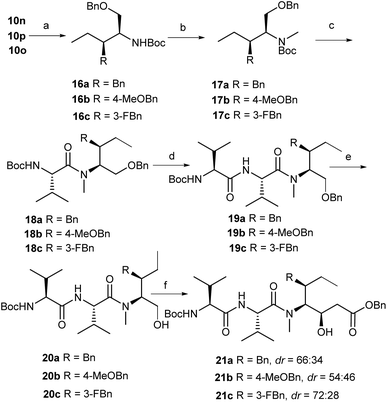
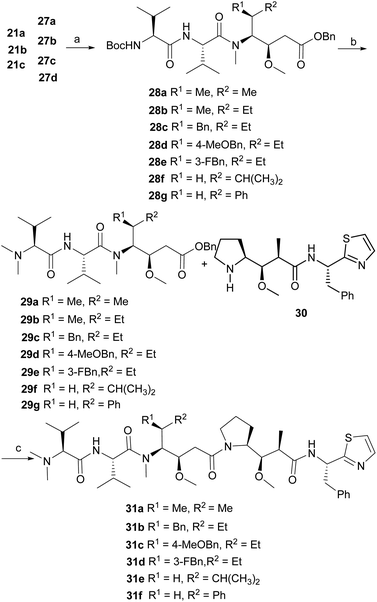
We turned our attention to utilize this diastereoselective approach in the synthesis of Dov-Val–Dil fragments of dolastatin 10 (1) analogues (Scheme 4). The deprotection (HCl/dioxane) of 10n and subsequent protection (Boc2O/TEA) gave ester 16a in 65% yield. The methylation of 16a with methyl trifluoromethanesulfonate (MeOTf)10c in the presence of lithium bis(trimethylsilyl)amide (LiHMDS) produced the desired compound 17a in 81% yield. The deprotection (TFA/DCM) of 17a and condensation (HATU/DIPEA) with N-Boc-L-Val-OH gave the desired amide 18a in 94% yield. The crude amine salt, prepared by the removal of the Boc group in 18a (TFA), was directly coupled with N-Boc-L-Val-OH (HATU/DIPEA) to give tripeptide 19a in 87% yield. The hydrogenation (Pd/C, H2) of 19a gave alcohol 20a in 82% yield. Upon the Dess–Martin oxidation of the hydroxy group in 20a, the subsequent addition with benzyl acetate in the presence of lithium diisopropylamide (LDA) gave the desired alcohol 21a in 73% yield with low diastereoselectivity (dr = 66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 34). Following the similar synthetic sequence described above, two analogues of Dov-Val–Dil fragment precursors 21b and 21c (dr = 54
34). Following the similar synthetic sequence described above, two analogues of Dov-Val–Dil fragment precursors 21b and 21c (dr = 54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 46 for 21b, dr = 72
46 for 21b, dr = 72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28 for 21c) in 24% and 32% respective overall yields were successfully achieved in parallel.
28 for 21c) in 24% and 32% respective overall yields were successfully achieved in parallel.
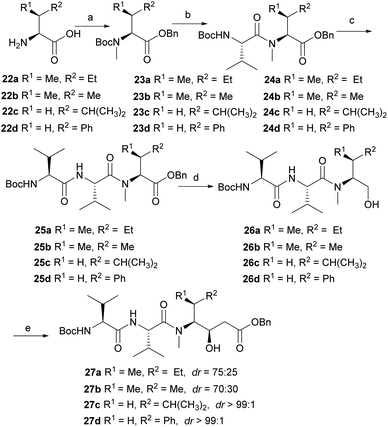
To obtain more diverse fragments of Dov-Val–Dil, several commercial amino acids 22a, 22b, 22c and 22d were also selected as the starting material. As shown in Scheme 5, continuous sequential protection of amino acids gave esters 23a, 23b, 23c and 23d in 40%, 42%, 41%, and 40% respective yields. Then through the key condensation and asymmetric addition as described in Scheme 4, four other analogues of Dov-Val–Dil fragment precursors 27a–27d (dr = 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 for 27a, dr = 70
25 for 27a, dr = 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 for 27b, dr > 99
30 for 27b, dr > 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for 27c, dr > 99
1 for 27c, dr > 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for 27d) in 44%, 33%, 27%, 25% respective overall yields were also successfully obtained in parallel.
1 for 27d) in 44%, 33%, 27%, 25% respective overall yields were also successfully obtained in parallel.
With a series of Dov-Val–Dil fragment precursors in hand, we then turned our attention to synthesize the dolastatin 10 (1) analogues. As shown in Scheme 6, 27b was firstly methylated with methyl trifluoromethanesulfonate (MeOTf) in the presence of bis(trimethylsilyl)amine lithium (LiHMDS) to give protected dipeptide 28a in 55% yield. The removal (TFA/DCM) of Boc in 28a and subsequent dimethylation20 (40% HCHO, Na(BH3)CN) gave Dov-Val–Dil fragment 29a in 70% yield. Upon the hydrogenation (Pd/C, H2) of Bn in 29a, the crude acid was directly amidated with our previously prepared Dap–Doe amine 3013d in the presence of HATU and DIPEA to give dolastatin 10 analogue 31a as a viscous liquid, which was further treated with acetone/hexane to give a white powder {mp 94–95 °C; [α]22D = −214.4 (c 0.125, CHCl3)} in 50% isolated yield. Finally, following a similar synthetic sequence described above, five other analogues of dolastatin 10 31b {[α]26D = −39.2 (c 0.25, CHCl3)}, 31c {[α]26D = −35.2 (c 0.25, CHCl3)}, 31d {[α]26D = −41.5 (c 1.00, CHCl3)}, 31e {[α]23D = −5.6 (c 0.50, CHCl3)}, and 31f {[α]23D = −26.4 (c 0.125, CHCl3)}were successfully achieved in 25%, 22%, 27%, 14%, and 12% respective overall yields in parallel. The structures of analogues 31a, 31b, 31c, 31d, 31e and 31f were overall confirmed by spectroscopic data.
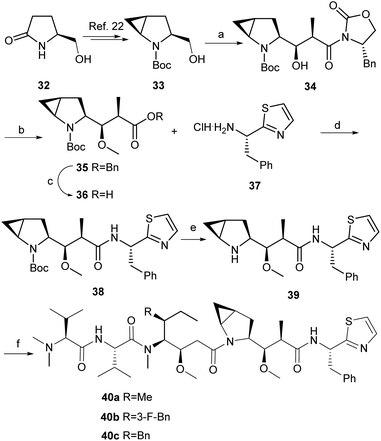
In light of our interest in diversity-oriented synthesis,21 we decided to prepare several analogues of dolastatin 10 containing a cyclopropane skeleton. As shown in Scheme 7, the cyclopropanated amino alcohol 33 was easily prepared from the commercialized (S)-(+)-5-hydroxymethyl-2-pyrrolidinone 32 by a known procedure.22 The hydroxy group in 33 was oxidized to the corresponding aldehyde without further purification, which was directly converted to compound 34 with two stereocenters according to a known procedure using the well-established Evans’ asymmetric aldol methodology23 in 60% yield with excellent diastereoselectivity (dr > 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Hydrolysis (LiOH, H2O2), protection (BnBr/K2CO3) and following methylation (MeOTf/LiHMDS) produced the protected ether 35 in 81% yield. The hydrogenation (Pd/C, H2) of 35 gave crude acid 36 without further purification, which was directly coupled with our previously prepared chiral salt 37
1). Hydrolysis (LiOH, H2O2), protection (BnBr/K2CO3) and following methylation (MeOTf/LiHMDS) produced the protected ether 35 in 81% yield. The hydrogenation (Pd/C, H2) of 35 gave crude acid 36 without further purification, which was directly coupled with our previously prepared chiral salt 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13d in the presence of HATU and DIPEA to generate 38 in 75% yield. Upon the deprotection (TFA, DCM) of Boc in 38, the crude amine was directly condensed separately with free acids of 29b, 29c and 29e to obtain three analogues of dolastatin 10 40a {[α]24D = −38.0 (c 0.10, CHCl3)}, 40b {[α]26D = −22.0 (c 0.25, CHCl3)}, and 40c {[α]24D = −30.2 (c 0.50, CHCl3)} in 31%, 28%, and 45% respective yields in parallel. The structures of analogues 40a, 40b and 40c were overall confirmed by spectroscopic data.
13d in the presence of HATU and DIPEA to generate 38 in 75% yield. Upon the deprotection (TFA, DCM) of Boc in 38, the crude amine was directly condensed separately with free acids of 29b, 29c and 29e to obtain three analogues of dolastatin 10 40a {[α]24D = −38.0 (c 0.10, CHCl3)}, 40b {[α]26D = −22.0 (c 0.25, CHCl3)}, and 40c {[α]24D = −30.2 (c 0.50, CHCl3)} in 31%, 28%, and 45% respective yields in parallel. The structures of analogues 40a, 40b and 40c were overall confirmed by spectroscopic data.
Conclusions
In summary, a diastereoselective approach to obtain amino alcohols 10 which were easily converted to non-natural amino acids through SmI2-induced radical addition of chiral imines 8 with 2-(benzyloxymethylsulfonyl)pyridine 9 has been developed. Moreover, this novel approach was used to prepare the key fragment-Dil of dolastatin 10 analogues. Using this strategy, nine dolastatin 10 analogues 31a, 31b, 31c, 31d, 31e, 31f, 40a, 40b and 40c have been synthesized. It is worth mentioning that several analogues containing divergent Dil subunits have been synthesized for the first time. Further chemistry and related biological data will be published in due course.Experimental
General
THF was distilled from sodium/benzophenone. The reactions were monitored by thin layer chromatography (TLC) on glass plates coated with silica gel with a fluorescent indicator. Flash chromatography was performed on silica gel (300–400 mesh). Optical rotations were measured on a polarimeter with a sodium lamp. HRMS were measured on an LTQ-Orbitrap-XL apparatus. IR spectra were recorded using a film on a Fourier transform infrared spectrometer. NMR spectra were recorded at 400 MHz or 600 MHz, and chemical shifts are reported in δ (ppm) referenced to the appropriate residual solvent peaks unless otherwise noted.General procedure for the synthesis of 8a–8u
To a solution of (S)-2-methyl-2-propane-sulfinamide (606 mg, 5.00 mmol) and aldehyde (5.00 mmol) in DCM (100 mL) was added anhydrous cupric sulfate (1.59 g, 10 mmol) and PPTS (63 mg, 0.25 mmol) in one portion, and the mixture was stirred for 24 h. The resulting mixture was filtered, and the filtrate was concentrated to give the crude product, which was purified by flash chromatography on silica gel (PE/EA = 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give the title product (8a–8u).
1) to give the title product (8a–8u).
(S,E)-N-Butylidene-2-methylpropane-2-sulfinamide (8a)
Colorless oil (572 mg, 65%); [α]25D +267 (c 1.00, CHCl3), lit.24{[α]20D +303.5 (c 1.00, CHCl3)}; IR (film): νmax 2961, 1622, 1363, 1085, 587 cm−1; 1H NMR (400 MHz, CDCl3) δ 8.07 (dd, J = 5.2, 4.4 Hz, 1H), 2.53–2.48 (m, 2H), 1.71–1.65 (m, 2H), 1.20 (s, 9H), 1.00 (t, J = 7.6 Hz, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 169.6, 56.5, 38.0, 22.3, 18.9, 13.8 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C8H18NOS+, 176.1104, found: 176.1102.(S,E)-N-Hexylidene-2-methylpropane-2-sulfinamide (8b)
Colorless oil (755 mg, 74%); [α]25D +282 (c 1.00, CHCl3), lit.25{[α]D +240.3 (c 1.00, CHCl3)}; IR (film): νmax 2957, 1622, 1363, 1087, 583 cm−1; 1H NMR (400 MHz, CDCl3) δ 8.10–8.03 (m, 1H), 2.55–2.47 (m, 2H), 1.67–1.58 (m, 2H), 1.37–1.32 (m, 4H), 1.20 (s, 9H), 0.94–0.86 (m, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 169.9, 56.6, 36.2, 31.5, 25.3, 22.5, 14.0 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C10H22NOS+, 204.1417, found: 204.1419.(S,E)-2-Methyl-N-octylidenepropane-2-sulfinamide (8c)
Colorless oil (720 mg, 62%); [α]25D +207 (c 1.00, CHCl3), lit.24{[α]20D +177.0 (c 1.00, CHCl3)}; IR (film): νmax 2926, 1621, 1455, 1087, 583 cm−1; 1H NMR (400 MHz, CDCl3) δ 8.07 (dd, J = 5.2, 4.4 Hz, 1H), 2.54–2.49 (m, 2H), 1.67–1.57 (m, 2H), 1.38–1.26 (m, 8H), 1.20 (s, 9H), 0.88 (t, J = 6.8 Hz, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 169.9, 56.5, 36.2, 31.7, 29.3, 29.1, 25.6, 22.7, 22.4, 14.1 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C12H26NOS+, 232.1730, found: 232.1730.(S,E)-2-Methyl-N-(3-methylbutylidene)propane-2-sulfinamide (8d)
Colorless oil (789 mg, 83%); [α]25D +282 (c 1.00, CHCl3); IR (film): νmax 2959, 1621, 1363, 1085, 586 cm−1; 1H NMR (400 MHz, CDCl3) δ 8.06 (dd, J = 5.2, 4.4 Hz, 1H), 2.44–2.38 (m, 2H), 2.13–2.01 (m, 1H), 1.21 (s, 9H), 1.02–1.00 (m, 3H), 1.00–0.98 (m, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 169.4, 56.5, 45.0, 26.2, 22.6, 22.5, 22.4 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C9H20NOS+, 190.1260, found: 190.1262.(S,E)-N-(3,3-Dimethylbutylidene)-2-methylpropane-2-sulfinamide (8e)
Colorless oil (887 mg, 87%); [α]25D +267 (c 1.00, CHCl3); IR (film): νmax 2958, 1619, 1364, 1092, 672 cm−1; 1H NMR (400 MHz, CDCl3) δ 8.12 (dd, J = 10.8, 5.4 Hz, 1H), 2.45–2.38 (m, 2H), 1.23–1.19 (m, 9H), 1.05–1.01 (m, 9H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 169.0, 56.7, 49.9, 31.5, 29.9, 22.6 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C10H22NOS+, 204.1417, found: 204.1416.(S,E)-N-(2-Ethylbutylidene)-2-methylpropane-2-sulfinamide (8f)
Colorless oil (898 mg, 88%); [α]25D +279 (c 1.00, CHCl3); IR (film): νmax 2963, 1619, 1458, 1086, 686 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.91 (dd, J = 6.0, 2.0 Hz, 1H), 2.42–2.33 (m, 1H), 1.64–1.57 (m, 4H), 1.23–1.21 (m, 9H), 0.94–0.89 (m, 6H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 173.3, 56.5, 48.8, 24.6, 22.5, 11.7, 11.6 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C10H22NOS+, 204.1417, found: 204.1416.(S,E)-N-((R)-2-Ethylpent-4-enylidene)-2-methylpropane-2-sulfinamide (8g)
Colorless oil (799 mg, 74%); [α]25D +270 (c 1.00, CHCl3); IR (film): νmax 2963, 1619, 1363, 1086, 915 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.96–7.90 (m, 1H), 5.81–5.68 (m, 1H), 5.12–5.01 (m, 2H), 2.60–2.49 (m, 1H), 2.40–2.27 (m, 2H), 1.66–1.58 (m, 2H), 1.20 (s, 9H), 0.94 (t, J = 7.6 Hz, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 172.4, 135.4, 117.0, 56.5, 46.7, 35.8, 24.5, 22.4, 11.4 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C11H22NOS+, 216.1417, found: 216.1415.(S,E)-N-((Z)-Hept-4-enylidene)-2-methylpropane-2-sulfinamide (8h)
Colorless oil (929 mg, 86%); [α]25D +247 (c 1.00, CHCl3); IR (film): νmax 2961, 1622, 1456, 1086, 583 cm−1; 1H NMR (400 MHz, CDCl3) δ 8.09–8.04 (m, 1H), 5.48–5.39 (m, 1H), 5.38–5.28 (m, 1H), 2.62–2.55 (m, 2H), 2.40–2.33 (m, 2H), 2.10–2.01 (m, 2H), 1.21–1.18 (m, 9H), 1.00–0.94 (m, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 169.2, 133.4, 126.9, 56.6, 36.3, 23.2, 22.4, 20.7, 14.3 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C11H22NOS+, 216.1417, found: 216.1414.(S,E)-N-(2,2-Dimethylpropylidene)-2-methylpropane-2-sulfinamide (8i)
Colorless oil (799 mg, 84%); [α]25D +287 (c 1.00, CHCl3), lit.26{[α]D +267.1 (c 0.70, CHCl3)}; IR (film): νmax 2963, 1620, 1474, 1363, 1086, 586 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.92 (brs, 1H), 1.18 (s, 9H), 1.16 (s, 9H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 175.6, 56.4, 37.9, 26.7, 22.3 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C9H20NOS+, 190.1260, found: 190.1262.(S,E)-2-Methyl-N-(2,2,5-trimethylhex-4-enylidene)propane-2-sulfinamide (8j)
Colorless oil (781 mg, 64%); [α]25D +212 (c 1.00, CHCl3); IR (film): νmax 2965, 1619, 1385, 1089, 588 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.93–7.90 (m, 1H), 5.15–5.07 (m, 1H), 2.23–2.16 (m, 2H), 1.70–1.68 (m, 3H), 1.62–1.60 (m, 3H), 1.20–1.18 (m, 9H), 1.14–1.12 (m, 6H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 175.7, 134.5, 119.6, 56.6, 42.1, 38.5, 26.1, 24.6, 24.3, 22.4, 18.0 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C13H26NOS+, 244.1730, found: 244.1731.(S,E)-N-(Cyclopropylmethylene)-2-methylpropane-2-sulfinamide (8k)
Colorless oil (714 mg, 82%); [α]25D +410 (c 1.00, CHCl3), lit.27{[α]D +332 (c 0.50, CH2Cl2)}; IR (film): νmax 2960, 1616, 1363, 1080, 695 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.46 (d, J = 7.6 Hz, 1H), 2.02–1.96 (m, 1H), 1.19 (s, 9H), 1.12–1.07 (m, 2H), 0.98–0.94 (m, 2H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 171.8, 56.7, 22.3, 17.6, 8.6, 8.5 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C8H16NOS+, 174.0947, found: 174.0945.(S,E)-N-(Cyclopentylmethylene)-2-methylpropane-2-sulfinamide (8l)
Colorless oil (717 mg, 71%); [α]25D +261 (c 1.00, CHCl3); IR (film): νmax 2956, 1619, 1474, 1084, 584 cm−1; 1H NMR (400 MHz, CDCl3) δ 8.01–7.98 (m, 1H), 2.99–2.92 (m, 1H), 1.94–1.85 (m, 2H), 1.73–1.65 (m, 6H), 1.20–1.18 (m, 9H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 172.6, 56.5, 45.7, 30.0, 29.9, 25.6, 25.6, 22.4 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C10H20NOS+, 202.1260, found: 202.1262.(S,E)-tert-Butyl 4-((2-methylpropan-2-ylsulfinamido)methyl)piperidine-1-carboxylate (8m)
Colorless oil (1.14 g, 72%); [α]25D +138 (c 1.00, CHCl3); IR (film): νmax 3444, 2975, 1692, 1423, 1170 cm−1; 1H NMR (400 MHz, CDCl3) δ 8.01 (d, J = 4.0 Hz, 1H), 4.15–4.02 (m, 2H), 2.94–2.82 (m, 2H), 2.67–2.56 (m, 1H), 1.92–1.84 (m, 2H), 1.59–1.49 (m, 2H), 1.46 (s, 9H), 1.19 (s, 9H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 170.8, 154.8, 79.8, 56.7, 42.2, 28.5, 28.4, 22.4 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C15H29N2O3S+, 317.1893, found: 317.1899.(S)-N-((R,E)-2-Benzylbutylidene)-2-methylpropane-2-sulfinamide (8n)
Colorless oil (1.16 g, 87%); [α]25D +143 (c 1.00, CHCl3); IR (film): νmax 2961, 1620, 1495, 1363, 1124, 699 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.97–7.88 (m, 1H), 7.30–7.21 (m, 2H), 7.19–7.12 (m, 3H), 2.93–2.81 (m, 3H), 1.68–1.56 (m, 2H), 1.06–0.99 (m, 9H), 0.99–0.91 (m, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 172.2, 139.2, 129.1, 128.5, 126.3, 56.6, 48.9, 38.1, 25.0, 22.3, 11.6 ppm; HRMS (ESI) m/z: [M + H]+ calcd for C15H24NOS+, 266.1573, found: 266.1576.(S)-N-((R,E)-2-(3-Fluorobenzyl)butylidene)-2-methylpropane-2-sulfinamide (8o)
Colorless oil (1.16 g, 82%); [α]25D +141 (c 1.00, CHCl3); IR (film): νmax 2963, 1619, 1589, 1488, 1363, 1083, 693 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.91 (d, J = 5.6 Hz, 1H), 7.25–7.16 (m, 1H), 6.94 (d, J = 7.2 Hz, 1H), 6.91–6.83 (m, 2H), 2.91–2.78 (m, 3H), 1.69–1.57 (m, 2H), 1.05 (s, 9H), 0.95 (t, J = 7.6 Hz, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 171.8, 163.0 (d, J = 244.3 Hz), 141.9 (d, J = 7.2 Hz), 130.0 (d, J = 8.3 Hz), 124.8 (d, J = 2.2 Hz), 116.0 (d, J = 20.9 Hz), 113.3 (d, J = 20.9 Hz), 56.7, 48.7, 37.8, 25.1, 22.3, 11.6 ppm; 19F NMR (376 MHz, CDCl3) δ −113.6 ppm; HRMS (ESI) m/z: [M + H]+ calcd for C15H23FNOS+, 284.1479, found: 284.1480.(S)-N-((R,E)-2-(4-Methoxybenzyl)butylidene)-2-methylpropane-2-sulfinamide (8p)
Colorless oil (1.23 g, 83%); [α]25D +130 (c 1.00, CHCl3); IR (film): νmax 3447, 2960, 1698, 1661, 1513, 1363, 1247, 1180 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.91 (d, J = 5.6 Hz, 1H), 7.10–7.05 (m, 2H), 6.82–6.77 (m, 2H), 3.77 (s, 3H), 2.88–2.80 (m, 1H), 2.80–2.73 (m, 2H), 1.65–1.55 (m, 2H), 1.05 (s, 9H), 0.94 (t, J = 7.6 Hz, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 172.5, 158.3, 131.3, 130.1, 114.1, 56.7, 55.4, 49.2, 37.4, 25.0, 22.4, 11.7 ppm; HRMS (ESI) m/z: [M + H]+ calcd for C16H26NO2S+, 296.1679, found: 296.1680.(S,E)-N-((R)-2-(4-tert-Butylbenzyl)butylidene)-2-methylpropane-2-sulfinamide (8q)
Colorless oil (1.21 g, 75%); [α]25D +104 (c 1.00, CHCl3); IR (film): νmax 2961, 1620, 1363, 1086, 589 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.91–7.87 (m, 1H), 7.28–7.24 (m, 2H), 7.12–7.07 (m, 2H), 2.84–2.81 (m, 3H), 1.66–1.58 (m, 2H), 1.28 (s, 9H), 0.99 (s, 9H), 0.95 (t, J = 7.6 Hz, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 172.4, 149.1, 136.0, 128.8, 125.4, 56.5, 49.0, 37.8, 34.4, 31.4, 25.3, 22.3, 11.6 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C19H32NOS+, 322.2199, found: 322.2203.(S,E)-2-Methyl-N-(3-(5-methylfuran-2-yl)propylidene)propane-2-sulfinamide (8r)
Yellow oil (944 mg, 78%); [α]25D +208 (c 1.00, CHCl3); IR (film): νmax 2957, 1623, 1363, 1086, 780 cm−1; 1H NMR (400 MHz, CDCl3) δ 8.11 (dd, J = 4.0, 3.2 Hz, 1H), 5.90–5.87 (m, 1H), 5.84–5.82 (m, 1H), 2.96–2.92 (m, 2H), 2.88–2.84 (m, 2H), 2.23 (s, 3H), 1.16 (s, 9H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 168.3, 152.2, 150.7, 106.2, 106.0, 56.7, 34.5, 23.9, 22.3, 13.5 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C12H20NO2S+, 242.1209, found: 242.1211.(S,E)-2-Methyl-N-(3-phenylpropylidene)propane-2-sulfinamide (8s)
Colorless oil (964 mg, 81%); [α]25D +196 (c 1.00, CHCl3); IR (film): νmax 2958, 1622, 1453, 1079, 699 cm−1; 1H NMR (400 MHz, CDCl3) δ 8.13–8.08 (m, 1H), 7.30–7.25 (m, 2H), 7.22–7.17 (m, 3H), 2.99–2.94 (m, 2H), 2.88–2.83 (m, 2H), 1.12 (s, 9H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 168.5, 140.3, 128.6, 128.4, 126.3, 56.6, 37.5, 31.4, 22.3 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C13H20NOS+, 238.1260, found: 238.1261.(S,E)-2-Methyl-N-(3-(3-(trifluoromethyl)phenyl)propylidene)propane-2-sulfinamide (8t)
Colorless oil (1.03 g, 67%); [α]25D +145 (c 1.00, CHCl3); IR (film): νmax 2961, 1623, 1328, 1124, 1075, 703 cm−1; 1H NMR (400 MHz, CDCl3) δ 8.13–8.08 (m, 1H), 7.50–7.45 (m, 2H), 7.43–7.38 (m, 2H), 3.08–3.04 (m, 2H), 2.94–2.89 (m, 2H), 1.11 (s, 9H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 167.8, 141.4, 131.9, 131.0 (d, J = 31.9 Hz), 129.1, 125.2 (d, J = 3.6 Hz), 124.2 (d, J = 270.7 Hz), 123.3 (d, J = 3.5 Hz), 56.7, 37.0, 31.0, 22.3 ppm; 19F NMR (376 MHz, CDCl3) δ −62.6 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C14H19F3NOS+, 306.1134, found: 306.1130.(S,E)-2-Methyl-N-((S)-2-methylbutylidene)propane-2-sulfinamide (8u)
Colorless oil (865 mg, 91%); [α]25D +296 (c 1.00, CHCl3), lit.28{[α]27D +208.7 (c 1.00, CHCl3)}; IR (film): νmax 2964, 2929, 1620, 1457, 1086 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.91–7.89 (m, 1H), 2.53–2.45 (m, 1H), 1.65–1.57 (m, 1H), 1.47–1.37 (m, 1H), 1.13 (s, 9H), 1.08–1.05 (m, 3H), 0.91–0.86 (m, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 173.3, 56.5, 41.6, 26.7, 22.3, 16.5, 11.6 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C9H20NOS+, 190.1260, found: 190.1261.(R,E)-2-Methyl-N-((S)-2-methylbutylidene)propane-2-sulfinamide (8v)
To a solution of (S)-(−)-2-methyl-1-butanol (176 mg, 2.00 mmol) in DCM (8 mL) was added Dess–Martin periodinane (1.70 g, 4.00 mmol). After being stirred for 30 min, the reaction mixture was carefully quenched with a saturated aqueous solution of NaHCO3 and solid Na2S2O3. The resulting mixture was extracted with DCM (20 mL × 3) and the combined organic layers were washed with brine, dried over MgSO4, filtered and concentrated to give a crude aldehyde without further purification. To a solution of the crude aldehyde in DCM (10 mL) was added (R)-2-methyl-2-propane-sulfinamide (266 mg, 2.20 mmol), anhydrous cupric sulfate (636 mg, 4.00 mmol) and PPTS (25 mg, 0.10 mmol), and after being stirred for 24 h, the reaction mixture was filtered and concentrated. The residue was purified by flash chromatography on silica gel (PE/EA = 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give the imine 8v. Colorless oil (282 mg, 75%, two steps); [α]25D −268 (c 1.00, CHCl3); IR (film): νmax 2964, 2875, 1621, 1086, 587 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.96 (dd, J = 5.2, 4.4 Hz, 1H), 2.58–2.50 (m, 1H), 1.71–1.64 (m, 1H), 1.52–1.47 (m, 1H), 1.21–1.19 (m, 9H), 1.16–1.13 (m, 3H), 0.97–0.92 (m, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 173.5, 56.5, 41.7, 26.7, 22.4, 16.5, 11.6 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C9H20NOS+, 190.1260, found: 190.1261.
1) to give the imine 8v. Colorless oil (282 mg, 75%, two steps); [α]25D −268 (c 1.00, CHCl3); IR (film): νmax 2964, 2875, 1621, 1086, 587 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.96 (dd, J = 5.2, 4.4 Hz, 1H), 2.58–2.50 (m, 1H), 1.71–1.64 (m, 1H), 1.52–1.47 (m, 1H), 1.21–1.19 (m, 9H), 1.16–1.13 (m, 3H), 0.97–0.92 (m, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 173.5, 56.5, 41.7, 26.7, 22.4, 16.5, 11.6 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C9H20NOS+, 190.1260, found: 190.1261.
(S,E)-2-Methyl-N-(4-methylpentan-2-ylidene)propane-2-sulfinamide (8w)
To a solution of 4-methyl-2-pentanone (350 mg, 5 mmol) in THF (14 mL) was added (S)-2-methyl-2-propane-sulfinamide (462 mg, 3.85 mmol) and Ti(OEt)4 (1.45 mL, 7.00 mmol). After the mixture was refluxed for 12 h, water was added. The resulting mixture was extracted with EtOAc (60 mL × 3), and the combined organic layers were dried over MgSO4, filtered and concentrated. The residue was purified by flash chromatography on silica gel (PE/EA = 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give the imine 8w. Colorless oil (504 mg, 50%); [α]25D +160 (c 1.00, CHCl3); IR (film): νmax 2957, 1620, 1463, 1387, 1074 cm−1; 1H NMR (400 MHz, CDCl3) δ 2.33–2.30 (m, 3H), 2.29–2.27 (m, 1H), 2.15–2.08 (m, 1H), 1.25 (s, 9H), 1.01–0.97 (m, 1H), 0.97–0.95 (m, 3H), 0.95–0.93 (m, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 185.2, 56.2, 52.5, 25.7, 23.3, 22.6, 22.5, 22.2 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C10H22NOS+, 204.1417, found: 204.1419.
1) to give the imine 8w. Colorless oil (504 mg, 50%); [α]25D +160 (c 1.00, CHCl3); IR (film): νmax 2957, 1620, 1463, 1387, 1074 cm−1; 1H NMR (400 MHz, CDCl3) δ 2.33–2.30 (m, 3H), 2.29–2.27 (m, 1H), 2.15–2.08 (m, 1H), 1.25 (s, 9H), 1.01–0.97 (m, 1H), 0.97–0.95 (m, 3H), 0.95–0.93 (m, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 185.2, 56.2, 52.5, 25.7, 23.3, 22.6, 22.5, 22.2 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C10H22NOS+, 204.1417, found: 204.1419.
