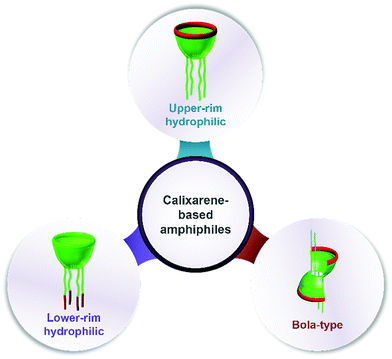
Assembling features of calixarene-based amphiphiles and supra-amphiphiles
Han-Wen
Tian
,
Yan-Cen
Liu
and
Dong-Sheng
Guo
 *
*
College of Chemistry, Key Laboratory of Functional Polymer Materials (Ministry of Education), State Key Laboratory of Elemento-Organic Chemistry, Nankai University, Tianjin 300071, China. E-mail: dshguo@nankai.edu.cn
First published on 27th September 2019
Abstract
Macrocyclic amphiphiles as an emerging family of artificial amphiphiles have gained considerable attention in recent years on account of their fascinating recognition and assembly properties. Benefiting from a preorganized framework, facile modification and host–guest recognition ability, calixarenes have been widely used to fabricate self-assemblies of both amphiphiles and supra-amphiphiles. In this review, we organized hundreds of reported amphiphilic calixarenes based on their structures and systematically summarized assembling features of calixarene-based amphiphiles and supra-amphiphiles. For amphiphilic calixarenes, the size and conformation of skeletons significantly affect their assembly behaviors, such as lower critical aggregation concentration (CAC) and more diverse morphology than conventional amphiphiles. Besides, we also focus on emerging topics like uniformity, compactness, and kinetic properties of calixarene aggregation. For supra-amphiphiles, the binding affinities of calixarenes endow them with the ability to induce guest assembly. In addition, complexation of guests also improves amphiphilic calixarene aggregation. The obtained assemblies not only possess the advantages of low CAC and compact packing, but also respond to various stimuli. Finally, we pointed out several research topics of calixarene-based amphiphiles and supra-amphiphiles to be further developed in the future, such as the relationship between molecular structures and assembly properties, crosslinking, co-assembly, and utilization of cavities. We hope this review could be a guidance for studying amphiphilic assemblies based on calixarenes and other macrocyclic compounds.
1. Introduction
Amphiphiles are molecules that contain both a hydrophobic component and a hydrophilic component connected by covalent bonds.1 Inspired by nature, synthetic amphiphilic molecules enrich the concept of amphiphiles. Based on the number and properties of polar head(s)/hydrophobic tail(s) as well as their manner of connection, amphiphiles are classified as conventional amphiphiles (single head/single tail), bolaamphiphiles, gemini amphiphiles, double and triple chain amphiphiles, catanionic amphiphiles, amphiphilic polymers, etc.2 Owing to their unique structures, amphiphiles can assemble into aggregates such as micelles, vesicles, lyotropic liquid crystals, 2D monolayers and 3D multilayers,1 resulting in important biological functions and various applications in our daily life and industry.3As an emerging family of artificial amphiphiles, macrocyclic amphiphiles have gained considerable attention in recent years on account of their fascinating recognition and assembly properties.3,4 Just as their name implies, macrocyclic amphiphiles are obtained by introducing hydrophilic groups and lipophilic groups to the preorganized scaffold. They incorporate both bola-type and gemini-type amphiphiles into a single molecule from the viewpoint of structural characteristics. Besides, the unique advantage of macrocyclic amphiphiles is the host–guest recognition. Macrocyclic amphiphiles are deemed as “surfactants with host–guest recognition sites”,5 whose macrocyclic binding sites are distributed on the surface of the amphiphilic assembly. Up to now, cyclodextrin,6 calixarene7 and pillararene8,9 have been the commonly used compounds to construct macrocyclic amphiphiles.
On the other hand, by combining supramolecular chemistry and amphiphiles, supra-amphiphiles have attracted widespread attention of scientists.10 In contrast to amphiphiles based on covalent bonds, supra-amphiphiles refer to amphiphiles constructed on the basis of noncovalent interactions or dynamic covalent bonds, which are very useful in the fabrication of nanomaterials with a high degree of structural complexity. Functional groups can be attached to supra-amphiphiles by employing various noncovalent interactions, greatly avoiding tedious covalent syntheses. Moreover, the dynamic and reversible nature of noncovalent interactions endows the resultant supramolecular architectures with excellent stimuli-responsive features. Due to their unique advantages, supra-amphiphiles are being widely and actively investigated in materials and biomedical sciences nowadays.11,12
Calixarenes are the third generation of macrocyclic compounds composed of phenolic units linked by methylene groups at the o-positions of phenolic hydroxyl groups. Their history dates back to the late nineteenth century, but they did not receive wide attention for a long time until Gutsche and coworkers studied calixarenes as mimic enzymes.13 Calixarenes have several sites for derivation and their sizes can be adjusted. Moreover, chemical modification, especially with water soluble groups, could significantly enhance their binding affinity. Benefiting from these properties, calixarenes have been described as macrocycles which have “(almost) unlimited possibilities”14 and have been widely used to fabricate amphiphiles and supra-amphiphiles. There have been a couple of reviews about calixarene-based amphiphiles and supra-amphiphiles. In 2010, Helttunena and Shahgaldian summarized self-assembly of amphiphilic calixarenes and resorcinarenes in water, and classified aggregates by their morphology.15 Later, Garcia-Rio and coworkers published a review which focuses on a promising series of calixarene, p-sulfonatocalixarene,16 while Klymchenko and coworkers focused on amphiphilic calixarenes as gene delivery vehicles.17 Recently, Guo and coworkers discussed assembly behaviors of calixarene-based amphiphiles and supra-amphiphiles, and focused on their applications in drug delivery and protein recognition.18 Up to now, calixarene-based amphiphiles and supra-amphiphiles have been widely used in many fields such as sensing,19 adsorption and extraction,20,21 catalysis,22 inorganic–organic hybrid materials,23 preparation of chiral materials24 and photoluminescent materials,25 and biomedical applications.18,26–35
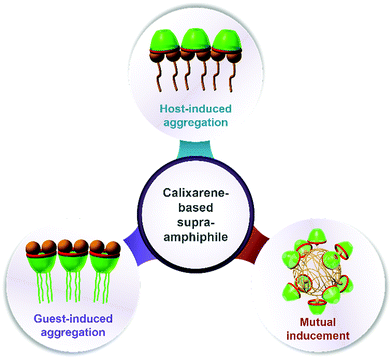
In this review, we will summarize calixarene-based amphiphiles and supra-amphiphiles reported up to now and focus our special attention on their assembling features in aqueous solution. The structure of this review will be such that we first summarize and comprehensively list chemical structures of amphiphilic calixarenes, including upper-rim hydrophilic amphiphiles, lower-rim hydrophilic amphiphiles and bola-type amphiphiles, followed by their assembling features. Next we summarize the self-assemblies of calixarene-based supra-amphiphiles and their assembling features, focusing on complexation-induced aggregation (guest-induced aggregation of host, host-induced aggregation of guest, and mutual inducement).
2. Calixarene-based amphiphiles
2.1 Fabricating amphiphilic calixarenes by covalent modification
Calixarenes possess several sites which are easily modified, such as an upper rim, lower rim, and methylene bridge. As a result, more than four hundred amphiphilic calixarenes were obtained by simply modifying hydrophilic or hydrophobic groups on scaffolds. Most works focused on amphiphilic calixarenes in the cone conformation,36 in which hydrophilic and hydrophobic groups are decorated on opposite rims, resulting in upper-rim hydrophilic amphiphiles and lower-rim hydrophilic amphiphiles (Scheme 1). Moreover, the adjustable conformation of calixarenes makes it easy to modify them on the basis of an alternate conformation, or stabilize the alternate conformation after modification. The obtained compounds are bola-type amphiphiles. We comprehensively list these three classes of amphiphilic calixarenes reported up to now in Schemes 2–4 and Tables 1–3 in order to facilitate readers for following this field and further studies.| Compound | Ref. | Compound | Ref. | Compound | Ref. | Compound | Ref. | Compound | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 186 | 49, 187 and 188 | 236 | 208 | 286 | 235 and 236 | 336 | 243 | 386 | 245 |
| 187 | 37 and 49 | 237 | 200 | 287 | 235 and 236 | 337 | 243 and 244 | 387 | 245 |
| 188 | 194 | 238 | 200, 204 and 223 | 288 | 237 | 338 | 244 | 388 | 245 |
| 189 | 197 | 239 | 204 and 227 | 289 | 247 | 339 | 244 | 389 | 245 |
| 190 | 197 | 240 | 200 | 290 | 247 | 340 | 248 | 390 | 245 |
| 191 | 197 | 241 | 200 and 223 | 291 | 249 | 341 | 244 | 391 | 245 |
| 192 | 190, 197 and 201 | 242 | 200 | 292 | 232 and 250 | 342 | 244 | 392 | 245 |
| 193 | 203 | 243 | 191 | 293 | 250 | 343 | 244 | 393 | 245 |
| 194 | 210 | 244 | 191 | 294 | 251–254 | 344 | 244 | 394 | 245 |
| 195 | 199 and 212 | 245 | 191 | 295 | 257 | 345 | 245 | 395 | 245 |
| 196 | 203 | 246 | 191 | 296 | 257 | 346 | 245 | 396 | 245 |
| 197 | 193 | 247 | 191 | 297 | 49 | 347 | 245 | 397 | 245 |
| 198 | 92 | 248 | 191 | 298 | 49 | 348 | 245 | 398 | 245 |
| 199 | 113 and 198 | 249 | 191 | 299 | 49 | 349 | 245 | 399 | 245 |
| 200 | 198 | 250 | 191 | 300 | 49 | 350 | 245 | 400 | 245 |
| 201 | 113 | 251 | 191 | 301 | 49 | 351 | 245 | 401 | 245 |
| 202 | 45, 198 and 230 | 252 | 191 | 302 | 246 | 352 | 245 | 402 | 262 |
| 203 | 45 | 253 | 209 | 303 | 246 | 353 | 245 | 403 | 262 |
| 204 | 113 and 198 | 254 | 211 | 304 | 246 | 354 | 245 | 404 | 262 |
| 205 | 238 | 255 | 213 | 305 | 259 | 355 | 245 | 405 | 262 |
| 206 | 189 | 256 | 213 | 306 | 246 | 356 | 245 | 406 | 262 |
| 207 | 189 | 257 | 213 | 307 | 246 | 357 | 245 | 407 | 22 |
| 208 | 189 | 258 | 213 | 308 | 246 | 358 | 245 | ||
| 209 | 198 | 259 | 213 | 309 | 263 | 359 | 245 | ||
| 210 | 102 | 260 | 213 | 310 | 264 | 360 | 245 | ||
| 211 | 198 | 261 | 194 and 229 | 311 | 242 | 361 | 244 | ||
| 212 | 202 and 203 | 262 | 194 and 229 | 312 | 246 | 362 | 244 | ||
| 213 | 207 | 263 | 234 | 313 | 246 | 363 | 244 | ||
| 214 | 207 | 264 | 234 | 314 | 246 | 364 | 244 | ||
| 215 | 207 | 265 | 234 and 241 | 315 | 246 | 365 | 245 | ||
| 216 | 190, 207 and 215–220 | 266 | 192 | 316 | 246 | 366 | 245 | ||
| 217 | 190, 207, 215, 216, 219, 221 and 222 | 267 | 192 | 317 | 246 | 367 | 245 | ||
| 218 | 190, 207 and 215–219 | 268 | 196 | 318 | 195 | 368 | 245 | ||
| 219 | 190, 193, 201, 207, 215, 216, 225 and 226 | 269 | 196 | 319 | 255 | 369 | 256 | ||
| 220 | 207 and 216 | 270 | 196 | 320 | 255 | 370 | 258 | ||
| 221 | 207 and 216 | 271 | 196 | 321 | 49 | 371 | 258 | ||
| 222 | 231 | 272 | 206 | 322 | 49 | 372 | 245 | ||
| 223 | 233 | 273 | 206 | 323 | 244 | 373 | 245 | ||
| 224 | 231 | 274 | 206 | 324 | 244 | 374 | 245 | ||
| 225 | 239 and 240 | 275 | 214 | 325 | 259 | 375 | 245 | ||
| 226 | 190 | 276 | 214 | 326 | 260 and 261 | 376 | 245 | ||
| 227 | 193 | 277 | 214 | 327 | 244 | 377 | 245 | ||
| 228 | 195 | 278 | 224 | 328 | 244 | 378 | 245 | ||
| 229 | 199 | 279 | 224 | 329 | 244 | 379 | 245 | ||
| 230 | 200 | 280 | 228 | 330 | 244 | 380 | 245 | ||
| 231 | 200 | 281 | 228 | 331 | 248 | 381 | 245 | ||
| 232 | 200, 204 and 205 | 282 | 188 and 232 | 332 | 198 | 382 | 245 | ||
| 233 | 208 | 283 | 235–237 | 333 | 243 and 244 | 383 | 245 | ||
| 234 | 208 | 284 | 235–237 | 334 | 243 | 384 | 245 | ||
| 235 | 208 | 285 | 235 and 236 | 335 | 243 | 385 | 245 |
| Compound | Ref. | Compound | Ref. | Compound | Ref. | Compound | Ref. |
|---|---|---|---|---|---|---|---|
| 408 | 40 | 420 | 265 | 432 | 266 | 444 | 267 |
| 409 | 40 | 421 | 265 | 433 | 266 | 445 | 267 |
| 410 | 230 | 422 | 268 | 434 | 266 | 446 | 269 |
| 411 | 230 | 423 | 270 | 435 | 271 | 447 | 269 |
| 412 | 40 | 424 | 272 | 436 | 271 | 448 | 273 |
| 413 | 40 | 425 | 272 | 437 | 271 | 449 | 273 |
| 414 | 40 | 426 | 269 and 272 | 438 | 271 | 450 | 273 |
| 415 | 230 | 427 | 268 | 439 | 271 | 451 | 273 |
| 416 | 274 | 428 | 232 | 440 | 271 | 452 | 242 and 275–277 |
| 417 | 228 | 429 | 120 | 441 | 271 | 453 | 273 |
| 418 | 228 | 430 | 247 | 442 | 271 | — | — |
| 419 | 228 | 431 | 247 | 443 | 267 | — | — |
As we can see from the schemes, most amphiphilic calixarenes are based on calix[4]arene. Calix[5]arene, calix[6]arene, calix[8]arene, calix[9]arene, and thiacalix[4]arene are also involved. For upper-rim hydrophilic amphiphiles, almost all the common substitutions which are possible for phenols have been carried out at the upper rim. For example, a sulfonate group is widely introduced because of its excellent water-solubility and convenient one-step reaction. A nitro group and a halogen (or benzyl halide) group were also attached to the upper-rim by a one-step reaction. Further derivatization from them results in numerous functional groups such as the phosphate group, guanidinium group, carboxylic group, amino group, azide group and so on. It is noteworthy that carboxylic group and amino group could involve in amide condensation, and the azide group could react with an alkynyl compound. These well-established reactions provide the possibility of decorating calixarene with almost everything, such as cyclodextrin, PEG, saccharide, and cholesterol. On the other hand, the hydrophobic moieties of most upper-rim hydrophilic amphiphiles were introduced by alkyl halides reacting with phenolic hydroxyl at the lower rim. Meanwhile, similar nucleophilic substitution has been applied for PEG chains, resulting in lower-rim hydrophilic amphiphiles. The upper-rim attached hydrophobic chain of most lower-rim hydrophilic amphiphiles were introduced at the cyclic formation step, i.e., using p-alkylphenol as a reactant. Among them, the most popular p-alkylphenol is p-tert-butylphenol. In addition, the alkyl chain can be connected to the methylene, being introduced at the cyclic formation step as well. For bola-type amphiphiles, their conformations were usually controlled by template metal ions, for example, calix[4]arene tends to form alternative conformers in the presence of cesium carbonate. Since all these factors (skeleton, hydrophobic chain length, hydrophilic group, and conformation) affect the hydrophilic–hydrophobic balance, amphiphilic calixarenes with various assembly properties have been obtained by taking advantage of convenient synthesis.