General procedure for the synthesis of 10a–10u
A solution of 2-(benzyloxymethylsulfonyl)-pyridine 9 (395 mg, 1.50 mmol) in THF (1 ml) was dropped into the mixture of imine 8 (0.50 mmol) with SmI2 (30 ml, 3.00 mmol, 0.1 M in THF) at room temperature under an Ar atmosphere. The reaction mixture was quenched with H2O (0.2 ml), then the organic layer was washed with brine, dried over MgSO4, filtered and concentrated, and the residue was purified by flash chromatography on silica gel (PE/EA = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give 10a–10u.
1) to give 10a–10u.
(S)-N-((S)-1-(Benzyloxy)pentan-2-yl)-2-methylpropane-2-sulfinamide (10a)
Colorless oil (99 mg, 67%); [α]20D +45.0 (c 1.00, CHCl3); IR (film): νmax 2956, 2869, 1589, 1454, 1387, 1362, 1114, 1056 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.37–7.27 (m, 5H), 4.60 (d, J = 12.0 Hz, 1H), 4.48 (d, J = 12.0 Hz, 1H), 3.67–3.62 (m, 2H), 3.56–3.48 (m, 1H), 3.47–3.36 (m, 1H), 1.63–1.53 (m, 1H), 1.52–1.44 (m, 1H), 1.43–1.28 (m, 2H), 1.22 (s, 9H), 0.90 (t, J = 7.2 Hz, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 138.3, 128.5, 127.9, 127.8, 73.4, 73.2, 55.9, 55.8, 35.2, 22.8, 22.7, 19.1, 14.1 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C16H28NO2S+, 298.1835, found: 298.1836.(S)-N-((S)-1-(Benzyloxy)heptan-2-yl)-2-methylpropane-2-sulfinamide (10b)
Colorless oil (104 mg, 64%); [α]21D +43.5 (c 1.00, CHCl3); IR (film): νmax 2954, 2928, 2859, 1454, 1362, 1098, 735, 698 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.38–7.27 (m, 5H), 4.65–4.45 (m, 2H), 3.68–3.60 (m, 1H), 3.56–3.48 (m, 1H), 3.46–3.32 (m, 2H), 1.73–1.61 (m, 1H), 1.60–1.48 (m, 1H), 1.42–1.37 (m, 1H), 1.35–1.26 (m, 5H), 1.22–1.20 (m, 9H), 0.93–0.83 (m, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 138.3, 138.2, 128.5, 127.9, 127.8, 73.4, 73.2, 56.6, 55.9, 55.8, 33.0, 32.8, 31.8, 25.7, 25.5, 22.8, 22.7, 22.6, 22.5, 14.2 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C18H32NO2S+, 326.2148, found: 326.2150.(S)-N-((S)-1-(Benzyloxy)nonan-2-yl)-2-methylpropane-2-sulfinamide (10c)
Colorless oil (92 mg, 52%); [α]21D +40.7 (c 1.00, CHCl3); IR (film): νmax 2924, 2855, 1454, 1362, 1113, 1057, 698 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.40–7.27 (m, 5H), 4.54 (d, J = 12.0 Hz, 1H), 4.50 (d, J = 12.0 Hz, 1H), 3.53–3.43 (m, 2H), 3.42–3.39 (m, 1H), 3.38–3.33 (m, 1H), 1.71–1.59 (m, 2H), 1.33–1.25 (m, 10H), 1.20 (s, 9H), 0.91–0.84 (m, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 138.3, 128.5, 127.8, 73.4, 73.3, 56.6, 55.9, 32.8, 31.9, 29.6, 29.3, 26.1, 22.8, 22.7, 14.2 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C20H36NO2S+, 354.2461, found: 354.2462.(S)-N-((S)-1-(Benzyloxy)-4-methylpentan-2-yl)-2-methylpropane-2-sulfinamide (10d)
Colorless oil (98 mg, 63%); [α]20D +35.0 (c 1.00, CHCl3); IR (film): νmax 2955, 2927, 2867, 1587, 1454, 1386, 1114, 698 cm−1; 1H NMR (400 MHz, CDCl3,) δ 7.39–7.28 (m, 5H), 4.60 (d, J = 12.0 Hz, 1H), 4.48 (d, J = 12.0 Hz, 1H), 3.69–3.64 (m, 1H), 3.64–3.59 (m, 1H), 3.55–3.48 (m, 1H), 3.47–3.44 (m, 1H), 1.84–1.65 (m, 1H), 1.62–1.46 (m, 2H), 1.23–1.18 (m, 9H), 0.94–0.86 (m, 6H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 138.3, 128.5, 127.9, 127.8, 74.0, 73.4, 73.3, 56.0, 54.6, 42.5, 41.7, 24.6, 23.3, 23.1, 22.8, 22.7, 22.3, 22.0 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C17H30NO2S+, 312.1992, found: 312.1993.(S)-N-((S)-1-(Benzyloxy)-4,4-dimethylpentan-2-yl)-2-methylpropane-2-sulfinamide (10e)
Colorless oil (105 mg, 65%); [α]21D +48.6 (c 1.00, CHCl3); IR (film): νmax 2953, 1587, 1474, 1454, 1388, 1117, 1069, 698 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.39–7.25 (m, 5H), 4.60 (d, J = 11.8 Hz, 1H), 4.49 (d, J = 11.8 Hz, 1H), 3.74–3.69 (m, 1H), 3.69–3.64 (m, 1H), 3.53–3.46 (m, 2H), 1.51–1.46 (m, 1H), 1.46–1.39 (m, 1H), 1.23–1.19 (m, 9H), 0.96–0.91 (m, 9H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 138.3, 128.5, 127.9, 127.8, 127.7, 75.4, 74.9, 73.3, 73.2, 55.8, 53.6, 53.0, 47.2, 45.6, 30.5, 30.1, 30.0, 22.8, 22.7 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C18H32NO2S+, 326.2148, found: 326.2149.(S)-N-((S)-1-(Benzyloxy)-3-ethylpentan-2-yl)-2-methylpropane-2-sulfinamide (10f)
Colorless oil (115 mg, 71%); [α]21D +26.7 (c 1.00, CHCl3); IR (film): νmax 2960, 2927, 1591, 1454, 1362, 1074, 1028 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.39–7.26 (m, 5H), 4.58 (d, J = 11.6 Hz, 1H), 4.49 (d, J = 11.6 Hz, 1H), 3.67–3.62 (m, 2H), 3.60 (d, J = 8.0 Hz, 1H), 3.46–3.37 (m, 1H), 1.57–1.49 (m, 1H), 1.46–1.38 (m, 2H), 1.36–1.26 (m, 2H), 1.24 (s, 9H), 0.90–0.82 (m, 6H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 138.3, 128.5, 127.9, 127.8, 73.2, 71.2, 57.6, 56.1, 42.8, 22.9, 21.9, 21.7, 11.7, 11.5 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C18H32NO2S+, 326.2148, found: 326.2148.(S)-N-((2S,3R)-1-(Benzyloxy)-3-ethylhex-5-en-2-yl)-2-methylpropane-2-sulfinamide (10g)
Colorless oil (98 mg, 58%); [α]21D +37.7 (c 1.00, CHCl3); IR (film): νmax 2958, 1638, 1454, 1100, 911, 736, 698 cm−1; 1H NMR (400 MHz, CDCl3, rotamers) δ 7.36–7.29 (m, 5H), 5.88–5.67 (m, 1H), 5.10–5.04 (m, 1H), 5.03–4.97 (m, 1H), 4.54 (d, J = 12.0 Hz, 1H), 4.48 (d, J = 12.0 Hz, 1H), 3.67–3.62 (m, 0.7H), 3.60–3.56 (m, 0.3H), 3.53–3.48 (m, 3H), 2.26–2.16 (m, 1H), 2.15–2.07 (m, 1H), 1.83–1.76 (m, 0.7H), 1.74–1.69 (m, 0.3H), 1.59–1.49 (m, 1H), 1.46–1.35 (m, 1H), 1.24–1.18 (m, 9H), 0.97–0.89 (m, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3, rotamers) δ 138.2, 137.2, 137.1, 128.5, 127.9, 127.8, 116.8, 116.5, 73.3, 73.2, 71.9, 71.0, 57.9, 57.5, 56.1, 56.0, 41.3, 41.2, 34.0, 33.9, 22.9, 22.7, 22.1, 11.7, 11.6 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C19H32NO2S+, 338.2148, found: 338.2149.(S)-N-((S,Z)-1-(Benzyloxy)oct-5-en-2-yl)-2-methylpropane-2-sulfinamide (10h)
Colorless oil (138 mg, 82%); [α]20D +41.4 (c 1.00, CHCl3); IR (film): νmax 2959, 2929, 1454, 1362, 1069, 735, 698 cm−1; 1H NMR (400 MHz, CDCl3, rotamers) δ 7.37–7.26 (m, 5H), 5.42–5.34 (m, 1H), 5.33–5.25 (m, 1H), 4.65–4.57 (m, 0.6H), 4.57–4.53 (m, 0.4H), 4.53–4.49 (m, 0.4H), 4.49–4.45 (m, 0.6H), 3.71–3.66 (m, 1H), 3.65–3.64 (m, 0.4H), 3.57–3.53 (m, 0.6H), 3.53–3.51 (m, 0.4H), 3.50–3.45 (m, 0.6H), 3.44–3.37 (m, 1H), 2.19–2.04 (m, 2H), 2.04–1.96 (m, 2H), 1.78–1.70 (m, 1H), 1.66–1.56 (m, 1H), 1.24–1.19 (m, 9H), 0.95 (t, J = 7.6 Hz, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3, rotamers) δ 138.2, 132.7, 132.6, 128.5, 128.2, 127.9, 127.8, 73.4, 73.3, 73.2, 56.2, 55.9, 55.8, 33.4, 32.7, 23.8, 23.6, 22.8, 22.7, 20.7, 14.4 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C19H32NO2S+, 338.2148, found: 338.2149.(S)-N-((S)-1-(Benzyloxy)-3,3-dimethylbutan-2-yl)-2-methylpropane-2-sulfinamide (10i)
Colorless oil (64 mg, 66%, dr = 62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 38); [α]21D +36.2 (c 1.00, CHCl3); IR (film): νmax 2955, 2868, 1586, 1474, 1388, 1115, 735, 698 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.38–7.27 (m, 5H), 4.61 (d, J = 11.6 Hz, 1H), 4.44 (d, J = 11.6 Hz, 1H), 3.95 (d, J = 8.0 Hz, 1H), 3.83–3.77 (m, 1H), 3.72–3.63 (m, 1H), 3.10–2.96 (m, 1H), 1.25 (s, 9H), 0.94 (s, 9H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 138.4, 128.4, 127.7, 127.6, 73.3, 71.1, 64.4, 56.4, 35.3, 27.4, 23.0 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C17H30NO2S+, 312.1992, found: 312.1997.
38); [α]21D +36.2 (c 1.00, CHCl3); IR (film): νmax 2955, 2868, 1586, 1474, 1388, 1115, 735, 698 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.38–7.27 (m, 5H), 4.61 (d, J = 11.6 Hz, 1H), 4.44 (d, J = 11.6 Hz, 1H), 3.95 (d, J = 8.0 Hz, 1H), 3.83–3.77 (m, 1H), 3.72–3.63 (m, 1H), 3.10–2.96 (m, 1H), 1.25 (s, 9H), 0.94 (s, 9H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 138.4, 128.4, 127.7, 127.6, 73.3, 71.1, 64.4, 56.4, 35.3, 27.4, 23.0 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C17H30NO2S+, 312.1992, found: 312.1997.
(S)-N-((R)-1-(Benzyloxy)-3,3-dimethylbutan-2-yl)-2-methylpropane-2-sulfinamide ((1R)-10i)
Colorless oil (39 mg, 25%); [α]21D +22.4 (c 1.00, CHCl3); IR (film): νmax 2955, 2867, 1473, 1454, 1364, 1117, 1070 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.40–7.28 (m, 5H), 4.56–4.47 (m, 2H), 3.63–3.57 (m, 1H), 3.55–3.50 (m, 1H), 3.50–3.45 (m, 1H), 3.64–3.57 (m, 1H), 3.56–3.50 (m, 1H), 3.18–3.09 (m, 1H), 1.24–1.18 (m, 7H), 1.05–0.99 (m, 9H) ppm; 13C NMR (100 MHz, CDCl3) δ 138.2, 128.5, 127.7, 73.3, 71.6, 64.7, 56.3, 34.3, 27.4, 22.8 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C17H30NO2S+, 312.1992, found: 312.1998.(S)-N-((S)-1-(Benzyloxy)-3,3,6-trimethylhept-5-en-2-yl)-2-methylpropane-2-sulfinamide (10j)
Colorless oil (70 mg, 57%, dr = 67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33); [α]20D +33.6 (c 0.50, CHCl3); IR (film): νmax 2960, 2923, 1587, 1453, 1385, 1180, 1113, 698 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.37–7.27 (m, 5H), 5.15 (dd, J = 8.0, 7.2 Hz, 1H), 4.61 (d, J = 11.8 Hz, 1H), 4.44 (d, J = 11.8 Hz, 1H), 3.96 (d, J = 8.8 Hz, 1H), 3.83–3.77 (m, 1H), 3.67 (dd, J = 9.8, 4.2 Hz, 1H), 3.15–3.07 (m, 1H), 2.00–1.95 (m, 2H), 1.73–1.69 (m, 3H), 1.57–1.53 (m, 3H), 1.25 (s, 9H), 0.93–0.88 (m, 6H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 138.5, 133.6, 128.5, 127.8, 127.6, 120.5, 73.4, 71.2, 63.1, 56.4, 39.0, 38.1, 26.3, 24.9, 24.4, 23.1, 18.1 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C21H36NO2S+, 366.2461, found: 366.2467.
33); [α]20D +33.6 (c 0.50, CHCl3); IR (film): νmax 2960, 2923, 1587, 1453, 1385, 1180, 1113, 698 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.37–7.27 (m, 5H), 5.15 (dd, J = 8.0, 7.2 Hz, 1H), 4.61 (d, J = 11.8 Hz, 1H), 4.44 (d, J = 11.8 Hz, 1H), 3.96 (d, J = 8.8 Hz, 1H), 3.83–3.77 (m, 1H), 3.67 (dd, J = 9.8, 4.2 Hz, 1H), 3.15–3.07 (m, 1H), 2.00–1.95 (m, 2H), 1.73–1.69 (m, 3H), 1.57–1.53 (m, 3H), 1.25 (s, 9H), 0.93–0.88 (m, 6H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 138.5, 133.6, 128.5, 127.8, 127.6, 120.5, 73.4, 71.2, 63.1, 56.4, 39.0, 38.1, 26.3, 24.9, 24.4, 23.1, 18.1 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C21H36NO2S+, 366.2461, found: 366.2467.
(S)-N-((R)-1-(Benzyloxy)-3,3,6-trimethylhept-5-en-2-yl)-2-methylpropane-2-sulfinamide ((1R)-10j)
Colorless oil (34 mg, 19%); [α]22D +20.4 (c 0.25, CHCl3); IR (film): νmax 2917, 2849, 1649, 1454, 1386, 1180, 698 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.37–7.28 (m, 5H), 5.26–5.15 (m, 1H), 4.50–4.48 (m, 2H), 3.64–3.59 (m, 2H), 3.54–3.49 (m, 1H), 3.25–3.20 (m, 1H), 2.06–2.00 (m, 2H), 1.73–1.70 (m, 3H), 1.59–1.57 (m, 3H), 1.19 (s, 9H), 1.00–0.98 (m, 3H), 0.98–0.96 (m, 3H) ppm; 13C NMR (100 MHz, CDCl3) δ 134.1, 128.5, 127.8, 127.7, 120.4, 73.3, 71.7, 63.8, 56.2, 38.1, 37.9, 26.3, 25.4, 24.9, 22.8, 18.2 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C21H36NO2S+, 366.2461, found: 366.2465.(S)-N-((S)-2-(Benzyloxy)-1-cyclopropylethyl)-2-methylpropane-2-sulfinamide (10k)
Colorless oil (60 mg, 61%, dr = 67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33); [α]20D +54.3 (c 1.00, CHCl3); IR (film): νmax 2923, 2862, 1587, 1454, 1387, 1117, 698 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.38–7.27 (m, 5H), 4.60 (d, J = 12.0 Hz, 1H), 4.49 (d, J = 12.0 Hz, 1H), 3.91 (d, J = 2.8 Hz, 1H), 3.69 (dd, J = 9.1, 3.6 Hz, 1H), 3.53 (dd, J = 9.1, 8.2 Hz, 1H), 2.81–2.74 (m, 1H), 1.23 (s, 9H), 0.82–0.73 (m, 1H), 0.66–0.59 (m, 1H), 0.50–0.43 (m, 2H), 0.24–0.14 (m, 1H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 138.1, 128.6, 127.8, 73.4, 73.0, 59.2, 55.4, 22.7, 13,1, 4.6, 2.3 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C16H26NO2S+, 296.1679, found: 296.1676.
33); [α]20D +54.3 (c 1.00, CHCl3); IR (film): νmax 2923, 2862, 1587, 1454, 1387, 1117, 698 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.38–7.27 (m, 5H), 4.60 (d, J = 12.0 Hz, 1H), 4.49 (d, J = 12.0 Hz, 1H), 3.91 (d, J = 2.8 Hz, 1H), 3.69 (dd, J = 9.1, 3.6 Hz, 1H), 3.53 (dd, J = 9.1, 8.2 Hz, 1H), 2.81–2.74 (m, 1H), 1.23 (s, 9H), 0.82–0.73 (m, 1H), 0.66–0.59 (m, 1H), 0.50–0.43 (m, 2H), 0.24–0.14 (m, 1H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 138.1, 128.6, 127.8, 73.4, 73.0, 59.2, 55.4, 22.7, 13,1, 4.6, 2.3 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C16H26NO2S+, 296.1679, found: 296.1676.
(S)-N-((R)-2-(Benzyloxy)-1-cyclopropylethyl)-2-methylpropane-2-sulfinamide ((1R)-10k)
Colorless oil (30 mg, 20%); [α]20D +34.2 (c 0.50, CHCl3); IR (film): νmax 2923, 1594, 1454, 1387, 1362, 1072 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.38–7.28 (m, 5H), 4.58 (d, J = 11.8 Hz, 1H), 4.54 (d, J = 11.8 Hz, 1H), 3.64–3.58 (m, 3H), 2.60–2.53 (m, 1H), 1.21 (s, 9H), 1.10–1.03 (m, 1H), 0.67–0.59 (m, 2H), 0.46–0.40 (m, 1H), 0.36–0.27 (m, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ 138.4, 128.5, 127.8, 127.7, 73.8, 73.4, 61.8, 55.8, 22.7, 14.3, 4.6, 4.4 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C16H26NO2S+, 296.1679, found: 296.1682.(S)-N-((S)-2-(Benzyloxy)-1-cyclopentylethyl)-2-methylpropane-2-sulfinamide (10l)
Colorless oil (53 mg, 61%, dr = 54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 46); [α]20D +19.4 (c 0.50, CHCl3); IR (film): νmax 2952, 1587, 1454, 1387, 1071, 735.6, 698 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.38–7.27 (m, 5H), 4.62 (d, J = 11.8 Hz, 1H), 4.46 (d, J = 11.8 Hz, 1H), 3.78 (d, J = 8.4 Hz, 1H), 3.69 (dd, J = 9.5, 3.8 Hz, 1H), 3.63 (dd, J = 9.5, 3.2 Hz, 1H), 3.19–3.11 (m, 1H), 2.21–2.06 (m, 1H), 1.85–1.71 (m, 1H), 1.62–1.56 (m, 2H), 1.56–1.50 (m, 2H), 1.24 (s, 9H), 1.22–1.20 (m, 1H), 1.20–1.10 (m, 2H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 138.4, 128.5, 127.9, 127.8, 73.4, 72.8, 61.6, 56.2, 42.8, 30.3, 29.8, 25.6, 25.4, 22.9 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C18H30NO2S+, 324.1992, found: 324.1993.
46); [α]20D +19.4 (c 0.50, CHCl3); IR (film): νmax 2952, 1587, 1454, 1387, 1071, 735.6, 698 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.38–7.27 (m, 5H), 4.62 (d, J = 11.8 Hz, 1H), 4.46 (d, J = 11.8 Hz, 1H), 3.78 (d, J = 8.4 Hz, 1H), 3.69 (dd, J = 9.5, 3.8 Hz, 1H), 3.63 (dd, J = 9.5, 3.2 Hz, 1H), 3.19–3.11 (m, 1H), 2.21–2.06 (m, 1H), 1.85–1.71 (m, 1H), 1.62–1.56 (m, 2H), 1.56–1.50 (m, 2H), 1.24 (s, 9H), 1.22–1.20 (m, 1H), 1.20–1.10 (m, 2H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 138.4, 128.5, 127.9, 127.8, 73.4, 72.8, 61.6, 56.2, 42.8, 30.3, 29.8, 25.6, 25.4, 22.9 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C18H30NO2S+, 324.1992, found: 324.1993.
(S)-N-((R)-2-(Benzyloxy)-1-cyclopentylethyl)-2-methylpropane-2-sulfinamide ((1R)-10l)
Colorless oil (46 mg, 28%); [α]22D +43.6 (c 0.25, CHCl3); IR (film): νmax 2952, 2920, 2865, 1650, 1453, 1362, 1066, 736, 698 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.38–7.29 (m, 5H), 4.58–4.44 (m, 2H), 3.63–3.46 (m, 3H), 3.25–3.14 (m, 1H), 2.26–2.10 (m, 1H), 1.97–1.81 (m, 1H), 1.80–1.70 (m, 1H), 1.60–1.50 (m, 3H), 1.43–1.29 (m, 2H),1.29–1.25 (m, 1H), 1.23–1.19 (m, 9H) ppm; 13C NMR (100 MHz, CDCl3) δ 138.4, 128.5, 127.9, 127.8, 76.8, 73.4, 73.3, 61.1, 56.0, 41.8, 30.2, 25.7, 25.3, 22.9, 22.8 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C18H30NO2S+, 324.1992, found: 324.1994.tert-Butyl-4-((S)-2-(benzyloxy)-1-(((S)-tert-butylsulfinyl)amino)ethyl)piperidine-1-carboxylate (10m)
Colorless oil (101 mg, 69%, dr = 67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33); [α]20D +10.1 (c 1.00, CHCl3); IR (film): νmax 3439, 2922, 1691, 1423, 1364, 1172, 1071, 772 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.39–7.28 (m, 5H), 4.58 (d, J = 11.8 Hz, 1H), 4.46 (d, J = 11.8 Hz, 1H), 4.25–3.97 (m, 2H), 3.73–3.60 (m, 3H), 3.18–3.02 (m, 1H), 2.72–2.54 (m, 2H), 1.86–1.73 (m, 2H), 1.56 (d, J = 11.2 Hz, 1H), 1.45 (s, 9H), 1.23 (s, 9H), 1.19–1.04 (m, 2H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 154.9, 138.0, 128.6, 128.0, 127.9, 79.5, 77.5, 77.2, 76.8, 73.5, 71.0, 56.2, 38.6, 28.9, 28.6, 22.7 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C23H39N2O4S+, 439.2625, found: 439.2624.
33); [α]20D +10.1 (c 1.00, CHCl3); IR (film): νmax 3439, 2922, 1691, 1423, 1364, 1172, 1071, 772 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.39–7.28 (m, 5H), 4.58 (d, J = 11.8 Hz, 1H), 4.46 (d, J = 11.8 Hz, 1H), 4.25–3.97 (m, 2H), 3.73–3.60 (m, 3H), 3.18–3.02 (m, 1H), 2.72–2.54 (m, 2H), 1.86–1.73 (m, 2H), 1.56 (d, J = 11.2 Hz, 1H), 1.45 (s, 9H), 1.23 (s, 9H), 1.19–1.04 (m, 2H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 154.9, 138.0, 128.6, 128.0, 127.9, 79.5, 77.5, 77.2, 76.8, 73.5, 71.0, 56.2, 38.6, 28.9, 28.6, 22.7 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C23H39N2O4S+, 439.2625, found: 439.2624.
tert-Butyl-4-((R)-2-(benzyloxy)-1-(((S)-tert-butylsulfinyl)amino)ethyl)piperidine-1-carboxylate ((1R)-10m)
Colorless oil (50 mg, 23%); [α]20D +27.1 (c 1.00, CHCl3); IR (film): νmax 3259, 2922, 1691, 1453, 1364, 1170, 698 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.38–7.30 (m, 5H), 4.55 (d, J = 12.0 Hz, 1H), 4.49 (d, J = 12.0 Hz, 1H), 4.26–4.08 (m, 2H), 3.61–3.55 (m, 1H), 3.54–3.45 (m, 2H), 3.24–3.10 (m, 1H), 2.76–2.58 (m, 2H), 1.97–1.79 (m, 2H), 1.67–1.61 (m, 1H), 1.47 (s, 9H), 1.36–1.23 (m, 2H), 1.20 (s, 9H) ppm; 13C NMR (100 MHz, CDCl3) δ 154.9, 138.0, 128.6, 128.0, 127.9, 79.5, 73.5, 71.0, 56.2, 38.6, 28.9, 28.6, 22.7 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C23H39N2O4S+, 439.2625, found: 439.2626.(S)-N-((2S,3R)-3-Benzyl-1-(benzyloxy)pentan-2-yl)-2-methylpropane-2-sulfinamide (10n)
Colorless oil (145 mg, 75%); [α]22D +12.5 (c 1.00, CHCl3); IR (film): νmax 2958, 2925, 2870, 1637, 1454, 1362, 1100, 1071, 735, 698 cm−1; 1H NMR (400 MHz, CDCl3, rotamers) δ 7.40–7.24 (m, 7H), 7.23–7.12 (m, 3H), 4.57–4.39 (m, 2H), 3.66–3.63 (m, 1H), 3.61–3.60 (m, 0.28H), 3.55–3.52 (m, 1H), 3.52–3.47 (m, 0.72H), 2.81–2.73 (m, 1H), 2.58–2.42 (m, 1H), 2.11–2.05 (m, 0.28H), 2.04–1.94 (m, 0.72H), 1.58–1.46 (m, 0.28H), 1.45–1.33 (m, 1H), 1.33–1.26 (m, 0.72H), 1.26–1.12 (m, 9H), 0.94–0.86 (m, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3, rotamers) δ 141.3, 138.2, 129.3, 129.2, 128.5, 127.9, 127.8, 127.7, 126.0, 73.3, 73.1, 71.8, 71.0, 57.3, 56.6, 56.1, 44.0, 43.6, 36.1, 36.0, 22.9, 22.7, 22.1, 11.7, 11.6 ppm; HRMS (ESI-Orbitrap) m/z: [M + Na]+ calcd for C23H33NO2SNa+, 410.2124, found: 410.2129.(S)-N-((2S,3R)-1-(Benzyloxy)-3-(3-fluorobenzyl)pentan-2-yl)-2-methylpropane-2-sulfinamide (10o)
Colorless oil (131 mg, 65%); [α]22D +12.2 (c 1.00, CHCl3); IR (film): νmax 2959, 1587, 1453, 1362, 1068, 743, 698 cm−1; 1H NMR (400 MHz, CDCl3, rotamers) δ 7.39–7.27 (m, 5H), 7.27–7.18 (m, 1H), 6.98–6.82 (m, 3H), 4.54 (d, J = 12.0 Hz, 1H), 4.43 (d, J = 12.0 Hz, 1H), 3.66–3.59 (m, 2.5H), 3.57–3.51 (m, 0.5H), 3.49–3.40 (m, 1H), 2.70 (dd, J = 13.6, 6.8 Hz, 1H), 2.47 (dd, J = 13.6, 6.8 Hz, 1H), 1.98 (d, J = 6.0 Hz, 1H), 1.46–1.35 (m, 1H), 1.35–1.27 (m, 1H), 1.25 (s, 7.5H), 1.18 (s, 1.5H), 0.89 (t, J = 7.4 Hz, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3, rotamers) δ 163.0 (d, J = 243.9 Hz), 143.9 (d, J = 2.0 Hz), 138.1, 129.9 (d, J = 8.2 Hz), 127.9, 127.8, 124.9, 115.9 (d, J = 20.7 Hz), 112.9 (d, J = 20.9 Hz), 73.4, 73.2, 71.7, 70.9, 57.2, 56.6, 56.1, 43.9, 43.5, 35.9, 35.8, 22.9, 22.7, 22.0, 11.5 ppm; 19F NMR (376 MHz, CDCl3) δ −114.0 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C23H33FNO2S+, 406.2211, found: 406.2214.(S)-N-((2S,3R)-1-(Benzyloxy)-3-(4-methoxybenzyl)pentan-2-yl)-2-methylpropane-2-sulfinamide (10p)
Colorless oil (170 mg, 82%); [α]20D +14.0 (c 1.00, CHCl3); IR (film): νmax 2957, 1611, 1512, 1246, 1179, 1071, 698 cm−1; 1H NMR (400 MHz, CDCl3, rotamers) δ 7.38–7.26 (m, 5H), 7.10–7.01 (m, 2H), 6.87–6.77 (m, 2H), 4.56–4.40 (m, 2H), 3.78 (s, 3H), 3.70–3.54 (m, 2H), 3.63–3.46 (m, 1.67H), 3.42–3.38 (m, 0.33H), 2.75–2.60 (m, 1H), 2.53–2.36 (m, 1H), 2.03–1.87 (m, 1H), 1.55–1.48 (m, 0.33H), 1.44–1.34 (m, 1H), 1.34–1.27 (m, 0.67H), 1.27–1.20 (m, 6H), 1.17–1.15 (m, 3H), 0.96–0.84 (m, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3, rotamers) δ 158.0, 157.9, 138.2, 133.2, 132.6, 130.2, 130.1, 128.5, 127.9, 127.8, 127.7, 113.9, 73.3, 73.1, 71.9, 71.1, 57.3, 56.6, 56.1, 56.0, 55.4, 44.2, 43.7, 35.1, 35.0, 22.9, 22.7, 22.6, 22.0, 11.8, 11.6 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C24H36NO3S+, 418.2410, found: 418.2412.(S)-N-((2S,3R)-1-(Benzyloxy)-3-(4-(tert-butyl)benzyl)pentan-2-yl)-2-methylpropane-2-sulfinamide (10q)
Colorless oil (145 mg, 66%); [α]22D +7.7 (c 1.00, CHCl3); IR (film): νmax 2959, 1587, 1455, 1363, 1073, 735, 698 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.36–7.25 (m, 7H), 7.10–7.04 (m, 2H), 4.52 (d, J = 11.6 Hz, 1H), 4.41 (d, J = 11.6 Hz, 1H), 3.65–3.62 (m, 1H), 3.59 (d, J = 8.4 Hz, 1H), 3.54–3.48 (m, 1H), 2.79–2.60 (m, 1H), 2.56–2.37 (m, 1H), 2.07–1.89 (m, 1H), 1.45–1.36 (m, 1H), 1.31 (s, 9H), 1.24 (s, 9H), 1.20–1.19 (m, 1H), 1.15–1.13 (m, 1H), 0.95–0.86 (m, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 148.8, 138.2, 138.1, 128.9, 128.5, 127.9, 127.8, 127.7, 125.4, 73.1, 71.1, 56.7, 56.1, 44.2, 35.5, 34.5, 31.5, 22.9, 22.7, 22.4, 11.7 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C27H42NO2S+, 444.2931, found: 444.2932.(S)-N-((S)-1-(Benzyloxy)-4-(5-methylfuran-2-yl)butan-2-yl)-2-methylpropane-2-sulfinamide (10r)
Colorless oil (98 mg, 54%); [α]21D +33.0 (c 1.00, CHCl3); IR (film): νmax 2922, 1570, 1454, 1362, 1072, 1021, 698 cm−1; 1H NMR (400 MHz, CDCl3, rotamers) δ 7.37–7.28 (m, 5H), 5.92–5.88 (m, 0.37H), 5.85–5.81 (m, 1.63H), 4.62–4.46 (m, 2H), 3.71–3.65 (m, 1H), 3.59–3.52 (m, 1H), 3.51–3.39 (m, 2H), 2.78–2.67 (m, 1H), 2.67–2.53 (m, 1H), 2.24 (s, 3H), 2.09–1.92 (m, 1H), 1.91–1.87 (m, 1H), 1.24–1.19 (m, 9H) ppm; 13C{1H} NMR (100 MHz, CDCl3, rotamers) δ 153.5, 153.3, 150.6, 138.2, 128.5, 127.9, 127.8, 106.2, 106.0, 105.9, 73.4, 73.3, 56.1, 56.0, 55.8, 31.8, 24.6, 22.8, 22.7, 13.6 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C20H30NO3S+, 364.1941, found: 364.1944.(S)-N-((S)-1-(Benzyloxy)-4-phenylbutan-2-yl)-2-methylpropane-2-sulfinamide (10s)
Colorless oil (114 mg, 64%); [α]20D +30.8 (c 0.50, CHCl3); IR (film): νmax 2924, 1602, 1454, 1363, 1071, 745, 698 cm−1; 1H NMR (400 MHz, CDCl3, rotamers) δ 7.37–7.30 (m, 5H), 7.30–7.27 (m, 2H), 7.20–7.13 (m, 3H), 4.59 (d, J = 12.0 Hz, 1H), 4.47 (d, J = 12.0 Hz, 1H), 3.73 (d, J = 7.6 Hz, 1H), 3.68 (dd, J = 9.4, 4.2 Hz, 1H), 3.56 (dd, J = 9.6, 4.4 Hz, 1H), 3.47–3.40 (m, 1H), 2.75–2.68 (m, 1H), 2.66–2.56 (m, 1H), 1.96–1.80 (m, 2H), 1.26–1.23 (m, 7.6H), 1.20–1.19 (m, 1.4H) ppm; 13C{1H} NMR (100 MHz, CDCl3, rotamers) δ 141.8, 138.2, 128.7, 128.6, 128.5, 128.0, 126.1, 73.4, 73.3, 56.1, 55.8, 35.2, 32.2, 22.9, 22.8, 22.7 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C21H30NO2S+, 360.1992, found: 360.1996.(S)-N-((S)-1-(Benzyloxy)-4-(3-(trifluoromethyl)phenyl)butan-2-yl)-2-methylpropane-2-sulfinamide (10t)
Colorless oil (119 mg, 56%); [α]19D +31.7 (c 1.00, CHCl3); IR (film): νmax 2921, 1454, 1328, 1163, 1123, 1073, 799, 701 cm−1; 1H NMR (400 MHz, CDCl3, rotamers) δ 7.47–7.39 (m, 3H), 7.39–7.36 (m, 1H), 7.35–7.28 (m, 5H), 4.63–4.55 (m, 0.68H), 4.53–4.52 (m, 0.32H), 4.52–4.50 (m, 0.32H), 4.49–4.45 (m, 0.68H), 3.79–3.69 (m, 1H), 3.68–3.66 (m, 0.32H), 3.60–3.56 (m, 0.68H), 3.54–3.45 (m, 1H), 3.44–3.36 (m, 1H), 2.89–2.83 (m, 0.32H), 2.81–2.73 (m, 1H), 2.71–2.61 (m, 0.68H), 2.08–1.95 (m, 1H), 1.94–1.86 (m, 1 H), 1.26 (s, 6H), 1.21 (s, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3, rotamers) δ 142.7, 142.5, 138.1, 132.0 (d, J = 23.6 Hz), 130.8 (d, J = 31.8 Hz), 129.0, 128.6, 127.9 (d, J = 5.6 Hz), 125.2 (d, J = 3.5 Hz), 123.0, 73.4, 73.3, 73.2, 56.2, 56.1, 56.0, 55.9, 34.9, 34.3, 32.1, 22.8, 22.7 ppm; 19F NMR (376 MHz, CDCl3) δ −62.6 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C22H29F3NO2S+, 428.1866, found: 428.1867.(S)-N-((2S,3S)-1-(Benzyloxy)-3-methylpentan-2-yl)-2-methylpropane-2-sulfinamide (10u)
Colorless oil (79 mg, 75%, dr = 68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 32); [α]20D +41.4 (c 1.00, CHCl3); IR (film): νmax 2959, 2924, 2874, 1591, 1454, 1362, 1074 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.38–7.28 (m, 5H), 4.59 (d, J = 12.0 Hz, 1H), 4.46 (d, J = 12.0 Hz, 1H), 3.69 (d, J = 7.2 Hz, 1H), 3.65–3.61 (m, 2H), 3.29–3.17 (m, 1H), 1.83–1.72 (m, 1H), 1.57–1.48 (m, 1H), 1.23 (s, 9H), 1.15–1.05 (m, 1H), 0.97–0.88 (m, 6H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 138.3, 128.5, 127.9, 127.8, 73.2, 70.7, 59.9, 56.0, 36.5, 25.5, 22.9, 15.1, 11.5 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C17H30NO2S+, 312.1992, found: 312.1997.