2.2 Assembling features of amphiphilic calixarenes
Reported CAC values of amphiphilic calixarenes are summarized in Table 4. It is easy to notice that a large proportion of amphiphilic calixarenes have quite low CACs (<1 mM) compared with common surfactants. For example, the CACs of sodium butyl benzene sulfonate, sodium hexyl benzene sulfonate, sodium octyl benzene sulfonate, and sodium dodecyl benzene sulfonate (SDBS) are 100 mM, 30 mM, 14 mM, and 1.5 mM, respectively. The CACs of the corresponding amphiphilic calix[4]arenes 3, 6, 8, and 9 are 3.2 mM, 0.488 mM, 0.085 mM, and 0.02 mM, respectively. As a reference compound, the CAC of the gemini-type SDBS derivate is 0.9 mM. If we consider generalized monomer concentration, the CAC of the gemini-type SDBS derivate is 1.8 mM monomer, which is similar to SDBS, while the CAC of calix[4]arene 9 is 0.08 mM monomer, which is 19 times lower than that of SDBS. The lower CAC of calixarene undoubtedly originates from the cyclic oligomeric structure. From the viewpoint of entropy, amphiphiles in the assemblies have a lower degree of freedom than that in bulky water, so the entropy of amphiphiles (not including water molecules) decreases during the assembly process. The oligomer structure of calixarene leads to much lower entropy loss than the corresponding monomer, resulting in lower CAC as well as a more sensitive response to the structural difference.279
| Compound | CAC (mM) | Conditiona | Methodb | Ref. |
|---|---|---|---|---|
| a The condition is 25 °C in pure water if no label. I is the ionic strength. EY is Eosin Y. b ITC: isothermal titration microcalorimetry, DOSY: diffusion-ordered spectroscopy, AFM: atomic force microscopy, NMR: nuclear magnetic resonance. c The nomenclature and values of CAC1, CAC2, and CAC3 were taken from the original literature without any change. | ||||
| 3 | 2.5 | 30 °C | Conductivity | 49 |
| 3 | 3.05 | 15 °C | ITC | 53 |
| 3 | 3.20 | ITC | 53 | |
| 3 | 3.40 | 35 °C | ITC | 53 |
| 3 | 3.73 | 45 °C | ITC | 53 |
| 3 | 4.16 | 55 °C | ITC | 53 |
| 3 | 3.18 | Conductivity | 54 | |
| 4 | (8.98 ± 2.69) × 10−2 | 10 mM NaCl | Fluorescence | 65 |
| 4 | (5.84 ± 4.49) × 10−2 | 15 mM NaCl | Fluorescence | 65 |
| 4 | 0.566 | 50 mM NaCl | Fluorescence | 66 |
| 6 | 0.54 | Fluorescence | 84 | |
| 6 | 0.32 | D2O | DOSY | 84 |
| 6 | 0.450 | 15 °C | ITC | 53 |
| 6 | 0.488 | ITC | 53 | |
| 6 | 0.520 | 35 °C | ITC | 53 |
| 6 | 0.600 | 45 °C | ITC | 53 |
| 6 | 0.689 | 55 °C | ITC | 53 |
| 6 | 0.491 | Conductivity | 54 | |
| 6 | 0.040 | 10 mM NaCl | Fluorescence | 64 |
| 7 | 0.020 | 10 mM NaCl | Fluorescence | 64 |
| 8 | 0.0700 | 15 °C | ITC | 53 |
| 8 | 0.0850 | ITC | 53 | |
| 8 | 0.0940 | 35 °C | ITC | 53 |
| 8 | 0.112 | 45 °C | ITC | 53 |
| 8 | 0.150 | 55 °C | ITC | 53 |
| 8 | 0.0911 | Conductivity | 54 | |
| 9 | 0.02 | Fluorescence | 109 | |
| 15 | 0.0285 | AFM | 129 | |
| 21 | 0.65 | pH 10 NaHCO3, I = 0.123 M | UV-vis | 38 |
| 31 | 0.045 | pH 10 NaHCO3, I = 0.123 M | UV-vis | 38 |
| 31 | 0.035 | pH 10 NaHCO3, I = 0.123 M | UV-vis | 38 |
| 34 | 1 | pH 6, 20 °C | Surface tension | 41 |
| 34 | 1.3 | pH 8, 20 °C | Surface tension | 41 |
| 35 | 1.6 | pH 6, 20 °C | Surface tension | 41 |
| 35 | 1.3 | pH 8, 20 °C | Surface tension | 41 |
| 36 | 1.3 | pH 6, 20 °C | Surface tension | 41 |
| 36 | 1 | pH 8, 20 °C | Surface tension | 41 |
| 37 | 1.2 | pH 6, 20 °C | Surface tension | 41 |
| 37 | 1.1 | pH 8, 20 °C | Surface tension | 41 |
| 38 | 1.3 | pH 6, 20 °C | Surface tension | 41 |
| 38 | 1.2 | pH 8, 20 °C | Surface tension | 41 |
| 39 | 1.2 | pH 6, 20 °C | Surface tension | 41 |
| 39 | 1 | pH 8, 20 °C | Surface tension | 41 |
| 40 | 1.2 | pH 6, 20 °C | Surface tension | 41 |
| 40 | 1.1 | pH 8, 20 °C | Surface tension | 41 |
| 41 | 1.2 | pH 6, 20 °C | Surface tension | 41 |
| 41 | 0.2 | pH 8, 20 °C | Surface tension | 41 |
| 42 | 0.1 | pH 6, 20 °C | Surface tension | 41 |
| 42 | 0.4 | pH 8, 20 °C | Surface tension | 41 |
| 43 | 0.5 | pH 6, 20 °C | Surface tension | 41 |
| 43 | 0.1 | pH 8, 20 °C | Surface tension | 41 |
| 44 | 0.1 | pH 6, 20 °C | Surface tension | 41 |
| 44 | 0.1 | pH 8, 20 °C | Surface tension | 41 |
| 46 | 0.023 | Conductivity | 91 | |
| 46 | 0.056 | pH 7 Na+/K+ PB | Fluorescence | 91 |
| 46 | 0.037 | pH 9 Na+ borate | Fluorescence | 91 |
| 46 | 0.041 | pH 7 Na+/K+ PB | Fluorescence | 91 |
| 46 | 0.0021 | pH 9 Na+ borate | Fluorescence | 91 |
| 46 | 0.073 | pH 7 Na+/K+ PB | Fluorescence | 91 |
| 46 | 0.052 | pH 9 Na+ borate | Fluorescence | 91 |
| 46 | 0.04 | pH 7 Na+/K+ PB | Fluorescence | 91 |
| 46 | 0.043 | pH 9 Na+ borate, | Fluorescence | 91 |
| 47 | ≤0.04 | I = 0.07 M | Fluorescence | 98 |
| 49 | 0.2 | D2O | 1H NMR | 112 |
| 57 | 0.01 | 17 °C | Surface tension | 132 |
| 57 | 0.01 | 30 °C | Fluorescence | 132 |
| 57 | 3.8 | 1H NMR | 127 | |
| 58 (I−) | 8.7 | 1H NMR | 127 | |
| 58 (Cl−) | 0.00879 | 22–23 °C | Surface tension | 75 |
| 58 (Cl−) | 9.8 | 22–23 °C; 30 °C | UV-vis, osmolality, surface tension | 134 |
| 62 | 0.0052 | Fluorescence | 46 | |
| 64 | 0.37 | Fluorescence | 72 | |
| 64 | 0.067 | 20 mM Tris, pH 7.4 | Fluorescence | 72 |
| 64 | 0.081 | 20 mM Tris, pH 7.4, 150 mM NaCl | Fluorescence | 72 |
| 64 | 4 | UV-vis | 73 | |
| 64 | 0.12 | 0.05 mM NaCl | UV-vis | 73 |
| 64 | 0.367 | 22–23 °C | Surface tension | 75 |
| 64 | 0.39 | Fluorescence | 77 | |
| 64 | 0.068 | 20 mM PB, pH 7.4 | Fluorescence | 77 |
| 64 | 0.064 | 20 mM PB, pH 7.4, 150 mM NaCl | Fluorescence | 77 |
| 65 | 0.026 | Fluorescence | 77 | |
| 65 | 0.0098 | 20 mM PB, pH 7.4 | Fluorescence | 77 |
| 65 | 0.0044 | 20 mM PB, pH 7.4, 150 mM NaCl | Fluorescence | 77 |
| 66 | 0.048 | Fluorescence | 72 | |
| 66 | 0.0062 | 20 mM Tris, pH 7.4 | Fluorescence | 72 |
| 66 | 0.003 | 20 mM Tris, pH 7.4, 150 mM NaCl | Fluorescence | 72 |
| 66 | 0.019 | Fluorescence | 77 | |
| 66 | 0.0044 | 20 mM PB, pH 7.4 | Fluorescence | 77 |
| 66 | 0.0027 | 20 mM PB, pH 7.4, 150 mM NaCl | Fluorescence | 77 |
| 66 | 0.0478 | 22–23 °C | Surface tension | 75 |
| 67 | 0.008 | Fluorescence | 101 | |
| 69 | 0.00075 | Fluorescence | 77 | |
| 69 | 0.001 | 20 mM PB, pH 7.4 | Fluorescence | 77 |
| 72 | 0.017 | Fluorescence | 77 | |
| 72 | 0.0036 | 20 mM PB, pH 7.4 | Fluorescence | 77 |
| 72 | 0.0030 | 20 mM PB, pH 7.4, 150 mM NaCl | Fluorescence | 77 |
| 72 | 0.014 | 20 mM acetate, pH 5 | Fluorescence | 77 |
| 73 | 1.4 | 1H NMR | 127 | |
| 74 | 0.01 | Fluorescence | 72 | |
| 74 | 0.0029 | 20 mM Tris, pH 7.4 | Fluorescence | 72 |
| 74 | 0.0018 | 20 mM Tris, pH 7.4, 150 mM NaCl | Fluorescence | 72 |
| 82 | 0.33 | Fluorescence | 48 | |
| 91 | 0.11 | 50 mM NaCl, pH 3.0 | Fluorescence | 122 |
| 91 | 0.042 | 50 mM NaCl, pH 8.0 | Fluorescence | 122 |
| 91 | 0.11 | pH 8.0 Tris–HCl, 50 mM NaCl | Fluorescence | 123 |
| 91 | 0.11 | pH 3.0, 50 mM NaCl | UV-vis | 124 |
| 94 | 0.0040 | 50 mM NaCl, pH 3.0 | Fluorescence | 122 |
| 97 | 0.0029 | 50 mM NaCl, pH 3.0 | Fluorescence | 122 |
| 98 | 0.0045 | MES pH 6.5 | Fluorescence | 136 |
| 98 | 0.0050 | MES pH 6.5 | Fluorescence | 136 |
| 99 | 0.0028 | MES pH 6.5 | Fluorescence | 136 |
| 99 | 0.0050 | MES pH 6.5 | Fluorescence | 136 |
| 101 | (4.4 ± 0.2) × 10−3 | 50 mM NaCl, pH 3.0 | Fluorescence | 140 |
| 101 | (1.0 ± 0.1) × 10−3 | 50 mM NaCl, pH 8.3 | Fluorescence | 140 |
| 101 | (1.8 ± 0.2) × 10−3 | pH 10, 50 mM NaCl | Fluorescence | 140 |
| 101 | 0.0044 | pH 3.2 | — | 63 |
| 101 | 0.0010 | pH 7.5 | — | 63 |
| 101 | 0.0018 | pH 10 | — | 63 |
| 105 | 0.0064 | MES pH 6.5 | Fluorescence | 136 |
| 105 | 0.79 | MES pH 6.5 | Fluorescence | 136 |
| 106 | 0.0048 | MES pH 6.5 | Fluorescence | 136 |
| 106 | 0.16 | MES pH 6.5 | Fluorescence | 136 |
| 108 | (1.8 ± 0.2) × 10−3 | 150 mM NaCl | Fluorescence | 167 |
| 109 | (5.0 ± 1.0) × 10−4 | 150 mM NaCl | Fluorescence | 167 |
| 124 (D) | 0.13 | pH 8.0 Tris–HCl, 50 mM NaCl | Fluorescence | 123 |
| 124 (L) | 0.10 | pH 8.0 Tris–HCl, 50 mM NaCl | Fluorescence | 123 |
| 125 | 0.00154 | 50 mM NaCl | Fluorescence | 157 |
| 126 | 0.025 | Fluorescence, surface tension | 159 | |
| 131 | 0.21 | 37 °C | Relaxivity | 278 |
| 132 | 2.3 | Relaxivity | 175 | |
| 133 | 0.12 | Relaxivity | 175 | |
| 140 | 0.019 | Fluorescence | 142 | |
| 141 | 0.015 | Fluorescence | 142 | |
| 142 | 0.013 | Fluorescence | 146 | |
| 142 | 0.013 | Fluorescence | 142 | |
| 143 | 0.00014 | 50 mM NaCl | Fluorescence | 150 |
| 144 | 0.00027 | 50 mM NaCl | Fluorescence | 150 |
| 145 | 0.0045 | 50 mM NaCl | Fluorescence | 150 |
| 158 | 0.0055 | Fluorescence | 46 | |
| 160 | 1 | 30 °C | Conductivity | 49 |
| 162 | 0.5 | 30 °C | Surface tension | 5 |
| 162 | 0.67 | 30 °C | Conductivity | 5 |
| 162 | 0.5 | 30 °C | Fluorescence | 5 |
| 162 | 0.636 | 15 °C | ITC | 53 |
| 162 | 0.751 | ITC | 53 | |
| 162 | 0.850 | 35 °C | ITC | 53 |
| 162 | 0.904 | 45 °C | ITC | 53 |
| 162 | 0.957 | 55 °C | ITC | 53 |
| 162 | 0.734 | Conductivity | 54 | |
| 163 (micelle) | 0.6 | DLS | 154 | |
| 163 (domain) | 1 × 10−4 | DLS | 154 | |
| 163 (nanoassociate) | 1 × 10−6 | DLS | 154 | |
| 164 | 0.1 | UV-vis | 155 | |
| 166 | 0.1 | 30 °C | Surface tension | 49 |
| 166 | 0.16 | 30 °C | Conductivity | 49 |
| 170 | (7.9 ± 0.5) × 10−3 | Fluorescence | 173 | |
| 171 | (8.0 ± 0.2) × 10−3 | Fluorescence | 173 | |
| 173 | 0.5 | 30 °C | Surface tension | 49 |
| 173 | 0.7 | 30 °C | Conductivity | 49 |
| 175 | 0.700 | 15 °C | ITC | 53 |
| 175 | 0.750 | ITC | 53 | |
| 175 | 0.810 | 35 °C | ITC | 53 |
| 175 | 0.894 | 45 °C | ITC | 53 |
| 175 | 0.994 | 55 °C | ITC | 53 |
| 175 | 0.729 | Conductivity | 54 | |
| 186 | 0.56 | 30 °C | Surface tension | 49 |
| 186 | 0.55 | 30 °C | Conductivity | 49 |
| 223 | 0.0005 | UV-vis | 257 | |
| 230 | 0.4 | 10% DMF aqueous | Surface tension | 200 |
| 231 | CAC1: 0.95; CAC2: 5.0c | 10% DMF aqueous | Surface tension | 200 |
| 232 | CAC2: 2.2; CAC3: 80c | Surface tension | 200 | |
| 232 | 2 | 205 | ||
| 233 | 2.2 | 208 | ||
| 234 | 2.1 | 208 | ||
| 236 | 2.1 | 208 | ||
| 237 | 0.1 | 10% DMF aqueous | Surface tension | 200 |
| 238 | CAC1: 0.6; CAC2: 3.8; CAC3: 75c | Surface tension | 200 | |
| 238 | 6.5 | Viscosity | 200 | |
| 238 | CAC1: 0.95; CAC2: 6.0; CAC3: 60c | 10% DMF aqueous | Surface tension | 200 |
| 238 | 27 | 10% DMF aqueous | Viscosity | 200 |
| 239 | 2.1 | 204 | ||
| 239 | 2.7 | 227 | ||
| 240 | CAC1: 0.2; CAC2: 2.0; CAC3: 16c | Surface tension | 200 | |
| 240 | 78 | Viscosity | 200 | |
| 240 | CAC1: 0.95; CAC2: 7.6; CAC3: 60c | 10% DMF aqueous | Surface tension | 200 |
| 240 | CAC2: 36; CAC3: 65c | 10% DMF aqueous | Viscosity | 200 |
| 241 | CAC1: 0.18; CAC2: 4.5c | Surface tension | 200 | |
| 241 | 5.5 | Viscosity | 200 | |
| 242 | CAC1: 0.13; CAC2: 0.9c | Surface tension | 200 | |
| 242 | 2.5 | Viscosity | 200 | |
| 261 | 0.51 | Fluorescence | 229 | |
| 262 | 0.22 | Fluorescence | 229 | |
| 294 | 0.64 | D2O | DOSY | 252 |
| 294 | 1.15 | D2O | DOSY | 254 |
| 296 | 0.0045 | UV-vis | 257 | |
| 298 | 0.43 | 30 °C | Conductivity | 49 |
| 299 | 0.61 | 30 °C | Surface tension | 49 |
| 299 | 0.58 | 30 °C | Conductivity | 49 |
| 300 | 0.21 | 30 °C | Surface tension | 49 |
| 300 | 0.25 | 30 °C | Conductivity | 49 |
| 305 | 0.00579 | Fluorescence | 259 | |
| 322 | 0.15 | 30 °C | Surface tension | 49 |
| 322 | 0.40 | 30 °C | Conductivity | 49 |
| 325 | 0.00305 | Fluorescence | 259 | |
| 407 | CAC1: 0.19; CAC2: 6.9c | Surface tension | 22 | |
| 416 | 0.016 | Fluorescence | 274 | |
| 432 | (91 ± 5) × 10−3 | pH 7.4 Tris, pyrene | Fluorescence | 266 |
| 432 | (2.0 ± 0.1) × 10−3 | pH 7.4 Tris, EY | Fluorescence | 266 |
| 433 | (59 ± 3) × 10−3 | pH 7.4 Tris, pyrene | Fluorescence | 266 |
| 433 | (2.6 ± 0.2) × 10−3 | pH 7.4 Tris, EY | Fluorescence | 266 |
| 434 | (33 ± 2) × 10−3 | pH 7.4 Tris, pyrene | Fluorescence | 266 |
| 434 | (2.0 ± 0.1) × 10−3 | pH 7.4 Tris, EY | Fluorescence | 266 |
| 443 | 0.024 | Fluorescence | 267 | |
| 444 | 0.025 | Fluorescence | 267 | |
| 445 | 0.009 | Fluorescence | 267 | |
For example, CACs decrease more rapidly with longer alkyl chains of amphiphilic sulfonatocalix[n]arenes (SCnAs) than that of sodium benzene sulfonate surfactants, as Basilio and co-workers proposed. They systematically investigated the relationship between CACs and the hydrophobic chain length of amphiphilic SCnAs 3, 6, and 8 from the viewpoint of thermodynamics by ITC in detail, and obtained their free energy of micellization 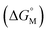 .53,54 They proposed that the
.53,54 They proposed that the 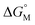 is the sum of contributions of each part of the molecule to the total free energy, such as ionic groups and counterions, aromatic rings, oxygen atoms that connect the aromatic rings to the alkyl chains, methylene group of the bridges, methylene groups of the alkyl chains, and terminal methyl groups of the chains. Among these, the free energy of transferring a methyl group from water to the micellar interior
is the sum of contributions of each part of the molecule to the total free energy, such as ionic groups and counterions, aromatic rings, oxygen atoms that connect the aromatic rings to the alkyl chains, methylene group of the bridges, methylene groups of the alkyl chains, and terminal methyl groups of the chains. Among these, the free energy of transferring a methyl group from water to the micellar interior 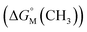 is equal to that of methylene groups
is equal to that of methylene groups 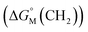 add a constant (which can be represented by
add a constant (which can be represented by 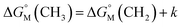 , in which k is a constant). Therefore, the overall contribution of alkyl chains equals the free energy change for transferring one CH2 unit from the aqueous medium to the micellar interior
, in which k is a constant). Therefore, the overall contribution of alkyl chains equals the free energy change for transferring one CH2 unit from the aqueous medium to the micellar interior 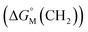 multiplied by the carbon number of the alkyl chain, while other parts remain constant despite the change in carbon number. So the slope of
multiplied by the carbon number of the alkyl chain, while other parts remain constant despite the change in carbon number. So the slope of 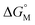 against carbon number is
against carbon number is 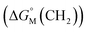 .53,54
.53,54
Since 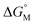 is proportional to log CAC, we plotted log 4CAC of four amphiphilic SC4As with 4, 6, 8, 12 carbons and log CAC of the corresponding monomer, versus the number of carbon atoms in the hydrophobic chain (Fig. 1), and the slope of linear fitting is proportional to
is proportional to log CAC, we plotted log 4CAC of four amphiphilic SC4As with 4, 6, 8, 12 carbons and log CAC of the corresponding monomer, versus the number of carbon atoms in the hydrophobic chain (Fig. 1), and the slope of linear fitting is proportional to 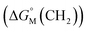 . Results show a negative slope which reflects that the hydrophobic interaction contributes more favourably to the micellization process in the presence of longer alkyl chains. More importantly, the slope of calixarenes (−0.27) is lower than the value generally observed for single-chain surfactants (−0.22), which means
. Results show a negative slope which reflects that the hydrophobic interaction contributes more favourably to the micellization process in the presence of longer alkyl chains. More importantly, the slope of calixarenes (−0.27) is lower than the value generally observed for single-chain surfactants (−0.22), which means 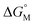 decreases more rapidly with longer alkyl chains of amphiphilic SCnAs than that of sodium sulfonate surfactants. This may be due to the existence of intramolecular interactions between the alkyl chains of the free monomers.53
decreases more rapidly with longer alkyl chains of amphiphilic SCnAs than that of sodium sulfonate surfactants. This may be due to the existence of intramolecular interactions between the alkyl chains of the free monomers.53
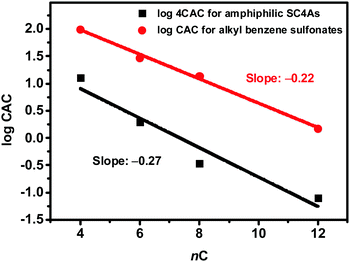 | ||
| Fig. 1 Variation of the CAC with the number of carbons (nC) per alkyl chain for amphiphilic SC4As and alkyl benzene sulfonates. | ||
Similar to the difference between monomers and oligomers, in general, larger size of the skeleton results in a lower degree of entropic cost,279 thus should lead to lower CAC, which is indeed supported by some reported values. For example, Shinkai and co-workers reported that in amphiphilic SCnAs 3, 160, 173, which have 4, 6, and 8 repeat units respectively, the CAC values decrease from 2.5 mM to 1.0 mM and then to 0.7 mM with increasing ring size.49 Zhao and co-workers synthesized amphiphilic calix[6]arene 305 and calix[8]arene 325 by introducing acetoxyls into the hydroxyls of calixarenes.259 They interpreted the decrease in CAC (5.79–3.05 μM) with increasing phenyl groups (6–8) as strengthening of the hydrophobic interactions. However, other examples didn’t show any significant trend. For instance, Xu and coworkers found the CAC of choline-modified calix[5]arene 158 (5.5 μM) is slightly higher than that of the corresponding calix[4]arene, 62 (5.2 μM).46 Shinkai and coworkers reported that the CACs of lower-rim sulfonic group modified 186, 299 and 322 are 0.55 mM, 0.58 mM and 0.40 mM by conductivity at 30 °C, respectively.49 These unexpected phenomena may be explained by the shape of the skeleton and the conformation in bulk solution. As an example of conformation influencing CAC, Basilio and co-workers studied amphiphilic SCnAs 6, 162 and 175. The CAC values increase (from 0.488 to 0.750 mM) with increasing number of monomeric units (from 4 to 8).54 The calix[4]arene derivative, which is preorganized into the cone conformation, is favourable for the formation of globular aggregates. The calix[6]arene and calix[8]arene derivatives do not adopt cone conformations in bulk solution. Further thermodynamic studies show that changing these conformations to the more favourable cone conformer in the aggregates implied an energetic cost that contributed to making the micellization Gibbs free energy 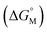 less efficient. The other example related to conformations was reported by Arimori and coworkers.132 CAC of amphiphilic calix[4]arene 412 in a cone conformation is 10 μM whereas in a 1,3-alternate conformation, 412 could not aggregate at a concentration of even up to 10 mM. This difference implies that the conformation of calixarene is a critical factor of CAC.
less efficient. The other example related to conformations was reported by Arimori and coworkers.132 CAC of amphiphilic calix[4]arene 412 in a cone conformation is 10 μM whereas in a 1,3-alternate conformation, 412 could not aggregate at a concentration of even up to 10 mM. This difference implies that the conformation of calixarene is a critical factor of CAC.
On the other hand, similar to conventional surfactants, CACs of amphiphilic calixarenes are directly related to their hydrophobic/hydrophilic groups and affected by environments (temperature, pH, ionic strength and solvent).
For example, Rodik and co-workers synthesized a series of choline modified amphiphilic calixarenes 64–66 and 69 bearing various lengths of alkyl chains at the lower rim.77 They found that CACs decreased (390–0.75 μM) with the increase in the chain length (3–16 CH2 units). And we have already discussed before that CACs decrease more rapidly with longer alkyl chains.
Introducing extra interactions such as hydrogen bonds is an efficient way to enhance assembly, decreasing the CAC. Consoli and co-workers synthesized two amphiphilic calix[4]arenes 261 and 262 decorated with nucleotides at the lower rim.229 The CAC of 262 bearing adenine nucleotides (0.22 mM) is lower than that of 261 bearing thymine nucleotides (0.51 mM), which is consistent with the capacity of adenine to establish stronger stacking interactions with respect to thymine nucleobase.
High salt concentration could reduce electrostatic repulsion of like charges at the hydrophilic head group, resulting in a lower CAC value. Rodik and co-workers synthesized amphiphilic calix[4]arenes 64 and 66 bearing cationic choline groups at the upper rim and alkyl chains at the lower rim.72 Their CACs were found to be decreased from pure water (0.37 mM for 64 and 0.048 mM for 66) to 20 mM Tris buffer (0.067 mM for 64 and 0.0062 mM for 66). Mchedlov-Petrossyan and co-workers found that the CAC of 64 decreased (4–0.12 mM) with increasing concentration of NaCl as well.73
Similarly, the pH value could change the protonation states of hydrophilic heads, influencing charge interactions, resulting in a CAC change. Fujii and co-workers synthesized a new amphiphilic calix[4]arene 91 with hydrophilic amino end groups.122 With pH increasing from 3 (below the pKa of amino group) to 8 (above the pKa of amino group), the CAC decreased (from 0.11 to 0.042 mM). Further, they prepared a new calix[4]arene-based lipid 101 containing glutamic acid as the hydrophilic group.63,140 The α-amine and the γ-carboxylic acid groups of the glutamic acid moiety allowed a continuous change in the state of the head group from cationic to zwitterionic and then to anionic with increasing pH. The CAC at pH 7.5 (1.0 μM) was lower than that obtained under other pH conditions (4.4 μM at pH 3.2 and 1.8 μM at pH 10) because the intermolecular electrostatic repulsions are cancelled by the zwitterionic nature.
| Compound | Morphologya | Radiusb (nm) | Conditionc | Methodd | Ref. |
|---|---|---|---|---|---|
| a NP: nanoparticle, SLN: solid lipid nanoparticle. b Radii are obtained from papers directly or calculated from diameters. Part of data which is not shown in papers clearly is read from figures which contain these data. Rs is the radius of the shell. Rg is the gyration radius. Rh is the hydrodynamic radius. c The condition is 25 °C in pure water if no label. CA is the corresponding amphiphilic calixarene. d SLS: static light scattering, cryo-TEM: cryogenic transmission electron microscopy, HR TEM: high resolution transmission electron microscopy, NTA: nanoparticle tracking analysis method, PGSE: pulse gradient spin echo, FE-SEM: field-emission scanning electron microscopy, EF-TEM: energy-filtered transmission electron microscopy, OPM: optical polarization microscopy, FFF-MALS: field flow fractionation combined with multi-angle light scattering. | |||||
| 3 | Ellipsoidal micelle | Minor: 1.15, major: 6.6 (prolate); 3.5 (oblate) | D2O | DOSY | 54 |
| 4 | Spherical micelle | R s: 2.13; Rg: 1.64 ± 0.02 | 10 mM NaCl | SAXS | 65 |
| 4 | Spherical micelle | R s: 2.19; Rg: 1.73 ± 0.03 | 15 mM NaCl | SAXS | 65 |
| 6 | Ellipsoidal micelle | Major: 4.6 | D2O | DOSY | 84 |
| 6 | Ellipsoidal micelle | Minor: 1.40, major: 8.9 (prolate); 4.6 (oblate) | D2O | DOSY | 54 |
| 6 | Spherical micelle | R s: 2.40; Rg: 1.97 | 10 mM NaCl | SAXS | 64 |
| 7 | Spherical micelle | R s: 2.65; Rg: 2.15 | 10 mM NaCl | SAXS | 64 |
| 8 | Ellipsoidal micelle | Minor: 1.65, major: 7.8 (prolate); 4.3 (oblate) | D2O | DOSY | 54 |
| 8 | Spherical micelle | R s: 2.10 | 10 mM NaCl | SAXS | 64 |
| 9 | Micelle | 6.9 | DLS, AFM | 109 | |
| 11 | Micelle | 2.1–2.8 | Various pH | DLS | 114 |
| 12 | SLN | 85 | DLS, AFM | 126 | |
| 12 | SLN | 78 ± 1 | 0.1 mM NaCl | DLS, AFM | 126 |
| 12 | SLN | 81 ± 2 | 1 mM NaCl | DLS, AFM | 126 |
| 12 | SLN | 81 ± 1 | 15 mM NaCl | DLS, AFM | 126 |
| 12 | SLN | 80 ± 1 | 145 mM NaCl | DLS, AFM | 126 |
| 12 | SLN | 66 ± 1 | 0.1 mM KCl | DLS, AFM | 126 |
| 12 | SLN | 76 ± 3 | 1 mM KCl | DLS, AFM | 126 |
| 12 | SLN | 86 ± 1 | 5 mM KCl | DLS, AFM | 126 |
| 12 | SLN | 83 ± 2 | 140 mM KCl | DLS, AFM | 126 |
| 12 | SLN | 85 ± 1 | 145 mM KCl | DLS, AFM | 126 |
| 12 | SLN | 64 ± 1 | 0.1 mM CaCl2 | DLS, AFM | 126 |
| 12 | SLN | 60 ± 1 | 0.5 mM CaCl2 | DLS, AFM | 126 |
| 12 | SLN | 59 ± 1 | 1 mM CaCl2 | DLS, AFM | 126 |
| 12 | SLN | 105 ± 1 | 2 mM CaCl2 | DLS, AFM | 126 |
| 12 | SLN | 371 ± 13 | 2.5 mM CaCl2 | DLS, AFM | 126 |
| 12 | SLN | 1206 ± 1179 | 3 mM CaCl2 | DLS, AFM | 126 |
| 12 | SLN | 1429 ± 433 | 4 mM CaCl2 | DLS, AFM | 126 |
| 12 | SLN | 1560 ± 485 | 5 mM CaCl2 | DLS, AFM | 126 |
| 12 | SLN | 815 ± 1393 | 145 mM CaCl2 | DLS, AFM | 126 |
| 12 | SLN | 74 ± 2 | 0.1 mM MgCl2 | DLS, AFM | 126 |
| 12 | SLN | 64 ± 1 | 0.5 mM MgCl2 | DLS, AFM | 126 |
| 12 | SLN | 62 ± 1 | 1 mM MgCl2 | DLS, AFM | 126 |
| 12 | SLN | 104 ± 1 | 2 mM MgCl2 | DLS, AFM | 126 |
| 12 | SLN | 141 ± 6 | 2.5 mM MgCl2 | DLS, AFM | 126 |
| 12 | SLN | 507 ± 25 | 3 mM MgCl2 | DLS, AFM | 126 |
| 12 | SLN | 1048 ± 497 | 5 mM MgCl2 | DLS, AFM | 126 |
| 12 | SLN | 1247 ± 435 | 10 mM MgCl2 | DLS, AFM | 126 |
| 12 | SLN | 1141 ± 647 | 20 mM MgCl2 | DLS, AFM | 126 |
| 12 | SLN | 962 ± 1314 | 30 mM MgCl2 | DLS, AFM | 126 |
| 12 | SLN | 757 ± 493 | 145 mM MgCl2 | DLS, AFM | 126 |
| 13 | SLN | 69 | DLS, AFM | 126 | |
| 13 | SLN | 69 ± 1 | 0.1 mM NaCl | DLS, AFM | 126 |
| 13 | SLN | 69 ± 1 | 1 mM NaCl | DLS, AFM | 126 |
| 13 | SLN | 68 ± 2 | 15 mM NaCl | DLS, AFM | 126 |
| 13 | SLN | 66 ± 2 | 145 mM NaCl | DLS, AFM | 126 |
| 13 | SLN | 70 ± 1 | 0.1 mM KCl | DLS, AFM | 126 |
| 13 | SLN | 68 ± 1 | 1 mM KCl | DLS, AFM | 126 |
| 13 | SLN | 65 ± 1 | 5 mM KCl | DLS, AFM | 126 |
| 13 | SLN | 66 ± 1 | 140 mM KCl | DLS, AFM | 126 |
| 13 | SLN | 66 ± 2 | 145 mM KCl | DLS, AFM | 126 |
| 13 | SLN | 69 ± 3 | 0.1 mM CaCl2 | DLS, AFM | 126 |
| 13 | SLN | 58 ± 2 | 0.5 mM CaCl2 | DLS, AFM | 126 |
| 13 | SLN | 52 ± 1 | 1 mM CaCl2 | DLS, AFM | 126 |
| 13 | SLN | 191 ± 6 | 2 mM CaCl2 | DLS, AFM | 126 |
| 13 | SLN | 525 ± 22 | 2.5 mM CaCl2 | DLS, AFM | 126 |
| 13 | SLN | 1117 ± 467 | 3 mM CaCl2 | DLS, AFM | 126 |
| 13 | SLN | 1345 ± 860 | 4 mM CaCl2 | DLS, AFM | 126 |
| 13 | SLN | 1239 ± 1172 | CaCl2 5 mM | DLS, AFM | 126 |
| 13 | SLN | 1567 ± 3019 | 145 mM CaCl2 | DLS, AFM | 126 |
| 13 | SLN | 95 ± 65 | 0.1 mM MgCl2 | DLS, AFM | 126 |
| 13 | SLN | 64 ± 1 | 0.5 mM MgCl2 | DLS, AFM | 126 |
| 13 | SLN | 60 ± 2 | 1 mM MgCl2 | DLS, AFM | 126 |
| 13 | SLN | 136 ± 2 | 2 mM MgCl2 | DLS, AFM | 126 |
| 13 | SLN | 229 ± 20 | 2.5 mM MgCl2 | DLS, AFM | 126 |
| 13 | SLN | 837 ± 30 | 3 mM MgCl2 | DLS, AFM | 126 |
| 13 | SLN | 1365 ± 399 | 5 mM MgCl2 | DLS, AFM | 126 |
| 13 | SLN | 650 ± 650 | 10 mM MgCl2 | DLS, AFM | 126 |
| 13 | SLN | 1425 ± 256 | 20 mM MgCl2 | DLS, AFM | 126 |
| 13 | SLN | 848 ± 1237 | 30 mM MgCl2 | DLS, AFM | 126 |
| 13 | SLN | 1242 ± 625 | 145 mM MgCl2 | DLS, AFM | 126 |
| 18 | Vesicle | 54 ± 3 | 10 mM PB 7.2, 154 mM NaCl | DLS | 43 |
| 24 | Mixture of large or small vesicles, distorted vesicles and rod-like micelles | R h: 43; Rg: 58 | 0.1 M NH3 aqueous | SLS, DLS, cryo-TEM | 42 |
| 24 | Liquid crystal | — | 5 N NH3 aqueous | OPM | 42 |
| 24 | Mixture of SLNs and lipid layers | 100 (SLN) | PCS, AFM | 67 | |
| 25 | Spherical aggregate | 100–125 | pH 3 | FE-SEM, EF-TEM | 23 |
| 25 | Necklace-like aggregate | 250 | pH 7 | FE-SEM, EF-TEM | 23 |
| 26 | Nanofiber | 750 | H2O–EtOH 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
DLS, FE-SEM, TEM | 90 |
| 29 | Multilamellar vesicle | 84 ± 24 | DLS, FE-SEM, TEM | 111 | |
| 34 | Micelle | — | pH 6 | — | 41 |
| 34 | Micelle | — | pH 8 | — | 41 |
| 35 | Micelle | — | pH 6 | — | 41 |
| 35 | Micelle | — | pH 8 | — | 41 |
| 36 | Micelle | — | pH 6 | — | 41 |
| 36 | Micelle | — | pH 8 | — | 41 |
| 37 | Micelle | — | pH 6 | — | 41 |
| 37 | Micelle | — | pH 8 | — | 41 |
| 38 | Micelle | — | pH 6 | — | 41 |
| 38 | Micelle | — | pH 8 | — | 41 |
| 39 | Micelle | — | pH 6 | — | 41 |
| 39 | Micelle | — | pH 8 | — | 41 |
| 40 | Micelle | — | pH 6 | — | 41 |
| 40 | Micelle | — | pH 8 | — | 41 |
| 41 | Micelle | — | pH 6 | — | 41 |
| 41 | Micelle | — | pH 8 | — | 41 |
| 42 | Micelle | — | pH 6 | — | 41 |
| 42 | Micelle | — | pH 8 | — | 41 |
| 43 | Micelle | — | pH 6 | — | 41 |
| 43 | Micelle | — | pH 8 | — | 41 |
| 44 | Micelle | — | pH 6 | — | 41 |
| 44 | Micelle | — | pH 8 | — | 41 |
| 46 | Mixture of rod-like and spherical micelles | 3.6 (rod-like); 4.2 (spherical) | Na+ and K+ PB, pH 7 | Cryo-TEM | 91 |
| 46 | Spherical micelle | 3.2 | Na+ borate, pH 9 | Cryo-TEM | 91 |
| 46 | Membrane with a uniform pattern of pores | — | pH 4 | Cryo-TEM | 91 |
| 47 | — | 3 | pH 7.2 | PGSE NMR | 97 |
| 47 | Hollow spherical cage | 3.8 | 27 mM Na+ and K+ | Cryo-TEM | 97 |
| 47 | Micelle | 4.0–4.5 | 27 mM K+, pH 7.0 | Cryo-TEM | 99 |
| 47 | Micelle | 3.2–3.7 | 27 mM Na+ and K+, pH 7.0 | Cryo-TEM | 99 |
| 49 | — | 3.27 | D2O | DOSY | 112 |
| 51 | SLN | 73 ± 3 | DLS | 117 | |
| 57 | Micellar aggregate | 1–2 | 30 °C | DLS | 132 |
| 58 | Vesicle | 40–650 | DLS, TEM | 134 | |
| 60 | Liquid crystal | — | OPM | 42 | |
| 61 | Micelle | 2.7 ± 0.3 | 10 mM PB, pH 7.2, 154 mM NaCl | DLS | 43 |
| 62 | Vesicle | 75 | DLS | 46 | |
| 63 | Micelle | 2.5 | 75 mM Na2SO4 | DLS | 60 |
| 64 | Micelle | ∼2 | DLS | 71 | |
| 64 | — | ∼2 | DLS, TEM | 73 | |
| 64 | Micelle | 1.47 | 20 mM Tris, pH 7.4 | DLS | 77 |
| 65 | Micelle | 2.74 | 20 mM Tris, pH 7.4 | DLS | 77 |
| 66 | Micelle | 3.2 | DLS | 72 | |
| 66 | Micelle | 2.82 | 20 mM Tris, pH 7.4 | DLS | 77 |
| 67 | Micelle | 20 | DLS | 101 | |
| 67 | Micelle | 3.69 | 20 mM Tris, pH 7.4 | DLS | 77 |
| 68 | — | 150–200 | 20 mM Tris, pH 7.4 | DLS | 77 |
| 69 | Micelle | 4.14 | 20 mM Tris, pH 7.4 | DLS | 77 |
| 72 | Micelle | 3.04 | 20 mM Tris, pH 7.4 | DLS | 77 |
| 72 | Micelle | 3.28 | 20 mM acetate, pH 5.0 | DLS | 77 |
| 74 | Micelle | 3.2 | DLS | 72 | |
| 75 | Spherical micelle | — | 50 mM NaCl | SAXS | 63 |
| 76 | Spherical micelle | — | 50 mM NaCl | SAXS | 63 |
| 77 | Spherical micelle | — | 50 mM NaCl | SAXS | 63 |
| 77 | Spherical micelle | — | 100 mM NaCl | SAXS | 63 |
| 77 | Spherical micelle | — | 200–300 mM NaCl | SAXS | 63 |
| 77 | Mixture of various micellar shapes | — | >400 mM NaCl | SAXS | 63 |
| 78 | Spherical micelle | — | 50 mM NaCl | SAXS | 63 |
| 79 | Spherical micelle | — | 50 mM NaCl | SAXS | 63 |
| 82 | Micelle | 14 | 0.33 mM | DLS | 48 |
| 82 | Vesicle or aggregate of micelles | 70 | 1 mM | DLS | 48 |
| 86 | SLN | 95 | PCS | 93 | |
| 91 | Spherical micelle | — | pH < 6, 50 mM NaCl | AFM | 122 |
| 91 | Mixture of rod-like and spherical micelles | — | 3 < pH < 8, 50 mM NaCl | AFM | 122 |
| 91 | Connected network | — | pH = 10, 50 mM NaCl | AFM | 122 |
| 91 | Cylindrical micelle | — | pH 8.0, 50 mM NaCl | AFM | 122 |
| 91 | Spherical micelle | 2.05 | pH 4.2, 50 mM NaCl | SAXS | 122 |
| 91 | Cylindrical micelle | 1.68 | pH 7.5, 50 mM NaCl | SAXS | 122 |
| 91 | Micelle | R g: 1.67 | — | SAXS | 125 |
| 91 | Spherical micelle | R h: 2.05; Rg: 1.47 ± 0.11 | 50 mM NaCl, pH 3.0 | SAXS | 124 |
| 92 | Spherical micelle | R h: 2.10; Rg: 1.58 ± 0.15 | 50 mM NaCl, pH 3.0 | SAXS | 124 |
| 93 | Spherical micelle | R h: 2.25; Rg: 1.94 ± 0.17 | 50 mM NaCl, pH 3.0 | SAXS | 124 |
| 94 | Spherical micelle | — | pH < 6, 50 mM NaCl | AFM | 122 |