32); [α]20D +41.4 (c 1.00, CHCl3); IR (film): νmax 2959, 2924, 2874, 1591, 1454, 1362, 1074 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.38–7.28 (m, 5H), 4.59 (d, J = 12.0 Hz, 1H), 4.46 (d, J = 12.0 Hz, 1H), 3.69 (d, J = 7.2 Hz, 1H), 3.65–3.61 (m, 2H), 3.29–3.17 (m, 1H), 1.83–1.72 (m, 1H), 1.57–1.48 (m, 1H), 1.23 (s, 9H), 1.15–1.05 (m, 1H), 0.97–0.88 (m, 6H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 138.3, 128.5, 127.9, 127.8, 73.2, 70.7, 59.9, 56.0, 36.5, 25.5, 22.9, 15.1, 11.5 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C17H30NO2S+, 312.1992, found: 312.1997.
(S)-N-((2R,3S)-1-(Benzyloxy)-3-methylpentan-2-yl)-2-methylpropane-2-sulfinamide ((2R)-10u)
Colorless oil (37 mg, 24%); [α]21D +44.2 (c 1.00, CHCl3); IR (film): νmax 2959, 2926, 2872, 1590, 1454, 1362, 698 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.38–7.28 (m, 5H), 4.54 (d, J = 12 Hz, 1H), 4.48 (d, J = 12 Hz, 1H), 3.52–3.44 (m, 2H), 3.43–3.38 (m, 1H), 3.30 (d, J = 6.0 Hz, 1H), 1.83–1.78 (m, 1H), 1.62–1.47 (m, 1H), 1.31–1.26 (m, 1H), 1.19 (s, 9H), 0.96–0.88 (m, 6H) ppm; 13C NMR (100 MHz, CDCl3) δ 138.3, 128.5, 127.8, 73.3, 72.0, 59.4, 56.0, 36.4, 26.2, 22.7, 14.6, 11.8 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C17H30NO2S+, 312.1992, found: 312.1993.(R)-N-((2R,3S)-1-(Benzyloxy)-3-methylpentan-2-yl)-2-methylpropane-2-sulfinamide (10v)
A solution of 2-(benzyloxymethylsulfonyl)-pyridine 9 (395 mg, 1.50 mmol) in THF was dropped into the mixture of imine 8v (94 mg, 0.50 mmol) with SmI2 (30 ml, 3.00 mmol, 0.1 M in THF) at room temperature under an Ar atmosphere. The reaction mixture was quenched with H2O (0.20 ml), then the organic layer was washed with brine, dried over MgSO4, filtered and concentrated, and the residue was purified by flash chromatography on silica gel (PE/EA = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give 10v. Colorless oil (99 mg, 80%, dr = 80
1) to give 10v. Colorless oil (99 mg, 80%, dr = 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20); [α]20D −38.4 (c 1.00, CHCl3); IR (film): νmax 2959, 2924, 2873, 1454, 1362, 1067, 736, 698 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.39–7.27 (m, 5H), 4.57 (d, J = 12.0 Hz, 1H), 4.50 (d, J = 12.0 Hz, 1H), 3.69–3.62 (m, 1H), 3.62–3.57 (m, 1H), 3.49 (d, J = 8.0 Hz, 1H), 3.40–3.30 (m, 1H), 1.79–1.68 (m, 1H), 1.50–1.42 (m, 1H), 1.23 (s, 9H), 1.17–1.10 (m, 1H), 0.91–0.85 (m, 6H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 138.3, 128.5, 127.9, 127.8, 73.3, 71.5, 59.6, 56.2, 36.7, 26.1, 22.9, 14.9, 11.8 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C17H30NO2S+, 312.1992, found: 312.1995.
20); [α]20D −38.4 (c 1.00, CHCl3); IR (film): νmax 2959, 2924, 2873, 1454, 1362, 1067, 736, 698 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.39–7.27 (m, 5H), 4.57 (d, J = 12.0 Hz, 1H), 4.50 (d, J = 12.0 Hz, 1H), 3.69–3.62 (m, 1H), 3.62–3.57 (m, 1H), 3.49 (d, J = 8.0 Hz, 1H), 3.40–3.30 (m, 1H), 1.79–1.68 (m, 1H), 1.50–1.42 (m, 1H), 1.23 (s, 9H), 1.17–1.10 (m, 1H), 0.91–0.85 (m, 6H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 138.3, 128.5, 127.9, 127.8, 73.3, 71.5, 59.6, 56.2, 36.7, 26.1, 22.9, 14.9, 11.8 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C17H30NO2S+, 312.1992, found: 312.1995.
(R)-N-((2S,3S)-1-(Benzyloxy)-3-methylpentan-2-yl)-2-methylpropane-2-sulfinamide ((2S)-10v)
Colorless oil (25 mg, 16%); [α]20D −26.4 (c 1.00, CHCl3); IR (film): νmax 2959, 2924, 2873, 1454, 1362, 1067, 698 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.39–7.27 (m, 5H), 4.53 (d, J = 12.2 Hz, 1H), 4.49 (d, J = 12.2 Hz, 1H), 3.55–3.47 (m, 2H), 3.44 (d, J = 6.4 Hz, 1H), 3.30–3.25 (m, 1H), 1.84–1.76 (m, 1H), 1.63–1.51 (m, 1H), 1.20 (s, 9H), 1.18–1.12 (m, 1H), 0.98–0.86 (m, 6H) ppm; 13C NMR (100 MHz, CDCl3) δ 138.3, 128.5, 127.8, 73.4, 71.6, 60.4, 56.0, 37.2, 25.6, 22.7, 15.5, 11.6 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C17H30NO2S+, 312.1992, found: 312.1995.(S)-N-(1-(Benzyloxy)-2,4-dimethylpentan-2-yl)-2-methylpropane-2-sulfinamide (10w)
A solution of 2-(benzyloxymethylsulfonyl)-pyridine 9 (395 mg, 1.50 mmol) in THF was dropped into the mixture of imine 8w (102 mg, 0.50 mmol) with SmI2 (30 ml, 3.00 mmol, 0.1 M in THF) at room temperature under an Ar atmosphere. The reaction mixture was quenched with H2O (0.20 ml), then the organic layer was washed with brine, dried over MgSO4, filtered and concentrated, and the residue was purified by flash chromatography on silica gel (PE/EA = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give 10w. Colorless oil (84 mg, 52%); [α]23D +36.0 (c 0.25, CHCl3); IR (film): νmax 2955, 1455, 1362, 1200, 1099, 919, 736, 698 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.39–7.27 (m, 5H), 4.61 (d, J = 12.0 Hz, 1H), 4.49 (d, J = 12.0 Hz, 1H), 3.72–3.69 (m, 1H), 3.45–3.68 (m, 2H), 1.75–1.70 (m, 1H), 1.52–1.42 (m, 2H), 1.34–1.30 (m, 3H), 1.20 (s, 9H), 0.96–0.88 (m, 6H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 137.6, 127.5, 126.8, 126.7, 77.5, 72.5, 57.8, 54.7, 47.1, 24.3, 24.2, 22.7, 21.9, 21.5 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C18H32NO2S+, 326.2148, found: 326.2149.
1) to give 10w. Colorless oil (84 mg, 52%); [α]23D +36.0 (c 0.25, CHCl3); IR (film): νmax 2955, 1455, 1362, 1200, 1099, 919, 736, 698 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.39–7.27 (m, 5H), 4.61 (d, J = 12.0 Hz, 1H), 4.49 (d, J = 12.0 Hz, 1H), 3.72–3.69 (m, 1H), 3.45–3.68 (m, 2H), 1.75–1.70 (m, 1H), 1.52–1.42 (m, 2H), 1.34–1.30 (m, 3H), 1.20 (s, 9H), 0.96–0.88 (m, 6H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 137.6, 127.5, 126.8, 126.7, 77.5, 72.5, 57.8, 54.7, 47.1, 24.3, 24.2, 22.7, 21.9, 21.5 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C18H32NO2S+, 326.2148, found: 326.2149.
tert-Butyl (S)-(1-(benzyloxy)-4-methylpentan-2-yl)carbamate (11)
A cooled (0 °C) solution of 10d (1.00 g, 3.22 mmol) in MeOH (14 mL) was treated with a solution of HCl/dioxane (2.80 mL). After being stirred for 30 min, the reaction mixture was concentrated. The resulting mixture was diluted with DCM (13 mL), and Boc2O (1.18 g, 6.44 mmol) and TEA (1.80 mL, 12.88 mmol) were added. After being stirred overnight, the mixture was quenched with a saturated aqueous solution of NH4Cl and extracted with DCM (30 mL × 3). The combined organic layers were washed with brine, dried over MgSO4, filtered and concentrated, and the residue was purified by flash chromatography on silica gel (PE/EA = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give 11. Colorless oil (662 mg, 67%, two steps); [α]23D −34.6 (c 1.00, CHCl3); IR (film): νmax 3348, 2956, 1713, 1498, 1365, 1171, 1101, 698 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.38–7.27 (m, 5H), 4.67 (d, J = 7.6 Hz, 1H), 4.55 (d, J = 12.2 Hz, 1H), 4.48 (d, J = 12.2 Hz, 1H), 3.89–3.77 (m, 1H), 3.50–3.41 (m, 2H), 1.68–1.56 (m, 1H), 1.44 (s, 9H), 1.42–1.35 (m, 2H), 0.94–0.89 (m, 6H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 155.7, 138.5, 128.5, 127.7, 127.6, 79.1, 73.3, 72.6, 48.7, 41.4, 28.6, 25.0, 23.1, 22.5 ppm; HRMS (ESI-Orbitrap) m/z: [M + Na]+ calcd for C18H29NO3Na+, 330.2040, found: 330.2038.
1) to give 11. Colorless oil (662 mg, 67%, two steps); [α]23D −34.6 (c 1.00, CHCl3); IR (film): νmax 3348, 2956, 1713, 1498, 1365, 1171, 1101, 698 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.38–7.27 (m, 5H), 4.67 (d, J = 7.6 Hz, 1H), 4.55 (d, J = 12.2 Hz, 1H), 4.48 (d, J = 12.2 Hz, 1H), 3.89–3.77 (m, 1H), 3.50–3.41 (m, 2H), 1.68–1.56 (m, 1H), 1.44 (s, 9H), 1.42–1.35 (m, 2H), 0.94–0.89 (m, 6H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 155.7, 138.5, 128.5, 127.7, 127.6, 79.1, 73.3, 72.6, 48.7, 41.4, 28.6, 25.0, 23.1, 22.5 ppm; HRMS (ESI-Orbitrap) m/z: [M + Na]+ calcd for C18H29NO3Na+, 330.2040, found: 330.2038.
tert-Butyl (S)-(1-hydroxy-4-methylpentan-2-yl)carbamate (12)
Compound 11 (500 mg, 1.52 mmol) and 10% Pd/C (50 mg) were stirred in MeOH (50 mL) for 4 h under a H2 atmosphere. Then the mixture was filtered to give a crude alcohol, which was purified by flash chromatography on silica gel (PE/EA = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give 12. Colorless oil (267 mg, 81%); [α]23D −20.3 (c 1.00, CHCl3); IR (film): νmax 3348, 2956, 1713, 1171, 1101, 698 cm−1; 1H NMR (400 MHz, CDCl3) δ 4.68–4.53 (m, 1H), 3.77–3.62 (m, 1H), 3.67–3.60 (m, 1H), 3.54–3.42 (m, 1H), 2.83–2.60 (m, 1H), 1.68–1.62 (m, 1H), 1.44 (s, 9H), 1.34–1.27 (m, 2H), 0.97–0.87 (m, 6H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 156.7, 79.8, 66.7, 51.2, 40.7, 28.5, 24.9, 23.2, 22.3 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C11H24NO3+, 218.1681, found: 218.1685.
1) to give 12. Colorless oil (267 mg, 81%); [α]23D −20.3 (c 1.00, CHCl3); IR (film): νmax 3348, 2956, 1713, 1171, 1101, 698 cm−1; 1H NMR (400 MHz, CDCl3) δ 4.68–4.53 (m, 1H), 3.77–3.62 (m, 1H), 3.67–3.60 (m, 1H), 3.54–3.42 (m, 1H), 2.83–2.60 (m, 1H), 1.68–1.62 (m, 1H), 1.44 (s, 9H), 1.34–1.27 (m, 2H), 0.97–0.87 (m, 6H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 156.7, 79.8, 66.7, 51.2, 40.7, 28.5, 24.9, 23.2, 22.3 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C11H24NO3+, 218.1681, found: 218.1685.
(tert-Butoxycarbonyl)-L-leucine (13)
To a cooled (0 °C) solution of 12 (180 mg, 0.83 mmol) in DCM (4 mL) was slowly added Dess–Martin periodinane (880 mg, 2.08 mmol). After being stirred for 30 min, the reaction mixture was carefully quenched with a saturated aqueous solution of NaHCO3 and solid Na2S2O3. The resulting mixture was extracted with DCM (20 mL × 3) and the combined organic layers were washed with brine, dried over MgSO4, filtered and concentrated to give a crude aldehyde without further purification. A solution of the above crude aldehyde in t-BuOH/2-methyl-2-butene (1/3 mL) was treated with a solution of NaClO2 (224 mg, 2.49 mmol) and NaH2PO4·2H2O (388 mg, 2.49 mmol) in water (3 mL). After being stirred for 8 h, the reaction mixture was extracted with EtOAc (30 mL × 3) and the combined organic layers were washed with brine, dried over MgSO4, filtered and concentrated to give a crude acid. The residue was purified by flash chromatography on silica gel (DCM/MeOH = 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–50
1–50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give 13. Colorless oil (134 mg, 70%, two steps); [α]22D −29.0 (c 1.00, DMF); IR (film): νmax 3387, 2956, 1714, 1649, 1251, 1019, 750, 697 cm−1; 1H NMR (400 MHz, CDCl3) δ 4.94–4.79 (m, 1H), 4.35–4.25 (m, 1H), 1.79–1.63 (m, 2H), 1.57–1.50 (m, 1H), 1.44 (s, 9H), 0.98–0.91 (m, 6H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 177.7, 155.9, 80.4, 52.1, 41.4, 28.4, 24.9, 23.0, 21.9 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C11H22NO4+, 232.1543, found: 232.1549.
1) to give 13. Colorless oil (134 mg, 70%, two steps); [α]22D −29.0 (c 1.00, DMF); IR (film): νmax 3387, 2956, 1714, 1649, 1251, 1019, 750, 697 cm−1; 1H NMR (400 MHz, CDCl3) δ 4.94–4.79 (m, 1H), 4.35–4.25 (m, 1H), 1.79–1.63 (m, 2H), 1.57–1.50 (m, 1H), 1.44 (s, 9H), 0.98–0.91 (m, 6H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 177.7, 155.9, 80.4, 52.1, 41.4, 28.4, 24.9, 23.0, 21.9 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C11H22NO4+, 232.1543, found: 232.1549.
tert-Butyl ((2S,3R)-1-(benzyloxy)-3-(3-fluorobenzyl)pentan-2-yl)carbamate (14)
To a cooled (0 °C) solution of 10o (1.90 g, 4.69 mmol) in MeOH (20 mL) was added dropwise a solution of HCl/dioxane (4.00 mL). After being stirred for 30 min, the reaction mixture was concentrated. The resulting mixture was diluted with DCM (20 mL), and then Boc2O (2.04 g, 9.38 mmol) and TEA (2.6 ml, 18.76 mmol) were added. After stirring overnight, the mixture was quenched with a saturated aqueous solution of NH4Cl and extracted with DCM (50 mL × 3). The combined organic layers were washed with brine, dried over MgSO4, filtered and concentrated to give a crude middle compound without further purification. Above crude middle compound and 10% Pd/C (190 mg) were stirred in MeOH (100 mL) for 4 h under a H2 atmosphere, and filtered to give a crude alcohol, which was purified by flash chromatography on silica gel (PE/EA = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give 14. Colorless oil (1.28 g, 88%, three steps); [α]22D −6.4 (c 1.00, CHCl3); IR (film): νmax 3356, 2967, 1689, 1589, 1488, 1366, 1170 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.26–7.19 (m, 1H), 6.98–6.93 (m, 1H), 6.91–6.85 (m, 2H), 4.84–4.63 (m, 1H), 3.77–3.68 (m, 1H), 3.67–3.59 (m, 2H), 2.75 (dd, J = 13.6, 4.2 Hz, 1H), 2.70–2.64 (m, 0.3H), 2.60 (d, J = 6.8 Hz, 1H), 2.57–2.42 (m, 0.7H), 1.90–1.79 (m, 1H), 1.45 (s, 9H), 1.40–1.28 (m, 2H), 0.97–0.88 (m, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 163.0 (d, J = 243.9 Hz), 156.8, 143.6, 129.9 (d, J = 8.0 Hz), 124.8 (d, J = 8.1 Hz), 115.9 (d, J = 20.7 Hz), 113.0 (d, J = 20.9 Hz), 79.9, 64.5, 54.5, 54.3, 43.2, 42.5, 36.5, 35.9, 28.5, 22.6, 11.8, 10.8 ppm; 19F NMR (376 MHz, CDCl3) δ −113.6 ppm; HRMS (ESI-Orbitrap) m/z: [M + Na]+ calcd for C17H26FNO3Na+ (M + Na)+, 334.1789, found: 334.1789.
1) to give 14. Colorless oil (1.28 g, 88%, three steps); [α]22D −6.4 (c 1.00, CHCl3); IR (film): νmax 3356, 2967, 1689, 1589, 1488, 1366, 1170 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.26–7.19 (m, 1H), 6.98–6.93 (m, 1H), 6.91–6.85 (m, 2H), 4.84–4.63 (m, 1H), 3.77–3.68 (m, 1H), 3.67–3.59 (m, 2H), 2.75 (dd, J = 13.6, 4.2 Hz, 1H), 2.70–2.64 (m, 0.3H), 2.60 (d, J = 6.8 Hz, 1H), 2.57–2.42 (m, 0.7H), 1.90–1.79 (m, 1H), 1.45 (s, 9H), 1.40–1.28 (m, 2H), 0.97–0.88 (m, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 163.0 (d, J = 243.9 Hz), 156.8, 143.6, 129.9 (d, J = 8.0 Hz), 124.8 (d, J = 8.1 Hz), 115.9 (d, J = 20.7 Hz), 113.0 (d, J = 20.9 Hz), 79.9, 64.5, 54.5, 54.3, 43.2, 42.5, 36.5, 35.9, 28.5, 22.6, 11.8, 10.8 ppm; 19F NMR (376 MHz, CDCl3) δ −113.6 ppm; HRMS (ESI-Orbitrap) m/z: [M + Na]+ calcd for C17H26FNO3Na+ (M + Na)+, 334.1789, found: 334.1789.
(2S,3R)-2-Amino-3-(3-fluorobenzyl)pentanoic acid hydrochloride (15)
Dess–Martin periodinane (2.05 g, 4.83 mmol) was carefully added to a cooled (0 °C) solution of 14 (600 mg, 1.93 mmol) in DCM (8 mL) and stirred for 30 min. Then the reaction mixture was carefully quenched with a saturated aqueous solution of NaHCO3 and solid Na2S2O3. The mixture was extracted with DCM (30 mL × 3) and the combined organic layers were washed with brine, dried over MgSO4, filtered and concentrated to give a crude aldehyde without further purification. The above aldehyde was dissolved in t-BuOH/2-methyl-2-butene (2.3/7.0 mL) and NaClO2 (589 mg, 5.79 mmol) and NaH2PO4·2H2O (902 mg, 5.79 mmol) in water (7 mL) was added dropwise. After stirring for 8 h, the reaction mixture was extracted with EtOAc (30 mL × 3). The combined organic layers were washed with brine, dried over MgSO4, filtered and concentrated to give a crude acid without further purification. The above crude acid was dissolved in MeOH (8 mL) and treated with a solution of HCl/MeOH (2.00 mL, 3 M) for 2 h. The mixture was directly concentrated to give a crude salt, which was recrystallized to give 15. White solid (287 mg, 66%, three steps); mp 103.6–105.6 °C; [α]20D −33.6 (c 0.50, MeOH); IR (film): νmax 3421, 2966, 1732, 1589, 1489, 1253, 784, 691 cm−1; 1H NMR (400 MHz, CD3OD) δ 7.41–7.31 (m, 1H), 7.14–7.07 (m, 1H), 7.07–6.95 (m, 2H), 3.8–3.82 (m, 1H), 2.87–2.67 (m, 2H), 2.32–2.12 (m, 1H), 1.65–1.48 (m, 1H), 1.43–1.30 (m, 1H), 1.07–0.95 (m, 3H) ppm; 13C{1H} NMR (150 MHz, CD3OD) δ 171.0, 164.4 (d, J = 243.3 Hz), 143.3 (d, J = 7.1 Hz), 131.5 (d, J = 8.3 Hz), 126.2, 117.0 (d, J = 21.5 Hz), 113.2 (d, J = 21.2 Hz), 55.3, 44.8, 44.5, 36.7, 36.6, 23.4, 23.0, 12.1, 11.9 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C12H17FNO2+, 226.1238, found: 226.1239.General procedure for the synthesis of 16a, 16b and 16c
A cooled (0 °C) solution of 10n/10o/10p (10.12 mmol) in MeOH (40 mL) was treated with a solution of HCl/dioxane (8.00 mL) for 30 min. The reaction mixture was concentrated and the resulting mixture was dissolved in DCM (40 mL). Boc2O (4.41 g, 20.24 mmol) and TEA (5.63 mL, 40.48 mmol) were added. After being stirred overnight, the mixture was quenched with a saturated aqueous solution of NH4Cl and extracted with DCM (60 mL × 3). The combined organic layers were washed with brine, dried over MgSO4, filtered and concentrated, and the residue was purified by flash chromatography on silica gel (PE/EA = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give the title product (16a, 16b and 16c).
1) to give the title product (16a, 16b and 16c).
tert-Butyl ((2S,3R)-3-benzyl-1-(benzyloxy)pentan-2-yl)carbamate (16a)
Colorless oil (2.52 g, 65%, two steps); [α]23D −9.1 (c 3.00, CHCl3); IR (film): νmax 3446, 2965, 1699, 1496, 1365, 1170, 699 cm−1; 1H NMR (400 MHz, CDCl3, rotamers) δ 7.37–7.31 (m, 2H), 7.31–7.22 (m, 5H), 7.19–7.13 (m, 3H), 4.79–4.65 (m, 1H), 4.52–4.36 (m, 2H), 3.97–3.78 (m, 1H), 3.57–3.41 (m, 2H), 2.85–2.74 (m, 0.3H), 2.70–2.60 (m, 0.7H), 2.58–2.48 (m, 0.7H), 2.44–2.37 (m, 0.3H), 2.03–1.85 (m, 1H), 1.44 (s, 9H), 1.38–1.20 (m, 2H), 0.95–0.78 (m, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3, rotamers) δ 156.0, 155.8, 141.3, 138.3, 129.2, 129.1, 128.4, 128.3, 127.8, 127.7, 125.9, 79.1, 73.2, 73.0, 70.8, 70.4, 51.7, 51.5, 43.2, 42.4, 36.7, 35.8, 28.5, 22.3, 11.5, 10.6 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C24H34NO4+, 384.2533, found: 384.2536.tert-Butyl ((2S,3R)-1-(benzyloxy)-3-(4-methoxybenzyl)pentan-2-yl)carbamate (16b)
Colorless oil (3.13 g, 75%, two steps); [α]23D −12.7 (c 1.00, CHCl3); IR (film): νmax 3344, 2964, 1635, 1512, 1246, 1173, 698 cm−1; 1H NMR (400 MHz, CDCl3, rotamers) δ 7.37–7.26 (m, 5H), 7.12–7.05 (m, 2H), 7.85–7.78 (m, 2H), 4.79–4.65 (m, 0.8H), 4.53–4.51 (m, 0.2H), 4.50–4.36 (m, 2H), 3.92–3.80 (m, 1H), 3.78 (s, 3H), 3.57–3.42 (m, 2H), 2.77–2.71 (m, 0.2H), 2.63–2.52 (m, 0.8H), 2.52–2.45 (m, 0.8H), 2.42–2.30 (m, 0.2H), 1.95–1.82 (m, 1H), 1.44 (s, 9H), 1.34–1.17 (m, 2H), 0.98–0.81 (m, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3, rotamers) δ 157.9, 156.0, 155.8, 138.3, 133.3, 130.2, 130.1, 128.5, 127.7, 113.8, 79.2, 73.3, 73.1, 70.8, 70.5, 55.4, 51.4, 43.4, 35.7, 34.9, 28.6, 22.3, 11.6, 10.7 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C25H36NO4+, 414.2639, found: 414.2641.tert-Butyl ((2S,3R)-1-(benzyloxy)-3-(3-fluorobenzyl)pentan-2-yl)carbamate (16c)
Colorless oil (3.25 g, 76%, two steps); [α]22D −8.3 (c 1.00, CHCl3); IR (film): νmax 3447, 2964, 1699, 1614, 1488, 1390, 1248, 1099, 747 cm−1; 1H NMR (400 MHz, CDCl3, rotamers) δ 7.36–7.25 (m, 5H), 7.25–7.19 (m, 1H), 6.97–6.84 (m, 3H), 4.78–4.70 (m, 0.75H), 4.54–4.41 (m, 0.25H), 4.51–4.38 (m, 2H), 3.92–3.76 (m, 1H), 3.59–3.43 (m, 2H), 2.86–2.73 (m, 0.25H), 2.72–2.59 (m, 0.75H), 2.59–2.45 (m, 0.75H), 2.46–2.39 (m, 0.25H), 2.00–1.89 (m, 1H), 1.45 (s, 9H), 1.33–1.20 (m, 2H), 0.94–0.84 (m, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3, rotamers) δ 162.5 (d, J = 243.7 Hz), 156.0, 155.8, 144.1, 138.2, 129.7 (d, J = 8.3 Hz), 128.5, 127.8 (d, J = 4.0 Hz), 125.0, 124.8, 116.0 (d, J = 20.6 Hz), 112.8 (d, J = 20.9 Hz), 79.3, 73.3, 73.2, 70.7, 70.3, 51.8, 51.5, 43.1, 42.4, 36.5, 35.7, 29.8, 28.5, 22.3, 11.6, 10.5 ppm; 19F NMR (376 MHz, CDCl3) δ −114.1 ppm; HRMS (ESI-Orbitrap) m/z: [M + Na]+ calcd for C24H32FNO3Na+, 424.2258, found: 424.2258.General procedure for the synthesis of 17a, 17b and 17c
Compounds 16a/16b/16c (6.50 mmol) were stirred in HMPA (1.35 ml, 7.80 mmol) and THF (28 mL) at −78 °C, and then a solution of LiHMDS (7.80 ml, 7.80 mmol, 1 M in THF) was slowly added dropwise and stirred for 30 min. The reaction mixture was allowed to warm to −15 °C and MeOTf (1.47 ml, 13.00 mmol) was added. The resulting mixture was stirred for an additional 15 min. The reaction mixture was quenched with a saturated aqueous solution of NH4Cl and extracted with EtOAc (40 mL × 3). The combined organic layers were washed with brine, dried over MgSO4, filtered and concentrated, and the residue was purified by flash chromatography on silica gel (PE/EA = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give the desired product (17a, 17b and 17c).
1) to give the desired product (17a, 17b and 17c).
tert-Butyl ((2S,3R)-3-benzyl-1-(benzyloxy)pentan-2-yl)(methyl)carbamate (17a)
Colorless oil (2.09 g, 81%); [α]23D +4.8 (c 1.00, CHCl3); IR (film): νmax 3444, 2970, 1688, 1454, 1365, 1149, 737, 699 cm−1; 1H NMR (400 MHz, CDCl3, rotamers) δ 7.37–7.28 (m, 5H), 7.27–7.23 (m, 2H), 7.20–7.12 (m, 3H), 4.62–4.48 (m, 1H), 4.46–4.41 (m, 0.5H), 4.41–4.38 (m, 0.5H), 4.38–4.02 (m, 1H), 3.68–3.56 (m, 2H), 2.84–2.73 (m, 3H), 2.72–2.63 (m, 1H), 2.55–2.38 (m, 1H), 2.17–1.93 (m, 1H), 1.49–1.42 (m, 9H), 1.31–1.17 (m, 2H), 0.90–0.79 (m, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3, rotamers) δ 156.9, 156.5, 141.2, 140.7, 138.6, 138.4, 129.1, 128.5, 128.4, 127.7, 127.6, 126.0, 125.9, 125.8, 79.6, 79.4, 79.3, 72.9, 70.4, 70.2, 69.7, 69.5, 56.4, 56.1, 39.8, 39.4, 39.2, 36.0, 35.7, 35.6, 30.5, 29.8, 28.7, 28.6, 21.6, 21.2, 9.3, 8.7 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C25H36NO3+, 398.2690, found: 398.2688.tert-Butyl ((2S,3R)-1-(benzyloxy)-3-(4-methoxybenzyl)pentan-2-yl)(methyl)carbamate (17b)
Colorless oil (2.64 g, 95%); [α]24D +5.1 (c 1.00, CHCl3); IR (film): νmax 3448, 2966, 1680, 1512, 1246, 1149, 1037, 737 cm−1; 1H NMR (400 MHz, CDCl3, rotamers) δ 7.39–7.25 (m, 5H), 7.10–7.01 (m, 2H), 6.85–6.75 (m, 2H), 4.65–4.50 (m, 1H), 4.47–4.33 (m, 1H), 4.37–3.96 (m, 1H), 3.79 (s, 3H), 3.67–3.66 (m, 2H), 2.85–2.71 (m, 3H), 2.68–2.57 (m, 1H), 2.47–2.35 (m, 1H), 2.12–1.83 (m, 1H), 1.49–1.41 (m, 9H), 1.27–1.16 (m, 2H), 0.91–0.81 (m, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3, rotamers) δ 157.9, 156.5, 138.6, 138.4, 133.1, 132.7, 129.9, 128.5, 128.4, 127.7, 127.6, 113.8, 79.6, 79.2, 72.9, 70.4, 69.7, 55.4, 39.5, 39.3, 35.0, 34.6, 28.7, 28.6, 21.5, 21.1, 21.0, 9.3, 8.7 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C26H38NO4+, 428.2795, found: 428.2796.tert-Butyl-((2S,3R)-1-(benzyloxy)-3-(3-fluorobenzyl)pentan-2-yl)(methyl)carbamate (17c)
Colorless oil (2.51 g, 93%); [α]25D +1.3 (c 1.00, CHCl3); IR (film): νmax 3445, 2969, 1689, 1452, 1365, 1251, 1142, 697 cm−1; 1H NMR (400 MHz, CDCl3, rotamers) δ 7.38–7.26 (m, 5H), 7.24–7.16 (m, 1H), 6.97–6.78 (m, 3H), 4.62–4.48 (m, 1H), 4.47–4.35 (m, 1H), 4.37–4.00 (m, 1H), 3.68–3.53 (m, 2H), 2.85–2.70 (m, 3H), 2.68–2.5 8 (m, 1H), 2.56–2.37 (m, 1H), 2.17–1.98 (m, 1H), 1.49–1.42 (m, 9H), 1.32–1.15 (m, 2H), 0.92–0.78 (m, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3, rotamers) δ 163.0 (d, J = 243.8 Hz), 156.4, 156.3, 144.3, 143.8, 143.4, 138.4 (d, J = 19.1 Hz), 129.7, 128.5, 128.4, 127.7 (d, J = 7.3 Hz), 124.8, 115.9 (d, J = 20.6 Hz), 112.9 (d, J = 19.9 Hz), 79.7, 79.5, 79.3, 73.0, 70.5, 70.2, 69.7, 58.6, 56.3, 39.7, 39.3, 39.2, 35.8, 35.7, 35.4, 30.6, 29.8, 28.6, 21.5, 21.4, 21.2, 9.3, 8.8 ppm; 19F NMR (376 MHz, CDCl3) δ −113.8 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C25H35FNO3+, 416.2596, found: 416.2596.General procedure for the synthesis of 18a, 18b, 18c, 24a, 24b, 24c and 24d
Compounds 17a/17b/17c/23a/23b/23c/23d (5.00 mmol) were stirred in DCM (20 mL) at 0 °C and were treated with TFA (4.00 mL) for 2 h. The mixture was concentrated under reduced pressure and the residue was dissolved in DCM (20 mL). HATU (2.85 g, 7.50 mmol), DIPEA (2.61 mL, 15.00 mmol), and N-Boc-L-Val-OH (1.19 g, 5.50 mmol) were added in turn and the resulting mixture was stirred overnight. The reaction mixture was quenched with a saturated aqueous solution of NH4Cl and extracted with DCM (50 mL × 3). The combined organic layers were washed with brine, dried over MgSO4, filtered and concentrated, and the residue was purified by flash chromatography on silica gel (PE/EA = 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give the desired product (18a, 18b, 18c, 24a, 24b, 24c and 24d).
1) to give the desired product (18a, 18b, 18c, 24a, 24b, 24c and 24d).
tert-Butyl-((S)-1-(((2S,3R)-3-benzyl-1-(benzyloxy)pentan-2-yl)(methyl)amino)-3-methyl-1-oxobutan-2-yl)carbamate (18a)
Colorless oil (2.33 g, 94%, two steps); [α]23D +10.0 (c 1.00, CHCl3); IR (film): νmax 3434, 2966, 1636, 1495, 1171, 739, 699 cm−1; 1H NMR (400 MHz, CDCl3, rotamers) δ 7.37–7.30 (m, 2H), 7.30–7.23 (m, 5H), 7.20–7.06 (m, 3H), 5.41–5.15 (m, 1H), 4.84–4.66 (m, 1H), 4.59–4.50 (m, 1H), 4.47–4.36 (m, 2H), 3.66–3.52 (m, 2H), 3.13–2.98 (m, 3H), 2.83–2.80 (m, 0.5H), 2.72–2.67 (m, 0.5H), 2.56–2.45 (m, 1H), 2.21–2.07 (m, 1H), 2.03–1.87 (m, 1H), 1.45–1.40 (m, 9H), 1.31–1.26 (m, 1H), 1.22–1.12 (m, 1H), 0.93–0.85 (m, 6H), 0.79 (t, J = 7.4 Hz, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3, rotamers) δ 173.4, 156.2, 140.8, 138.1, 129.1, 128.5, 128.4, 128.0, 127.9, 127.8, 126.1, 79.5, 73.2, 73.1, 69.8, 55.7, 38.8, 38.3, 35.9, 35.4, 31.3, 31.1, 28.5, 28.4, 21.5, 20.5, 19.7, 17.7, 17.0, 9.4, 8.7 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C30H45N2O4+, 497.3374, found: 497.3370.tert-Butyl-((S)-1-(((2S,3R)-1-(benzyloxy)-3-(4-methoxybenzyl)pentan-2-yl)(methyl)amino)-3-methyl-1-oxobutan-2-yl)carbamate (18b)
Colorless oil (2.10 g, 80%, two steps); [α]24D +8.0 (c 1.00, CHCl3); IR (film): νmax 3438, 2965, 1636, 1512, 1365, 1247, 1175, 1109 cm−1; 1H NMR (400 MHz, CDCl3, rotamers) δ 7.38–7.23 (m, 5H), 7.13–7.01 (m, 2H), 6.87–6.77 (m, 2H), 5.42–5.13 (m, 1H), 4.83–4.69 (m, 0.76H), 4.56–4.53 (m, 0.24H), 4.52–4.43 (m, 1H), 4.43–4.27 (m, 2H), 3.78 (s, 3H), 3.65–3.50 (m, 2H), 3.13–2.89 (m, 3H), 2.81–2.78 (m, 0.24H), 2.68–2.59 (m, 0.76H), 2.54–2.42 (m, 1H), 2.19–1.88 (m, 2H), 1.41 (s, 9H), 1.24–1.13 (m, 2H), 0.96–0.83 (m, 6H), 0.82–0.74 (m, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3, rotamers) δ 173.4, 157.9, 156.2, 138.1, 132.7, 130.0, 128.5, 128.4, 128.0, 127.9, 127.8, 113.8, 79.4, 73.1, 69.7, 55.7, 55.3, 38.9, 34.8, 31.1, 28.5, 28.4, 21.3, 19.6, 17.7, 9.5 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C31H47N2O5+, 527.3480, found: 527.3480.tert-Butyl ((S)-1-(((2S,3R)-1-(benzyloxy)-3-(3-fluorobenzyl)pentan-2-yl)(methyl)amino)-3-methyl-1-oxobutan-2-yl)carbamate (18c)
Colorless oil (2.18 g, 85%, two steps); [α]26D +6.7 (c 1.00, CHCl3, rotamers); IR (film): νmax 3320, 2965, 1708, 1637, 1488, 1390, 1172, 774 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.38–7.27 (m, 5H), 7.26–7.23 (m, 1H), 6.94–6.82 (m, 3H), 5.40–5.14 (m, 1H), 4.83–4.66 (m, 0.7H), 4.56–4.53 (m, 0.3H), 4.50–4.42 (m, 1H), 4.42–4.29 (m, 2H), 3.63–3.48 (m, 2H), 3.13–2.96 (m, 3H), 2.83–2.81 (m, 0.3H), 2.73–2.65 (m, 0.7H), 2.58–2.48 (m, 1H), 2.20–2.06 (m, 1H), 2.02–1.87 (m, 1H), 1.41 (s, 9H), 1.27–1.14 (m, 2H), 0.97–0.87 (m, 6H), 0.85–0.75 (m, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3, rotamers) δ 173.3, 162.9 (d, J = 244.0 Hz), 156.0, 143.3 (d, J = 7.0 Hz), 137.8, 129.7 (d, J = 8.1 Hz), 128.4, 128.3, 127.9, 127.7, 127.6, 124.6, 115.7 (d, J = 20.6 Hz), 112.9 (d, J = 20.8 Hz), 79.4, 73.1, 69.7, 55.6, 38.6, 35.5, 31.0, 28.4, 28.3, 21.3, 19.5, 17.6, 9.3 ppm; 19F NMR (376 MHz, CDCl3) δ −113.8 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C30H44FN2O4+, 515.3280, found: 515.3281.Benzyl N-((tert-butoxycarbonyl)-L-valyl)-N-methyl-L-isoleucinate (24a)13d
Colorless oil (1.78 g, 82%, two steps).Benzyl N-((tert-butoxycarbonyl)-L-valyl)-N-methyl-L-valinate (24b)
Colorless oil (1.47 g, 70%, two steps); [α]23D −80.8 (c 1.00, CHCl3); IR (film) νmax 2965, 1736, 1654, 1497, 1366, 1175, 1130, 698 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.35–7.31 (m, 5H), 5.25–5.20 (m, 1H), 5.17–5.13 (m, 1H), 5.07–5.03 (m, 1H), 5.00 (d, J = 10.8 Hz, 1H), 4.35 (dd, J = 9.2, 6.8 Hz, 1H), 2.99–2.96 (m, 3H), 2.27–2.17 (m, 1H), 1.88–1.78 (m, 1H), 1.41 (s, 9H), 1.03–1.00 (m, 3H), 0.98–0.88 (m, 1H), 0.86–0.82 (m, 8H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 173.6, 170.8, 156.1, 135.6, 128.7, 128.6, 128.6, 79.6, 66.8, 61.6, 55.5, 31.4, 31.2, 28.4, 27.2, 20.0, 19.4, 18.7, 17.7 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C23H37N2O5+, 421.2697, found: 421.2694.Benzyl N-((tert-butoxycarbonyl)-L-valyl)-N-methyl-L-leucinate (24c)
Colorless oil (1.67 g, 77%, two steps); [α]23D −39.9 (c 1.00, CHCl3); IR (film) νmax 2960, 1738, 1711, 1498, 1456, 1367, 1265, 694 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.38–7.30 (m, 5H), 5.43 (dd, J = 10.8, 5.2 Hz, 1H), 5.20–5.05 (m, 3H), 4.39 (dd, J = 9.2, 6.4 Hz, 1H), 2.99–2.95 (m, 3H), 1.95–1.87 (m, 1H), 1.80–1.70 (m, 2H), 1.52–1.45 (m, 1H), 1.42 (s, 9H), 0.94–0.88 (m, 9H), 0.87–0.84 (m, 3H) ppm; 13C{1H} NMR (CDCl3, 100 MHz) δ 173.5, 171.7, 156.1, 135.5, 128.7, 128.6, 79.6, 67.2, 55.4, 54.6, 37.0, 31.3, 31.1, 28.4, 24.8, 23.4, 21.5, 19.5, 17.6 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C24H39N2O5+, 435.2854, found: 435.2849.Benzyl N-((tert-butoxycarbonyl)-L-valyl)-N-methyl-L-phenylalaninate (24d)
Colorless oil (1 g, 68%, two steps); [α]23D −72.4 (c 1.00, CHCl3); IR (film) νmax 2968, 1741, 1649, 1498, 1407, 1366, 1012, 696 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.35–7.32 (m, 3H), 7.31–7.28 (m, 2H), 7.25–7.16 (m, 5H), 5.41 (dd, J = 9.6, 5.6 Hz, 1H), 5.22–5.18 (m, 1H), 5.11–5.06 (m, 1H), 5.06–5.02 (m, 1H), 4.32 (dd, J = 9.2, 6.0 Hz, 1H), 3.40 (dd, J = 14.4, 5.6 Hz, 1H), 2.99 (dd, J = 14.4, 10.0 Hz, 1H), 2.90–2.86 (m, 3H), 1.90–1.80 (m, 1H), 1.40 (s, 9H), 0.90–0.86 (m, 3H), 0.84–0.80 (m, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 172.9, 170.6, 155.8, 136.9, 135.4, 129.0, 128.7, 128.6, 128.6, 126.9, 79.5, 67.3, 58.4, 55.1, 34.7, 32.8, 31.3, 28.5, 19.6, 17.2 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C27H37N2O5+, 469.2697, found: 469.2692.General procedure for the synthesis of 19a, 19b, 19c, 25a, 25b, 25c and 25d
Compounds 18a/18b/18c/24a/24b/24c/24d (3.20 mmol) were dissolved in DCM (16 mL) at 0 °C and treated with TFA (3.20 mL) for 2 h. The mixture was concentrated under reduced pressure and the residue was dissolved in DCM (16 mL). HATU (1.82 g, 4.8 mmol), DIPEA (1.67 ml, 9.6 mmol), and N-Boc-L-Val-OH (0.76 g, 3.52 mmol) were added in turn and the resulting mixture was stirred overnight. The reaction mixture was quenched with a saturated aqueous solution of NH4Cl and extracted with DCM (20 mL × 3). The combined organic layers were washed with brine, dried over MgSO4, filtered and concentrated, and the residue was purified by flash chromatography on silica gel (PE/EA = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give the desired product (19a, 19b, 19c, 25a, 25b, 25c and 25d).
1) to give the desired product (19a, 19b, 19c, 25a, 25b, 25c and 25d).
tert-Butyl ((S)-1-(((S)-1-(((2S,3R)-3-benzyl-1-(benzyloxy)pentan-2-yl)(methyl)amino)-3-methyl-1-oxobutan-2-yl)amino)-3-methyl-1-oxobutan-2-yl)carbamate (19a)
Colorless oil (1.66 g, 87%, two steps); [α]23D −6.4 (c 1.00, CHCl3); IR (film): νmax 3444, 2963, 1633, 1517, 1454, 1174, 739, 699 cm−1; 1H NMR (400 MHz, CDCl3, rotamers) δ 7.35–7.29 (m, 3H), 7.29–7.24 (m, 5H), 7.19 (d, J = 7.2 Hz, 1H), 7.15–7.10 (m, 2H), 6.69–6.55 (m, 1H), 5.13–5.08 (m, 0.7H), 4.94–4.87 (m, 0.3H), 4.79–4.75 (m, 1H), 4.53–4.47 (m, 0.3H), 4.45–4.30 (m, 1.7H), 3.99–3.84 (m, 1H), 3.69–362 (m, 0.3H), 3.60–3.52 (m, 1.7H), 3.16–2.97 (m, 3H), 2.84–2.76 (m, 0.3H), 2.75–2.64 (m, 0.7H), 2.59–2.48 (m, 1H), 2.25–2.17 (m, 0.3H), 2.10–1.97 (m, 2.7H), 1.45–1.43 (m, 9H), 1.04–0.94 (m, 2H), 0.92–0.85 (m, 12H), 0.75 (t, J = 7.2 Hz, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3, rotamers) δ 172.4, 171.5, 155.8, 140.7, 138.0, 129.0, 128.5, 128.4, 128.0, 127.8, 126.1, 79.8, 73.3, 73.2, 69.6, 63.2, 60.1, 58.6, 54.2, 38.7, 35.8, 31.5, 31.3, 30.9, 28.4, 21.5, 19.6, 19.3, 17.7, 9.3 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C35H54N3O5+, 596.4058, found: 596.4059.tert-Butyl ((S)-1-(((S)-1-(((2S,3R)-1-(benzyloxy)-3-(4-methoxybenzyl)pentan-2-yl)(methyl)amino)-3-methyl-1-oxobutan-2-yl)amino)-3-methyl-1-oxobutan-2-yl)carbamate (19b)
Colorless oil (1.72 g, 86%, two steps); [α]23D −4.7 (c 1.00, CHCl3); IR (film): νmax 3325, 2962, 1650, 1512, 1455, 1247, 1176, 1039 cm−1; 1H NMR (400 MHz, CDCl3, rotamers) δ 7.36–7.26 (m, 5H), 7.07–6.98 (m, 2H), 6.82–6.77 (m, 2H), 6.62–6.47 (m, 1H), 5.13–5.03 (m, 1H), 4.77 (dd, J = 8.8, 6.8 Hz, 1H), 4.52–4.43 (m, 1H), 4.40–4.32 (m, 1H), 3.98–3.86 (m, 1H), 3.78 (s, 3H), 3.65–3.51 (m, 2H), 3.15–2.97 (m, 2.7H), 2.84–2.79 (m, 0.3H), 2.76–2.70 (m, 0.3H), 2.66–2.59 (m, 0.7H), 2.54–2.41 (m, 1H), 2.20–1.96 (m, 3H), 1.45–1.42 (m, 9H), 1.15–0.95 (m, 2H), 0.93–0.85 (m, 12H), 0.82–0.72 (m, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3, rotamers) δ 172.4, 171.5, 158.0, 155.8, 138.1, 132.6, 130.0, 128.5, 128.4, 127.9, 127.8, 113.9, 79.8, 73.1, 69.6, 60.2, 55.4, 54.2, 38.8, 34.8, 31.5, 31.3, 28.4, 21.4, 20.1, 19.6, 19.3, 18.0, 17.7 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C36H56N3O6+, 626.4164, found: 626.4164.tert-Butyl ((S)-1-(((S)-1-(((2S,3R)-1-(benzyloxy)-3-(3-fluorobenzyl)pentan-2-yl)(methyl)amino)-3-methyl-1-oxobutan-2-yl)amino)-3-methyl-1-oxobutan-2-yl)carbamate (19c)
Colorless oil (1.73 g, 88%, two steps); [α]26D −11.2 (c 1.00, CHCl3); IR (film): νmax 3296, 2962, 1693, 1630, 1524, 1417, 1249, 1174 cm−1; 1H NMR (400 MHz, CDCl3, rotamers) δ 7.37–7.26 (m, 5H), 7.26–7.24 (m, 1H), 7.23–7.17 (m, 1H), 6.94–6.81 (m, 3H), 6.63–6.48 (m, 1H), 5.10–5.06 (m, 0.7H), 4.79–4.70 (m, 1.3H), 4.50–4.34 (m, 2H), 3.98–3.87 (m, 1H), 3.68–3.48 (m, 2H), 3.14–3.01 (m, 2.7H), 2.85–2.81 (m, 0.3H), 2.81–2.72 (m, 0.3H), 2.71–2.61 (m, 0.7H), 2.57–2.45 (m, 1H), 2.22–2.15 (m, 0.3H), 2.14–1.98 (m, 2.7H), 1.45–1.42 (m, 9H), 1.27–1.04 (m, 2H), 0.93–0.85 (m, 12H), 0.82–0.71 (m, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3, rotamers) δ 172.6, 172.4, 171.5, 163.0 (d, J = 243.9 Hz), 155.8, 143.3 (d, J = 6.8 Hz), 137.9, 129.9, 129.8 (d, J = 8.0 Hz), 128.5, 128.4, 128.0, 127.9, 127.8, 124.7, 115.8 (d, J = 20.8 Hz), 113.0 (d, J = 20.8 Hz), 79.8, 73.2, 69.7, 60.1, 54.1, 38.5, 35.6, 31.5, 31.3, 28.4, 21.4, 20.5, 20.1, 19.6, 19.3, 18.0, 17.7, 17.2, 9.2, 8.6 ppm; 19F NMR (376 MHz, CDCl3) δ −114.0 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C35H53FN3O5+, 614.3964, found: 614.3967.Benzyl N-(tert-butoxycarbonyl)-L-valyl-L-valyl-N-methyl-L-isoleucinate (25a)
Colorless oil (1.60 g, 94%, two steps); [α]22D −88.1 (c 1.00, CHCl3); IR (film) νmax 3322, 2964, 1738, 1690, 1525, 1179, 845 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.35–7.30 (m, 5H), 6.50–6.43 (m, 1H), 5.22–5.04 (m, 4H), 4.75–4.69 (m, 1H), 3.93–3.88 (m, 1H), 3.00–2.97 (m, 3H), 2.08–1.97 (m, 2H), 1.96–1.88 (m, 1H), 1.45–1.42 (m, 9H), 1.30–1.24 (m, 1H), 1.03–0.96 (m, 1H), 0.95–0.93 (m, 3H), 0.92–0.88 (m, 6H), 0.85–0.80 (m, 9H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 172.6, 171.6, 170.8, 155.8, 135.6, 128.7, 128.7, 128.6, 79.9, 66.8, 60.5, 60.1, 54.0, 33.1, 31.5, 31.4, 31.2, 28.4, 24.9, 19.4, 19.2, 18.0, 17.8, 15.9, 10.8 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C29H48N3O6+, 534.3538, found: 534.3531.Benzyl N-(tert-butoxycarbonyl)-L-valyl-L-valyl-N-methyl-L-valinate (25b)
Colorless oil (1.53 g, 92%, two steps); [α]23D −92.0 (c 1.00, CHCl3); IR (film) νmax 3322, 2964, 1738, 1692, 1525, 1366, 1178, 845 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.35–7.30 (m, 5H), 6.53–6.40 (m, 1H), 5.24–5.18 (m, 1H), 5.08–5.02 (m, 2H), 4.98 (d, J = 10.4 Hz, 1H), 4.71 (dd, J = 8.8, 7.2 Hz, 1H), 3.94–3.88 (m, 1H), 2.97 (brs, 3H), 2.26–2.18 (m, 1H), 2.08–2.00 (m, 1H), 1.95–1.86 (m, 1H), 1.43 (s, 9H), 1.01–0.99 (m, 3H), 0.97–0.92 (m, 1H), 0.90–0.87 (m, 5H), 0.85–0.82 (m, 6H), 0.80–0.77 (m, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 172.5, 171.5, 170.5, 155.7, 135.4, 128.6, 128.5, 128.5, 79.8, 66.7, 61.5, 60.0, 53.9, 31.3, 31.3, 31.0, 28.3, 27.0, 19.8, 19.3, 19.2, 18.6, 17.8, 17.7 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C28H46N3O6+, 520.3381, found: 520.3377.Benzyl N-(tert-butoxycarbonyl)-L-valyl-L-valyl-N-methyl-L-leucinate (25c)
Colorless oil (1.54 g, 90%, two steps); [α]21D −53.6 (c 1.00, CHCl3); IR (film) νmax 3321, 2959, 1739, 1689, 1525, 1367, 1179, 833 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.32–7.29 (m, 5H), 6.53–6.46 (m, 1H), 5.42 (dd, J = 10.8, 5.2 Hz, 1H), 5.17–5.05 (m, 3H), 4.76 (dd, J = 8.4, 7.2 Hz, 1H), 3.91 (dd, J = 8.4, 6.8 Hz, 1H), 2.97–2.95 (m, 3H), 2.09–2.02 (m, 1H), 2.02–1.96 (m, 1H), 1.79–1.67 (m, 2H), 1.43 (s, 9H), 1.41–1.35 (m, 1H), 0.92–0.88 (m, 12H), 0.87–0.84 (m, 6H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 172.5, 171.5, 171.4, 155.7, 135.3, 128.6, 128.5, 79.8, 67.1, 60.0, 54.4, 53.8, 36.8, 31.3, 31.1, 31.1, 28.3, 24.7, 23.3, 21.3, 19.4, 19.1, 17.9, 17.5 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C29H48N3O6+, 534.3538, found: 534.3530.Benzyl N-(tert-butoxycarbonyl)-L-valyl-L-valyl-N-methyl-L-phenylalaninate (25d)
Colorless oil (1.58 g, 87%, two steps); [α]23D −66.2 (c 0.50, CHCl3); IR (film) νmax 3321, 2963, 1739, 1525, 1366, 1247, 696 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.36–7.33 (m, 3H), 7.31–7.28 (m, 2H), 7.25–7.20 (m, 2H), 7.19–7.14 (m, 3H), 6.41–6.36 (m, 1H), 5.48 (dd, J = 10.4, 5.6 Hz, 1H), 5.19–5.09 (m, 2H), 5.02–4.98 (m, 1H), 4.66 (dd, J = 8.8, 5.6 Hz, 1H), 3.84 (dd, J = 8.0, 6.8 Hz, 1H), 3.42 (dd, J = 14.8, 5.6 Hz, 1H), 2.98 (dd, J = 14.8, 10.4 Hz, 1H), 2.89–2.87 (m, 3H), 2.00–1.88 (m, 2H), 1.44–1.43 (m, 9H), 0.90–0.87 (m, 3H), 0.85–0.79 (m, 9H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 172.0, 171.4, 170.5, 155.8, 136.6, 135.4, 128.8, 128.7, 128.6, 128.6, 127.0, 79.9, 67.4, 60.1, 58.0, 53.6, 34.5, 32.5, 31.5, 31.1, 28.4, 19.7, 19.4, 18.0, 17.2 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C32H46N3O6+, 568.3381, found: 568.3377.General procedure for the synthesis of 20a, 20b and 20c
Compounds 19a/19b/19c (2.60 mmol) and 10% Pd/C (800 mg) were stirred in MeOH (50 mL) for 4 h under a H2 atmosphere. Then, the mixture was filtered to give a crude alcohol, which was purified by flash chromatography on silica gel (PE/EA = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give the title product (20a, 20b and 20c).