| 94 | Vesicular micelle | — | pH = 8, 50 mM NaCl | AFM | 122 |
| 94 | Mixture of rod-like and spherical micelles | — | pH = 6, 50 mM NaCl | AFM | 122 |
| 94 | Cylindrical micelle | — | pH 6.3, 50 mM NaCl | AFM | 122 |
| 94 | Spherical micelle | 2.75 | pH 4.3, 50 mM NaCl | SAXS | 122 |
| 94 | Cylindrical micelle | 2.0 | pH 6.3, 50 mM NaCl | SAXS | 122 |
| 94 | Plate micelle | 1.91 | pH 7.8, 50 mM NaCl | SAXS | 122 |
| 94 | Spherical micelle | R h: 2.75; Rg: 2.52 ± 0.19 | 50 mM NaCl, pH 3.0 | SAXS | 124 |
| 95 | Cylindrical micelle | R h: 2.20; Rg: 1.70 ± 0.10 | 50 mM NaCl, pH 3.0 | SAXS | 124 |
| 96 | Cylindrical micelle | R h: 2.35; Rg: 1.87 ± 0.13 | 50 mM NaCl, pH 3.0 | SAXS | 124 |
| 97 | Rod-like micelle | — | pH < 6, 50 mM NaCl | AFM | 122 |
| 97 | Cylindrical micelle | 2.40 | pH 4.7, 50 mM NaCl | SAXS | 122 |
| 98 | — | 88 ± 6 | 50 mM MES, pH 6.5 | DLS, SLS | 136 |
| 99 | — | 70 ± 27 | 50 mM MES, pH 6.5 | DLS, SLS | 136 |
| 100 | Dot-like micelle | 2.25 | 50 mM NaCl, pH 3.0 | SAXS, AFM | 139 |
| 101 | Spherical micelle | 2.15 | 50 mM NaCl, pH 3.0 | SAXS | 140 |
| 101 | Spherical micelle | 2.35 | 50 mM NaCl, pH 5.4 | SAXS | 140 |
| 101 | Finite cylindrical micelle | 1.90 | 50 mM NaCl, pH 6.2 | SAXS | 140 |
| 101 | Infinite cylindrical micelle | 1.80 | 50 mM NaCl, pH 7.4 | SAXS | 140 |
| 101 | Infinite cylindrical micelle | 1.80 | 50 mM NaCl, pH 8.3 | SAXS | 140 |
| 101 | Finite cylindrical micelle | 1.98 | 50 mM NaCl, pH 9.2 | SAXS | 140 |
| 101 | Spherical micelle | 2.38 | 50 mM NaCl, pH 10 | SAXS | 140 |
| 102 | Spherical micelle | — | 50 mM NaCl | SAXS | 63 |
| 105 | — | 97 ± 2 | 50 mM MES, pH 6.5 | DLS, SLS | 136 |
| 106 | — | 77 ± 9 | 50 mM MES, pH 6.5 | DLS, SLS | 136 |
| 108 | Micelle | R g: 1.76 | 150 mM NaCl | DLS, SAXS, AFM | 167 |
| 109 | Micelle | R g: 2.46 | 150 mM NaCl | DSL, SAXS, AFM | 167 |
| 123 | — | 10.1 ± 0.8 | PBS | DLS | 149 |
| 124 | Vesicle | 10–30 | 50 mM NaCl/Tris–HCl, pH 7–8 | DLS, TEM | 123 |
| 125 | Cylindrical micelle | R h: 2.50 | 50 mM NaCl, pH 7.0, 25 °C | SAXS | 157 |
| 125 | Spherical micelle | R h: 2.85; Rg: 2.81 | 50 mM NaCl, pH 7.0, 40 °C | SAXS | 157 |
| 125 | Spherical micelle | R h: 2.72; Rg: 2.73 | 50 mM NaCl, pH 12, 25 °C | SAXS | 157 |
| 125 | Spherical micelle | R h: 2.58; Rg: 1.80 | 50 mM NaCl, pH 12, 40 °C | SAXS | 157 |
| 126 | Globular micelle | R h: 3; Rg: 2.43 | DLS, SAXS | 159 | |
| 127 | SLN | 65 | DLS, AFM | 163 | |
| 127 | Nanosphere | 65 ± 1 | DLS, cryo-TEM | 164 | |
| 127 | Nanocapsule | 60 ± 1 | DLS, cryo-TEM | 164 | |
| 128 | SLN | 95 | DLS, AFM | 163 | |
| 128 | Nanosphere | 95 ± 1 | DLS, cryo-TEM | 164 | |
| 128 | Nanocapsule | 76 ± 1 | DLS, cryo-TEM | 164 | |
| 129 | Vesicle | 25–50 | DLS, TEM | 169 | |
| 130 | Fiber | 25 (radius), several micrometers long | DLS, TEM | 169 | |
| 131 | Micelle | 2.2 | DLS | 278 | |
| 137 | Vesicle | 100 | DLS, TEM, FE-SEM | 185 | |
| 138 | Vesicle | 18 | DLS, TEM, FE-SEM | 185 | |
| 138 | Micelle | 3 | pH 5 | DLS, TEM | 185 |
| 139 | Micelle | 3 | DLS, TEM | 185 | |
| 140 | Micelle | 2.9 | DLS | 142 | |
| 140 | Micelle | 2.8 | TEM | 142 | |
| 140 | Irregular NP | 7–16 | TEM | 142 | |
| 141 | Micelle | 3.5 | DLS | 142 | |
| 141 | Micelle | 3.6 | TEM | 142 | |
| 141 | Sole like NP | 13–43 | TEM | 142 | |
| 141 | Solid micelle | 3.6 | TEM | 143 | |
| 142 | Irregular NP | 8–50 | TEM | 146 | |
| 142 | Mixture of micelles and NPs | 3 (micelle); 40 (NP) | HR TEM | 146 | |
| 142 | Mixture of micelles and NPs | 3 (micelle); 25 (NP) | Cryo-TEM | 146 | |
| 142 | Micelle | 1.8 | DLS | 146 | |
| 142 | Micelle | 1.8 | DLS | 142 | |
| 142 | Micelle | 2.5 | TEM, HR TEM, cryo-TEM | 142 | |
| 142 | Irregular NP | 8–50 | TEM, HR TEM, cryo-TEM | 142 | |
| 142 | Solid micelle | 2.5 | TEM | 143 | |
| 143 | Micelle | R h: 2.90; Rg: 2.26 ± 0.14 | 50 mM NaCl | SAXS | 150 |
| 144 | Micelle | R h: 3.60; Rg: 2.63 ± 0.12 | 50 mM NaCl | SAXS | 150 |
| 145 | Micelle | R h: 4.10; Rg: 3.75 ± 0.34 | 50 mM NaCl | SAXS | 150 |
| 147 | Spherical micelle | — | 50 mM NaCl | SAXS | 63 |
| 148 | — | 118 | PCS | 168 | |
| 154 | — | 88 | 10 mM HEPES, pH 8.0 | DLS | 179 |
| 158 | Vesicle | 40 | DLS | 46 | |
| 162 | Ellipsoidal micelle | Minor: 2.10, major: 3.45 | SAXS | 147 | |
| 162 | Ellipsoidal micelle | Minor: 1.40, major: 6.6 (prolate); 3.7 (oblate) | D2O | DOSY | 54 |
| 163 | Micelle | Minor: 3.25, major: 10.675 | SAXS | 147 | |
| 163 | Micelle (>0.6 mM) | ∼123–190 | DLS, NTA | 154 | |
| 163 | Domain (100–0.1 μM) | ∼70–190 | DLS, NTA | 154 | |
| 163 | Nanoassociate (10–1 nM) | ∼160–195 | DLS, NTA | 154 | |
| 164 | Vesicle | 25–125 | pH 7.8 | TEM, AFM, DLS | 155 |
| 164 | Vesicle | ≤50 | pH 7.8, sonicate 1 h | AFM | 155 |
| 164 | Vesicle | 25 | pH 6.5 | TEM | 155 |
| 164 | Vesicle | 225 | pH 8.5 | TEM, DLS | 155 |
| 164 | Micelle | 1.3 | 1 mM AgClO4 | TEM | 155 |
| 168 | — | 30–100 | 0.1 mM | DLS, TEM | 73 |
| 168 | — | 33–250 | 0.2 mM | DLS, TEM | 73 |
| 168 | — | 40–150; 250–350 | 0.6 mM | DLS, TEM | 73 |
| 168 | — | 50–350 | 0.8 mM | DLS, TEM | 73 |
| 168 | — | 55–400 | 1.0 mM | DLS, TEM | 73 |
| 168 | — | 35–55; 80–200 | 4.0 mM | DLS, TEM | 73 |
| 168 | — | 65–350 | 6.0 mM | DLS, TEM | 73 |
| 168 | — | 75–400 | 8.0 mM | DLS, TEM | 73 |
| 168 | — | 35–60; 150–250 | 10.0 mM | DLS, TEM | 73 |
| 170 | Vesicle | 60 ± 15 | 8 μM | Cryo-TEM, DLS | 173 |
| 170 | Vesicle | 50, 230 | 20 μM | Cryo-TEM, DLS | 173 |
| 171 | Flattened bilayer | 108 | 7.4 μM | Cryo-TEM, DLS | 173 |
| 171 | Flattened bilayer | 150 | 15 μM | Cryo-TEM, DLS | 173 |
| 175 | Ellipsoidal micelle | Minor: 1.40, major: 7.3 (prolate); 4.0 (oblate) | D2O | DOSY | 54 |
| 184 | Vesicle | 150 ± 50 | pH 4.5–12 | SLS, DLS | 156 |
| 184 | Micelle | 5 | pH 3 | SLS, DLS | 156 |
| 185 | Vesicle | 150 ± 50 | pH 4.5–12 | SLS, DLS | 156 |
| 185 | Micelle | 5 | pH 3 | SLS, DLS | 156 |
| 192 | SLN | 130 | PBS | PCS | 190 |
| 193 | Mixture of micelles and large aggregates | 12; 49; 133 | Acetate pH 4.1 | DLS, TEM | 203 |
| 193 | Mixture of micelles and large aggregates | 8–10 | Borate pH 8.6 | DLS, TEM | 203 |
| 196 | Mixture of spherical micelles and large aggregates | 9–10 | HCl pH 1.2; phthalate pH 3; acetate pH 4.1 | DLS, TEM | 203 |
| 196 | Mixture of spherical micelles and large aggregates | 20–30 | Borate pH 8.6 | DLS, TEM | 203 |
| 196 | Mixture of spherical micelles, bilayers and cylindrical micelles | — | PB pH 12 | DLS, TEM | 203 |
| 197 | SLN | 97 | PCS | 193 | |
| 198 | Mixture of tubular and ribbon-like aggregates | 4–10 (radius), 8–100 (length) | 1 mM | Cryo-TEM | 92 |
| 198 | Fiber | Liquid crystalline lamellar phase | 30% THF aqueous | Cryo-TEM | 92 |
| 199 | — | — | AFM | 198 | |
| 200 | — | — | AFM | 198 | |
| 205 | Vesicle | 100–250 | EtOH | TEM, AFM | 238 |
| 212 | Vesicle | 34, 125 | DLS, TEM | 202 | |
| 212 | Fiber | 109 | MeOH | DLS, TEM | 202 |
| 216 | SLN | 170 | PBS | PCS | 190 |
| 217 | SLN | 177 | PBS | PCS | 190 |
| 218 | SLN | 175 | PBS | PCS | 190 |
| 219 | SLN | 74 | Produced using THF | PCS, AFM | 225 and 226 |
| 219 | SLN | 74 | Produced using EtOH | PCS | 226 |
| 219 | SLN | 98 | Produced using acetone | PCS | 226 |
| 219 | SLN | 107 | Produced using MeOH | PCS | 226 |
| 219 | SLN | 114 | 0.2 g L−1 | PCS | 226 |
| 219 | SLN | 132 | 0.3 g L−1 | PCS | 226 |
| 219 | SLN | 136 | 0.4 g L−1 | PCS | 226 |
| 219 | SLN | 130 | 0.5 g L−1 | PCS | 226 |
| 219 | SLN | 81 | 3% THF in production | PCS | 226 |
| 219 | SLN | 86 | 4% THF in production | PCS | 226 |
| 219 | SLN | 108 | 5% THF in production | PCS | 226 |
| 219 | SLN | 103 | 6% THF in production | PCS | 226 |
| 219 | SLN | 103 | 8% THF in production | PCS | 226 |
| 219 | SLN | 110 | 10% THF in production | PCS | 226 |
| 219 | SLN | 75 | 0.1 M NaCl | PCS | 226 |
| 219 | SLN | 80 | 0.1 M NaI | PCS | 226 |
| 219 | SLN | 83 | 0.1 M CH3CO2Na | PCS | 226 |
| 219 | SLN | 75 | 0.1 M NaHCO3 | PCS | 226 |
| 219 | SLN | 73 | 0.1 M KNO3 | PCS | 226 |
| 219 | SLN | 93 | 0.1 M KH2PO4 | PCS | 226 |
| 219 | SLN | 65 | PCS | 215 | |
| 219 | SLN | 175 | PBS | PCS | 190 |
| 219 | SLN | 75 | PCS, AFM | 193 | |
| 219 | SLN | 69 | PCS | 225 | |
| 219 | SLN | 70 | Carbopol 980 aqueous | PCS | 225 |
| 219 | SLN | 73 | Carbopol 2020 aqueous | PCS | 225 |
| 219 | SLN | 83 | Hyaluronic acid aqueous | PCS | 225 |
| 219 | SLN | 76 | Xanthane aqueous | PCS | 225 |
| 223 | Vesicle | 25–35, 100 | TEM | 233 | |
| 223 | Lamellar-like vesicle | 25–500 | MeOH | TEM | 233 |
| 223 | Fiber | — | CHCl3 | TEM | 233 |
| 223 | Inverted micelle | 13 | Perfluorohexane | TEM | 233 |
| 223 | Circular assembly | 50 (radius), 25–30 (height) | AFM | 257 | |
| 224 | SLN | 74 | 0.1 M NaH2PO4 | PCS | 226 |
| 224 | SLN | 73 | 0.1 M KCl | PCS | 226 |
| 226 | SLN | 163 | PBS | PCS | 190 |
| 227 | SLN | 92 | PCS | 193 | |
| 231 | — | 140–200 | 0.8–7 mM, 10% DMF aqueous | DLS | 200 |
| 232 | Micelle | R g: 25.6 | 0.1 mM | SAXS | 205 |
| 232 | Micelle | R g: 30.5 | 0.5 mM | SAXS | 205 |
| 232 | Micelle | R g: 35.2 | 1.0 mM | SAXS | 205 |
| 232 | Micelle | 8–6 | 0.1–10 mM | DLS | 205 |
| 237 | — | 62–130 | 0.09–3 mM, 10% DMF aqueous | DLS | 200 |
| 238 | — | 14–8 | 0.25–16 mM | DLS | 200 |
| 239 | Mixture of micelle-like aggregates and layers | 4 | DLS | 227 | |
| 240 | — | 8–4 | 0.15–16 mM | DLS | 200 |
| 241 | — | 9–5 | 0.15–16 mM | DLS | 200 |
| 242 | — | 15–5 | 0.25–20 mM | DLS | 200 |
| 247 | Spherical particle | R h: 107.1; Rg: 120.4 | DLS, SLS, AFM | 191 | |
| 248 | Spherical particle | R h: 108.0; Rg: 120.3 | DLS, SLS, AFM | 191 | |
| 249 | Spherical particle | R h: 98.6; Rg: 122.0 | DLS, SLS, AFM | 191 | |
| 250 | Spherical particle | R h: 121.7; Rg: 135.0 | DLS, SLS, AFM | 191 | |
| 251 | Spherical particle | R h: 106.8; Rg: 131.0 | DLS, SLS, AFM | 191 | |
| 252 | Spherical particle | R h: 77.7; Rg: 91.0 | DLS, SLS, AFM | 191 | |
| 255 | — | 14.3 | DLS, TEM | 213 | |
| 256 | — | 5.5 | DLS, TEM | 213 | |
| 257 | — | 79.5 | DLS, TEM | 213 | |
| 258 | — | 10.3 | DLS, TEM | 213 | |
| 259 | — | 34.7 | DLS, TEM | 213 | |
| 260 | — | 17.7 | DLS, TEM | 213 | |
| 261 | Mixture of grape-like superstructures and nonlinear chains | 60–85, 600–900 (length) | SEM, TEM | 229 | |
| 262 | Mixture of micelles and large spherical aggregates | 100–130; 350 | DLS, SEM, TEM | 229 | |
| 278 | Sheet-like monolayer | 1.3 | AFM, TEM | 224 | |
| 278 | Bundles of sticks | 1.4–16.6 (radius), 500–3500 (length) | DMSO–H2O 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
AFM, TEM | 224 |
| 278 | Vesicle | 25 | Acetone–H2O 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
AFM, TEM | 224 |
| 279 | Linear or dot-like aggregate | — | AFM, TEM | 224 | |
| 279 | Vesicle | 25 | DMSO–H2O 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
AFM, TEM | 224 |
| 282 | — | 241 ± 30 | 3 mM | DLS | 232 |
| 282 | — | 240 ± 37 | 0.3 mM | DLS | 232 |
| 282 | — | 84 ± 4 | 30 μM | DLS | 232 |
| 282 | — | 131 ± 13 | 3 μM | DLS | 232 |
| 283 | — | 87 ± 11 | 3 mM | DLS | 232 |
| 283 | — | 227 ± 47 | 0.3 mM | DLS | 232 |
| 283 | — | 203 ± 15 | 30 μM | DLS | 232 |
| 283 | — | 415 ± 33 | 3 μM | DLS | 232 |
| 284 (Br−) | Particle | 90.5 ± 11.5 | DLS | 237 | |
| 284 (NO3−) | Particle | 30.7 ± 8.4 | DLS | 235 | |
| 284 (NO3−) | Particle | 24 ± 3 | DLS | 236 | |
| 286 | Particle | 7.3 ± 1.3 | DLS | 235 | |
| 286 | Particle | 8 ± 1 | DLS | 236 | |
| 287 | Particle | 1.9 ± 0.4 | DLS | 235 | |
| 289 | Spherical particle | 70.8 | 5 mM Tris–HCl, pH 7.5, 1 mM (CA) | DLS | 247 |
| 289 | Spherical particle | 80.8 | 5 mM Tris–HCl, pH 7.5, 0.8 mM (CA) | DLS | 247 |
| 289 | Spherical particle | 104.5 | 5 mM Tris–HCl, pH 7.5, 0.1 mM (CA) | DLS | 247 |
| 289 | Spherical particle | 194.8 | 5 mM Tris–HCl, pH 7.5, 10 μM (CA) | DLS | 247 |
| 289 | Spherical particle | 244.4 | 5 mM Tris–HCl, pH 7.5, 1 μM (CA) | DLS | 247 |
| 290 | Spherical particle | 37.7 | 5 mM Tris–HCl, pH 7.5, 1 mM (CA) | DLS | 247 |
| 290 | Spherical particle | 72.8 | 5 mM Tris–HCl, pH 7.5, 0.8 mM (CA) | DLS | 247 |
| 290 | Spherical particle | 100.6 | 5 mM Tris–HCl, pH 7.5, 0.1 mM (CA) | DLS | 247 |
| 290 | Spherical particle | 122.4 | 5 mM Tris–HCl, pH 7.5, 10 μM (CA) | DLS, TEM | 247 |
| 290 | Spherical particle | 226.9 | 5 mM Tris–HCl, pH 7.5, 1 μM (CA) | DLS | 247 |
| 292 | — | 211 ± 37 | 0.3 mM | DLS | 250 |
| 292 | — | 168 ± 24 | 30 μM | DLS | 250 |
| 292 | — | 159 ± 102 | 3 μM | DLS | 250 |
| 292 | — | 99 ± 3 | 0.3 mM (CA), 0.3 mM Ag+ | DLS | 250 |
| 292 | — | 174 ± 61 | 30 μM (CA), 30 μM Ag+ | DLS | 250 |
| 292 | — | 218 ± 77 | 3 μM (CA), 3 μM Ag+ | DLS | 250 |
| 292 | — | 166 ± 12 | 0.3 mM (CA), 0.3 mM Ag+ | DLS | 250 |
| 292 | — | 265 ± 135 | 3 mM | DLS | 232 |
| 292 | — | 211 ± 37 | 0.3 mM | DLS | 232 |
| 292 | — | 168 ± 24 | 30 μM | DLS | 232 |
| 292 | — | 159 ± 102 | 3 μM | DLS | 232 |
| 293 | — | 378 ± 47 | 0.3 mM | DLS | 250 |
| 293 | — | 265 ± 135 | 30 μM | DLS | 250 |
| 293 | — | 118 ± 103 | 3 μM | DLS | 250 |
| 293 | — | 195 ± 42 | 30 μM (CA), 30 μM Ag+ | DLS | 250 |
| 293 | — | 189 ± 31 | 3 μM (CA), 3 μM Ag+ | DLS | 250 |
| 294 | Micelle | (17.0 ± 1.3)–(24.4 ± 1.4) | 2–10 mM, D2O | DOSY, AFM | 252 |
| 294 | Mixture of premicelles and large aggregates | 100; 300 | 0.5 mM | DOSY, DLS | 254 |
| 294 | Mixture of aggregate | 50–100; 25 | AFM | 254 | |
| 296 | Homogeneous assembly | 30–40 (radius), 3–5 (height) | AFM | 257 | |
| 305 | Spherical particle | 111.2 ± 17.4. | PBS pH 7.4 | DLS, TEM | 259 |
| 311 | Vesicle | — | H2O–EtOH 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
TEM | 242 |
| 325 | Spherical particle | 89.8 ± 14.8 | PBS pH 7.4 | DLS, TEM | 259 |
| 370 | — | 100–110 | 0.33–10 g L−1 | DLS | 258 |
| 371 | — | R h: 100–300; Rg: 70–140 | 0.8 mg L−1–1.2 g L−1 | DLS | 258 |
| 402 | SLN | 108 | DLS | 262 | |
| 402 | SLN | 74 | Produced using acetone | DLS | 262 |
| 402 | SLN | 215 | Produced using EtOH | DLS | 262 |
| 402 | SLN | 106 | 0.2 g L−1 | DLS | 262 |
| 402 | SLN | 125 | 0.3 g L−1 | DLS | 262 |
| 402 | SLN | 123 | 0.4 g L−1 | DLS | 262 |
| 402 | SLN | 139 | 0.5 g L−1 | DLS | 262 |
| 402 | SLN | 117 | 5% glycerol | DLS | 262 |
| 402 | SLN | 99 | 10% glycerol | DLS | 262 |
| 402 | SLN | 114 | 15% glycerol | DLS | 262 |
| 402 | SLN | 102 | 20% glycerol | DLS | 262 |
| 402 | SLN | 104 | 25% glycerol | DLS | 262 |
| 403 | SLN | 72 | DLS | 262 | |
| 404 | SLN | 70 | DLS | 262 | |
| 405 | SLN | 50 | DLS | 262 | |
| 406 | SLN | 41 | DLS | 262 | |
| 407 | — | R h: 4.242; Rg: 3.288 ± 0.044 | 0.5 mM | DLS, SAXS | 22 |
| 407 | — | R h: 3.296; Rg: 2.555 ± 0.128 | 1 mM | DLS, SAXS | 22 |
| 407 | — | R h: 34.06; Rg: 2.640 ± 0.014 | 5 mM | DLS, SAXS | 22 |
| 407 | — | R h: 4.074; Rg: 3.158 ± 0.002 | 10 mM | DLS, SAXS | 22 |
| 407 | — | 5; 100 | DLS | 22 | |
| 415 | Particle | 3 ± 0.2; 10 ± 1 | DLS | 236 | |
| 420 | Tube | 15 (radius), 70–300 (length) | SEM | 265 | |
| 421 | Tube | 106 (radius), 108 (length) | SEM, AFM | 265 | |
| 422 | Vesicle | 370 ± 28 | 10 mM Tris, pH 7.4 | DLS | 268 |
| 422 | Vesicle | 158 ± 3 | 10 mM Tris, pH 7.4, 65 °C | DLS | 268 |
| 422 | Vesicle | 268 ± 13 | 10 mM Tris, pH 7.4, irradiated | DLS | 268 |
| 422 | Vesicle | 133 ± 5 | 10 mM Tris, pH 7.4, 65 °C, irradiated | DLS | 268 |
| 427 | Vesicle | 440 ± 40 | 10 mM Tris, pH 7.4 | DLS | 268 |
| 427 | Vesicle | 130 ± 10 | 10 mM Tris, pH 7.4, 65 °C | DLS | 268 |
| 427 | Vesicle | 245 ± 40 | 10 mM Tris, pH 7.4, irradiated | DLS | 268 |
| 427 | Vesicle | 120 ± 1 | 10 mM Tris, pH 7.4, 65 °C, irradiated | DLS | 268 |
| 428 | — | 150 ± 13 | 3 mM | DLS | 232 |
| 428 | — | 71 ± 16 | 0.3 mM | DLS | 232 |
| 428 | — | 136 ± 38 | 30 μM | DLS | 232 |
| 428 | — | 100 ± 19 | 3 μM | DLS | 232 |
| 429 | SLN | 66 | DLS, SEM | 120 | |
| 430 | Spherical particle | 49.9 | 5 mM Tris–HCl, pH 7.5, 1 mM (CA) | DLS | 247 |
| 430 | Spherical particle | 52.2 | 5 mM Tris–HCl, pH 7.5, 0.8 mM (CA) | DLS | 247 |
| 430 | Spherical particle | 103.7 | 5 mM Tris–HCl, pH 7.5, 0.1 mM (CA) | DLS | 247 |
| 430 | Spherical particle | 119.8 | 5 mM Tris–HCl, pH 7.5, 10 μM (CA) | DLS | 247 |
| 430 | Spherical particle | 448.0 | 5 mM Tris–HCl, pH 7.5, 1 μM (CA) | DLS | 247 |
| 431 | Spherical particle | 46.4 | 5 mM Tris–HCl, pH 7.5, 1 mM (CA) | DLS | 247 |
| 431 | Spherical particle | 69.9 | 5 mM Tris–HCl, pH 7.5, 0.8 mM (CA) | DLS | 247 |
| 431 | Spherical particle | 212.9 | 5 mM Tris–HCl, pH 7.5, 0.1 mM (CA) | DLS | 247 |
| 431 | Spherical particle | 412.3 | 5 mM Tris–HCl, pH 7.5, 10 μM (CA) | DLS | 247 |
| 431 | Spherical particle | 502.0 | 5 mM Tris–HCl, pH 7.5, 1 μM (CA) | DLS | 247 |
| 432 | — | 62 ± 9 | 50 mM Tris, pH 7.4 | DLS | 266 |
| 433 | — | 66 ± 2 | 50 mM Tris, pH 7.4 | DLS | 266 |
| 434 | — | 62 ± 1 | 50 mM Tris, pH 7.4 | DLS | 266 |
| 443 | Vesicle | 42 ± 2 | 0.025 mM | DLS | 267 |
| 443 | Vesicle | 47 ± 1 | 0.05 mM | DLS | 267 |
| 443 | Vesicle | 53 ± 1 | 0.1 mM | DLS | 267 |
| 443 | Vesicle | 50 ± 2 | 0.25 mM | DLS | 267 |
| 443 | Vesicle | 42 ± 1 | 0.5 mM | DLS | 267 |
| 443 | Vesicle | 64 ± 2 | 0.75 mM | DLS | 267 |
| 443 | Vesicle | 62 ± 2 | 1.0 mM | DLS | 267 |
| 444 | Vesicle | 31 ± 1 | 0.01 mM | DLS | 267 |
| 444 | Vesicle | 24 ± 1 | 0.025 mM | DLS | 267 |
| 444 | Vesicle | 26 ± 1 | 0.05 mM | DLS | 267 |
| 444 | Vesicle | 25 ± 1 | 0.1 mM | DLS | 267 |
| 444 | Vesicle | 24 ± 1 | 0.25 mM | DLS | 267 |
| 444 | Vesicle | 25 ± 1 | 0.5 mM | DLS | 267 |
| 444 | Vesicle | 31 ± 1 | 0.75 mM | DLS | 267 |
| 444 | Vesicle | 31 ± 1 | 1.0 mM | DLS | 267 |
| 448 | Vesicle | 40 | H2O–EtOH 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
TEM, SEM, AFM, DLS | 273 |
| 449 | Vesicle | 70 | H2O–EtOH 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
TEM, SEM, AFM, DLS | 273 |
| 449 | Vesicle | 46 | H2O–EtOH 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
TEM, SEM, AFM, DLS | 273 |
| 450 | Spherical aggregate | 60–90 | H2O–EtOH 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
TEM, SEM, AFM, DLS | 273 |
| 450 | Tubular aggregate | 28 (radius) | H2O–EtOH 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
TEM, SEM, AFM, DLS | 273 |
| 451 | Spherical aggregate | 60–90 | H2O–EtOH 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
TEM, SEM, AFM, DLS | 273 |
| 451 | Tubular aggregate | 28 (radius) | H2O–EtOH 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
TEM, SEM, AFM, DLS | 273 |
| 452 | Vesicle | 145 | H2O–EtOH 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
TEM, AFM, DLS | 242 |
| 452 | Mixture of vesicles and fibers | — | H2O–EtOH 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
SEM, TEM | 242 |
| 452 | Fiber | 50–100 (radius), 104 (length) | H2O–EtOH 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
TEM, SEM, AFM | 242 |
| 452 | Nanotube | 30–40 | 0.5 g L−1 HAuCl4, H2O–EtOH 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
TEM | 275 |
| 452 | Nanotube | — | 0.5 g L−1 AgNO3, H2O–EtOH 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
TEM | 275 |
| 453 | Tubular aggregate | — | H2O–EtOH 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
TEM, SEM, AFM, DLS | 273 |
Compared with corresponding conventional surfactants, self-assemblies of amphiphilic calixarenes show diverse morphologies. For example, sodium dodecyl sulphate (SDS) forms spherical micelles with an average radius of 2.09 nm.280 Its corresponding calixarene, amphiphilic sulfonatocalix[4]arene 9, forms micelles with an average radius of 6.9 nm109 and the hexameric derivative 163 forms aggregates with various sizes (radii from about 70 nm to 195 nm) depending on the concentration.154 For positively charged surfactants, dodecylguanidine hydrochloride forms micelles as well,281 while the amphiphilic guanidinium-modified calix[4]arene 51 is able to form SLN with an average radius of 73 nm.117 Such great differences mainly come from their unique skeletons. It is well known that the critical packing parameter (CPP) proposed by Israelachivili and coworkers is a parameter to estimate the morphology of an amphiphilic assembly.282 Definition of the CPP value is P = VH/(a0lc), where VH is the volume occupied by hydrophobic groups in the assembly core, a0 is cross-sectional area occupied by the hydrophilic group at the assembly–solution interface, and lc is the chain length of the hydrophobic group in the assembly core. However, the CPP is hard to precisely apply in the case of macrocyclic amphiphiles. Various sizes and conformations of skeletons result in complicated, unpredictable, and diverse morphologies.
For example, Zhao and coworkers reported fully carboxylic acid modified amphiphilic calix[6]arene 305 and calix[8]arene 325.259 The mean radius of aggregates of 305 (111.2 ± 17.4 nm) is larger than that of 325 (89.8 ± 14.8 nm). Similarly, Xu and coworkers investigated choline modified amphiphilic calix[4]arene 62 and calix[5]arene 158.46 Despite their similar CAC values, the average radius of vesicles of 62 (75 nm) is almost two fold that of 158 (40 nm) (Fig. 2). The explanation of decreasing diameter with a larger skeleton is controversial; it may be related to an enhanced hydrophobic effect, different symmetry, or lower entropy loss. Exceptionally, Basilio and coworkers reported that an ellipsoidal micelle of amphiphilic sulfonatocalix[8]arene 175 has a longer main semiaxis (7.3 nm) than that of calix[6]arene 162 (6.6 nm), which is the result of a more flexible conformation of calix[8]arene.54
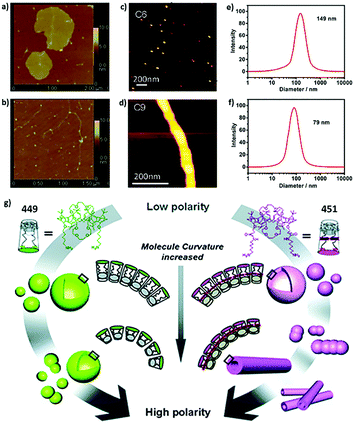 | ||
| Fig. 2 AFM images of (a) 278 and (b) 279 in pure water. Reprinted with permission from ref. 224. Copyright 2012 from Science China Press and Springer-Verlag Berlin Heidelberg. AFM images of (c) 94 and (d) 97 in 50 mM NaCl, pH 3. Reprinted with permission from ref. 122. Copyright 2012 from American Chemical Society. DLS data of (e) 62 and (f) 158 in water. Reprinted with permission from ref. 46. Copyright 2016 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. (g) Schematic representation for morphology transitions in self-assembly of 449 and 451 with changes in medium polarity. Reprinted with permission from ref. 273. Copyright 2011 from Royal Society of Chemistry. | ||
Conformation is also an important factor. Stoikov's group reported a series of quaternary ammonium-modified amphiphilic calix[4]arenes which have the same decoration and different conformations (283, 289 and 290 in cone conformation and 428, 430 and 431 in 1,3-alternate conformation).232,247 At a concentration of 0.3 mM, the radius of assembly of 283 (227 nm) is larger than that of 428 (71 nm). At a concentration of 1 mM, the radius of assembly of 289 (70.8 nm) is larger than that of 430 (49.9 nm) while the radius of assembly of 290 (37.7 nm) is smaller than that of 431 (46.4 nm). We assume that conformations of skeletons in assemblies affect the curvature and sizes of assemblies due to different aggregation modes.
Certainly, the length of hydrophobic chains and the structure of hydrophilic head groups could affect the morphologies of aggregates. For instance, increasing the hydrophobic chain from 6 carbons to 9 carbons causes different morphologies of amphiphilic aminocalix[4]arenes 94 and 97 assemblies, which are micelle and cylinder respectively (Fig. 2).122 For a series of amphiphilic calixarenes that show similar aggregation morphologies, some of them present a trend in size. Jebors and coworkers reported several SLNs assembled by acylcalix[9]arenes with different lengths of the carbon chain (402–406). The sizes of their aggregates decrease with increasing carbon atoms (radii decrease from 108 nm to 41 nm).262 Similarly, Burilov and coworkers studied two kinds of amine modified amphiphilic calixarenes with 4 carbons (98 and 105) and 8 carbons (99 and 106) as the hydrophobic chain, respectively.13698 and 99 are completely modified whereas 105 and 106 are partially modified. Aggregates of 98 and 105 show larger radii (88 nm and 97 nm, respectively) than those of 99 and 106 (70 nm and 77 nm, respectively), respectively. In principle, more and longer hydrophobic chains lead to a stronger hydrophobic effect, resulting in more compact packing, which leads to smaller aggregates. However, there are contrary examples such as a series of cyclodextrin modified amphiphilic calixarenes 127 and 128, whose aggregate size increases with increasing carbon chain (radii increase from 65 nm to 95 nm).163,164 This phenomenon may be related to the large volume of cyclodextrin. Besides, the aggregates of bola-type amphiphilic calixarenes 432–434 are of the same size, although they bear 4-carbon chains, 8-carbon chains, and 14-carbon chains, respectively.266 In brief, we just find limited examples of hydrophobic chains affecting the morphology with a certain trend, while in many cases, morphologies vary irregularly with chain length.