1) to give the title product (20a, 20b and 20c).
tert-Butyl ((S)-1-(((S)-1-(((2S,3R)-3-benzyl-1-hydroxypentan-2-yl)(methyl)amino)-3-methyl-1-oxobutan-2-yl)amino)-3-methyl-1-oxobutan-2-yl)carbamate (20a)
Colorless oil (1.08 g, 82%); [α]24D −11.1 (c 1.00, CHCl3); IR (film): νmax 3434, 2965, 1625, 1496, 1417, 1300, 1247, 745 cm−1; 1H NMR (400 MHz, CDCl3, rotamers) δ 7.84–7.70 (m, 0.5H), 7.38–7.24 (m, 3H), 7.23–7.16 (m, 2H), 7.15–7.08 (m, 1H), 7.07–6.87 (m, 0.5H), 5.25–5.05 (m, 1H), 4.87–4.67 (m, 1H), 4.67–4.47 (m, 1H), 4.27–4.15 (m, 0.5H), 4.15–4.02 (m, 0.5H), 4.02–3.91 (m, 1H), 3.72–3.46 (m, 1H), 3.16–2.93 (m, 3H), 2.85–2.78 (m, 0.5H), 2.75–2.70 (m, 0.5H), 2.58–2.53 (m, 0.5H), 2.51–2.33 (m, 0.5H), 2.11–1.96 (m, 3H), 1.42 (s, 9H), 1.26–1.06 (m, 2H), 1.00–0.84 (m, 12H), 0.83–0.68 (m, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3, rotamers) δ 174.0, 173.6, 172.2, 171.8, 140.6, 140.5, 129.5, 129.0, 128.7, 128.5, 126.2, 79.9, 61.8, 61.3, 59.8, 58.9, 58.7, 55.2, 54.6, 39.1, 38.7, 35.9, 35.6, 32.0, 31.6, 31.5, 31.2, 31.3, 28.4, 21.5, 19.9, 19.4, 19.3, 18.4, 18.1, 17.9, 9.1, 8.6 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C28H48N3O5+, 506.3589, found: 506.3590.tert-Butyl-((S)-1-(((S)-1-(((2S,3R)-1-hydroxy-3-(4-methoxybenzyl)pentan-2-yl)(methyl)amino)-3-methyl-1-oxobutan-2-yl)amino)-3-methyl-1-oxobutan-2-yl)carbamate (20b)
Colorless oil (1.11 g, 80%); [α]23D −13.7 (c 1.00, CHCl3); IR (film): νmax 3326, 2963, 1624, 1513, 1466, 1366, 1246, 1039 cm−1; 1H NMR (400 MHz, CDCl3, rotamers) δ 7.17–7.09 (m, 1H), 7.09–7.01 (m, 2H), 6.90–6.84 (m, 0.5H), 6.84–6.80 (m, 2H), 6.75–6.69 (m, 0.5H), 5.18–5.07 (m, 1H), 4.83–4.70 (m, 1H), 4.60–4.54 (m, 0.5H), 4.49–4.42 (m, 0.5H), 4.07–4.03 (m, 0.5H), 4.00–3.95 (m, 0.5H), 3.93–3.85 (m, 1H), 3.79 (s, 3H), 3.69–3.59 (m, 1H), 3.14–3.01 (m, 3H), 2.70–2.59 (m, 1H), 2.56–2.50 (m, 1H), 2.38–2.32 (m, 0.5H), 2.12–2.08 (m, 0.5H), 2.06–1.96 (m, 2H), 1.45–1.42 (m, 9H), 1.25–1.08 (m, 2H), 0.97–0.88 (m, 12H), 0.85–0.77 (m, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3, rotamers) δ 173.9, 173.6, 172.2, 171.8, 158.0, 155.9, 132.4, 132.3, 130.5, 129.9, 114.0, 79.9, 61.9, 61.3, 59.9, 58.7, 55.4, 55.2, 54.6, 39.2, 38.8, 34.9, 34.6, 31.7, 31.3, 31.1, 28.4, 20.5, 19.9, 19.5, 19.4, 18.0, 17.9, 9.2, 8.6 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C29H50N3O6+, 536.3694, found: 536.3695.tert-Butyl ((S)-1-(((S)-1-(((2S,3R)-3-(3-Fluorobenzyl)-1-hydroxypentan-2-yl)(methyl)amino)-3-methyl-1-oxobutan-2-yl)amino)-3-methyl-1-oxobutan-2-yl)carbamate (20c)
Colorless oil (1.13 g, 83%); [α]26D −12.4 (c 0.50, CHCl3); IR (film): νmax 3292, 2964, 1623, 1525, 1417, 1366, 1173, 779 cm−1;1H NMR (400 MHz, CDCl3, rotamers) δ 7.46–7.27 (m, 1H), 7.26–7.18 (m, 1H), 7.04–6.83 (m, 3H), 6.83–6.60 (m, 1H), 5.19–4.95 (m, 1H), 4.87–4.67 (m, 1H), 4.64–4.39 (m, 1H), 4.16–3.94 (m, 1H), 3.93–3.83 (m, 1H), 3.74–3.51 (m, 1H), 3.15–3.01 (m, 3H), 2.77–2.65 (m, 1H), 2.58–2.45 (m, 1H), 2.13–2.03 (m, 2H), 2.02–1.81 (m, 1H), 1.47–1.41 (m, 9H), 1.25–1.06 (m, 2H), 1.03–0.91(m, 12H), 0.76 (t, J = 7.2 Hz, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3, rotamers) δ 173.6, 171.8, 169.1, 163.0 (d, J = 245.4 Hz), 155.9, 143.1, 130.0 (d, J = 8.1 Hz), 124.7, 115.8 (d, J = 20.6 Hz), 113.2 (d, J = 21.0 Hz), 80.0, 62.0, 61.4, 60.0, 59.5, 54.7, 38.9, 35.7, 32.2, 31.8, 31.4, 31.1, 28.4, 21.5, 19.3, 18.0, 9.2 ppm; 19F NMR (376 MHz, CDCl3) δ −113.7 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C28H47FN3O5+, 524.3494, found: 524.3497.General procedure for the synthesis of 21a, 21b, 21c, 27a, 27b, 27c and 27d
Compounds 20a/20b/20c/26a/26b/26c/26d (1.50 mmol) in DCM (8 mL) were added to Dess–Martin periodinane (672 mg, 3.00 mmol) and stirred for 30 min at room temperature. The mixture was carefully quenched with a solution of saturated aqueous NaHCO3 and solid Na2S2O3. The resulting mixture was extracted with DCM (30 mL × 3), and the combined organic layers were washed with brine, and then dried and concentrated to give the aldehyde without further purification. To a solution of benzyl acetate (0.51 ml, 3.60 mmol) in THF was added dropwise a solution of LDA (1.73 ml, 3.45 mmol, 2 M in THF) at −78 °C and stirred for 30 min. The solution of the above aldehyde in THF (2 ml) was added dropwise, and the reaction mixture was stirred for 30 min at −78 °C. The mixture was quenched with a saturated aqueous solution of NH4Cl and extracted with EtOAc (20 mL × 3). The combined organic layers were washed with brine, dried over MgSO4, filtered and concentrated, and the residue was purified by flash chromatography on silica gel (PE/EA = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give the title product (21a, 21b, 21c, 27a, 27b, 27c and 27d).
1) to give the title product (21a, 21b, 21c, 27a, 27b, 27c and 27d).
Benzyl (6S,9S,12S,13R)-13-hydroxy-6,9-diisopropyl-2,2,11-trimethyl-4,7,10-trioxo-12-((R)-1-phenylbutan-2-yl)-3-oxa-5,8,11-triazapentadecan-15-oate (21a)
Colorless oil (519 mg, 53%, dr = 66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 34, two steps); [α]24D −10.6 (c 1.00, CHCl3); IR (film): νmax 3439, 2963, 1636, 1496, 1390, 1301, 1167, 748 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.39–7.32 (m, 5H), 7.31–7.27 (m, 1H), 7.27–7.22 (m, 2H), 7.20–7.14 (m, 3H), 6.75 (d, J = 8.0 Hz, 1H), 5.16–5.11 (m, 2H), 5.08 (d, J = 8.4 Hz, 1H), 4.78 (dd, J = 8.0, 7.2 Hz, 1H), 4.46–4.39 (m, 1H), 3.97 (dd, J = 8.0, 7.2 Hz, 1H), 3.10–3.01 (m, 3H), 2.96–2.87 (m, 1H), 2.57–2.48 (m, 3H), 2.35–2.21 (m, 1H), 2.07–1.98 (m, 2H), 1.44 (s, 9H), 1.38–1.26 (m, 2H), 1.14–1.04 (m, 1H), 1.02–0.96 (m, 3H), 0.98–0.87 (m, 9H), 0.76 (t, J = 7.2 Hz, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3,) δ 173.3, 172.3, 171.7, 155.9, 140.8, 135.7, 129.1, 128.7, 128.6, 128.5, 128.4, 126.1, 79.9, 69.5, 66.8, 60.2, 54.3, 40.3, 39.2, 36.2, 31.3, 31.2, 28.4, 22.2, 19.9, 19.4, 18.0, 17.6, 10.1 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C37H56N3O7+, 654.4113, found: 654.4114.
34, two steps); [α]24D −10.6 (c 1.00, CHCl3); IR (film): νmax 3439, 2963, 1636, 1496, 1390, 1301, 1167, 748 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.39–7.32 (m, 5H), 7.31–7.27 (m, 1H), 7.27–7.22 (m, 2H), 7.20–7.14 (m, 3H), 6.75 (d, J = 8.0 Hz, 1H), 5.16–5.11 (m, 2H), 5.08 (d, J = 8.4 Hz, 1H), 4.78 (dd, J = 8.0, 7.2 Hz, 1H), 4.46–4.39 (m, 1H), 3.97 (dd, J = 8.0, 7.2 Hz, 1H), 3.10–3.01 (m, 3H), 2.96–2.87 (m, 1H), 2.57–2.48 (m, 3H), 2.35–2.21 (m, 1H), 2.07–1.98 (m, 2H), 1.44 (s, 9H), 1.38–1.26 (m, 2H), 1.14–1.04 (m, 1H), 1.02–0.96 (m, 3H), 0.98–0.87 (m, 9H), 0.76 (t, J = 7.2 Hz, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3,) δ 173.3, 172.3, 171.7, 155.9, 140.8, 135.7, 129.1, 128.7, 128.6, 128.5, 128.4, 126.1, 79.9, 69.5, 66.8, 60.2, 54.3, 40.3, 39.2, 36.2, 31.3, 31.2, 28.4, 22.2, 19.9, 19.4, 18.0, 17.6, 10.1 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C37H56N3O7+, 654.4113, found: 654.4114.
Benzyl(6S,9S,12S,13R)-13-hydroxy-6,9-diisopropyl-12-((R)-1-(4-methoxyphenyl)butan-2-yl)-2,2,11-trimethyl-4,7,10-trioxo-3-oxa-5,8,11-triazapentadecan-15-oate (21b)
Colorless oil (625 mg, 61%, dr = 54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 46, two steps); [α]24D −10.7 (c 1.00, CHCl3); IR (film): νmax 3433, 2963, 1626, 1512, 1366, 1247, 1173, 697 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.39–7.31 (m, 5H), 7.20–7.10 (m, 1H), 7.10–7.02 (m, 2H), 6.88–6.81 (m, 1H), 6.81–6.69 (m, 2H), 5.15–5.12 (m, 2H), 5.11–5.00 (m, 1H), 4.78 (dd, J = 7.2, 6.4 Hz, 1H), 4.47–4.39 (m, 1H), 4.42–3.95 (m, 1H), 3.78 (s, 3H), 3.10–2.98 (m, 3H), 2.90–2.79 (m, 1H), 2.57–2.48 (m, 2H), 2.48–2.40 (m, 1H), 2.26–2.14 (m, 1H), 2.10–1.96 (m, 2H), 1.44 (s, 9H), 1.37–1.22 (m, 2H), 1.12–1.03 (m, 1H), 1.02–0.96 (m, 3H), 0.93–0.85 (m, 9H), 0.78–0.75 (m, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 173.4, 172.3, 171.7, 158.0, 155.8, 135.7, 132.7, 130.0, 128.7, 128.5, 128.4, 113.9, 79.9, 69.5, 66.8, 60.1, 58.6, 55.3, 54.3, 40.4, 39.1, 35.2, 31.3, 31.2, 28.4, 22.2, 19.9, 19.3, 18.6, 18.1, 17.6, 10.1 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C38H58N3O8+, 684.4218, found: 684.4219.
46, two steps); [α]24D −10.7 (c 1.00, CHCl3); IR (film): νmax 3433, 2963, 1626, 1512, 1366, 1247, 1173, 697 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.39–7.31 (m, 5H), 7.20–7.10 (m, 1H), 7.10–7.02 (m, 2H), 6.88–6.81 (m, 1H), 6.81–6.69 (m, 2H), 5.15–5.12 (m, 2H), 5.11–5.00 (m, 1H), 4.78 (dd, J = 7.2, 6.4 Hz, 1H), 4.47–4.39 (m, 1H), 4.42–3.95 (m, 1H), 3.78 (s, 3H), 3.10–2.98 (m, 3H), 2.90–2.79 (m, 1H), 2.57–2.48 (m, 2H), 2.48–2.40 (m, 1H), 2.26–2.14 (m, 1H), 2.10–1.96 (m, 2H), 1.44 (s, 9H), 1.37–1.22 (m, 2H), 1.12–1.03 (m, 1H), 1.02–0.96 (m, 3H), 0.93–0.85 (m, 9H), 0.78–0.75 (m, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 173.4, 172.3, 171.7, 158.0, 155.8, 135.7, 132.7, 130.0, 128.7, 128.5, 128.4, 113.9, 79.9, 69.5, 66.8, 60.1, 58.6, 55.3, 54.3, 40.4, 39.1, 35.2, 31.3, 31.2, 28.4, 22.2, 19.9, 19.3, 18.6, 18.1, 17.6, 10.1 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C38H58N3O8+, 684.4218, found: 684.4219.
Benzyl(6S,9S,12S,13R)-12-((R)-1-(3-fluorophenyl)butan-2-yl)-13-hydroxy-6,9-diisopropyl-2,2,11-trimethyl-4,7,10-trioxo-3-oxa-5,8,11-triazapentadecan-15-oate (21c)
Colorless oil (725 mg, 72%, dr = 72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28, two steps); [α]26D −11.8 (c 0.50, CHCl3); IR (film): νmax 3323, 2963, 1626, 1524, 1386, 1169, 773 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.42–7.32 (m, 5H), 7.25–7.17 (m, 1H), 6.99–6.95 (m, 1H), 6.94–6.82 (m, 2H), 6.61 (d, J = 8.8 Hz, 1H), 5.18–5.11 (m, 2H), 5.05 (d, J = 8.4 Hz, 1H), 4.77 (dd, J = 7.2, 6.4 Hz, 1H), 4.42–4.32 (m, 1H), 3.99–3.88 (m, 1H), 3.09–3.01 (m, 3H), 2.98–2.87 (m, 1H), 2.56–2.51 (m, 2H), 2.51–2.44 (m, 1H), 2.37–2.20 (m, 1H), 2.07–2.00 (m, 2H), 1.41 (s, 9H), 1.39–1.27 (m, 2H), 1.16–1.04 (m, 1H), 1.01–0.94 (m, 3H), 0.94–0.83 (m, 9H), 0.77 (t, J = 7.2 Hz, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 173.2, 172.4, 171.7, 162.5 (d, J = 243.7 Hz), 155.9, 135.6, 129.8 (d, J = 8.3 Hz), 128.7, 128.6, 128.5, 124.8, 115.9 (d, J = 20.7 Hz), 113.0 (d, J = 20.8 Hz), 80.0, 69.5, 66.9, 60.2, 54.3, 40.4, 39.3, 36.0, 31.3, 31.1, 28.4, 22.2, 20.0, 19.4, 18.0, 17.5, 10.2 ppm; 19F NMR (376 MHz, CDCl3) δ −113.8 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C37H55FN3O7+, 672.4019, found: 672.4017.
28, two steps); [α]26D −11.8 (c 0.50, CHCl3); IR (film): νmax 3323, 2963, 1626, 1524, 1386, 1169, 773 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.42–7.32 (m, 5H), 7.25–7.17 (m, 1H), 6.99–6.95 (m, 1H), 6.94–6.82 (m, 2H), 6.61 (d, J = 8.8 Hz, 1H), 5.18–5.11 (m, 2H), 5.05 (d, J = 8.4 Hz, 1H), 4.77 (dd, J = 7.2, 6.4 Hz, 1H), 4.42–4.32 (m, 1H), 3.99–3.88 (m, 1H), 3.09–3.01 (m, 3H), 2.98–2.87 (m, 1H), 2.56–2.51 (m, 2H), 2.51–2.44 (m, 1H), 2.37–2.20 (m, 1H), 2.07–2.00 (m, 2H), 1.41 (s, 9H), 1.39–1.27 (m, 2H), 1.16–1.04 (m, 1H), 1.01–0.94 (m, 3H), 0.94–0.83 (m, 9H), 0.77 (t, J = 7.2 Hz, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 173.2, 172.4, 171.7, 162.5 (d, J = 243.7 Hz), 155.9, 135.6, 129.8 (d, J = 8.3 Hz), 128.7, 128.6, 128.5, 124.8, 115.9 (d, J = 20.7 Hz), 113.0 (d, J = 20.8 Hz), 80.0, 69.5, 66.9, 60.2, 54.3, 40.4, 39.3, 36.0, 31.3, 31.1, 28.4, 22.2, 20.0, 19.4, 18.0, 17.5, 10.2 ppm; 19F NMR (376 MHz, CDCl3) δ −113.8 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C37H55FN3O7+, 672.4019, found: 672.4017.
Benzyl(6S,9S,12S,13R)-12-((S)-sec-butyl)-13-hydroxy-6,9-diisopropyl-2,2,11-trimethyl-4,7,10-trioxo-3-oxa-5,8,11-triazapentadecan-15-oate (27a)
Colorless oil (545 mg, 63%, dr = 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25, two steps); [α]23D −21.4 (c 1.00, CHCl3); IR (film) νmax 3302, 2964, 1693, 1525, 1417, 1297, 870 cm−1; 1H NMR (400 MHz, CDCl3, rotamers) δ 7.37–7.32 (m, 5H), 7.34–7.30 (m, 1H), 6.87–6.71 (m, 1H), 5.17–5.12 (m, 2H), 5.10–5.05 (m, 1H), 4.77 (d, J = 9.2, 7.2 Hz, 1H), 4.40–4.32 (m, 1H), 4.03–3.94 (m, 1H), 3.73–3.48 (m, 1H), 3.04–3.02 (m, 2.7H), 2.79–2.78 (m, 0.3H), 2.58–2.52 (m, 2H), 2.07–1.99 (m, 2H), 1.96–1.85 (m, 1H), 1.44–1.42 (m, 9H), 1.42–1.36 (m, 2H), 1.00–0.97 (m, 6H), 0.92–0.88 (m, 9H), 0.83 (t, J = 7.4 Hz, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3, rotamers) δ 173.6, 172.3, 171.7, 155.8, 135.7, 128.7, 128.5, 128.5, 128.4, 79.9, 69.8, 66.8, 60.0, 54.3, 39.2, 33.6, 31.4, 31.3, 28.4, 26.1, 19.8, 19.3, 18.1, 18.0, 17.7, 17.5, 16.2, 11.1 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C31H52N3O7+, 578.3800, found: 578.3796.
25, two steps); [α]23D −21.4 (c 1.00, CHCl3); IR (film) νmax 3302, 2964, 1693, 1525, 1417, 1297, 870 cm−1; 1H NMR (400 MHz, CDCl3, rotamers) δ 7.37–7.32 (m, 5H), 7.34–7.30 (m, 1H), 6.87–6.71 (m, 1H), 5.17–5.12 (m, 2H), 5.10–5.05 (m, 1H), 4.77 (d, J = 9.2, 7.2 Hz, 1H), 4.40–4.32 (m, 1H), 4.03–3.94 (m, 1H), 3.73–3.48 (m, 1H), 3.04–3.02 (m, 2.7H), 2.79–2.78 (m, 0.3H), 2.58–2.52 (m, 2H), 2.07–1.99 (m, 2H), 1.96–1.85 (m, 1H), 1.44–1.42 (m, 9H), 1.42–1.36 (m, 2H), 1.00–0.97 (m, 6H), 0.92–0.88 (m, 9H), 0.83 (t, J = 7.4 Hz, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3, rotamers) δ 173.6, 172.3, 171.7, 155.8, 135.7, 128.7, 128.5, 128.5, 128.4, 79.9, 69.8, 66.8, 60.0, 54.3, 39.2, 33.6, 31.4, 31.3, 28.4, 26.1, 19.8, 19.3, 18.1, 18.0, 17.7, 17.5, 16.2, 11.1 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C31H52N3O7+, 578.3800, found: 578.3796.
Benzyl (6S,9S,12S,13R)-13-hydroxy-6,9,12-triisopropyl-2,2,11-trimethyl-4,7,10-trioxo-3-oxa-5,8,11-triazapentadecan-15-oate (27b)
Colorless oil (481 mg, 57%, dr = 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, two steps); [α]23D +4.0 (c 0.50, CHCl3); IR (film) νmax 2971, 2920, 1668, 1396, 1202, 1135, 936, 870 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.39–7.34 (m, 5H), 7.34–7.31 (m, 1H), 5.21–5.13 (m, 3H), 4.79 (dd, J = 8.8, 7.2 Hz, 1H), 4.36 (dd, J = 12.0, 6.4 Hz, 1H), 4.10–3.95 (m, 1H), 3.08–3.03 (m, 3H), 2.59–2.50 (m, 2H), 2.25–2.11 (m, 1H), 2.20–1.98 (m, 3H), 1.45–1.43 (m, 9H), 1.04–1.01 (m, 3H), 1.00–0.98 (m, 3H), 0.95–0.92 (m, 3H), 0.91–0.88 (m, 6H), 0.86–0.83 (m, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 173.7, 172.2, 155.9, 135.7, 128.7, 128.5, 128.5, 128.4, 80.0, 69.8, 66.8, 60.1, 54.5, 39.3, 31.3, 31.2, 28.4, 27.4, 20.5, 20.4, 19.7, 19.3, 18.2, 17.8 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C30H50N3O7+, 564.3643, found: 564.3646.
30, two steps); [α]23D +4.0 (c 0.50, CHCl3); IR (film) νmax 2971, 2920, 1668, 1396, 1202, 1135, 936, 870 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.39–7.34 (m, 5H), 7.34–7.31 (m, 1H), 5.21–5.13 (m, 3H), 4.79 (dd, J = 8.8, 7.2 Hz, 1H), 4.36 (dd, J = 12.0, 6.4 Hz, 1H), 4.10–3.95 (m, 1H), 3.08–3.03 (m, 3H), 2.59–2.50 (m, 2H), 2.25–2.11 (m, 1H), 2.20–1.98 (m, 3H), 1.45–1.43 (m, 9H), 1.04–1.01 (m, 3H), 1.00–0.98 (m, 3H), 0.95–0.92 (m, 3H), 0.91–0.88 (m, 6H), 0.86–0.83 (m, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 173.7, 172.2, 155.9, 135.7, 128.7, 128.5, 128.5, 128.4, 80.0, 69.8, 66.8, 60.1, 54.5, 39.3, 31.3, 31.2, 28.4, 27.4, 20.5, 20.4, 19.7, 19.3, 18.2, 17.8 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C30H50N3O7+, 564.3643, found: 564.3646.
Benzyl(6S,9S,12S,13R)-13-hydroxy-12-isobutyl-6,9-diisopropyl-2,2,11-trimethyl-4,7,10-trioxo-3-oxa-5,8,11-triazapentadecan-15-oate (27c)
Colorless oil (563 mg, 65%, two steps); [α]23D −31.6 (c 1.00, CHCl3); IR (film) νmax 3319, 2961, 1690, 1525, 1417, 1172, 771 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.39–7.30 (m, 5H), 6.69–6.58 (m, 1H), 5.15–5.11 (m, 2H), 5.10–5.04 (m, 1H), 4.87–4.74 (m, 2H), 4.09–4.01 (m, 1H), 3.96–3.91 (m, 1H), 3.08–3.04 (m, 2H), 3.00–2.99 (m, 1H), 2.53–2.49 (m, 1H), 2.12–2.00 (m, 3H), 1.78–1.58 (m, 2H), 1.44–1.43 (m, 9H), 1.40–1.34 (m, 1H), 1.03–0.99 (m, 2H), 0.98–0.95 (m, 3H), 0.94–0.93 (m, 2H), 0.91–0.88 (m, 9H), 0.84–0.81 (m, 2H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 199.1, 198.9, 173.3, 172.8, 172.7, 172.6, 171.8, 171.6, 155.8, 135.6, 128.7, 128.5, 128.5, 128.4, 80.0, 79.9, 70.4, 66.8, 63.3, 60.1, 54.2, 54.0, 38.9, 37.4, 35.1, 34.5, 33.3, 31.5, 31.4, 31.3, 31.3, 31.1, 28.4, 25.0, 24.9, 24.0, 23.4, 21.6, 19.9, 19.8, 19.6, 19.3, 18.0, 17.8, 17.6, 17.5 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C31H52N3O7+, 578.3800, found: 578.3795.Benzyl(6S,9S,12S,13R)-12-benzyl-13-hydroxy-6,9-diisopropyl-2,2,11-trimethyl-4,7,10-trioxo-3-oxa-5,8,11-triazapentadecan-15-oate (27d)
Colorless oil (651 mg, 71%, two steps); [α]23D −32.8 (c 1.00, CHCl3); IR (film) νmax 3333, 2959, 1633, 1533, 1180, 937, 844 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.39–7.33 (m, 5H), 7.26–7.10 (m, 5H), 6.61–6.48 (m, 1H), 5.18–5.09 (m, 2H), 5.08–5.02 (m, 1H), 4.63–4.54 (m, 1H), 4.32–4.10 (m, 2H), 3.94–3.83 (m, 1H), 3.34–3.12 (m, 1H), 3.08–2.96 (m, 1H), 2.94–2.74 (m, 3H), 2.57–2.42 (m, 2H), 2.03–1.95 (m, 1H), 1.91–1.81 (m, 1H), 1.45–1.40 (m, 9H), 0.92–0.88 (m, 3H), 0.87–0.78 (m, 9H), 0.48–0.34 (m, 1H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 172.7, 172.7, 172.6, 172.3, 171.6, 171.5, 155.8, 138.3, 138.2, 135.6, 135.5, 129.4, 128.9, 128.9, 128.7, 128.7, 128.6, 128.5, 128.4, 128.4, 127.7, 127.1, 126.9, 126.6, 79.9, 70.1, 69.6, 66.9, 66.8, 63.6, 60.2, 60.0, 54.0, 53.1, 39.0, 34.1, 32.6, 31.1, 30.7, 30.4, 29.2, 28.4, 20.2, 19.3, 18.0, 17.9, 17.0, 16.7 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C34H50N3O7+, 612.3643, found: 612.3645.General procedure for the synthesis of 23a, 23b, 23c and 23d
Compounds 22a/22b/22c/22d (20.00 mmol) were stirred in H2O/dioxane (35 ml/30 ml), then NaOH (1.60 g, 40.00 mmol) and Boc2O (4.80 g, 22.00 mmol) were added and the mixture was stirred overnight at room temperature. The reaction mixture was quenched with a saturated aqueous solution of NH4Cl and extracted with EtOAc (50 mL × 1). The water phase was acidified with hydrochloric acid to pH = 2–3 and the resulting mixture was extracted with EtOAc (100 mL × 3). The combined organic layers were washed with brine, dried over MgSO4, filtered and concentrated to give a crude acid without further purification. To a cooled (0 °C) solution of the above crude acid in THF (70 mL) was added NaH (1.23 g, 51.30 mmol) three portions and stirred for 30 min. MeI (4.36 mL, 70.00 mmol) was slowly added dropwise and stirred for 48 h. The mixture was quenched with a saturated aqueous solution of NH4Cl and extracted with EtOAc (50 mL × 3). The combined organic layers were washed with brine, dried over MgSO4, filtered and concentrated to give a crude acid without further purification. A solution of the above acid in DMSO (70 mL) was treated with K2CO3 (4.24 g, 40.00 mmol) and BnBr (2.37 mL, 20.00 mmol) overnight. The reaction mixture was quenched with a saturated aqueous solution of NH4Cl and extracted with EtOAc (100 mL × 3). The combined organic layers were washed with water and brine, dried over MgSO4, filtered and concentrated, and the residue was purified by flash chromatography on silica gel (PE/EA = 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give the desired product (23a, 23b, 23c and 23d).
1) to give the desired product (23a, 23b, 23c and 23d).
Benzyl N-(tert-butoxycarbonyl)-N-methyl-L-isoleucinate (23a)13d
Colorless oil (2.68 g, 40%, three steps).Benzyl N-(tert-butoxycarbonyl)-N-methyl-L-valinate (23b)
Colorless oil (2.70 g, 42%, three steps); [α]23D −67.3 (c 1.00, CHCl3); IR (film) νmax 2967, 1739, 1455, 1392, 1258, 1144, 774 cm−1; 1H NMR (400 MHz, CDCl3, rotamers) δ 7.38–7.28 (m, 5 H), 5.19–5.11 (m, 2H), 4.51 (d, J = 10.4 Hz, 0.5 H), 4.15 (d, J = 10.4 Hz, 0.5 H), 2.85–2.76 (m, 3H), 2.25–2.13 (m, 1H), 1.46–1.41 (m, 9H), 0.97–0.94 (m, 3H), 0.91–0.87 (m, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3, rotamers) δ 171.5, 171.1, 156.4, 155.8, 136.0, 135.8, 128.7, 128.6, 128.4, 128.2, 128.1, 80.4, 80.1, 66.5, 66.3, 65.2, 63.4, 30.7, 30.6, 28.5, 27.9, 27.7, 20.1, 19.9, 19.2, 18.9 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C18H28NO4+, 322.2013, found: 322.2009.Benzyl N-(tert-butoxycarbonyl)-N-methyl-L-leucinate (23c)
Colorless oil (2.75 g, 41%, three steps); [α]21D −29.3 (c 1.00, CHCl3); IR (film) νmax 2959, 1698, 1455, 1391, 1367, 1151, 972, 848 cm−1; 1H NMR (400 MHz, CDCl3, rotamers) δ 7.37–7.31 (m, 5H), 5.16–5.13 (m, 2H), 4.93 (dd, J = 8.8, 7.2 Hz, 0.5H), 4.63 (dd, J = 10.8, 4.8 Hz, 0.5H), 2.80–2.74 (m, 3H), 1.78–1.67 (m, 2H), 1.60–1.51 (m, 1H), 1.46–1.40 (m, 9H), 0.96–0.91 (m, 6H) ppm; 13C{1H} NMR (CDCl3, 100 MHz, rotamers) δ 172.5, 172.3, 156.4, 155.8, 136.0, 135.8, 128.7, 128.6, 128.4, 128.3, 128.1, 128.0, 80.4, 80.0, 66.7, 66.6, 57.4, 56.2, 38.0, 37.5, 30.6, 30.5, 28.5, 28.4, 25.0, 24.7, 23.4, 23.4, 21.4, 21.3 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C19H30NO4+, 336.2169, found: 336.2162.Benzyl N-(tert-butoxycarbonyl)-N-methyl-L-phenylalaninate (23d)
Colorless oil (2.95 g, 40%, three steps); [α]23D −47.5 (c 1.00, CHCl3); IR (film) νmax 1743, 1693, 1455, 1142, 849, 696 cm−1; 1H NMR (400 MHz, CDCl3, rotamers) δ 7.36–7.31 (m, 5H), 7.29–7.26 (m, 1.5H), 7.24–7.15 (m, 3.5H), 5.22–5.12 (m, 2H), 4.95 (dd, J = 10.4, 5.2 Hz, 0.5H), 4.62 (dd, J = 10.8, 4.4 Hz, 0.5H), 3.37–3.26 (m, 1H), 3.08–2.99 (m, 1H), 2.72–2.67 (m, 3H), 1.37–1.29 (m, 9H) ppm; 13C{1H} NMR (100 MHz, CDCl3, rotamers) δ 171.4, 171.1, 155.9, 155.1, 137.7, 137.5, 135.8, 135.6, 129.1, 129.1, 128.7, 128.7, 128.5, 128.3, 128.2, 126.8, 126.6, 80.4, 80.1, 67.0, 66.9, 61.7, 59.9, 35.6, 35.1, 32.6, 32.3, 28.4, 28.3 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C22H28NO4+, 370.2013, found: 370.2007.General procedure for the synthesis of 26a, 26b, 26c and 26d
To a solution of 25a/25b/25c/25d (2.70 mmol) in THF (11 ml) was added dropwise LiHBEt3 (8.10 ml, 8.10 mmol, 1 M in THF) at 0 °C before gradually warming to room temperature, and the reaction mixture was stirred overnight. The reaction mixture was quenched with a saturated aqueous solution of NH4Cl and extracted with EtOAc (20 mL × 3). The combined organic layers were washed with brine, dried over MgSO4, filtered and concentrated. The residue was purified by flash chromatography on silica gel (PE/EA = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give the title product (26a, 26b, 26c and 26d).
1) to give the title product (26a, 26b, 26c and 26d).
tert-Butyl-((S)-1-(((S)-1-(((2S,3S)-1-hydroxy-3-methylpentan-2-yl)(methyl)amino)-3-methyl-1-oxobutan-2-yl)amino)-3-methyl-1-oxobutan-2-yl)carbamate (26a)
Colorless oil (1.04 g, 90%); [α]23D −30.6 (c 0.50, CHCl3); IR (film) νmax 3290, 2964, 1693, 1366, 1175, 1016, 870 cm−1; 1H NMR (400 MHz, CDCl3, rotamers) δ 7.49–7.44 (m, 0.75H), 6.77–6.73 (m, 0.25H), 5.15–5.12 (m, 0.75H), 5.01–4.97 (m, 0.25H), 4.83–4.77 (m, 1H), 4.36–4.28 (m, 1H), 4.17–4.11 (m, 1H), 3.97–3.82 (m, 2H), 3.61–3.50 (m, 1H), 3.02–3.01 (m, 2.25H), 2.94–2.87 (m, 1H), 2.78–2.77 (m, 0.75H), 2.09–2.01 (m, 2H), 1.69–1.62 (m, 1H), 1.59–1.47 (m, 1H), 1.44–1.42 (m, 9H), 1.02–1.00 (m, 3H), 0.94–0.91 (m, 12H), 0.80 (t, J = 7.4 Hz, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3, rotamers) δ 173.7, 171.8, 155.9, 79.9, 64.5, 61.5, 61.3, 60.3, 59.8, 54.6, 54.2, 53.6, 35.4, 32.8, 31.9, 31.7, 31.4, 30.9, 28.4, 27.6, 25.7, 20.3, 19.4, 19.3, 19.2, 18.4, 18.2, 18.1, 16.3, 15.9, 11.9, 10.5 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C22H44N3O5+, 430.3276, found: 430.3273.tert-Butyl-((S)-1-(((S)-1-(((S)-1-hydroxy-3-methylbutan-2-yl)(methyl)amino)-3-methyl-1-oxobutan-2-yl)amino)-3-methyl-1-oxobutan-2-yl)carbamate (26b)
Colorless oil (1.01 g, 90%); [α]20D −37.9 (c 1.00, CHCl3); IR (film) νmax 3302, 2964, 1693, 1533, 1367, 1247, 844 cm−1; 1H NMR (400 MHz, CDCl3, rotamers) δ 7.74–7.65 (m, 0.76H), 7.10–7.03 (m, 0.24H), 5.23–5.02 (m, 1H), 4.84–4.74 (m, 1H), 4.36–4.28 (m, 0.76H), 4.25–4.19 (m, 0.76H), 4.02–3.96 (m, 0.24H), 3.91–3.84 (m, 1H), 3.75–3.68 (m, 0.24H), 3.59–3.40 (m, 1H), 3.24–3.10 (m, 1H), 3.04–3.02 (m, 2.24H), 2.80–2.79 (m, 0.76H), 2.16–2.06 (m, 0.76H), 2.03–1.94 (m, 1.24H), 1.86–1.76 (m, 1H), 1.44–1.41 (m, 9H), 1.04–1.01 (m, 3H), 0.98–0.90 (m, 13H), 0.81–0.79 (m, 2H) ppm; 13C{1H} NMR (100 MHz, CDCl3, rotamers) δ 173.8, 173.2, 172.8, 171.9, 155.9, 80.1, 79.8, 65.4, 62.8, 61.3, 60.5, 60.2, 59.7, 54.6, 54.2, 32.0, 31.7, 31.5, 31.0, 30.9, 28.6, 28.4, 27.5, 27.0, 21.2, 21.0, 20.3, 20.1, 20.0, 19.3, 19.2, 19.2, 18.5, 18.2, 18.1 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C21H42N3O5+, 416.3119, found: 416.3120.tert-Butyl-((S)-1-(((S)-1-(((S)-1-hydroxy-4-methylpentan-2-yl)(methyl)amino)-3-methyl-1-oxobutan-2-yl)amino)-3-methyl-1-oxobutan-2-yl)carbamate (26c)
Colorless oil (695 mg, 60%); [α]22D −24.5 (c 1.00, CHCl3); IR (film) νmax 3293, 2959, 1696, 1535, 1468, 1366, 1173, 757 cm−1; 1H NMR (400 MHz, CDCl3, rotamers) δ 7.65–7.57 (m, 0.73H), 6.90–6.82 (m, 0.27H), 5.20–5.15 (m, 0.73H), 5.07–5.01 (m, 0.27H), 4.88–4.70 (m, 2H), 4.27–4.12 (m, 1H), 4.00–3.84 (m, 0.27H), 3.68–3.56 (m, 1H), 3.54–3.41 (m, 1H), 3.25–3.03 (m, 0.73H), 2.98–2.97 (m, 2.27H), 2.79–2.78 (m, 0.73H), 2.11–2.05 (m, 0.73H), 2.04–1.96 (m, 1.27H), 1.93–1.74 (m, 0.73H), 1.62–1.56 (m, 0.27H), 1.44–1.41 (m, 9H), 1.39–1.36 (m, 1H), 1.21–1.11 (m, 1H), 1.02–1.00 (m, 2H), 0.99–0.95 (m, 3H), 0.95–0.93 (m, 3H), 0.92–0.87 (m, 7H), 0.87–0.84 (m, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3, rotamers) δ 173.6, 172.8, 172.7, 171.7, 155.8, 80.0, 79.7, 63.1, 62.4, 60.0, 59.6, 57.2, 54.5, 54.3, 54.0, 39.1, 36.6, 31.9, 31.7, 31.3, 30.9, 29.7, 28.3, 26.9, 24.9, 24.8, 23.5, 23.3, 22.8, 22.0, 19.7, 19.2, 19.0, 18.4, 18.2, 18.1, 17.9 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C22H44N3O5+, 430.3276, found: 430.3277.tert-Butyl-((S)-1-(((S)-1-(((S)-1-hydroxy-3-phenylpropan-2-yl)(methyl)amino)-3-methyl-1-oxobutan-2-yl)amino)-3-methyl-1-oxobutan-2-yl)carbamate (26d)
Colorless oil (738 mg, 59%); [α]23D −57.8 (c 1.00, CHCl3); IR (film) νmax 3310, 2965, 1686, 1523, 1366, 1247, 760 cm−1; 1H NMR (400 MHz, CDCl3, rotamers) δ 7.32–7.27 (m, 1H), 7.25–7.22 (m, 1H), 7.21–7.15 (m, 3H), 7.01–6.95 (m, 0.5H), 6.77–6.72 (m, 0.5H), 5.13–5.08 (m, 0.5H), 5.05–5.02 (m, 0.5H), 4.72–4.64 (m, 1H), 4.51–4.46 (m, 0.5H), 4.43–4.36 (m, 0.5H), 4.06–3.99 (m, 0.5H), 3.95–3.88 (m, 0.5H), 3.73–3.69 (m, 1.5H), 3.61–3.52 (m, 0.5H), 3.16–3.03 (m, 1H), 2.97–2.96 (m, 1.5H), 2.91–2.90 (m, 1.5H), 2.89–2.83 (m, 1H), 2.81–2.77 (m, 1H), 2.03–1.95 (m, 1H), 1.68–1.58 (m, 1H), 1.44–1.41 (m, 9H), 0.97–0.94 (m, 1.5H), 0.92–0.89 (m, 4.5H), 0.88–0.86 (m, 3H), 0.78–0.75 (m, 1.5H), 0.48–0.45 (m, 1.5H) ppm; 13C{1H} NMR (100 MHz, CDCl3, rotamers) δ 173.0, 172.8, 172.8, 171.7, 155.9, 137.8, 137.7, 129.2, 129.0, 128.9, 128.6, 127.1, 126.7, 80.2, 79.9, 62.7, 62.4, 61.3, 60.2, 59.9, 59.7, 54.6, 54.2, 36.0, 34.5, 32.7, 31.6, 31.3, 30.9, 30.8, 28.4, 28.4, 19.7, 19.4, 19.3, 19.2, 18.2, 18.0, 17.8 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C25H42N3O5+, 464.3119, found: 464.3117.General procedure for the synthesis of 28a, 28b, 28c, 28d, 28e, 28f and 28g
Compounds 21a/21b/21c/27a/27b/27c/27d (0.49 mmol) and HMPA (94 μL, 0.54 mmol) were stirred in THF (2 mL) at −78 °C, then a solution of LiHMDS (0.49 ml, 0.49 mmol, 1 M in THF) was slowly added dropwise and stirred for 30 min. The reaction mixture was warmed to −15 °C and MeOTf (111 μL, 0.98 mmol) was added and stirred for an additional 15 min. The reaction mixture was quenched with a saturated aqueous solution of NH4Cl and extracted with EtOAc (20 mL × 3). The combined organic layers were washed with brine, dried over MgSO4, filtered and concentrated, and the residue was purified by flash chromatography on silica gel (PE/EA = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give the title product (28a, 28b, 28c, 28d, 28e, 28f and 28g).
1) to give the title product (28a, 28b, 28c, 28d, 28e, 28f and 28g).
Benzyl-(6S,9S,12S,13R)-6,9,12-triisopropyl-13-methoxy-2,2,11-trimethyl-4,7,10-trioxo-3-oxa-5,8,11-triazapentadecan-15-oate (28a)
Colorless oil (156 mg, 55%); [α]23D −40.0 (c 0.50, CHCl3); IR (film) νmax 3288, 2964, 1739, 1525, 1417, 1390, 1170, 698 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.38–7.33 (m, 5H), 6.56–6.49 (m, 1H), 5.20–5.08 (m, 3H), 4.78–4.64 (m, 2H), 3.97–3.90 (m, 2H), 3.33–3.29 (m, 3H), 2.99–2.76 (m, 3H), 2.60–2.44 (m, 2H), 2.07–1.99 (m, 2H), 1.43 (s, 9H), 1.01–0.97 (m, 6H), 0.93–0.88 (m, 9H), 0.83–0.79 (m, 3H) ppm; 13C{1H} NMR (150 MHz, CDCl3) δ 172.4, 170.9, 170.9, 155.1, 135.1, 127.9, 127.8, 127.7, 79.1, 77.8, 77.3, 66.1, 66.0, 63.8, 59.3, 57.8, 57.6, 57.3, 53.5, 52.8, 36.6, 36.1, 31.0, 30.6, 30.6, 30.5, 27.7, 26.3, 20.2, 19.8, 19.4, 19.3, 18.9, 18.5, 17.2, 17.0, 15.9 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C31H52N3O7+, 578.3800, found: 578.3799.Benzyl-(6S,9S,12S,13R)-12-((S)-sec-butyl)-6,9-diisopropyl-13-methoxy-2,2,11-trimethyl-4,7,10-trioxo-3-oxa-5,8,11-triazapentadecan-15-oate (28b)13d
Colorless oil (153 mg, 53%).Benzyl-(6S,9S,12S,13R)-6,9-diisopropyl-13-methoxy-2,2,11-trimethyl-4,7,10-trioxo-12-((R)-1-phenylbutan-2-yl)-3-oxa-5,8,11-triazapentadecan-15-oate (28c)
Colorless oil (252 mg, 77%); [α]23D −9.7 (c 1.00, CHCl3); IR (film): νmax 3322, 2964, 1626, 1455, 1390, 1367, 1169, 752 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.39–7.32 (m, 5H), 7.28–7.25 (m, 2H), 7.25–7.22 (m, 1H), 7.20–7.13 (m, 3H), 6.49 (d, J = 8.8 Hz, 1H), 5.18–5.08 (m, 2H), 5.08–5.05 (m, 1H), 5.02–4.90 (m, 1H), 4.80–4.72 (m, 1H), 4.12–3.97 (m, 1H), 3.97–3.85 (m, 1H), 3.35–3.32 (m, 3H), 3.02–2.96 (m, 3H), 2.80–2.72 (m, 1H), 2.61–2.42 (m, 3H), 2.09–1.95 (m, 3H), 1.44 (s, 9H), 1.16–1.02 (m, 1H), 1.02–0.95 (m, 3H), 0.92–0.85 (m, 9H), 0.76 (t, J = 7.0 Hz, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3,) δ 172.8, 171.6, 171.5, 155.8, 140.5, 135.8, 129.1, 128.7, 128.5, 126.2, 79.9, 78.0, 66.8, 60.1, 58.0, 54.2, 39.9, 37.4, 35.9, 31.3, 31.2, 28.4, 22.1, 19.8, 19.3, 18.0, 17.5, 9.8 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C38H58N3O7+, 668.4269, found: 668.4268.Benzyl (6S,9S,12S,13R)-6,9-diisopropyl-13-methoxy-12-(1-(4-methoxyphenyl)butan-2-yl)-2,2,11-trimethyl-4,7,10-trioxo-3-oxa-5,8,11-triazapentadecan-15-oate (28d)
Colorless oil (249 mg, 73%); [α]24D −7.5 (c 1.00, CHCl3); IR (film): νmax 3322, 2961, 1636, 1512, 1455, 1247, 1095, 751 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.41–7.29 (m, 5H), 7.09–7.04 (m, 2H), 6.83–6.77 (m, 2H), 6.48 (d, J = 8.4 Hz, 1H), 5.19–5.08 (m, 2H), 5.08–5.03 (m, 1H), 5.01–4.85 (m, 1H), 4.76 (dd, J = 7.6, 6.8 Hz, 1H), 4.08–3.94 (m, 1H), 3.91 (dd, J = 7.6, 6.8 Hz, 1H), 3.78 (s, 3H), 3.34–3.31 (m, 3H), 2.99–2.96 (m, 3H), 2.72–2.63 (m, 1H), 2.60–2.41 (m, 3H), 2.08–1.94(m, 3H), 1.43 (s, 9H), 1.30–1.02 (m, 1H), 1.02–0.96 (m, 3H), 0.93–0.85 (m, 9H), 0.76 (t, J = 7.2 Hz, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 172.8, 171.5, 158.1, 155.8, 135.8, 132.4, 130.0, 128.7, 128.5, 113.9, 79.9, 78.0, 66.8, 60.2, 58.0, 55.4, 54.2, 40.1, 37.4, 34.9, 31.3, 28.5, 22.0, 19.8, 19.3, 18.0, 17.5, 9.8 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C39H60N3O8+, 698.4375, found: 698.4376.Benzyl (6S,9S,12S,13R)-12-((R)-1-(3-fluorophenyl)butan-2-yl)-6,9-diisopropyl-13-methoxy-2,2,11-trimethyl-4,7,10-trioxo-3-oxa-5,8,11-triazapentadecan-15-oate (28e)
Colorless oil (225 mg, 67%); [α]26D −14.4 (c 1.00, CHCl3); IR (film): νmax 3314, 2963, 1635, 1488, 1390, 1249, 1168, 1095 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.42–7.28 (m, 5H), 7.21 (dd, J = 14.0, 6.8 Hz, 1H), 6.94 (d, J = 7.6 Hz, 1H), 6.90–6.83 (m, 2H), 6.50 (d, J = 8.8 Hz, 1H), 5.20–5.09 (m, 2H), 5.08–5.03 (m, 1H), 4.99–4.84 (m, 1H), 4.76 (dd, J = 7.2, 6.4 Hz, 1H), 4.06–3.95 (m, 1H), 3.92 (dd, J = 8.0, 7.2 Hz, 1H), 4.06–3.96 (m, 1H), 3.34–3.32 (m, 3H), 3.01–2.98 (m, 3H), 2.79–2.71 (m, 1H), 2.59–2.46 (m, 3H), 2.08–1.97 (m, 3H), 1.43 (s, 9H), 1.16–1.02 (m, 1H), 1.00–0.94 (m, 3H), 0.90–0.87 (m, 9H), 0.76 (t, J = 7.2 Hz, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 172.9, 171.6, 171.5, 163.0 (d, J = 243.8 Hz), 155.8, 143.2, 135.8, 129.9 (d, J = 8.4 Hz), 128.7, 128.5, 124.8, 115.9 (d, J = 20.7 Hz), 113.1 (d, J = 20.9 Hz), 79.9, 78.0, 66.8, 60.2, 58.0, 54.2, 40.0, 37.6, 35.8, 31.3, 31.2, 28.4, 22.1, 19.8, 19.3, 18.0, 17.5, 10.0 ppm; 19F NMR (376 MHz, CDCl3) δ −113.6 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C38H57FN3O7+, 686.4175, found: 686.4179.Benzyl(6S,9S,12S,13R)-12-isobutyl-6,9-diisopropyl-13-methoxy-2,2,11-trimethyl-4,7,10-trioxo-3-oxa-5,8,11-triazapentadecan-15-oate (28f)
Colorless oil (145 mg, 50%); [α]23D −74.8 (c 0.25, CHCl3); IR (film) νmax 3319, 2960, 1690, 1525, 1416, 1247, 1173, 770 cm−1; 1H NMR (400 MHz, CDCl3, rotamers) δ 7.37–7.33 (m, 5H), 6.52–6.45 (m, 1H), 5.17–5.09 (m, 2H), 5.10–5.04 (m, 1H), 4.78–4.73 (m, 1H), 3.98–3.84 (m, 1.33H), 3.70–3.55 (m, 1H), 3.45–3.36 (m, 0.67H), 3.33–3.31 (m, 3H), 2.97–2.94 (m, 3H), 2.58–2.40 (m, 2H), 2.12–1.90 (m, 3H), 1.44 (s, 9H), 1.39–1.34 (m, 1H), 0.97–0.94 (m, 3H), 0.91–0.88 (m, 10H), 0.87–0.84 (m, 3H), 0.82–0.79 (m, 2H) ppm; 13C{1H} NMR (100 MHz, CDCl3, rotamers) δ 172.5, 171.6, 171.6, 171.5, 155.8, 135.8, 128.7, 128.6, 128.5, 128.5, 128.0, 127.9, 80.1, 79.9, 66.7, 60.1, 58.5, 54.1, 31.3, 28.4, 24.8, 24.0, 23.9, 21.7, 21.5, 19.9, 19.6, 19.2, 18.0, 17.8, 17.6, 17.5 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C32H54N3O7+, 592.3956, found: 592.3953.Benzyl (6S,9S,12S,13R)-12-benzyl-6,9-diisopropyl-13-methoxy-2,2,11-trimethyl-4,7,10-trioxo-3-oxa-5,8,11-triazapentadecan-15-oate (28g)
Colorless oil (135 mg, 44%); [α]22D −25.3 (c 1.00, CHCl3); IR (film) νmax 3320, 2964, 1735, 1497, 1246, 1164, 772 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.37–7.32 (m, 5H), 7.34–7.29 (m, 1H), 7.23–7.18 (m, 2H), 7.16–7.10 (m, 3H), 6.43–6.35 (m, 1H), 5.16–5.13 (m, 2H), 5.04–4.98 (m, 1H), 4.55 (dd, J = 8.8, 4.8 Hz, 1H), 4.01–3.80 (m, 2H), 3.40–3.36 (m, 3H), 3.16 (dd, J = 14.8, 4.0 Hz, 1H), 2.93–2.75 (m, 3H), 2.66–2.51 (m, 2H), 2.03–1.92 (m, 1H), 1.90–1.81 (m, 1H), 1.44–1.41 (m, 9H), 0.99–0.92 (m, 1H), 0.91–0.88 (m, 3H), 0.85–0.80 (m, 9H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 172.5, 171.8, 171.2, 155.7, 138.1, 137.6, 135.7, 129.3, 128.8, 128.6, 128.5, 128.4, 128.4, 126.9, 126.5, 125.6, 79.9, 79.7, 79.5, 79.5, 66.7, 66.6, 62.6, 60.0, 59.9, 58.7, 58.5, 53.7, 53.0, 37.0, 33.8, 32.0, 31.1, 30.7, 30.4, 29.7, 28.3, 20.1, 19.5, 19.3, 19.2, 17.9, 17.8, 16.8, 16.3 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C35H52N3O7+, 626.3800, found: 626.3798.General procedure for the synthesis of 29a, 29b, 29c, 29d, 29e, 29f and 29g
A cooled (0 °C) solution of 28a/28b/28c/28d/28e/28f/28g (0.21 mmol) in DCM (1 mL) was treated with TFA (0.20 mL) for 2 h. The mixture was concentrated under reduced pressure and the residue was dissolved in CH3CN (1 mL), then a solution of 40% HCHO (354 μL, 1.68 mmol) and Na(BH3)CN (40 mg, 0.63 mmol) was added. After being stirred for 10 h, the reaction mixture was evaporated and extracted with EtOAc (20 mL × 3). The combined organic layers were washed with brine, dried over MgSO4, filtered and concentrated. The residue was purified by flash chromatography on silica gel (DCM/MeOH/NH4OH = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.01) to give the title product (29a, 29b, 29c, 29d, 29e, 29f and 29g).
0.01) to give the title product (29a, 29b, 29c, 29d, 29e, 29f and 29g).