Hydrophilic head affecting the aggregation morphology is an even more complicated topic. Factors such as volume, hydration energy, and interactions between hydrophilic head groups may give us a clue, but it is still difficult to predict aggregate morphologies from their structure. Here we just name several examples that may provide some ideas. Stoikov and coworkers235–237,247 synthesized several amphiphilic butylthiacalix[4]arenes with quaternary ammonium, as well as amide (289, 290), ester (287), benzene (284), or phthalimide (286) as hydrophilic groups. The 287 assembly has a significantly smaller radius (1.9 nm) than those of 284 (30.7 nm), 286 (7.3 nm), 289 (>70.8 nm, Tris buffer) and 290 (>37.7 nm, Tris buffer), owing to the small size of the ester. Similar results were reported by Shahgaldian and coworkers on diethylphosphate and phosphate modified calixarene, 192 (130 nm) and 226 (163 nm), respectively.190 On the other hand, Li and coworkers reported cyclodextrin modified calixarene 279 and 278, which possess one and two β-cyclodextrins respectively.224 In pure water, 279 forms small linear or dot like assemblies, whereas 278 shows large sheet like aggregations (Fig. 2). This phenomenon could be explained by hydrogen bonds between cyclodextrins. Besides, Klymchenko's group synthesized amphiphilic calixarenes modified by choline (66) and a N-(2-aminoethyl)-N,N-dimethylammonium group (72).76,77 DLS results showed the micelles of 72 (3.04 nm) are little larger than those of 66 (2.82 nm), probably due to the larger hydration shell of amino groups as compared to hydroxyls. However, there are some examples showing that the morphology is not significantly influenced by different hydrophilic head groups. For example, Burilov and coworkers compared assembly behavior of amphiphilic thiacalix[4]arenes bearing carboxyl (427) and sulfonic (422) groups, respectively; these two compounds show similar shape and size.268 Compounds 212, 196, and 193, reported by Martin and coworkers, which possess amine, aminodiacetate, and phosphate groups, respectively, present similar results.202,203
The morphologies of assemblies are largely related to experimental conditions283 such as concentration. For example, Padnya and coworkers reported a series of quaternary ammonium based thiacalix[4]arenes 289 and 290, whose aggregate sizes increase with decreasing concentration (radii increase from 70.8 nm to 244.4 nm and from 37.7 nm to 226.9 nm, respectively, with concentration decrease from 1 mM to 1 μM).247 The authors assume that this phenomenon can be explained by the existence of two kinds of aggregates, spherical aggregates and elongated self-associates.
On the other hand, properties of solvents may modulate the morphology of amphiphilic calixarene assemblies, mainly by influencing the interactions of hydrophilic head groups. For instance, the polarity of solvent affects hydrogen bonds, resulting in a change in the packing mode of some amphiphiles. Liang and coworkers synthesized amphiphilic calix[6]biscrowns 450 and 451 possessing amide groups, interacting with each other via hydrogen bonds, at the hydrophilic part.273 Their morphologies underwent a clear transition from spherical to tubular aggregates when the solvent polarity, i.e. content of water in water/ethanol solution, is increased (Fig. 2), whereas analogues 448 and 449, without any amide linkage, only showed a size decrease upon the same change in solvent polarity. Similarly, two cyclodextrin modified amphiphilic calix[4]arenes 278 and 279,224 which we have already mentioned before, also present different assembly patterns in different solvents. With decreasing polarity, the morphology of 279 transferred from dots to linear aggregates and then to vesicles; meanwhile, 278 changed from sheet-like to bundle-like aggregations. All these examples show that lower solvent polarity enhances hydrogen bonds between head groups, resulting in larger aggregates.
pH of the solution is a common factor to modulate assembly morphology, by influencing electrostatic interactions and hydrophobicity. For groups whose pKa values are in a regular range, such as amine, carboxyl acid, and pyridine, adjusting the pH could change the number of charges they possess. More like charges at the hydrophilic head cause stronger repulsion, resulting in a larger curvature. For example, Martin and coworkers reported a phosphate modified amphiphilic calix[4]arene 196, whose major assembly morphology is bilayer at pH 4.1 while small micelles at pH 12.203 On the other hand, Houmadi and coworkers reported a sulfonatocalix[6]arene 164 with imidazolyl groups at the upper rim.155 At pH 6.5, the average radius of its assembly is 25 nm, which is much smaller than those at pH 7.8 (25–125 nm) and pH 8.5 (225 nm). This phenomenon could be explained by protonation of imidazolyl group affecting the hydrophilic and hydrophobic balance.
Salt concentration influencing assembly is another interesting topic, and it is also related to further application in biological systems. Houel and coworkers did a systematic study on salt concentration affecting assembly using phosphonate-modified calix[4]arenes 12 and 13.126 In the presence of monovalent cations (Na+, K+), no apparent change in size was observed over a concentration range from 0.1 mM to 145 mM. In contrast, divalent cations could cause a significant size increase as the concentration is increased from 2 mM. The authors assumed that divalent cations have the ability to crosslink the assemblies.
| Compound | N agg | Conditiona | Methodb | Ref. |
|---|---|---|---|---|
| a The condition is 25 °C in pure water if no label. b AUC: analytical ultracentrifugation. AF4-MALS: multiangle light scattering coupled with asymmetric field flow fractionation. | ||||
| 4 | 4 | 10 mM NaCl | SAXS, AF4-MALS, AUC | 64 and 65 |
| 4 | 6 | 15 mM NaCl | SAXS, AF4-MALS, AUC | 65 |
| 6 | 17 | 10 mM NaCl | SAXS, AF4-MALS, AUC | 64 |
| 7 | 24 | 10 mM NaCl | SAXS, AF4-MALS, AUC | 64 |
| 46 | 12 | pH 7 | Cryo-TEM | 91 |
| 47 | 7 | 27 mM Na+ and K+ | Cryo-TEM | 97 |
| 75 | 8 | 50 mM NaCl | SAXS, AF4-MALS, AUC | 63 |
| 76 | 8 | 50 mM NaCl | SAXS, AF4-MALS, AUC | 63 |
| 77 | 12 | 50 mM NaCl | SAXS, AF4-MALS, AUC | 63 |
| 77 | 12 | 100 mM NaCl | SAXS | 63 |
| 77 | 20 | 200–300 mM NaCl | SAXS | 63 |
| 78 | 12 | 50 mM NaCl | SAXS, AF4-MALS, AUC | 63 |
| 79 | 20 | 50 mM NaCl | SAXS, AF4-MALS, AUC | 63 |
| 91 | 6 | 50 mM NaCl, pH 3.0 | SAXS, AF4-MALS, AUC | 122 and 124 |
| 92 | 12 | 50 mM NaCl, pH 3.0 | SAXS, AF4-MALS, AUC | 124 |
| 93 | 12 | 50 mM NaCl, pH 3.0 | SAXS, AF4-MALS, AUC | 124 |
| 94 | 12 | 50 mM NaCl, pH 3.0 | SAXS, AF4-MALS, AUC | 122 and 124 |
| 100 | 12 | 50 mM NaCl, pH 3.0 | SAXS, AFM, DLS | 139 |
| 101 | 6 | pH 3.2 | SAXS, AF4-MALS | 63 |
| 101 | 12 | pH 10 | SAXS, AF4-MALS | 63 |
| 101 | 6 | 50 mM NaCl, pH 3.0 | SAXS, FFF-MALS | 140 |
| 101 | 12 | 50 mM NaCl, pH 10 | SAXS, FFF-MALS | 140 |
| 102 | 8 | 50 mM NaCl | SAXS, AF4-MALS | 63 |
| 125 | 24 | 50 mM NaCl, pH 7.0, 40 °C | SAXS, AF4-MALS, AUC | 157 |
| 125 | 20 | 50 mM NaCl, pH 12 | SAXS, AF4-MALS, AUC | 157 |
| 125 | 21 | 50 mM NaCl, pH 12, 40 °C | SAXS, AF4-MALS, AUC | 157 |
| 143 | 20 | 50 mM NaCl | SAXS, AF4-MALS | 63 |
| 143 | 12 | 50 mM NaCl | SAXS, FFF-MALS | 150 |
| 144 | 8 | 50 mM NaCl | SAXS, FFF-MALS | 150 |
| 145 | 3–4 | 50 mM NaCl | SAXS, FFF-MALS | 150 |
| 146 | 12 | 50 mM NaCl | SAXS, AF4-MALS | 63 |
| 147 | 3.6 | 50 mM NaCl | AF4-MALS | 63 |
In 2004, Kellermann and coworkers reported the first completely uniform and structurally precise micelle, whose structure was determined by cryo-TEM and 3D reconstruction techniques.97 The micelle is formed spontaneously by exactly seven 47 molecules (Fig. 3), which is a T-shaped compound and with third generation dendritic heads. Later, they reported another uniform micelle formed by twelve 46 molecules, which is with second generation dendritic heads.91 Compared with molecule 47, the smaller space required by 46 allows denser packing, resulting in a larger Nagg value.
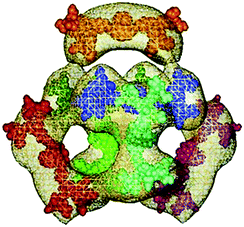 | ||
| Fig. 3 A structurally precise micelle formed by exactly seven 47 molecules is determined by 3D reconstruction techniques. Reprinted with permission from ref. 97. Copyright 2004 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
The Sakurai group systematically studied assembly behavior of a series of calixarene micelles, whose head groups, including sulfonate group,64,65 primary amine group,63,122,124 quaternary amine group,63 cysteine,139 glutamic acid,140 polyamidoamine,63 mono/disaccharides,63,157 PEG,63,150 and so on, were conjugated with calixarene at the upper rim by a click reaction. Using methods including SAXS, AUC, AF4-MALS and LS, the morphologies and Nagg values of these micelles were determined. They found that these Nagg values coincide with the vertex numbers of regular polyhedral structures when Nagg are less than 30, so they named these small micelles as platonic micelles since the regular polyhedral structures are called platonic solids. They proposed that the formation of platonic micelles is a result of the maximal coverage ratio, which means the ratio of the total area of the caps to the surface area of the sphere (Fig. 4).
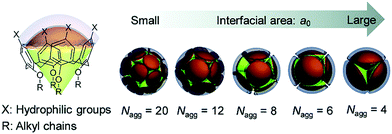 | ||
| Fig. 4 Chemical structure of a calix[4]arene-based amphiphile and the schematic illustration of the effect of the size of interfacial area on the Nagg of the micelles composed of the amphiphiles. Reprinted with permission from ref. 65. Copyright 2018 from Royal Society of Chemistry. | ||
It is clear that the equilibrium interfacial area between the hydrophilic and hydrophobic domains (a0) has a significant effect on the Nagg, which was proved by studying a series of amphiphilic calixarenes bearing PEGs with different molecular weights as hygrophilic head groups.150 Experiment results showed that amphiphile containing PEG of 550 g mol−1 forms a dodecamer while that of 1000 g mol−1 forms an octamer, since the former one has smaller a0. Both of the micelles are monodispersed; meanwhile, high molecular weight PEG (2000 g mol−1) leads to polydisperse micelles, because PEG 2000 exhibits a greater affinity for water and higher mobility than PEG 550 and 1000, resulting in a too large a0 to form stable monodisperse micelles.
The a0 value could be easily influenced by the solvent environment. As an example, high salt concentration decreases repulsion among head groups, resulting in smaller a0, thus leading to larger Nagg.63,65 Also, for head groups containing an amine or carboxyl group, pH variation may protonate or deprotonate them, resulting in a change in repulsion interaction among these head groups. Consequently, the a0 value increases with larger repulsion and vice versa, leading to different Nagg, or even a transition of morphology. For instance, amino-modified compounds 87 and 88 form spheres at pH 3.0 while form cylinders at higher pH.122
A more complicated example is the glutamic acid containing 100,140 since it allows a continuous change in the state of its head groups from cationic to zwitterionic and then to anionic with increasing pH, resulting in a morphological transformation from spherical to cylindrical and again to spherical. Their Naggs at pH 3.0 and 10 were determined as 6 and 12 respectively. The molecular modeling results showed that the glutamic acid moieties exhibited folded-back structures with deprotonated carboxylic acid, resulting in a smaller hydrophilic volume than that with the protonated amino groups, causing increased Nagg from pH 3.0 to pH 10. It is also noteworthy that its lc could also change during pH variation, because anions at pH 10 are more far away from the center of the molecule than cations at pH 3.0.
The ionized state change induced by pH change could also influence the hydrogen bond formation. Disaccharides containing 120 provides an interesting example.157 The micellar morphologies are cylindrical at pH 7.0 and micelles with Nagg of 20 at pH 12 (25 °C), due to the cleavage of the hydrogen bonds by deprotonation of the hydroxyl groups in the sugar molecules. Similarly, temperature also affects hydrogen bonds. As a result, compound 120 forms micelles with Nagg of 24 at 40 °C (pH 7), and forms micelles with Nagg of 12 at 40 °C (pH 12).
As we mentioned before, lc is also important to the Nagg of aggregates. According to packing parameter theory, Nagg is proportional to lc2, whose trend is consistent with experimental results of a series of quaternary amine group bearing calixarenes.63 As the number of carbons in the alkyl chain increases from 3 to 7, Nagg increases discretely from 8 to 12, and then to 20. However, a series of amine group bearing calixarenes show a different behavior.124 Their platonic micelles bearing butyl, heptyl, and hexyl chains remain at 12-mer. This phenomenon and discrete Nagg may indicate that the coverage ratio defined by the Tammes problem is more suitable than packing parameter theory in the case of investigating small micelles.
To test the universality of the platonic micelles, Sakurai's group also studied assembly behavior of a series of amphiphilic SC4As 4, 6–8.64,65Naggs of these micelles increase from 4 to 17 to 24 and then to cylindrical structures with increasing alkyl chain length from pentyl to hexyl to heptyl and then to octyl, respectively. Although the values of 17 and 24 do not agree with the vertex numbers of regular polyhedra, but they match the local maxima in the Thomson problem considering the Coulomb potential for the calculation of the best packing on a sphere with multiple identical spherical caps.
Due to their preorganized structures, amphiphilic calixarene assemblies show different compactness from that of the corresponding monomers. Shinkai and co-workers investigated the microviscosity of a series of amphiphilic calixarenes above their CACs by measuring P.49P values of a series of sulfonic group modified amphiphilic calixarenes 162, 299, 300, and 322 (0.033–0.106) are higher than those of SDS (0.020) and hexadecyltrimethylammonium chloride (0.016), which means more compact packing of calixarene micelles than conventional surfactant micelles.
Cho and co-workers reported that alanine-modified calix[4]arene 25 self-assembles into a hollow necklace-like structure at neutral pH.23 IR spectra showed strong hydrogen bonding between the carbonyl groups and the highly organized, closely packed hydrocarbon chains exhibiting sharp IR bands.
As an assembly property, compactness is also influenced by pH and salt concentration, which modulate head group interactions. For example, Becherer and co-workers reported that carboxylic group modified calix[4]arene 46 micelles are clearly smaller at pH 9 than those at neutral pH, which can be concluded that denser packing of the micelles occurs under basic conditions in comparison with the aggregates obtained at neutral pH.91 The required smaller space of head groups allows denser packing.
Xu and co-workers demonstrated choline-modified calixarene (62 and 158) based molecular light-harvesting platforms, whose spectrum tunability is affected by assembly compactness.46 When they increased the ionic strength of solvent, the ionic heads packed closer resulted in more compact aggregation, which led to higher energy-transfer efficiency and acceptor emission.
Although there have been only limited examples related to the compactness until now, we believe more and more systematic investigations will come up soon, since it is necessary for developing materials with better performance and reliability.
As Basilio and co-workers reported, in contrast to conventional surfactants, the exchange rate of amphiphilic SC4A 6 between solution and micelle is slow on the NMR time scale.84 The rate constants were determined by 2D EXSY experiments and these constants were found to be several orders of magnitude lower than those of conventional surfactants and comparable to those of other amphiphilic calixarenes and gemini surfactants. The explanation for this result presumably lies in the fact that the sole barrier felt by the amphiphile entering the micelle arises from long-range electrostatic repulsions due to the micellar charge. As 6 is a preorganized surfactant with four negative charges at the upper rim, this could increase the activation barrier and consequently slow down the rate constant for the association.
Takahashi and co-workers observed that quaternary ammonium modified 77 micelles transit from a dodecamer to an icosamer induced by a rapid increase in the NaCl concentration (cNaCl), using a stopped-flow device and time-resolved SAXS.133 The Nagg remained at 12 during the first 60 s after the increase in cNaCl, and then abruptly increased to 20 (Fig. 5). They speculated that the following kinetic process might take place: (1) the micelles with Nagg = 12 become metastable after the cNaCl increases to 290 mM. (2) Within the micelles, fluctuation of 77 takes place, providing sufficient space for the insertion of other 77 molecules in a process that might be very slow. (3) Once one 77 has been inserted into the metastable micelle, Nagg rapidly increases to 20.
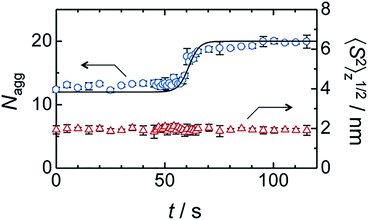 | ||
| Fig. 5 Time evolution of Nagg (blue circles) and the radius of gyration 〈S2〉z1/2 (red triangles). The solid curve for Nagg represents the fitted model curve. Reprinted with permission from ref. 133. Copyright 2017 from Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
Similar examples of amphiphilic calixarenes forming meta-stable “kinetic trap” states were reported, with more diverse morphologies.286 For example, Strobel and co-workers reported that carboxylatocalix[4]arene 24 self-assembles into vesicles and long thin features that could possibly be rod-like micelles in dilute solution according to light scattering and cryo-TEM experiments.42 Houmadi and co-workers reported that 164 self-assembles into vesicles in freshly prepared solution.155 Vesicles of similar size and shape but with larger membrane shells were observed by TEM in 1 day old solutions, whereas giant vesicles appear after 1 week.
Despite scant examples, we have enough reason to predict a prosperous development of amphiphilic calixarene assembly kinetics. In general, highly kinetic systems at or close to equilibrium show uniform aggregate shapes and sizes, whereas systems with slow kinetics exhibit rather broad shape and size distributions.42 Consequently, kinetics is critical for preparation procedures of amphiphilic calixarene assemblies. The tailored preparation process is needed for fabricating well-designed assemblies according to their kinetics features. Moreover, the slow assembling kinetics of amphiphilic calixarenes can be applied in extensive fields, such as controlled release in drug delivery systems and dissipative self-assembly systems.
3. Calixarene-based supra-amphiphiles
Amphiphilic macrocycles possess cavities, which endow them with binding affinity to various guests. Taking this advantage, guests could modulate the aggregation behavior of amphiphilic macrocycles. By host–guest interactions as well, non-amphiphilic macrocycles own the ability to influence the aggregate properties of some surfactants. The concept of “supra-amphiphiles” proposed by Zhang and co-workers covers those behaviors, describing amphiphiles constructed on the basis of non-covalent interactions or dynamic covalent bonds.10,287–292Specific to water soluble calixarenes, they interact with guests including dyes, drugs, and biomacromolecules by hydrophobic interactions, π–π interactions, electrostatic interactions and so on. Furthermore, their unique skeletons provide multivalent interaction sites. As a result, these guests efficiently affect the aggregation of amphiphilic calixarenes, and the aggregation behavior of these guests could be modulated by calixarenes conveniently. Based on the amphiphilicity of the host and guest molecules, the assembling features of calixarene-based supra-amphiphiles can be divided into guest-induced aggregation of host, host-induced aggregation of guest and mutual inducement (Scheme 5).
Chemical structures of host and guest molecules which have been used to construct supra-amphiphiles are summarized in Schemes 6, 7 and Tables 7, 8.
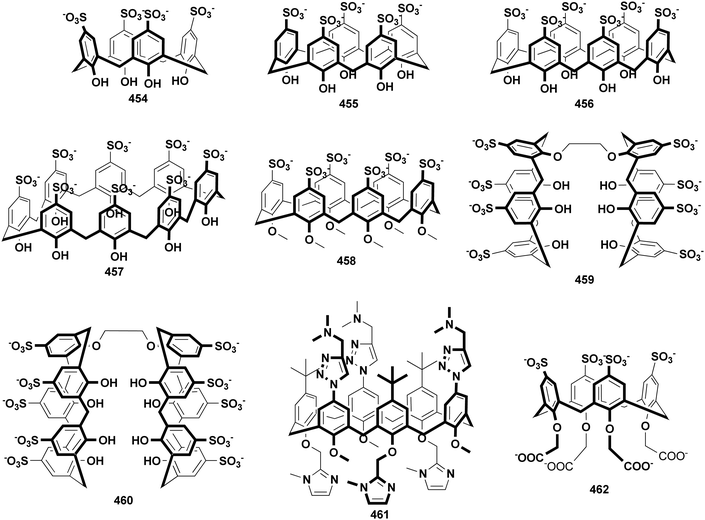 | ||
| Scheme 6 Structures of hydrophilic host molecules which have been used to construct supra-amphiphiles. | ||
| Compound | Ref. | Compound | Ref. | Compound | Ref. |
|---|---|---|---|---|---|
| 454 | 293–308 | 457 | 293, 296 and 307 | 460 | 309 |
| 455 | 298, 309 and 310 | 458 | 311–315 | 461 | 316 |
| 456 | 293, 296 and 317–319 | 459 | 303 and 320 | 462 | 321 |
| Compound | Ref. | Compound | Ref. | Compound | Ref. | Compound | Ref. |
|---|---|---|---|---|---|---|---|
| G1 | 322 | G14 | 305 | G27 | 306 | G40 | 252 |
| G2 | 322 | G15 | 302 | G28 | 293 | G41 | 317 |
| G3 | 322 | G16 | 320 | G29 | 295 | G42 | 307 and 317–319 |
| G4 | 322 | G17 | 300 | G30 | 296 | G43 | 317 |
| G5 | 316 | G18 | 300 | G31 | 55 | G44 | 310 |
| G6 | 138 | G19 | 300 | G32 | 162 | G45 | 88 |
| G7 | 57 | G20 | 304 | G33 | 162 | G46 | 161 |
| G8 | 311 | G21 | 298 | G34 | 173 | G47 | 161 |
| G9 | 312 | G22 | 303 | G35 | 297 | G48 | 301 |
| G10 | 311–315 | G23 | 308 | G36 | 162 | G49 | 161 |
| G11 | 294 and 319 | G24 | 319 | G37 | 162 | G50 | 266 |
| G12 | 311 | G25 | 319 | G38 | 309 | G51 | 211 |
| G13 | 299 | G26 | 319 | G39 | 309 | — | — |
3.1 Guest-induced aggregation of host
| Host | Guest | Morphology | Radius (nm) | CAC | Quantity (host![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) guest) guest) |
Conditiona | Method | Ref. |
|---|---|---|---|---|---|---|---|---|
| a The condition is 25 °C in pure water if no label. | ||||||||
| 3 | — | — | — | 3.18 mM | — | — | 55 | |
| 3 | G31 | — | — | 0.25 mM | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Fluorescence | 55 | |
| 3 | G7 | — | — | 1.4 mM | 3 mM (guest) | Conductivity | 57 | |
| 6 | — | Micelle | — | 330 μM | — | Fluorescence | 88 | |
| 6 | G45 | Mult-lamellar sphere | 81 | 35 μM | 50 μM (guest) | DLS, TEM | 88 | |
| 62 | — | Micelle | 2.7 | 0.9 mM | — | ITC, DLS | 138 | |
| 62 | G6 | Vesicle | 247 | 0.02 mM | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
UV-vis, DLS, SEM, TEM, AFM | 138 | |
| 111 | — | Micelle | 20–100 | 1.2 mM | — | pH 3.0 | CD, DLS, AFM, TEM, SEM | 161 |
| 111 | G46 | Branched fiber | 103–104 (length) | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
pH 3.0 | AFM, TEM, SEM | 161 |
| 111 | G49 | Twisted fiber | 103–3 × 103 (length) | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
pH 3.0 | AFM, TEM, SEM | 161 |
| 111 | G47 | Network | — | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
pH 3.0 | AFM, TEM, SEM | 161 |
| 111 | G32 | Rod-like fiber | — | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
pH 3.0 | AFM, SEM | 162 |
| 111 | G33 | Rod-like fiber | — | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
pH 3.0 | AFM, SEM | 162 |
| 111 | G36 | Network | 2 × 103–5 × 103 (length) | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
pH 3.0 | AFM, SEM | 162 |
| 111 | G37 | Network | — | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
pH 3.0 | AFM, SEM | 162 |
| 142 | — | Mixture of micelles and NPs | 2.5; 40 | 13 μM | — | DLS, HR TEM | 322 | |
| 142 | G4 | Mixture of hollow micelles and hollow rod-like micelles | 6 | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
DLS, HR TEM, cryo-TEM | 322 | |
| 142 | G3 | Mixture of micelles and NPs | 5 | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
DLS, HR TEM, cryo-TEM | 322 | |
| 143 | G1 | Hollow micelle | 5–10 | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
DLS, HR TEM, cryo-TEM | 322 | |
| 143 | G2 | Linear micelle | 3.8 (radius), 50–400 (length) | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
DLS, HR TEM, cryo-TEM | 322 | |
| 170 | — | Vesicle | 60 ± 15 | 7.9 ± 0.5 μM | — | Fluorescence, DLS | 173 | |
| 170 | G34 | Mixture of micelles, vesicles and super-aggregates | <10; >25; 50–100 | 7.2 ± 0.3 μM | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Fluorescence, DLS, cryo-TEM, TEM | 173 | |
| 254 | G51 | Vesicle | 40–155 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Dioxane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water 5 water 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
TEM, DLS | 211 | |
| 254 | G51 | Vesicle | 85 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Dioxane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water 5 water 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
TEM, DLS | 211 | |
| 254 | G51 | Spherical micelle | 65 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Dioxane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water 5 water 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
TEM, DLS | 211 | |
| 254 | G51 | Micelle | ∼150 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
Dioxane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water 5 water 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
TEM, DLS | 211 | |
| 254 | G51 | Mixture of network aggregates and spherical micelles | ∼200 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
Dioxane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water 5 water 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
TEM, DLS | 211 | |
| 432 | — | — | 62 ± 9 | 91 ± 5 μM | — | 50 mM Tris pH 7.4 | Fluorescence | 266 |
| 432 | G50 | — | 45 ± 3 | 2.0 ± 0.1 μM | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
50 mM Tris pH 7.4 | Fluorescence, DLS | 266 |
| 433 | — | — | 66 ± 2 | 59 ± 3 μM | — | 50 mM Tris pH 7.4 | Fluorescence | 266 |
| 433 | G50 | — | 53 ± 2 | 2.6 ± 0.2 μM | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
50 mM Tris pH 7.4 | Fluorescence, DLS | 266 |
| 434 | G50 | — | 58 ± 4 | 2.0 ± 0.1 μM | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
50 mM Tris pH 7.4 | Fluorescence, DLS | 266 |
| 434 | — | — | 62 ± 1 | 33 ± 2 μM | — | 50 mM Tris pH 7.4 | Fluorescence | 266 |
| 294 | — | Micelle | 2.44 | 0.64 mM | — | D2O | DOSY, AFM | 252 |
| 294 | G40 | Micelle | — | 0.35 mM | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
D2O | DOSY | 252 |
Aggregation of calixarenes bearing sulfonic groups at the lower rim could also be induced by cationic guests. Gattuso and coworkers synthesized an amphiphilic calix[5]arene 294, and DOSY results showed that its CAC was 0.64 mM.252 In the presence of G40, its CAC decreases to 0.35 mM.251,252 The inclusion of guests into the cavity of 294 is clearly proved by DOSY results, while some guests involved in the assembly are located outside the cavity. It is noteworthy that the CAC values of guests also decrease upon addition of 294.
Following the same principle, anionic guest induced cationic calixarene aggregation was also reported by Wang and coworkers.138 The CAC value of a quaternary ammonium-modified calix[4]arene 59 decreases by 45 times in the presence of ATP (G6). Similarly, Burilov and coworkers synthesized several ammonium modified amphiphilic thiacalix[4]arenes (432–434) and studied their aggregation behavior in the absence and presence of Eosin Y (G50).266 CAC values of all these thiacalix[4]arenes, no matter the length of the hydrophobic chain, showed a significant decrease (at least 15 folds) with addition of G50.
For example, 6 forms small micelles in the absence of a guest, while forms aggregates with 81 nm average radius in the presence of G45, which was proved by DLS, TEM, and significant Tyndall effect.88 Similar results were reported in the study of 59 in the presence of G6 (Fig. 6).138
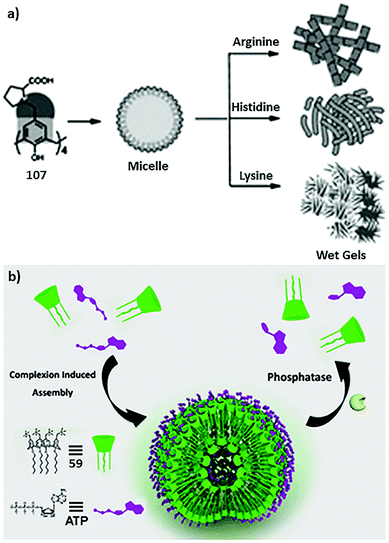 | ||
| Fig. 6 (a) Schematic illustration of gel generation from gelator 107 induced by basic amino acids. Reprinted with permission from ref. 161. Copyright 2011 from Royal Society of Chemistry. (b) Schematic illustration of the self-assembly of 59 with ATP (G6) and its phosphatase-response. Reprinted with permission from ref. 138. Copyright 2013 from Royal Society of Chemistry. | ||
Moreover, guest induced larger aggregates could further form hydrogels. Liu's group reported several hydrogels constructed by proline modified calix[4]arene 107, in the presence of different guests.161,162107 itself forms spherical aggregates with a wide size dispersion. However, upon mixing with G32, G33, G36, G37, G46, G47, and G49, the morphology of 107 changed to fibers, resulting in a binary hydrogel. AFM, SEM, and TEM results showed that the fiber shapes differed with guests. For example, the fibers of the 107–G46 are composed of long and branched fibers, while 107–G49 are shorter and twisted. And a denser network with stacks of rod-like nanofibers was observed in 107–G47 (Fig. 6).
3.2 Host-induced aggregation of guests
As we mentioned before, when a cationic surfactant G45 is used to promote aggregation of an anionic amphiphilic calixarene 6, the CAC value of G45 decreases as well. This mutually inducing phenomenon further provides us the idea that hydrophilic calixarenes should also have the ability to enhance aggregation of amphiphiles. In fact, calixarene-induced aggregation (CIA) was first reported in 2001,321 and has become more popular since 2009. The most typical hosts are SCnAs, which are capable of binding hundreds of guests with impressive affinity and promoting self-assembly of about 30 molecules in aqueous media. The concept of CIA was proposed in 2012, which means an appropriate concentration of SCnAs could lower some amphiphilic molecules’ CAC, enhance aggregate stability and compactness, and regulate the degree of order in the aggregates. This strategy could be applied to aromatic fluorescent dyes, surfactants, drugs, and biomacromolecules. Some of them have been summarized in previous reviews by García-Río16 and our group.3,323 Herein, instead of listing all the results in this field, we focus on the properties of CIA assembly with assistance of some typical works.From the viewpoint of intermolecular interactions, the hydrophobic interaction is the main driving force of conventional surfactant micelle formation, while electrostatic repulsion of its head group is unfavorable for assembly. SCnAs possessing multivalent negative charges could lower the potential energy of electrostatic repulsion efficiently. As a result, in the presence of SCnAs, surfactants tend to show lower CAC, more regular arrangement, and more compact packing (Table 10).
| Host | Guest | Morphology | Radius (nm) | CAC | Quantity (host![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) guest) guest) |
Conditiona | Method | Ref. |
|---|---|---|---|---|---|---|---|---|
| a The condition is 25 °C in pure water if no label. | ||||||||
| 461 | G5 | Vesicle | 150–300 | 74 μM | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
pH 6.8 | Surface tension, DLS, TEM | 316 |
| 461 | G5 | Vesicle | 220–310 | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
pH 4–11 | DLS | 316 |
| — | G8 | Micelle | — | 340 μM | — | Surface tension | 311 | |
| 458 | G8 | Micelle | — | 23 μM | 1 mM (host) | Surface tension | 311 | |
| — | G9 | Micelle | — | 310 mM | — | Surface tension | 311 | |
| 458 | G9 | Micelle | — | 25 mM | 1 mM (host) | Surface tension | 311 | |
| — | G10 | Micelle | 0.9 | 14 mM | — | Surface tension, fluorescence, DLS | 311 | |
| 458 | G10 | Micelle | 3.0 | 0.20 mM | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Surface tension, fluorescence, DLS | 311 | |
| 458 | G10 | Micelle | 3.3 | 0.20 mM | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Surface tension, fluorescence, DLS | 311 | |
| 458 | G10 | Micelle | 3.5 | 0.20 mM | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Surface tension, fluorescence, DLS | 311 | |
| 458 | G10 | Micelle | 6.1 | 0.20 mM | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
Surface tension, fluorescence, DLS | 311 | |
| 458 | G10 | Micelle | 3.0 | 0.20 mM | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
Surface tension, fluorescence, DLS | 311 | |
| 458 | G10 | Micelle | 1.6 | 0.20 mM | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 40 |
Surface tension, fluorescence, DLS | 311 | |
| 458 | G10 | Micelle | 1.1 | 0.20 mM | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 80 |
Surface tension, fluorescence, DLS | 311 | |
| 454 | G11 | Vesicle, small NP | 250–2500 | — | 50 mM, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 2.5 |
Nomarski light microscopy | 294 | |
| 454 | G11 | Vesicle | 57.2 ± 0.4 | — | 2 mM, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 2.5 |
DLS, TEM | 294 | |
| 456 | G11 | Spherical and ellipsoid NP | 46 | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6–7 6–7 |
DLS | 319 | |
| 456 | G11 | Supramolecular micelle | 3.4 ± 0.3 | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
15 mM NaCl, 10 °C | DLS | 319 |
| 456 | G11 | Supramolecular micelle | — | 20 μM | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 3 2, 3 |
50 mM NaCl | Fluorescence | 319 |
| — | G12 | — | — | 14 mM | — | Surface tension, conductivity | 313 | |
| 458 | G12 | Micelle | — | 0.16 mM | 0.1 mM (host) | Surface tension, conductivity | 313 | |
| 458 | G12 | Micelle | — | 0.16 mM | 0.5 mM (host) | Surface tension, conductivity | 313 | |
| 458 | G12 | — | — | 0.3 mM | 0.5 μM (host) | Surface tension | 315 | |
| 458 | G12 | — | — | 0.4 mM | 1 μM (host) | Surface tension | 315 | |
| 458 | G12 | — | — | 20 mM | 0.2 μM (host) | Fluorescence | 315 | |
| 458 | G12 | — | — | 0.3 mM | 2 μM (host) | Fluorescence | 315 | |
| 458 | G12 | — | — | 0.2 mM | 0.5 mM (host) | Fluorescence | 315 | |
| — | G13 | Micelle | 2.5 mM | — | — | 299 | ||
| 454 | G13 | — | — | 16 μM | 0.02 mM (host) | UV-vis | 299 | |
| 454 | G13 | — | — | 31 μM | 0.05 mM (host) | UV-vis | 299 | |
| 454 | G13 | — | — | 31 μM | 0.08 mM (host) | UV-vis | 299 | |
| 454 | G13 | Vesicle | 97 | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
UV-vis, DLS, TEM, SEM, cryo-TEM, HR TEM | 299 | |
| — | G14 | Micelle | 4.3 ± 0.7 | (162 ± 8) μM | — | 35 °C | Surface tension, NMR, light microscopy, cryo-TEM | 305 |
| 454 | G14 | Micelle | 30 ± 2 | (93 ± 2) mM | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 250 |
35 °C | Surface tension, NMR, light microscopy, cryo-TEM | 305 |
| 454 | G14 | Micelle | — | (13 ± 1) mM | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
35 °C | Surface tension, NMR, light microscopy, cryo-TEM | 305 |
| 454 | G14 | Micelle | 29 ± 3 | (6.5 ± 0.8) mM | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 70 |
35 °C | Surface tension, NMR, light microscopy, cryo-TEM | 305 |
| 454 | G14 | Mixture of bilayer-based tubules and vesicles | 12 ± 1 | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 25 |
35 °C | Surface tension, NMR, light microscopy, cryo-TEM | 305 |
| 454 | G14 | Mixture of bilayer-based tubules and vesicles | 6.0 ± 0.2 | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
35 °C | Surface tension, NMR, light microscopy, cryo-TEM | 305 |
| — | G15 | — | — | 6.0 mM | — | Fluorescence, surface tension | 302 | |
| 454 | G15 | — | — | 70 μM | 20 μM (host) | Fluorescence, surface tension | 302 | |
| 454 | G15 | — | — | 80 μM | 50 μM (host) | Fluorescence, surface tension | 302 | |
| 454 | G15 | — | — | 100 μM | 80 μM (host) | Fluorescence, surface tension | 302 | |
| 454 | G15 | Spherical aggregate | 196 | — | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
DLS, TEM, SEM | 302 | |
| 459 | G16 | Platelet-like micelle | 300 (length); 100 (width); 20 (height) | — | 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
25 °C | TEM, AFM | 320 |
| 459 | G16 | Cross-linked NP | — | — | 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
37 °C | DLS, TEM | 320 |
| — | G17 | — | — | 1 mM | — | — | 300 | |
| 454 | G17 | — | — | 2.5 μM | 0.02 mM (host) | UV-vis | 300 | |
| 454 | G17 | — | — | 5.3 μM | 0.04 mM (host) | UV-vis | 300 | |
| 454 | G17 | — | — | 7.3 μM | 0.06 mM (host) | UV-vis | 300 | |
| 454 | G17 | Vesicle | 66.2 | — | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
DLS, TEM, SEM, AFM | 300 | |
| — | G18 | — | — | 1 mM | — | — | 300 | |
| 454 | G18 | — | — | 3.1 μM | 0.02 mM (host) | UV-vis | 300 | |
| 454 | G18 | — | — | 3.2 μM | 0.04 mM (host) | UV-vis | 300 | |
| 454 | G18 | — | — | 3.7 μM | 0.06 mM (host) | UV-vis | 300 | |
| 454 | G18 | Vesicle | 62.3 | — | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
DLS, TEM, SEM, AFM | 300 | |
| — | G19 | — | — | 1 mM | — | — | 300 | |
| 454 | G19 | — | — | 3.7 μM | 0.02 mM (host) | UV-vis | 300 | |
| 454 | G19 | — | — | 5.0 μM | 0.04 mM (host) | UV-vis | 300 | |
| 454 | G19 | — | — | 6.6 μM | 0.06 mM (host) | UV-vis | 300 | |
| 454 | G19 | Vesicle | 49.0 | — | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
DLS, TEM, SEM, AFM | 300 | |
| — | G20 | — | — | 0.14 mM | — | Conductivity | 304 | |