Benzyl (3R,4S)-4-((S)-2-((S)-2-(dimethylamino)-3-methylbutanamido)-N,3-dimethylbutanamido)-3-methoxy-5-methylhexanoate (29a)
Colorless oil (74 mg, 70%, two steps); [α]23D −25.4 (c 0.50, CHCl3); IR (film) νmax 2961, 1738, 1517, 1455, 1163, 1098, 916 cm−1; 1H NMR (400 MHz, CDCl3, rotamers) δ 7.38–7.34 (m, 5H), 6.93–6.87 (m, 1H), 5.20–5.09 (m, 2H), 4.77–4.68 (m, 2H), 3.98–3.89 (m, 1H), 3.36–3.28 (m, 4H), 3.04–3.00 (m, 3H), 2.56–2.49 (m, 1H), 2.45–2.41 (m, 1H), 2.25–2.23 (m, 6H), 2.10–1.99 (m, 2H), 1.90–1.80 (m, 1H), 1.02–0.98 (m, 9H), 0.97–0.94 (m, 3H), 0.93–0.90 (m, 3H), 0.83–0.79 (m, 3H) ppm; 13C{1H} NMR (150 MHz, CDCl3, rotamers) δ 173.6, 172.0, 171.7, 135.9, 128.7, 128.5, 128.5, 78.0, 76.7, 66.8, 58.2, 58.0, 54.0, 43.1, 43.0, 37.3, 31.9, 31.0, 27.8, 27.0, 20.3, 20.2, 20.1, 19.8, 18.3, 17.8 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C28H48N3O5+, 506.3589, found: 506.3586.Benzyl (3R,4S,5S)-4-((S)-2-((S)-2-(dimethylamino)-3-methylbutanamido)-N,3-dimethylbutanamido)-3-methoxy-5-methylheptanoate (29b)13d
Colorless oil (76 mg, 70%, two steps).Benzyl (3R,4S,5R)-5-benzyl-4-((S)-2-((S)-2-(dimethylamino)-3-methylbutanamido)-N,3-dimethylbutanamido)-3-methoxyheptanoate (29c)
Colorless oil (96 mg, 77%, two steps); [α]25D −9.4 (c 1.00, CHCl3); IR (film): νmax 3336, 2961, 1623, 1454, 1164, 1084, 750, 669 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.39–7.32 (m, 5H), 7.29–7.24 (m, 2H), 7.20–7.14 (m, 3H), 6.92 (d, J = 9.2 Hz, 1H), 5.15 (d, J = 12.4 Hz, 1H), 5.08 (d, J = 12.4 Hz, 1H), 5.06–4.91 (m, 1H), 4.79 (dd, J = 8.8, 6.4 Hz, 1H), 4.08–3.94 (m, 1H), 3.35–3.31 (m, 3H), 3.04–2.98 (m, 3H), 2.79–2.70 (m, 1H), 2.62–2.47 (m, 3H), 2.43 (d, J = 6.4 Hz, 1H), 2.24–2.21 (m, 6H), 2.12–1.98 (m, 3H), 1.33–1.27 (m, 1H), 1.16–1.05 (m, 1H), 1.03–0.97 (m, 6H), 0.97–0.88 (m, 6H), 0.75 (t, J = 6.6 Hz, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3,) δ 173.3, 171.8, 171.5, 140.5, 135.9, 129.1, 128.7, 128.5, 126.1, 70.1, 66.8, 58.0, 53.8, 43.1, 37.4, 35.9, 31.0, 27.8, 22.0, 20.3, 20.0, 17.9, 9.7 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C35H54N3O5+, 596.4059, found: 596.4060.Benzyl (3R,4S,5R)-4-((S)-2-((S)-2-(dimethylamino)-3-methylbutanamido)-N,3-dimethylbutanamido)-3-methoxy-5-(4-methoxybenzyl)heptanoate (29d)
Colorless oil (117 mg, 89%, two steps); [α]25D −7.3 (c 1.00, CHCl3); IR (film): νmax 3446, 2960, 1657, 1512, 1247, 1084, 772 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.41–7.29 (m, 5H), 7.11–7.04 (m, 2H), 6.93 (d, J = 8.8 Hz, 1H), 6.83–6.77 (m, 2H), 5.15 (d, J = 12.2 Hz, 1H), 5.09 (d, J = 12.2 Hz, 1H), 5.02–4.91 (m, 1H), 4.79 (dd, J = 6.8, 6.0 Hz, 1H), 4.11–3.93 (m, 1H), 3.78 (s, 3H), 3.44–3.38 (m, 1H), 3.35–3.32 (m, 3H), 3.05–2.98 (m, 3H), 2.75–2.63 (m, 1H), 2.58–2.48 (m, 2H), 2.46–2.43 (m, 1H), 2.29–2.19 (m, 6H), 2.12–1.96 (m, 3H), 1.77–1.69 (m, 2H), 1.03–0.97 (m, 6H), 0.96–0.89 (m, 6H), 0.79–0.71 (m, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 173.3, 171.6, 158.0, 135.9, 132.4, 130.0, 128.7, 128.5, 113.9, 78.0, 76.6, 70.8, 66.8, 58.0, 55.4, 53.8, 43.0, 37.4, 34.9, 31.0, 27.8, 26.7, 22.0, 20.3, 20.0, 17.9, 9.7 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C36H56N3O6+, 626.4164, found: 626.4165.Benzyl (3R,4S,5R)-4-((S)-2-((S)-2-(dimethylamino)-3-methylbutanamido)-N,3-dimethylbutanamido)-5-(3-fluorobenzyl)-3-methoxyheptanoate (29e)
Colorless oil (117 mg, 91%, two steps); [α]25D −12.5 (c 1.00, CHCl3); IR (film): νmax 3443, 2962, 1624, 1454, 1124, 1084, 776 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.39–7.28 (m, 5H), 7.23–7.16 (m, 1H), 6.97–6.87 (m, 3H), 6.87–6.83 (m, 1H), 5.16 (d, J = 12.4 Hz, 1H), 5.10 (d, J = 12.4 Hz, 1H), 5.00–4.85 (m, 1H), 4.78 (dd, J = 6.8, 6.0 Hz, 1H), 4.07–3.87 (m, 1H), 3.36–3.29 (m, 3H), 3.07–3.00 (m, 3H), 2.78–2.70 (m, 1H), 2.58–2.47 (m, 3H), 2.44 (d, J = 6.4 Hz, 1H), 2.28–2.22 (m, 6H), 2.10–1.97 (m, 3H), 1.34–1.27 (m, 1H), 1.17–1.08 (m, 1H), 1.04–0.97 (m, 6H), 0.97–0.88 (m, 6H), 0.75 (t, J = 6.8 Hz, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 173.3, 171.8, 171.5, 163.0 (d, J = 243.9 Hz), 143.3 (d, J = 7.1 Hz), 135.8, 129.9 (d, J = 8.3 Hz), 128.7, 128.5, 124.8, 115.9 (d, J = 20.7 Hz), 113.0 (d, J = 20.9 Hz), 78.1, 76.6, 66.8, 58.0, 53.8, 43.1, 35.8, 30.9, 27.8, 22.1, 20.3, 20.0, 17.9, 9.9 ppm; 19F NMR (376 MHz, CDCl3) δ −113.6 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C35H53FN3O5+, 614.3964, found: 614.3967.Benzyl (3R,4S)-4-((S)-2-((S)-2-(dimethylamino)-3-methylbutanamido)-N,3-dimethylbutanamido)-3-methoxy-6-methylheptanoate (29f)
Colorless oil (71 mg, 65%, two steps); [α]25D −60.0 (c 0.50, CHCl3); IR (film) νmax 2959, 1738, 1456, 1197, 1103, 768 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.37–7.32 (m, 5H), 6.92–6.87 (m, 1H), 5.15–5.12 (m, 2H), 4.86–4.66 (m, 2H), 3.77–3.63 (m, 1H), 3.33–3.30 (m, 3H), 3.06–3.02 (m, 1H), 3.01–2.96 (m, 3H), 2.55–2.47 (m, 2H), 2.45–2.43 (m, 1H), 2.25–2.22 (m, 6H), 2.11–2.05 (m, 1H), 2.01–1.94 (m, 1H), 1.65–1.55 (m, 1H), 1.38–1.32 (m, 1H), 1.00–0.97 (m, 6H), 0.94–0.90 (m, 6H), 0.88–0.86 (m, 3H), 0.80–0.77 (m, 3H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 172.8, 171.7, 171.5, 135.7, 128.6, 128.4, 80.0, 76.5, 66.6, 58.4, 53.6, 53.4, 42.8, 37.7, 30.9, 27.7, 24.6, 24.6, 24.0, 21.3, 20.1, 19.9, 17.8, 17.8 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C29H50N3O5+, 520.3745, found: 520.3744.Benzyl-(3R,4S)-4-((S)-2-((S)-2-(dimethylamino)-3-methylbutanamido)-N,3-dimethylbutanamido)-3-methoxy-5-phenylpentanoate (29g)
Colorless oil (70 mg, 60%, two steps); [α]23D −21.2 (c 0.50, CHCl3); IR (film) νmax 2970, 1732, 1544, 1456, 1202, 936, 700 cm−1; 1H NMR (400 MHz, CDCl3, rotamers) δ 7.42–7.31 (m, 6H), 7.23–7.11 (m, 5H), 5.17–5.14 (m, 2H), 4.71–4.50 (m, 1H), 3.97–3.60 (m, 2H), 3.45–3.37 (m, 3H), 3.18–3.11 (m, 1H), 2.98–2.76 (m, 10H), 2.73–2.42 (m, 3H), 2.21–1.84 (m, 2H), 1.13–1.05 (m, 3H), 0.96–0.83 (m, 6H), 0.79–0.66 (m, 2H), 0.54–0.34 (m, 1H) ppm; 13C{1H} NMR (150 MHz, CDCl3, rotamers) δ 171.9, 171.4, 171.2, 166.5, 166.5, 162.2, 162.0, 138.1, 135.7, 135.7, 129.3, 129.0, 128.8, 128.7, 128.6, 127.1, 126.6, 117.1, 115.2, 80.0, 79.7, 72.0, 66.9, 66.8, 63.1, 59.0, 58.6, 53.9, 53.6, 36.9, 33.9, 29.6, 29.6, 28.9, 28.2, 28.1, 20.4, 20.1, 19.4, 19.4, 19.3, 17.0, 16.1 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C32H48N3O5+, 554.3589, found: 554.3583.General procedure for the synthesis of 31a, 31b, 31c, 31d, 31e and 31f
Compounds 29a/29c/29d/29e/29f/29g (0.12 mmol) and 10% Pd/C (40 mg) were stirred in MeOH (10 mL) for 4 h under a H2 atmosphere. Then, the mixture was filtered and concentrated to give a crude acid without further purification. HATU (68 mg, 0.18 mmol), DIPEA (63 μL, 0.36 mmol), and 30 (49 mg, 0.13 mmol) were added in turn. After being stirred for 12 h, the reaction mixture was quenched with a saturated aqueous solution of NH4Cl and extracted with DCM (10 mL × 3). The combined organic layers were washed with brine, dried over MgSO4, filtered and concentrated, and the residue was purified by flash chromatography on silica gel to give the title product (31a, 31b, 31c, 31d, 31e and 31f) as a viscous liquid, which was further treated with acetone/hexane to give a white powder.(S)-2-((S)-2-(Dimethylamino)-3-methylbutanamido)-N-((3S,4R)-4-methoxy-6-((S)-2-((1R,2R)-1-methoxy-2-methyl-3-oxo-3-(((S)-2-phenyl-1-(thiazol-2-yl)ethyl)amino)propyl)pyrrolidin-1-yl)-2-methyl-6-oxohexan-3-yl)-N,3-dimethylbutanamide (31a)
White powder (46 mg, 50%, three steps), mp 94–95 °C; [α]22D −214.4 (c 0.125, CHCl3); IR (film) νmax 3292, 2965, 1622, 1498, 1100, 830, 749 cm−1; 1H NMR (400 MHz, CDCl3, rotamers) δ 7.78–7.74 (m, 1H), 7.26–7.16 (m, 6H), 7.12–7.06 (m, 1H), 5.68–5.51 (m, 1H), 4.86–4.64 (m, 2H), 4.21–4.02 (m, 2H), 3.92–3.86 (m, 1H), 3.81–3.53 (m, 1H), 3.44–3.37 (m, 3H), 3.36–3.31 (m, 6H), 3.29–3.23 (m, 1H), 3.18 (s, 1H), 3.05 (s, 2H), 2.74–2.65 (m, 1H), 2.50–2.41 (m, 2H), 2.40–2.36 (m, 6H), 2.25–2.19 (m, 1H), 2.14–2.07 (m, 2H), 2.05–1.99 (m, 1H), 1.97–1.86 (m, 2H), 1.83–1.71 (m, 2H), 1.68–1.60 (m, 1H), 1.50–1.43 (m, 3H), 1.29–1.20 (m, 2H), 1.17–1.11 (m, 3H), 1.03–1.00 (m, 6H), 0.98–0.95 (m, 2H), 0.92–0.90 (m, 2H), 0.84–0.81 (m, 2H) ppm; 13C{1H} NMR (150 MHz, CDCl3, rotamers) δ 174.1, 173.4, 173.3, 171.9, 170.4, 170.1, 142.6, 137.0, 136.5, 129.5, 129.2, 128.8, 128.6, 127.2, 127.0, 119.4, 119.0, 85.7, 81.8, 78.5, 76.0, 64.6, 61.7, 60.6, 60.5, 59.3, 59.0, 58.7, 58.7, 58.2, 58.1, 55.8, 54.2, 53.6, 51.4, 47.8, 46.7, 44.9, 44.0, 43.8, 42.9, 42.8, 41.4, 41.3, 37.9, 36.3, 32.1, 31.1, 30.7, 29.8, 27.8, 27.2, 26.9, 25.9, 25.1, 24.8, 23.8, 20.8, 20.8, 20.4, 20.2, 20.1, 20.1, 19.8, 19.6, 18.4, 18.3, 17.9, 17.8, 15.4, 14.1, 12.7 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C41H67N6O6S+, 771.4837, found: 771.4836.(S)-N-((3R,4S,5R)-5-Benzyl-3-methoxy-1-((S)-2-((1R,2R)-1-methoxy-2-methyl-3-oxo-3-(((S)-2-phenyl-1-(thiazol-2-yl)ethyl)amino)propyl)pyrrolidin-1-yl)-1-oxoheptan-4-yl)-2-((S)-2-(dimethylamino)-3-methylbutanamido)-N,3-dimethylbutanamide (31b)
White powder (43 mg, 42%, three steps), mp 73.6–75.6 °C; [α]26D −39.2 (c 0.25, CHCl3); IR (film): νmax 3290, 2960, 1622, 1497, 1098, 749, 700 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.77–7.71(m, 1H), 7.26–7.21 (m, 5H), 7.20–7.04 (m, 6H), 7.00–6.92 (m, 1H), 5.63–5.53 (m, 1H), 5.02–4.86 (m, 1H), 4.82 (dd, J = 6.4, 5.6 Hz, 1H), 4.34–4.19 (m, 1H), 4.12–4.04 (m, 1H), 3.89 (d, J = 6.8 Hz, 1H), 3.44–3.42 (m, 1H), 3.38–3.36 (m, 2H), 3.33–3.33 (m, 2H), 3.30–3.28 (m, 1H), 3.21–3.17 (m, 1H), 3.08–3.05 (m, 2H), 2.83–2.76 (m, 1H), 2.53–2.41 (m, 4H), 2.28–2.22 (m, 6H), 2.12–2.00 (m, 4H), 1.95–1.90 (m, 1H), 1.84–1.74 (m, 2H), 1.68–1.64 (m, 6H), 1.44–1.32 (m, 2H), 1.15–1.12 (m, 3H), 1.04–0.98 (m, 6H), 0.96–0.92 (m, 6H), 0.77 (t, J = 7.0 Hz, 3H) ppm; 13C{1H} NMR (150 MHz, CDCl3) δ 173.9, 173.3, 171.7, 170.1, 142.6, 140.8, 137.1, 130.0, 129.2, 129.1, 128.8, 129.0, 128.4, 127.2, 126.9, 126.0, 119.4, 118.9, 85.7, 81.9, 60.5, 59.3, 58.3, 53.8, 52.7, 51.4, 47.7, 46.7, 43.9, 43.1, 41.5, 41.3, 40.3, 38.2, 35.7, 32.3, 31.0, 29.8, 27.8, 25.9, 25.1, 24.8, 23.8, 22.0, 20.4, 18.0, 13.9, 10.1 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C48H73N6O6S+, 861.5307, found: 861.5306.(S)-2-((S)-2-(Dimethylamino)-3-methylbutanamido)-N-((3R,4S,5R)-3-methoxy-1-((S)-2-((1R,2R)-1-methoxy-2-methyl-3-oxo-3-(((S)-2-phenyl-1-(thiazol-2-yl)ethyl)amino)propyl)pyrrolidin-1-yl)-5-(4-methoxybenzyl)-1-oxoheptan-4-yl)-N,3-dimethylbutanamide (31c)
White powder (36 mg, 34%), mp 72.5–74.5 °C; [α]26D −35.2 (c 0.25, CHCl3); IR (film): νmax 3290, 2962, 2874, 1623, 1512, 1453, 1247, 1100, 752 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.76–7.71 (m, 1H), 7.25–7.17 (m, 6H), 7.12–7.07 (m, 2H), 7.08–6.94 (m, 1H), 6.82–6.77 (m, 2H), 5.63–5.51 (m, 1H), 5.16–4.87 (m, 1H), 4.86–4.76 (m, 1H), 4.32–4.15 (m, 1H), 4.12–4.02 (m, 1H), 3.93–3.84 (m, 1H), 3.77 (s, 3H), 3.76–3.71 (m, 1H), 3.46–3.39 (m, 2H), 3.38–3.35 (m, 3H), 3.32–3.30 (m, 3H), 3.28–3.23 (m, 2H), 3.21–3.16 (m, 1H), 3.09–3.01 (m, 3H), 2.77–2.70 (m, 1H), 2.49–2.42 (m, 3H), 2.34–2.22 (m, 6H), 2.15–2.02 (m, 3H), 2.02–1.88 (m, 2H), 1.85–1.79 (m, 1H), 1.43–1.29 (m, 1H), 1.28–1.24 (m, 2H), 1.17–1.12 (m, 3H), 1.06–1.10 (m, 6H), 0.97–0.93 (m, 6H), 0.81–0.72 (m, 3H) ppm; 13C{1H} NMR (150 MHz, CDCl3) δ 173.9, 173.5, 171.8, 170.1, 157.9, 142.6, 137.1, 132.7, 130.1, 130.0, 129.6, 129.2, 128.8, 128.5, 126.9, 119.4, 118.9, 113.8, 85.7, 81.9, 70.8, 60.5, 59.3, 58.6, 58.3, 55.3, 53.8, 53.6, 52.7, 47.7, 44.9, 43.9, 43.1, 41.3, 38.2, 34.7, 31.0, 27.8, 25.1, 24.8, 23.7, 22.0, 20.3, 18.6, 18.0, 13.9, 10.1 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C49H75N6O7S+, 891.5413, found: 891.5415.(S)-2-((S)-2-(Dimethylamino)-3-methylbutanamido)-N-((3R,4S,5R)-5-(3-fluorobenzyl)-3-methoxy-1-((S)-2-((1R,2R)-1-methoxy-2-methyl-3-oxo-3-(((S)-2-phenyl-1-(thiazol-2-yl)ethyl)amino)propyl)pyrrolidin-1-yl)-1-oxoheptan-4-yl)-N,3-dimethylbutanamide (31d)
White powder (47 mg, 45%), mp 86.5–88.5 °C; [α]26D −41.5 (c 1.00, CHCl3); IR (film): νmax 3291, 2963, 1636, 1488, 1251, 1141, 1098, 697 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.76–7.71 (m, 1H), 7.26–7.17 (m, 6H), 7.12–7.05 (m, 1H), 7.02–6.96 (m, 2H), 6.92–6.83 (m, 2H), 5.61–5.54 (m, 1H), 4.94–4.84 (m, 1H), 4.80 (dd, J = 8.4, 7.6 Hz, 1H), 4.27–4.19 (m, 1H), 4.11–4.04 (m, 1H), 3.89 (d, J = 7.2 Hz, 1H), 3.44–3.42 (m, 1H), 3.37–3.35 (m, 3H), 3.34–3.32 (m, 3H), 3.31–3.29 (m, 1H), 3.28–3.24 (m, 1H), 3.18–3.17 (m, 1H), 3.08–3.05 (m, 2H), 2.82–2.75 (m, 1H), 2.54–2.40 (m, 4H), 2.34–2.26 (m, 6H), 2.16–2.02 (m, 4H), 1.96–1.88 (m, 2H), 1.84–1.73 (m, 2H), 1.72–1.61 (m, 2H), 1.43–1.33 (m, 1H), 1.28–1.24 (m, 1H), 1.17–1.13 (m, 3H), 1.06–1.00 (m, 6H), 0.97–0.93 (m, 6H), 0.82–0.72 (m, 3H) ppm; 13C{1H} NMR (150 MHz, CDCl3) δ 173.8, 173.2, 171.7, 170.3, 170.0 169.7, 162.9 (d, J = 243.8 Hz), 143.5, 142.6, 137.1, 136.6, 129.8 (d, J = 8.1 Hz), 129.5, 129.2, 128.8, 128.5, 127.2, 126.9, 124.9, 119.4, 118.9, 115.9 (d, J = 20.7 Hz), 113.0 (d, J = 20.9 Hz), 85.7, 81.9, 61.7, 60.5, 59.2, 58.9, 58.5, 58.3, 52.7, 51.4, 47.7, 46.7, 43.8, 43.1, 41.3, 40.4, 38.4, 35.6, 30.8, 27.8, 25.9, 25.1, 24.8, 23.7, 22.1, 20.3, 19.9, 18.0, 13.9, 10.3 ppm; 19F NMR (376 MHz, CDCl3) δ −113.9 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C48H72FN6O6S+, 879.5213, found: 879.5212.(S)-2-((S)-2-(Dimethylamino)-3-methylbutanamido)-N-((3R,4S)-3-methoxy-1-((S)-2-((1R,2R)-1-methoxy-2-methyl-3-oxo-3-(((S)-2-phenyl-1-(thiazol-2-yl)ethyl)amino)propyl)pyrrolidin-1-yl)-6-methyl-1-oxoheptan-4-yl)-N,3-dimethylbutanamide (31e)
White powder (40 mg, 42%), mp 108–110 °C; [α]23D −5.6 (c 0.50, CHCl3); IR (film) νmax 2914, 1959, 1682, 1453, 1200, 936, 870 cm−1; 1H NMR (600 MHz, CDCl3) δ 7.81–7.77 (m, 1H), 7.53–7.43 (m, 1H), 7.36–7.29 (m, 1H), 7.25–7.09 (m, 6H), 5.87–5.62 (m, 1H), 4.90–4.72 (m, 2H), 3.97–3.75 (m, 4H), 3.46–3.37 (m, 2H), 3.36–3.26 (m, 8H), 3.19–3.12 (m, 1H), 3.06–2.88 (m, 8H), 2.59–2.45 (m, 1H), 2.34–2.28 (m, 1H), 2.28–2.16 (m, 2H), 2.15–2.06 (m, 1H), 2.03–1.92 (m, 1H), 1.92–1.77 (m, 2H), 1.75–1.65 (m, 1H), 1.63–1.56 (m, 1H), 1.29–1.23 (m, 2H), 1.15–1.10 (m, 6H), 1.01–0.98 (m, 3H), 0.98–0.94 (m, 3H), 0.93–0.90 (m, 6H), 0.81–0.68 (m, 3H) ppm; 13C{1H} NMR (150 MHz, CDCl3) δ 172.5, 166.6, 162.3, 162.1, 161.8, 130.5, 129.5, 129.3, 128.8, 128.7, 127.3, 127.2, 125.8, 117.2, 115.3, 81.8, 81.6, 72.2, 51.6, 47.8, 47.7, 47.6, 47.6, 47.5, 47.3, 47.2, 44.4, 44.3, 44.3, 44.2, 44.1, 30.7, 29.9, 28.1, 27.9, 26.9, 26.0, 25.3, 24.1, 24.0, 23.9, 21.2, 21.1, 18.9, 18.8 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C42H69N6O6S+, 785.4994, found: 785.4999.(S)-2-((S)-2-(Dimethylamino)-3-methylbutanamido)-N-((2S,3R)-3-methoxy-5-((S)-2-((1R,2R)-1-methoxy-2-methyl-3-oxo-3-(((S)-2-phenyl-1-(thiazol-2-yl)ethyl)amino)propyl)pyrrolidin-1-yl)-5-oxo-1-phenylpentan-2-yl)-N,3-dimethylbutanamide (31f)
White powder (44 mg, 45%), mp 112–114 °C.; [α]23D −26.4 (c 0.125, CHCl3); IR (film) νmax 2916, 1957, 1650, 1453, 1203, 936, 919, 870 cm−1; 1H NMR (600 MHz, CDCl3, rotamers) δ 7.88–7.83 (m, 1H), 7.43–7.37 (m, 1H), 7.25–7.10 (m, 11H), 5.76–5.66 (m, 1H), 5.26–5.03 (m, 1H), 4.67–4.58 (m, 1H), 3.90–3.72 (m, 4H), 3.42–3.38 (m, 3H), 3.35–3.33 (m, 1H), 3.33–3.31 (m, 2H), 3.31–3.28 (m, 2H), 3.27–3.21 (m, 1H), 3.16–3.10 (m, 1H), 3.03–2.87 (m, 10H), 2.56–2.46 (m, 1H), 2.45–2.20 (m, 3H), 2.16–2.10 (m, 1H), 2.07–1.88 (m, 3H), 1.84–1.76 (m, 1H), 1.74–1.66 (m, 1H), 1.63–1.55 (m, 1H), 1.17–1.04 (m, 7H), 0.97–0.93 (m, 3H), 0.91–0.86 (m, 3H), 0.78–0.73 (m, 2H) ppm; 13C{1H} NMR (150 MHz, CDCl3, rotamers) δ 175.0, 173.5, 173.0, 171.5, 166.2, 161.8, 161.5, 161.2, 135.9, 129.4, 129.4, 129.3, 129.2, 129.1, 128.9, 128.8, 128.7, 128.6, 127.3, 126.7, 120.5, 118.7, 116.7, 114.8, 81.8, 72.1, 72.0, 61.7, 61.0, 60.6, 59.9, 59.6, 59.1, 54.5, 54.5, 54.4, 54.3, 51.7, 47.8, 47.8, 44.1, 41.2, 30.8, 29.9, 29.7, 29.7, 29.5, 28.2, 28.0, 27.9, 25.2, 25.1, 25.1, 24.5, 24.3, 20.4, 20.1, 19.0, 18.9, 17.1, 17.0, 15.5, 14.5, 14.3, 14.0 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C45H67N6O6S+, 818.4759, found: 818.4755.tert-Butyl(1S,3S,5S)-3-((1R,2R)-3-((S)-4-benzyl-2-oxooxazolidin-3-yl)-1-hydroxy-2-methyl-3-oxopropyl)-2-azabicyclo[3.1.0]hexane-2-carboxylate (34)
Compound 33 (1.74 g, 8.16 mmol) was stirred in DCM (30 ml). Then Dess–Martin periodinane (8.6 g, 20.4 mmol) was added and the resulting mixture was stirred for 30 min at room temperature. The reaction mixture was carefully quenched with a solution of saturated aqueous NaHCO3 and solid Na2S2O3. The mixture was extracted with DCM (50 mL × 3), and the combined organic layers were washed with brine, and dried and concentrated to give the aldehyde without further purification. To a stirred solution of (S)-4-benzyl-3-propionyloxazolidin-2-one (2.13 g, 8.98 mmol) in DCM (20 mL) at 0 °C was added dropwise n-Bu2BOTf ( 9.8 mL, 9.79 mmol, 1 M in DCM) and stirred for 10 min. TEA (1.47 mL, 10.61 mmol) was added dropwise and the reaction mixture was stirred for 1 h at room temperature. Then a solution of the above crude aldehyde in DCM was added dropwise, and the reaction mixture was stirred at −78 °C for 2 h before gradually warming to room temperature. Then the mixture was quenched with a solution of 30% aqueous H2O2 (15 mL) and MeOH (5 mL) at 0 °C. The resulting mixture was stirred at room temperature for additional 4 h and extracted with DCM (80 mL × 3). The combined organic layers were washed with brine, dried over MgSO4, filtered and concentrated, and the residue was purified by flash chromatography on silica gel (PE/EA = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give 34. Colorless oil (2.17 g, 60%, two steps); [α]24D −29.1 (c 1.00, CHCl3); IR (film): νmax 3443, 2976, 1782, 1617, 1405, 1211, 1117, 702 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.36–7.25 (m, 3H), 7.22–7.17 (m, 2H), 4.72–4.60 (m, 1H), 4.30–4.15 (m, 3H), 4.12–3.95 (m, 1H), 3.84–3.78 (m, 1H), 3.52–3.42 (m, 1H), 3.23 (dd, J = 13.2, 3.2 Hz, 1H), 2.77 (dd, J = 13.6, 9.6 Hz, 1H), 2.24–2.13 (m, 2H), 1.50 (s, 9H), 1.46–1.41 (m, 1H), 1.35–1.24 (m, 4H), 0.89–0.83 (m, 1H), 0.72–0.64 (m, 1H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 177.4, 155.4, 152.8, 135.2, 129.6, 129.1, 127.5, 66.2, 55.2, 40.8, 38.6, 37.9, 28.7, 27.5 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C24H33N2O6+, 445.2333, found: 445.2337.
1) to give 34. Colorless oil (2.17 g, 60%, two steps); [α]24D −29.1 (c 1.00, CHCl3); IR (film): νmax 3443, 2976, 1782, 1617, 1405, 1211, 1117, 702 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.36–7.25 (m, 3H), 7.22–7.17 (m, 2H), 4.72–4.60 (m, 1H), 4.30–4.15 (m, 3H), 4.12–3.95 (m, 1H), 3.84–3.78 (m, 1H), 3.52–3.42 (m, 1H), 3.23 (dd, J = 13.2, 3.2 Hz, 1H), 2.77 (dd, J = 13.6, 9.6 Hz, 1H), 2.24–2.13 (m, 2H), 1.50 (s, 9H), 1.46–1.41 (m, 1H), 1.35–1.24 (m, 4H), 0.89–0.83 (m, 1H), 0.72–0.64 (m, 1H) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 177.4, 155.4, 152.8, 135.2, 129.6, 129.1, 127.5, 66.2, 55.2, 40.8, 38.6, 37.9, 28.7, 27.5 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C24H33N2O6+, 445.2333, found: 445.2337.
tert-Butyl-(1S,3S,5S)-3-((1R,2R)-3-(benzyloxy)-1-methoxy-2-methyl-3-oxopropyl)-2-azabicyclo[3.1.0]hexane-2-carboxylate (35)
Compound 34 (0.95 g, 4.80 mmol) was dissolved in a mixture of THF and H2O (80 mL v/v = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Then a solution of 30% H2O2 (4 mL, 38.4 mmol) and LiOH·H2O (604 mg, 14.4 mmol) was added in one portion. After being stirred for 7 h, the mixture was acidified with hydrochloric acid to pH = 2–3 and the resulting mixture was extracted with EtOAc (100 mL × 3). The combined organic layers were dried over MgSO4, filtered and concentrated to give a crude compound, which was dissolved in DMF (20 mL). Then K2CO3 (950 mg, 9.6 mmol) and BnBr (0.57 mL, 4.8 mmol) were added. After being stirred for 4 h, the reaction mixture was diluted with water and extracted with EtOAc (50 mL × 3). The combined organic layers were washed with water and brine respectively, then dried over MgSO4, filtered and concentrated to give the crude secondary alcohol. To a cooled (−78 °C) solution of the secondary alcohol in HMPA (926 μL, 5.3 mmol) and THF (20 mL) was added a solution of LiHMDS (4.8 ml, 4.8 mmol, 1 M in THF) dropwise. After being stirred for 30 min, the reaction mixture was warmed to −15 °C and MeOTf (1.08 mL, 9.6 mmol) was added, and then the mixture was stirred for an additional 15 min. The reaction mixture was quenched with a saturated aqueous solution of NH4Cl and extracted with EtOAc (30 mL × 3). The combined organic layers were washed with brine, dried over MgSO4, filtered and concentrated. The residue was purified by flash chromatography on silica gel (PE/EA = 5
1). Then a solution of 30% H2O2 (4 mL, 38.4 mmol) and LiOH·H2O (604 mg, 14.4 mmol) was added in one portion. After being stirred for 7 h, the mixture was acidified with hydrochloric acid to pH = 2–3 and the resulting mixture was extracted with EtOAc (100 mL × 3). The combined organic layers were dried over MgSO4, filtered and concentrated to give a crude compound, which was dissolved in DMF (20 mL). Then K2CO3 (950 mg, 9.6 mmol) and BnBr (0.57 mL, 4.8 mmol) were added. After being stirred for 4 h, the reaction mixture was diluted with water and extracted with EtOAc (50 mL × 3). The combined organic layers were washed with water and brine respectively, then dried over MgSO4, filtered and concentrated to give the crude secondary alcohol. To a cooled (−78 °C) solution of the secondary alcohol in HMPA (926 μL, 5.3 mmol) and THF (20 mL) was added a solution of LiHMDS (4.8 ml, 4.8 mmol, 1 M in THF) dropwise. After being stirred for 30 min, the reaction mixture was warmed to −15 °C and MeOTf (1.08 mL, 9.6 mmol) was added, and then the mixture was stirred for an additional 15 min. The reaction mixture was quenched with a saturated aqueous solution of NH4Cl and extracted with EtOAc (30 mL × 3). The combined organic layers were washed with brine, dried over MgSO4, filtered and concentrated. The residue was purified by flash chromatography on silica gel (PE/EA = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give 35. Colorless oil (1.51 g, 81%, three steps); [α]23D +4.8 (c 0.50, CHCl3); IR (film): νmax 3443, 2075, 1637, 1404, 1172, 1088, 582 cm−1; 1H NMR (400 MHz, CDCl3, rotamers) δ 7.42–7.30 (m, 5H), 5.18–5.01 (m, 2H), 4.18–4.07 (m, 0.5H), 4.04–3.90 (m, 1H), 3.76–3.71 (m, 0.5H), 3.54–3.48 (m, 0.5H), 3.38–3.34 (m, 3.5H), 2.50–2.39 (m, 1H), 2.18–2.08 (m, 1H), 2.02–1.90 (m, 1H), 1.50–1.44 (m, 9H), 1.41–1.37 (m, 1H), 1.25–1.20 (m, 3H), 0.84 (d, J = 13.6 Hz, 1H), 0.70–0.60 (m, 1H) ppm; 13C{1H} NMR (100 MHz, CDCl3, rotamers) δ 174.4, 154.7, 135.9, 128.7, 128.4, 82.4, 81.2, 80.2, 79.5, 66.5, 63.6, 63.4, 61.0, 60.6, 43.4, 38.8, 38.5, 31.6, 30.3, 28.7, 28.0, 27.2, 14.0, 13.8 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C22H32NO5+, 390.2275, found: 390.2278.
1) to give 35. Colorless oil (1.51 g, 81%, three steps); [α]23D +4.8 (c 0.50, CHCl3); IR (film): νmax 3443, 2075, 1637, 1404, 1172, 1088, 582 cm−1; 1H NMR (400 MHz, CDCl3, rotamers) δ 7.42–7.30 (m, 5H), 5.18–5.01 (m, 2H), 4.18–4.07 (m, 0.5H), 4.04–3.90 (m, 1H), 3.76–3.71 (m, 0.5H), 3.54–3.48 (m, 0.5H), 3.38–3.34 (m, 3.5H), 2.50–2.39 (m, 1H), 2.18–2.08 (m, 1H), 2.02–1.90 (m, 1H), 1.50–1.44 (m, 9H), 1.41–1.37 (m, 1H), 1.25–1.20 (m, 3H), 0.84 (d, J = 13.6 Hz, 1H), 0.70–0.60 (m, 1H) ppm; 13C{1H} NMR (100 MHz, CDCl3, rotamers) δ 174.4, 154.7, 135.9, 128.7, 128.4, 82.4, 81.2, 80.2, 79.5, 66.5, 63.6, 63.4, 61.0, 60.6, 43.4, 38.8, 38.5, 31.6, 30.3, 28.7, 28.0, 27.2, 14.0, 13.8 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C22H32NO5+, 390.2275, found: 390.2278.
tert-Butyl-(1S,3S,5S)-3-((1R,2R)-1-methoxy-2-methyl-3-oxo-3-(((S)-2-phenyl-1-(thiazol-2-yl)ethyl)amino)propyl)-2-azabicyclo[3.1.0]hexane-2-carboxylate (38)
Compound 35 (1.48 g, 3.80 mmol) and 10% Pd/C (148 mg) were stirred in MeOH (60 mL) for 4 h under a H2 atmosphere. The mixture was filtered and concentrated to give a crude acid, which was dissolved in DCM (16 mL). HATU (2.17 g, 5.70 mmol), DIPEA (1.98 mL, 11.40 mmol), and 37 (912 mg, 3.80 mmol) were added and stirred overnight. The reaction mixture was quenched with a saturated aqueous solution of NH4Cl and extracted with DCM (30 mL × 3). The combined organic layers were washed with brine, dried over MgSO4, filtered and concentrated, and the residue was purified by flash chromatography on silica gel (PE/EA = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give 38. Colorless oil (1.38 g, 75%, two steps); [α]23D −39.2 (c 1.00, CHCl3); IR (film): νmax 3443, 2975, 2076, 1646, 1408, 1175, 1114 cm−1; 1H NMR (400 MHz, CDCl3, rotamers) δ 7.77–7.72 (m, 1H), 7.25–7.15 (m, 5H), 6.92–6.86 (m, 0.5H), 6.26–6.19 (m, 0.5H), 5.67–5.58 (m, 1H), 4.09–4.03 (m, 0.5H), 3.87–3.77 (m, 0.5H), 3.74–3.68 (m, 0.5H), 3.66–3.61 (m, 0.5H), 3.54–3.44 (m, 0.5H), 3.40–3.33 (m, 3H), 3.32–3.28 (m, 2H), 3.27–3.18 (m, 1.5H), 2.28–2.22(m, 0.5H), 2.14–2.05 (m,0.5H), 2.00–1.90 (m, 0.5H), 1.88–1.86 (m, 0.5H), 1.82–1.74 (m, 1H), 1.50 (s, 9H), 1.37–1.32 (m, 1H), 1.16–1.09 (m, 3H), 0.81–0.74 (m, 1H), 0.67–0.62 (m, 1H) ppm; 13C{1H} NMR (100 MHz, CDCl3, rotamers) δ 173.7, 173.2, 171.6, 171.0, 154.8, 142.6, 137.0, 136.6, 129.5, 129.4, 128.7, 128.6, 127.2, 127.0, 119.1, 118.9, 82.4, 81.1, 80.3, 79.7, 63.2, 62.9, 60.5, 52.4, 44.8, 44.4, 41.5, 38.9, 38.3, 28.7, 27.7, 26.5, 15.0, 14.6, 14.2, 13.9, 13.7 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C26H36N3O4S+, 486.2421, found: 486.2427.
1) to give 38. Colorless oil (1.38 g, 75%, two steps); [α]23D −39.2 (c 1.00, CHCl3); IR (film): νmax 3443, 2975, 2076, 1646, 1408, 1175, 1114 cm−1; 1H NMR (400 MHz, CDCl3, rotamers) δ 7.77–7.72 (m, 1H), 7.25–7.15 (m, 5H), 6.92–6.86 (m, 0.5H), 6.26–6.19 (m, 0.5H), 5.67–5.58 (m, 1H), 4.09–4.03 (m, 0.5H), 3.87–3.77 (m, 0.5H), 3.74–3.68 (m, 0.5H), 3.66–3.61 (m, 0.5H), 3.54–3.44 (m, 0.5H), 3.40–3.33 (m, 3H), 3.32–3.28 (m, 2H), 3.27–3.18 (m, 1.5H), 2.28–2.22(m, 0.5H), 2.14–2.05 (m,0.5H), 2.00–1.90 (m, 0.5H), 1.88–1.86 (m, 0.5H), 1.82–1.74 (m, 1H), 1.50 (s, 9H), 1.37–1.32 (m, 1H), 1.16–1.09 (m, 3H), 0.81–0.74 (m, 1H), 0.67–0.62 (m, 1H) ppm; 13C{1H} NMR (100 MHz, CDCl3, rotamers) δ 173.7, 173.2, 171.6, 171.0, 154.8, 142.6, 137.0, 136.6, 129.5, 129.4, 128.7, 128.6, 127.2, 127.0, 119.1, 118.9, 82.4, 81.1, 80.3, 79.7, 63.2, 62.9, 60.5, 52.4, 44.8, 44.4, 41.5, 38.9, 38.3, 28.7, 27.7, 26.5, 15.0, 14.6, 14.2, 13.9, 13.7 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C26H36N3O4S+, 486.2421, found: 486.2427.
General procedure for the synthesis of 40a, 40b and 40c
Compound 29b/29c/29e (0.1 mmol) and 10% Pd/C were stirred in MeOH (10 mL) for 4 h under a H2 atmosphere. The mixture was filtered and concentrated to give a crude acid without further purification. Compound 38 (53.4 mg, 0.11 mmol) and TFA (0.2 mL) were stirred in DCM (1 mL) for 2 h at 0 °C and the mixture was directly concentrated. The residue was dissolved in DCM (1 mL), HATU (57 mg, 0.15 mmol), and DIPEA (52 μL, 0.3 mmol) and the above crude acid was added. After being stirred for 12 h, the reaction mixture was quenched with a saturated aqueous solution of NH4Cl and extracted with DCM (20 mL × 3). The combined organic layers were washed with brine, dried over MgSO4, filtered and concentrated, and the residue was purified by flash chromatography on silica gel (DCM/MeOH = 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 25
1 to 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give the title product (40a, 40b and 40c) as a viscous liquid, which was further treated with acetone/hexane to give a white powder.
1) to give the title product (40a, 40b and 40c) as a viscous liquid, which was further treated with acetone/hexane to give a white powder.
(S)-2-((S)-2-(Dimethylamino)-3-methylbutanamido)-N-((3R,4S,5S)-3-methoxy-1-((1S,3S,5S)-3-((1R,2R)-1-methoxy-2-methyl-3-oxo-3-(((S)-2-phenyl-1-(thiazol-2-yl)ethyl)amino)propyl)-2-azabicyclo[3.1.0]hexan-2-yl)-5-methyl-1-oxoheptan-4-yl)-N,3-dimethylbutanamide (40a)
White powder (25 mg, 31%), mp 92.5–94.5 °C; [α]24D −38.0 (c 0.10, CHCl3); IR (film): νmax 3344, 2965, 1634, 1454, 1210, 1096, 698 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.77–7.72 (m, 1H), 7.24–7.18 (m, 5H), 7.13–7.06 (m, 1H), 5.61–5.51 (m, 1H), 4.93–4.72 (m, 2H), 4.40–4.32 (m, 1H), 4.24–4.13 (m, 1H), 3.89 (d, J = 8.0 Hz, 1H), 3.43–3.35 (m, 2H), 3.35–3.31 (m, 3H), 3.29–3.26 (m, 3H), 3.26–3.21 (m, 1H), 3.20–3.09 (m, 2H), 3.07–3.00 (m, 3H), 2.67–2.57 (m, 1H), 2.51–2.40 (m, 2H), 2.27–2.22 (m, 6H), 2.15–2.07 (m, 1H), 2.06–1.93 (m, 3H), 1.92–1.85 (m, 1H), 1.44–1.31 (m, 3H), 1.12–1.09 (m, 3H), 1.04–1.00 (m, 6H), 0.98–0.95 (m, 6H), 0.93–0.91 (m, 3H), 0.84–0.82 (m, 3H), 0.79–0.64 (m, 2H) ppm; 13C{1H} NMR (150 MHz, CDCl3) δ 173.6, 171.9, 165.5, 163.1, 158.4, 156.5, 142.6, 137.1, 132.4, 129.9, 129.6, 128.6, 127.0, 122.0, 121.9, 118.9, 118.8, 80.1, 63.3, 60.0, 58.0, 53.9, 53.3, 52.7, 52.5, 44.4, 43.0, 41.3, 38.3, 38.1, 33.4, 31.1, 27.8, 25.8, 20.2, 17.9, 16.4, 14.3 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C43H69N6O6S+, 797.4994, found: 797.4999.(S)-2-((S)-2-(Dimethylamino)-3-methylbutanamido)-N-((3R,4S,5R)-5-(3-fluorobenzyl)-3-methoxy-1-((1S,3S,5S)-3-((1R,2R)-1-methoxy-2-methyl-3-oxo-3-(((S)-2-phenyl-1-(thiazol-2-yl)ethyl)amino)propyl)-2-azabicyclo[3.1.0]hexan-2-yl)-1-oxoheptan-4-yl)-N,3-dimethylbutanamide (40b)
White powder (25 mg, 28%), mp 87.5–89.5 °C; [α]26D −22.0 (c 0.25, CHCl3); IR (film): νmax 3291, 2928, 1639, 1453, 1252, 1096, 774, 696 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.77–7.72 (m, 1H), 7.23–7.18 (m, 5H), 7.04–6.96 (m, 3H), 6.94–6.83 (m, 3H), 5.61–5.52 (m, 1H), 4.95–4.75 (m, 2H), 4.40–4.32 (m, 1H), 4.32–4.23 (m, 1H), 3.93–3.86 (m, 1H), 3.38–3.35 (m, 3H), 3.29–3.26 (m, 3H), 3.11–3.07 (m, 3H), 2.80–2.62 (m, 3H), 2.53–2.42 (m, 3H), 2.31–2.23 (m, 6H), 2.12–1.98 (m, 5H), 1.91–1.84 (m, 1H), 1.53–1.47 (m, 2H), 1.39–1.36 (m, 1H), 1.29–1.24 (m, 3H), 1.13–1.10 (m, 3H), 1.05–0.99 (m, 6H), 0.96–0.91 (m, 6H), 0.82–0.77 (m, 3H), 0.76–0.68 (m, 2H) ppm; 13C{1H} NMR (150 MHz, CDCl3) δ 173.5, 171.9, 169.5, 163.0 (d, J = 243.6 Hz), 143.6, 142.6, 137.0, 129.8 (d, J = 7.7 Hz), 129.5, 128.6, 127.0, 124.9, 118.9, 116.0 (d, J = 20.6 Hz), 112.9 (d, J = 20.9 Hz), 80.2, 63.3, 60.0, 58.0, 53.8, 52.6, 44.3, 43.1, 41.4, 40.7, 38.6, 38.3, 35.8, 30.9, 27.8, 25.7, 20.3, 18.0, 16.4, 14.3, 10.6 ppm; 19F NMR (376 MHz, CDCl3) δ −113.9 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C49H72FN6O6S+, 891.5213, found: 891.5211.(S)-N-((3R,4S,5R)-5-Benzyl-3-methoxy-1-((1S,3S,5S)-3-((1R,2R)-1-methoxy-2-methyl-3-oxo-3-(((S)-2-phenyl-1-(thiazol-2-yl)ethyl)amino)propyl)-2-azabicyclo[3.1.0]hexan-2-yl)-1-oxoheptan-4-yl)-2-((S)-2-(dimethylamino)-3-methylbutanamido)-N,3-dimethylbutanamide (40c)
White powder (39 mg, 45%), mp 76.5–78.5 °C; [α]24D −30.2 (c 0.50, CHCl3); IR (film): νmax 3423, 2930, 1637, 1535, 1454, 1096, 750, 700, cm−1; 1H NMR (400 MHz, CDCl3) δ 7.74–7.71 (m, 1H), 7.26–7.22 (m, 4H), 7.22–7.15 (m, 8H), 7.08 (d, J = 6.8 Hz, 1H), 6.95 (d, J = 9.2 Hz, 1H), 5.57 (dd, J = 14.0, 8.0 Hz, 1H), 5.02–4.86 (m, 1H), 4.83 (dd, J = 6.8, 6.0 Hz, 1H), 4.40–4.33 (m, 1H), 4.32–4.26 (m, 1H), 3.88 (d, J = 7.6 Hz, 1H), 3.48–3.38 (m, 1H), 3.37–3.35 (m, 3H), 3.32–3.28 (m, 1H), 3.28–3.26 (m, 3H), 3.10–3.07 (m, 3H), 2.82–2.75 (m, 1H), 2.72–2.58 (m, 1H), 2.55–2.49 (m, 1H), 2.44 (d, J = 6.4 Hz, 1H), 2.42–2.27 (m, 1H), 2.26–2.23 (m, 6H), 2.10–1.97 (m, 4H), 1.91–1.86 (m, 1H), 1.54–1.46 (m, 1H), 1.44–1.37 (m, 1H), 1.12–1.09 (m, 3H), 1.04–0.98 (m, 6H), 0.97–0.94 (m, 3H), 0.93–0.91 (m, 3H), 0.90–0.87 (m, 2H), 0.78 (t, J = 7.0 Hz, 3H), 0.75–0.67 (m, 2H) ppm; 13C{1H} NMR (150 MHz, CDCl3) δ 173.6, 173.3, 171.9, 171.7, 169.5, 142.6, 140.9, 137.0, 129.5, 129.2, 128.6, 128.4, 118.9, 80.1, 76.7, 63.3, 60.0, 58.0, 53.8, 52.6, 44.3, 43.1, 41.3, 38.4, 38.3, 35.9, 31.0, 29.8, 27.8, 25.7, 22.2, 20.3, 19.9, 18.0, 17.9, 16.4, 14.4, 10.3 ppm; HRMS (ESI-Orbitrap) m/z: [M + H]+ calcd for C49H73N6O6S+, 873.5307, found: 873.5309.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the National Natural Science Foundation of China (21772027 to B.-G. Wei, and 21702032 to C.-M. Si) and the Open Research Fund Program of Beijing Key Lab of Plant Resource Research and Development, BTBU (No. PRRD-2018-ZD1) for financial support.Notes and references
- (a) Y. Hamada and T. Shioiri, Recent Progress of the Synthetic Studies of Biologically Active Marine Cyclic Peptides and Depsipeptides, Chem. Rev., 2005, 105, 4441–4482 CrossRef CAS; (b) G.-M. Suarez-Jimenez, A. Burgos-Hernandez and J.-M. Ezquerra-Brauer, Bioactive Peptides and Depsipeptides with Anticancer Potential: Sources from Marine Animals, Mar. Drugs, 2012, 10, 963–986 CrossRef CAS.
- (a) W. Li, A. Schlecker and D. Ma, Total Synthesis of Antimicrobial and Antitumor Cyclic Depsipeptides, Chem. Commun., 2010, 46, 5403–5420 RSC; (b) L. A. Salvador-Reyes and H. Luesch, Biological Targets and Mechanisms of Action of Natural Products from Marine Cyanobacteria, Nat. Prod. Rep., 2015, 32, 478–503 RSC. For recent selected examples, see: (c) H. Luo, H. Yin, C. Tang, P. Wang and F. Liang, Synthesis of Cyclic Peptide Reniochalistatin E and Conformational isomers, Chin. Chem. Lett., 2018, 29, 1143–1146 CrossRef CAS; (d) K. C. Nicolaou, R. D. Erande, J. Yin, D. Vourloumis, M. Aujay, J. Sandoval, S. Munneke and J. Gavrilyuk, Improved Total Synthesis of Tubulysins and Design, Synthesis, and Biological Evaluation of New Tubulysins with Highly Potent Cytotoxicities against Cancer Cells as Potential Payloads for Antibody–Drug Conjugates, J. Am. Chem. Soc., 2018, 140, 3690–3711 CrossRef CAS PubMed; (e) M. Noden, R. Moreira, E. Huang, A. Yousef, M. Palmer and S. D. Taylor, Total Synthesis of Paenibacterin and Its Analogues, J. Org. Chem., 2019, 84, 5339–5347 CrossRef CAS.
- (a) K. L. McPhail, J. Correa, R. G. Linington, J. Gonzalez, E. Ortega-Barria, T. L. Capson and W. H. Gerwick, Antimalarial Linear Lipopeptides from a Panamanian Strain of the Marine Cyanobacterium Lyngbya majuscula, J. Nat. Prod., 2007, 70, 984–988 CrossRef CAS PubMed; (b) W. Cai, L. A. Salvador-Reyes, W. Zhang, Q.-Y. Chen, S. Matthew, R. Ratnayake, S. J. Seo, S. Dolles, D. J. Gibson, V. J. Paul and H. Luesch, Apratyramide, a Marine-Derived Peptidic Stimulator of VEGF-A and Other Growth Factors with Potential Application in Wound Healing, ACS Chem. Biol., 2018, 13, 91–99 CrossRef CAS; (c) A. Stoye, A. Juillard, A. H. Tang, J. Legac, J. Gut, K. L. White, S. A. Charman, P. J. Rosenthal, G. E. R. Grau, N. H. Hunt and R. J. Payne, Falcipain Inhibitors Based on the Natural Product Gallinamide A Are Potent in Vitro and in Vivo Antimalarials, J. Med. Chem., 2019, 62, 5562–5578 CrossRef CAS; (d) I. Momose, T. Onodera, H. Doi, H. Adachi, M. Iijima, Y. Yamazaki, R. Sawa, Y. Kubota, M. Igarashi and M. Kawada, Leucinostatin Y: A Peptaibiotic Produced by the Entomoparasitic Fungus Purpureocillium lilacinum 40-H-28, J. Nat. Prod., 2019, 82, 1120–1127 CrossRef CAS.
- (a) T. Jauset and M.-E. Beaulieu, Bioactive Cell Penetrating Peptides and Proteins in Cancer: A Bright Future Ahead, Curr. Opin. Pharmacol., 2019, 47, 133–140 CrossRef CAS; (b) K. Fosgerau and T. Hoffmann, Peptide Therapeutics: Current Status and Future Directions, Drug Discovery Today, 2015, 20, 122–128 CrossRef CAS; (c) G. Guidotti, L. Brambilla and D. Rossi, Cell-Penetrating Peptides: From Basic Research to Clinics, Trends Pharmacol. Sci., 2017, 38, 406–424 CrossRef CAS; (d) B. Negi, D. Kumar and D. S. Rawat, Marine Peptides As Anticancer Agents: A Remedy To Mankind By Nature, Curr. Protein Pept. Sci., 2017, 18, 885–904 CrossRef CAS.
- (a) G. R. Pettit, The Dolastatins, Prog. Chem. Org. Nat. Prod., 1997, 70, 1–79 CAS; (b) J. Poncet, The dolastatins, A Family of Promising Antineoplastic agents, Curr. Pharm. Des., 1999, 5, 139–162 CAS; (c) E. Flahive and J. K. Srirangam, in Anticancer Agents from Natural Products, ed. G. M. Cragg, D. G. I. Kingston and D. M. Newman, CRC Press/Taylor & Francis Group, Boca Raton, FL, 2nd edn, 2012, pp. 263–290 Search PubMed.
- (a) G. R. Pettit, Y. Kamano, C. Dufresne, R. L. Cerny, C. L. Herald and J. M. Schmidt, Isolation and Structure of the Cytostatic Linear Depsipeptide Dolastatin 15, J. Org. Chem., 1989, 54, 6005–6006 CrossRef CAS; (b) G. R. Pettit, Y. Kamano, H. Kizu, C. Dufresne, C. L. Herald, R. J. Bontems, J. M. Schmidt, F. E. Boettner and R. A. Nieman, Antineoplastic agents. 173. Isolation and Structure of the Cell Growth Inhibitory Depsipeptides Dolastatins 11 and 12, Heterocycles, 1989, 28, 553–558 CrossRef CAS; (c) G. R. Pettit, Y. Kamano, C. L. Herald, Y. Fujii, H. Kizu, M. R. Boyd, F. E. Boettner, D. L. Doubek and J. M. Schmidt, et al., Isolation of Dolastatins 10-15 from the Marine Mollusc Dolabella auricularia, Tetrahedron, 1993, 49, 9151–9170 CrossRef CAS.
- (a) G. R. Pettit, Y. Kamano, C. L. Herald, C. Dufresne, R. L. Cerny, D. L. Herald, J. M. Schmidt and H. Kizu, Antineoplastic agent. 174. Isolation and Structure of the Cytostatic Depsipeptide Dolastatin 13 from the Sea hare Dolabella auricularia, J. Am. Chem. Soc., 1989, 111, 5015–5017 CrossRef CAS; (b) G. R. Pettit, Y. Kamano, C. L. Herald, C. Dufresne, R. B. Bates, J. M. Schmidt, R. L. Cerny and H. Kizu, Antineoplastic agents. 190. Isolation and Structure of the Cyclodepsipeptide Dolastatin 14, J. Org. Chem., 1990, 55, 2989–2990 CrossRef CAS; (c) L. M. Nogle and W. H. Gerwick, Isolation of four New Cyclic Depsipeptides, Antanapeptins A-D, and Dolastatin 16 from a Madagascan Collection of Lyngbya majuscula, J. Nat. Prod., 2002, 65, 21–24 CrossRef CAS.
- (a) R. Bai, G. R. Pettit and E. Hamel, Binding of Dolastatin 10 to Tubulin at A Distinct Site for Peptide Antimitotic agents near the Exchangeable Nucleotide and Vinca Alkaloid Sites, J. Biol. Chem., 1990, 265, 17141–17149 CAS; (b) G. R. Pettit, N. Melody and J.-C. Chapuis, Antineoplastic Agents. 603. Quinstatins: Exceptional Cancer Cell Growth Inhibitors, J. Nat. Prod., 2017, 80, 692–698 CrossRef CAS; (c) T.-T. Liang, Q. Zhao, S. He, F.-Z. Mu, W. Deng and B.-N. Han, Modeling Analysis of Potential Target of Dolastatin 16 by Computational Virtual Screening, Chem. Pharm. Bull., 2018, 66, 602–607 CrossRef CAS.
- (a) G. R. Pettit, Y. Kamano, C. L. Herald, A. A. Tuinman, F. E. Boettner, H. Kizu, J. M. Schmidt, L. Baczynskyj, K. B. Tomer and R. J. Bontems, The isolation and Structure of A Remarkable Marine Animal Antineoplastic Constituent: Dolastatin 10, J. Am. Chem. Soc., 1987, 109, 6883–6885 CrossRef CAS; (b) G. R. Pettit, S. B. Singh, F. Hogan, P. Lloyd-Williams, D. L. Herald, D. D. Burkett and P. J. Clewlow, Antineoplastic agents. Part 189. The Absolute Configuration and Synthesis of Natural (−)-Dolastatin 10, J. Am. Chem. Soc., 1989, 111, 5463–5465 CrossRef CAS; (c) R. Bai, R. E. Schwartz, J. A. Kepler, G. R. Pettit and E. Hamel, Characterization of the Interaction of Cryptophycin 1 with Tubulin: Binding in the Vinca domain, Competitive Inhibition of Dolastatin 10 binding, and An Unusual Aggregation Reaction, Cancer Res., 1996, 56, 4398–4406 CAS.
- For selected examples, see: (a) K. Tomioka, M. Kanai and K. Koga, An Expeditious Synthesis of Dolastatin 10, Tetrahedron Lett., 1991, 32, 2395–2398 CrossRef CAS; (b) T. Shioiri, K. Hayashi and Y. Hamada, Stereoselective Synthesis of Dolastatin 10 and its Congeners, Tetrahedron, 1993, 49, 1913–1924 CrossRef CAS; (c) C. Mordant, S. Reymond, H. Tone, D. Lavergne, R. Touati, B. Ben Hassine, V. Ratovelomanana-Vidal and J.-P. Genet, Total Synthesis of Dolastatin 10 through Ruthenium-catalyzed Asymmetric Hydrogenations, Tetrahedron, 2007, 63, 6115–6123 CrossRef CAS; (d) J. Dugal-Tessier, S. D. Barnscher, A. Kanai and B. A. Mendelsohn, Synthesis and Evaluation of Dolastatin 10 Analogues Containing Heteroatoms on the Amino Acid Side Chains, J. Nat. Prod., 2017, 80, 2484–2491 CrossRef CAS; (e) M. Akaiwa, T. Martin and B. A. Mendelsohn, Synthesis and Evaluation of Linear and Macrocyclic Dolastatin 10 Analogues Containing Pyrrolidine Ring Modifications, ACS Omega, 2018, 3, 5212–5221 CrossRef CAS; (f) S. Yokosaka, A. Izawa, C. Sakai, E. Sakurada, Y. Morita and Y. Nishio, Synthesis and Evaluation of Novel Dolastatin 10 Derivatives for Versatile Conjugations, Bioorg. Med. Chem., 2018, 26, 1643–1652 CrossRef CAS.
- (a) A. Maderna and C. A. Leverett, Recent Advances in the Development of New Auristatins: Structural Modifications and Application in Antibody Drug Conjugates, Mol. Pharmaceutics, 2015, 12, 1798–1812 CrossRef CAS; (b) B. A. Mendelsohn, S. D. Barnscher, J. T. Snyder, Z. An, J. M. Dodd and J. Dugal-Tessier, Investigation of Hydrophilic Auristatin Derivatives for Use in Antibody Drug Conjugates, Bioconjugate Chem., 2017, 28, 371–381 CrossRef CAS; (c) K. C. Nicolaou and S. Rigol, The Role of Organic Synthesis in the Emergence and Development of Antibody–Drug Conjugates as Targeted Cancer Therapies, Angew. Chem., Int. Ed., 2019, 58, 11206–11241 CrossRef CAS.
- (a) G. R. Pettit and M. P. Grealish, A Cobalt-Phosphine Complex Directed Reformatskii Approach to a Stereospecific Synthesis of the Dolastatin 10 Unit Dolaproine (Dap), J. Org. Chem., 2001, 66, 8640–8642 CrossRef CAS; (b) P. K. Gajula, J. Asthana, D. Panda and T. K. Chakraborty, A Synthetic Dolastatin 10 Analogue Suppresses Microtubule Dynamics, Inhibits Cell Proliferation, and Induces Apoptotic Cell Death, J. Med. Chem., 2013, 56, 2235–2245 CrossRef CAS.
- (a) M. Li, P. Han, Z.-Y. Mao, W. Zhou, C.-M. Si, J. Xiong, B.-G. Wei and J.-F. Hu, Studies toward Asymmetric Synthesis of Hoiamides A and B, Tetrahedron Lett., 2016, 57, 5620–5623 CrossRef CAS; (b) L.-P. Shao, C.-M. Si, Z.-Y. Mao, W. Zhou, T. F. Molinski, B.-G. Wei and G.-Q. Lin, Synthesis and Structure Revision of Symplocin A, Org. Chem. Front., 2017, 4, 995–1004 RSC; (c) Z.-Y. Mao, C.-M. Si, Y.-W. Liu, H.-Q. Dong, B.-G. Wei and G.-Q. Lin, Divergent Synthesis of Revised Apratoxin E, 30-epi-Apratoxin E, and 30S/30R-Oxoapratoxin E, J. Org. Chem., 2017, 82, 10830–10845 CrossRef CAS; (d) W. Zhou, X.-D. Nie, Y. Zhang, C.-M. Si, Z. Zhou, X. Sun and B.-G. Wei, A Practical Approach to Asymmetric Synthesis of Dolastatin 10, Org. Biomol. Chem., 2017, 15, 6119–6131 RSC.
- (a) G.-Q. Lin, M.-H. Xu, Y.-W. Zhong and X.-W. Sun, An Advance on Exploring N-tert-Butanesulfinyl Imines in Asymmetric Synthesis of Chiral Amines, Acc. Chem. Res., 2008, 41, 831–840 CrossRef CAS PubMed; (b) M. T. Robak, M. A. Herbage and J. A. Ellman, Synthesis and Applications of tert-Butanesulfinamide, Chem. Rev., 2010, 110, 3600–3740 CrossRef CAS PubMed.
- G. Liu, D. A. Cogan, T. D. Owens, T. P. Tang and J. A. Ellman, Synthesis of Enantiomerically Pure N-tert-Butanesulfinyl Imines (tert-Butanesulfinimines) by the Direct Condensation of tert-Butanesulfinamide with Aldehydes and Ketones, J. Org. Chem., 1999, 64, 1278–1284 CrossRef CAS.
- (a) T. Skrydstrup, T. Jespersen, J.-M. Beau and M. Bols, A New and Convenient Benzyloxyalkylating Agent induced by Samarium diiodide, Chem. Commun., 1996, 515–516 RSC; (b) B.-G. Wei, J. Chen and P.-Q. Huang, A New Approach for the Asymmetric Syntheses of 2-epi-Deoxoprosopinine and Aza-sugar Derivatives, Tetrahedron, 2006, 62, 190–198 CrossRef CAS.
- (a) D. B. Dess and J. C. Martin, Readily Accessible 12-I-5 Oxidant for the Conversion of Primary and Secondary Alcohols to Aldehydes and Ketones, J. Org. Chem., 1983, 48, 4155–4156 CrossRef CAS; (b) D. B. Dess and J. C. Martin, A Useful 12-I-5 Triacetoxyperiodinane (the Dess-Martin Periodinane) for the Selective Oxidation of Primary or Secondary Alcohols and A Variety of Related 12-I-5 Species, J. Am. Chem. Soc., 1991, 113, 7277–7287 CrossRef CAS.
- (a) B. S. Bal, W. E. Childers and H. W. Pinnick, Oxidation of α,β-Unsaturated Aldehydes, Tetrahedron, 1981, 37, 2091–2096 CrossRef CAS; (b) F. Glaus, D. Dedić, P. Tare, V. Nagaraja, L. Rodrigues, J. A. Aínsa, J. Kunze, G. Schneider, R. C. Hartkoorn, S. T. Cole and K.-H. Altmann, Total Synthesis of Ripostatin B and Structure–Activity Relationship Studies on Ripostatin Analogs, J. Org. Chem., 2018, 83, 7150–7172 CrossRef CAS.
- S. Kokinaki, L. Leondiadis and N. Ferderigos, A Novel and Efficient Method for Cleavage of Phenacylesters by Magnesium Reduction with Acetic Acid, Org. Lett., 2005, 7, 1723–1724 CrossRef CAS.
- K. C. Nicolaou, J. Yin, D. Mandal, R. D. Erande, P. Klahn, M. Jin, D. Vourloumis, M. Aujay, J. Sandoval, J. Gavrilyuk and D. Vourloumis, Total Synthesis and Biological Evaluation of Natural and Designed Tubulysins, J. Am. Chem. Soc., 2016, 138, 1698–1708 CrossRef CAS.
- (a) C.-M. Si, Z.-Y. Mao, Z. Zhou, Z.-T. Du and B.-G. Wei, Divergent Synthesis of L-685,458 and its Analogues involving One-pot Intramolecular Tandem Sequence Reaction, Tetrahedron, 2015, 71, 9396–9402 CrossRef CAS; (b) Y.-W. Liu, Z.-Y. Mao, R.-J. Ma, J.-H. Yan, C.-M. Si and B.-G. Wei, Divergent Syntheses of L-733, 060 and CP-122721 from Functionalized Pieridinones Made by One-pot Tandem Cyclization, Tetrahedron, 2017, 73, 2100–2108 CrossRef CAS; (c) X.-M. Wang, Y.-W. Liu, Q.-E. Wang, Z. Zhou, C.-M. Si and B.-G. Wei, A Divergent Method to Key Unit of Tubulysin V through One-pot Diastereoselective Mannich process of N,O-acetal with Ketone, Tetrahedron, 2019, 75, 260–268 CrossRef CAS.
- G. Wang, C. A. James, N. A. Meanwell, L. G. Hamann and M. Belema, A Scalable Synthesis of (1R,3S,5R)-2-(tert-Butoxycarbonyl)-2-azabicyclo[3.1.0]hexane-3-carboxylic Acid, Tetrahedron Lett., 2013, 54, 6722–6724 CrossRef CAS.
- (a) D. A. Evans, H. P. Ng, J. S. Clark and D. L. Rieger, Diastereoselective Anti Aldol Reactions of Chiral Ethyl Ketones. Enantioselective Processes for the Synthesis of Polypropionate Natural Products, Tetrahedron, 1992, 48, 2127–2142 CrossRef CAS; (b) G. Ehrlich and C. B. W. Stark, Synthesis of Cytospolide Analogues and Late-State Diversification Thereof, J. Org. Chem., 2019, 84, 3132–3147 CrossRef CAS.
- S.-T. Ruan, J.-M. Luo, Y. Du and P.-Q. Huang, Asymmetric Vinylogous Mannich Reactions: A Versatile Approach to Functionalized Heterocycles, Org. Lett., 2011, 13, 4938–4941 CrossRef CAS.
- K. W. Kells and J. M. Chong, Addition of Bu3SnLi to tert-Butanesulfinimines as an Efficient Route to Chiral, Nonracemic α-Aminoorganostannanes, Org. Lett., 2003, 5, 4215–4218 CrossRef CAS.
- M. S. Maji, R. Fröhlich and A. Studer, Desymmetrization of Metallated Cyclohexadienes with Chiral N-tert-Butanesulfinyl Imines, Org. Lett., 2008, 10, 1847–1850 CrossRef CAS.
- T. Moragas, I. Churcher, W. Lewis and R. A. Stockman, Asymmetric Synthesis of Trisubstituted Aziridines via Aza-Darzens Reaction of Chiral Sulfinimines, Org. Lett., 2014, 16, 6290–6293 CrossRef CAS.
- Q. Yao and C. Yuan, Enantioselective Synthesis of H-Phosphinic Acids Bearing Natural Amino Acid Residues, J. Org. Chem., 2013, 78, 6962–6974 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9qo01292c |
| This journal is © the Partner Organisations 2020 |