| 454 | G20 | — | — | 0.125 mM | 50 μM (host) | UV-vis | 304 | |
| 454 | G20 | Multilamellar sphere | R h: 141, Rg: 108 | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
DLS, SLS, TEM | 304 | |
| 454 | G21 | Mixtures of fibers and something larger | 96 | — | — | DLS | 298 | |
| 454 | G21 | Mixture of fibers and columnar stacks | 100 | — | — | DLS, TEM, SEM, AFM | 298 | |
| — | G22 | Spherical aggregate | 55 | 0.14 mM | — | Surface tension, DLS, TEM, AFM | 303 | |
| 454 | G22 | — | — | 7.0 μM | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Surface tension | 303 | |
| 454 | G22 | Multilamellar spherical aggregate | 30 | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
DLS, TEM, AFM | 303 | |
| 459 | G22 | Spherical and linear aggregate | 60 | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
DLS, TEM, AFM | 303 | |
| — | G23 | — | 240 | 90 μM | — | DLS | 308 | |
| 454 | G23 | — | 72 | 0.8 μM | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
DLS | 308 | |
| 456 | G24 | Spherical and oblate lamellar NP | 15–48 | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2–7 2–7 |
DLS | 319 | |
| 456 | G24 | Supramolecular micelle | 2.4 ± 0.2 | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
50 mM NaCl, 15 °C | DLS | 319 |
| 456 | G24 | Supramolecular micelle | — | 16 μM | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
50 mM NaCl | Fluorescence | 319 |
| 456 | G25 | Spherical lamellar NP | 20–39 | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2–6 2–6 |
DLS | 319 | |
| 456 | G26 | Spherical NP | 25–41 | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2–6 2–6 |
DLS | 319 | |
| 454 | G27 | — | — | 0.078 mM | 0.05 mM | UV-vis | 306 | |
| 454 | G27 | — | — | 0.062 mM | 0.02 mM | UV-vis | 306 | |
| 454 | G27 | — | — | 0.103 mM | 0.08 mM | UV-vis | 306 | |
| 454 | G27 | Spherical NP | 133.36 | — | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
DLS, TEM | 306 | |
| — | G35 | — | — | 20 mM | — | — | 297 | |
| 454 | G35 | — | — | 0.02 mM | 0.02 mM (host) | UV-vis | 297 | |
| 454 | G35 | — | — | 0.04 mM | 0.05 mM (host) | UV-vis | 297 | |
| 454 | G35 | — | — | 0.07 mM | 0.08 mM (host) | UV-vis | 297 | |
| 454 | G35 | Vesicle | 181 | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
DLS, TEM, SEM | 297 | |
| 460 | G38 | Fiber-like | 104 (length) | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
0.1 M PB, pH 7.2 | AFM, SEM | 309 |
| 460 | G39 | Flake-like | 1.5–1.7 (height) | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
0.1 M PB, pH 7.2 | AFM, SEM | 309 |
| 455 | G39 | NP | 0.8–0.9 | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.1 M PB, pH 7.2 | AFM | 309 |
| 456 | G41 | NP | 65 ± 10 | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
DLS | 317 | |
| 456 | G41 | NP | 88 ± 28 | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
DLS | 317 | |
| 456 | G41 | NP | 80–190 | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3–120 3–120 |
DLS | 317 | |
| 456 | G42 | NP | 80 ± 30 | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
DLS | 317 | |
| 456 | G42 | NP | 75–150 | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–200 1–200 |
DLS | 317 | |
| 456 | G42 | Lamellar spherical aggregate | 65–100 | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
DLS, cryo-TEM, SANS | 317 | |
| 456 | G42 | Multilayered spherical aggregate | 35–75 | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
DLS, cryo-TEM, SANS | 317 | |
| — | G42 | Micelle | 2.3 ± 0.2 | — | — | 20 mM NaCl | DLS | 318 |
| 456 | G42 | NP | 33–100 | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
0–110 mM NaCl | DLS | 318 |
| 456 | G42 | NP | 33–119 | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
0–110 mM NaCl | DLS | 318 |
| 456 | G42 | NP | 25–87 | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
0–40 mM NaCl | DLS | 318 |
| 456 | G42 | Supramolecular micelle | 3.0 ± 0.2 | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
40–53 mM NaCl | DLS | 318 |
| 456 | G42 | NP | 28–69 | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
0–25 mM NaCl | DLS | 318 |
| 456 | G42 | Supramolecular micelle | 3.0 ± 0.2 | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
25–33 mM NaCl | DLS | 318 |
| 456 | G42 | NP | 33–94 | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
0–15 mM NaCl | DLS | 318 |
| 456 | G42 | Supramolecular micelle | 3.0 ± 0.2 | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
15–17 mM NaCl | DLS | 318 |
| 456 | G42 | Supramolecular micelle – NP | 3–250 | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
15 mM NaCl, 27–33 °C | DLS | 318 |
| 456 | G42 | Supramolecular micelle – NP | 3–250 | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
50 mM NaCl, 33–38 °C | DLS | 318 |
| 456 | G42 | NP | 80 | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
15 mM NaCl, 20 °C | DLS | 318 |
| 456 | G42 | Supramolecular micelle | 3 | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
15 mM NaCl, 25 °C | DLS | 318 |
| 456 | G42 | NP | 185 | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
15 mM NaCl, 30 °C | DLS | 318 |
| 456 | G42 | NP | 36 ± 10 | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
Cryo-TEM | 318 | |
| 456 | G42 | Supramolecular micelle | <8 | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
53 mM NaCl | Cryo-TEM | 318 |
| — | G42 | Micelle | — | 720 μM | — | 50 mM NaCl | Fluorescence | 318 |
| 456 | G42 | — | — | 15 μM | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
50 mM NaCl | Fluorescence | 318 |
| 456 | G42 | — | — | 15 μM | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
50 mM NaCl | Fluorescence | 318 |
| 456 | G42 | — | — | 15 μM | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
50 mM NaCl | Fluorescence | 318 |
| 456 | G42 | — | — | 15 μM | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.6 3.6 |
50 mM NaCl | Fluorescence | 318 |
| 454 | G42 | NP | 100 | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
0–0.08 mM NaCl | DLS, cryo-TEM | 307 |
| 454 | G42 | NP | 100 | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
0–0.07 mM NaCl | DLS, cryo-TEM | 307 |
| 457 | G42 | NP | 23–45 | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2–9 2–9 |
DLS | 307 | |
| 457 | G42 | NP | 45 | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.8–8.8 6.8–8.8 |
0–50 mM NaCl | DLS | 307 |
| 457 | G42 | Supramolecular micelle – NP | 2.5–350 | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.9 4.9 |
30 mM NaCl, 45–55 °C | DLS | 307 |
| 457 | G42 | Supramolecular micelle – NP | 2.5–320 | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.9 4.9 |
50 mM NaCl, 50–55 °C | DLS | 307 |
| 457 | G42 | NP – supramolecular micelle | 45–2.5 | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.8 5.8 |
0–50 mM NaCl | DLS | 307 |
| 457 | G42 | NP – supramolecular micelle | 38–2.5 | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.9 3.9 |
0–50 mM NaCl | DLS | 307 |
| 457 | G42 | — | — | 15 μM | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.0 2.0 |
50 mM NaCl | Fluorescence | 307 |
| 457 | G42 | — | — | 12 μM | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.9 5.9 |
50 mM NaCl | Fluorescence | 307 |
| 456 | G43 | NP | 55 ± 13 | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
DLS | 317 | |
| 456 | G43 | NP | 60–90 | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–200 1–200 |
DLS | 317 | |
| — | G44 | — | — | 0.27 mM | — | Fluorescence | 310 | |
| 455 | G44 | — | — | 0.07 mM | 0.02 mM (host) | Fluorescence | 310 | |
| 455 | G44 | — | — | 0.09 mM | 0.05 mM (host) | Fluorescence | 310 | |
| 455 | G44 | — | — | 0.08 mM | 0.08 mM (host) | Fluorescence | 310 | |
| 455 | G44 | Vesicle | 49.4 | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
DLS, TEM, SEM | 310 | |
Harangozo and coworkers reported a nanoparticle consisting of G42 and SC6A (456).307 Small angle neutron scattering (SANS) and cryo-TEM results indicated that the diameter of the multilayered nanoparticles was around 160 nm. Interestingly, they found that the nanoparticles have a tendency to transform into supramolecular micelles (around 6 nm diameter) in the presence of NaCl (Fig. 7c), which may be due to additional ions interfering the hydrate structure around the hydrophobic chains and the cross-sectional area of this supra-amphiphile.
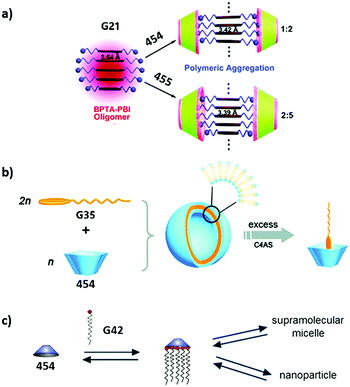 | ||
| Fig. 7 (a) Schematic illustration of the complex-induced aggregation of BPTA-PBI (G21) by 454 and 455. Reprinted with permission from ref. 298. Copyright 2012 from Royal Society of Chemistry. (b) Schematic representation of the construction of a supramolecular binary vesicle based on the host–guest complexation of 454 with G35. Reprinted with permission from ref. 297. Copyright 2011 from American Chemical Society. (c) Illustration of 454 induced G42 formation of nanoparticles and supramolecular micelles. Reprinted with permission from ref. 307. Copyright 2016 from American Chemical Society. | ||
Besides the size of assemblies, SCnA could also modulate their shape. Guo and coworkers studied the morphology of the SC5A 455 and G21 aggregates.298 The TEM image of free G21 showed some irregular arrangement without a specific topological structure, while nano-rod structures with an average length of 220 nm appeared in the presence of 455. These rods are considered to be composed of bundles of fibers, resulting from the hierarchical assembly of calixarenes (Fig. 7).
However, measuring XRD is not suitable for every assembly. So the García-Río group and the Biczok group took hydrophobicity of assembly as a parameter for compactness comparison. They assumed that a more hydrophobic assembly means more compactness.
Basilio and coworkers measured the hydrophobicity of dodecyltrimethylammonium bromide (G10) assembly by measuring the hydrolysis rate of two kinetic probes.314 Hydrolysis rates at different surfactant concentrations were plotted and fitted, from which the binding constant of the kinetic probe between the micelles and the bulk water (KSm) was obtained. The KSm value of G10 in the absence of 458 is smaller than that in the presence of 458, which means the addition of 458 leads to stronger hydrophobicity of aggregates.
Biczok and coworkers investigated the polarity of interfacial layers by using a fluorescent probe, from whose maximum fluorescence emission wavelength, the local polarity around the probe can be determined.307 The polarity results were in the order 456–G42 supramolecular micelle <457–G42 supramolecular micelle <G42 micelle, which indicated more compact stacking of micelles.
3.3 Mutual inducement
In addition to the aforementioned two complexation-induced aggregation strategies, mutual inducement involves a larger range of host and guest molecules to fabricate supra-amphiphiles. For example, amphiphilic hosts and amphiphilic guests generate new supra-amphiphiles with different CACs and morphologies.251,304,324 Hydrophilic hosts and hydrophobic drugs form supra-amphiphiles which can be applied in medical diagnosis and treatment.325,326 Hydrophilic hosts and polymers, especially biomacromolecules like protein, DNA, and chitosan, are able to mutually induce aggregation.77,205,227,304,327–330 Supra-amphiphiles consisting of various kinds of host and guest molecules not only enrich the supra-amphiphile concept but also promise potential applications such as drug delivery and gene transfection.4. Conclusion and outlook
In this review, we systematically summarized the assembling features of calixarene-based amphiphiles and supra-amphiphiles. Hundreds of amphiphilic calixarenes were fabricated by facile covalent modification, including upper-rim hydrophilic amphiphiles, lower-rim hydrophilic amphiphiles and bola-type amphiphiles. Compared with conventional amphiphiles, amphiphilic calixarenes usually show lower CACs and more diverse morphologies. Moreover, amphiphilic calixarenes are able to form uniform assemblies with precise Naggs. The assemblies of amphiphilic calixarenes pack more compactly than those of common surfactants and their assembling kinetics is much slower. The preorganized framework of calixarenes is the most important factor to influence these unique assembly properties. In general, a larger number of repeat units lead to higher assembling tendency when the conformation is fixed, and the cone conformation is more beneficial to aggregation than the alternative conformation.On the other hand, benefiting from their cavities, aggregation of amphiphilic calixarenes could be induced by various guests. Similarly, hydrophilic calixarenes possess the ability to enhance the assembly of amphiphilic guests. These induced aggregation phenomena are due to additional attractive interactions decreasing the repulsion of head groups of amphiphiles. Construction of such supra-amphiphiles avoids tedious synthesis, and the obtained assemblies also bear the properties of low CAC, regular morphology, and compact packing.
With prosperous development, there are still some promising objectives and challenges for the development of this area. First, the fundamental studies of assembly behaviors, such as the relationship between molecular structures and assembly properties, compactness and kinetics, need to be systematical investigated urgently. Up to now, hundreds of amphiphilic calixarenes as well as their CACs and morphologies have been reported, but there is no appropriate rule to describe how these properties depend on structures, which is extremely important to rational design of amphiphilic calixarenes with superior performance. On the other hand, compactness and kinetics behavior are featured properties of amphiphilic calixarenes, but only demonstrated in limited works. More attention is needed in the future because they are not only the important fundamental topics, but also related to further applications. For example, compact vesicles provide reliable platforms for capsuling cargo and constructing fluorescence materials with high efficiency,46,180 while the kinetics feature is critical for preparation procedures of these materials.
Second, crosslinking represents a convenient avenue to obtain assemblies with better stability. For instance, Shulov and coworkers proposed a new platform for bioimaging by cyanine 3 and cyanine 5 corona crosslinked calixarene micelles.60 The obtained protein-sized nanoparticles present excellent stability in various environments, showing a high fluorescence signal to noise ratio without dye leakage. Crosslinking can also be employed on the basis of supra-amphiphiles. Peng and coworkers constructed a novel supramolecular crosslinked vesicle by post-modification of a dynamic SC4A-(dodecyloxybenzyl)tripropargylammonium vesicle with the “click” reaction.331 The obtained vesicle is stable enough in diverse and complex surroundings and can be disrupted with specific chemical stimuli to realize controlled release. These pioneering works demonstrate the advantages of the crosslinking strategy, which may inspire more fascinating applications in the future.
Third, by combining amphiphilic calixarenes with various kinds of other amphiphiles, such as conventional surfactants, phospholipids or macrocyclic amphiphiles, the obtained co-assemblies exhibit different assembly behaviors. Co-assembled amphiphiles may introduce attractive interactions between hydrophilic head groups of amphiphilic calixarenes, leading to lower CACs and various morphologies.66 Moreover, calixarene conformations may be regulated by co-assembling, resulting in better binding affinity.86 Besides, co-assembly of different amphiphilic macrocycles enables heteromultivalent recognition.179
Last but not least, although the cavities of calixarenes are well utilized in supra-amphiphiles, they have not attracted much attention in calixarene amphiphilic assemblies up to now. Actually, binding and assembling abilities complement each other and influence each other. As we mentioned above, aggregation enhances the binding ability by regulating the calixarene conformation.86 Furthermore, by utilizing the host–guest recognition cavity of calixarenes on the assembly surface, the morphology of the assembly can be controlled by guests, non-covalent modification of specific functional groups can be performed, and multidimensional and hierarchical self-assemblies can be achieved.
In summary, aiming on these objectives and challenges will lead to a deep understanding of the assembling features of amphiphiles and supra-amphiphiles based on calixarenes, and also enrich their construction motifs and strategies, which are essential to develop functional materials based on calixarenes. Moreover, although this review focused on calixarenes, the conclusions are also transferable to other macrocyclic amphiphiles and supra-amphiphiles.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the NSFC (51873090 and 21672112), the Fundamental Research Funds for the Central Universities and the Innovation Project of Hebei Province (19241303D), which are gratefully acknowledged.Notes and references
- Y. Chang, Y. Jiao, H. E. Symons, J. F. Xu, C. F. J. Faul and X. Zhang, Chem. Soc. Rev., 2019, 48, 989–1003 RSC.
- A. Sorrenti, O. Illa and R. M. Ortuno, Chem. Soc. Rev., 2013, 42, 8200–8219 RSC.
- Y.-C. Liu, H.-W. Sun and D.-S. Guo, Curr. Org. Chem., 2018, 22, 2127–2149 CrossRef CAS.
- Y. Yao, R.-B. Zhao, Y.-J. Shi, Y. Cai, J. Chen, S.-Y. Sun, W. Zhang and R.-K. Tang, Chem. Commun., 2018, 54, 8068–8071 RSC.
- S. Shinkai, S. Mori, H. Koreishi, T. Tsubaki and O. Manabe, J. Am. Chem. Soc., 1986, 108, 2409–2416 CrossRef CAS.
- G. Varan, C. Varan, N. Erdogar, A. A. Hincal and E. Bilensoy, Int. J. Pharm., 2017, 531, 457–469 CrossRef CAS PubMed.
- J. Gao and D.-S. Guo, Sci. Sin.: Chim., 2019, 49, 811–820 Search PubMed.
- H. Zhang, Z. Liu and Y. Zhao, Chem. Soc. Rev., 2018, 47, 5491–5528 RSC.
- S.-Y. Sun, M. Geng, L. Huang, Y.-M. Chen, M.-P. Cen, D. Lu, A.-W. Wang, Y. Wang, Y.-J. Shi and Y. Yao, Chem. Commun., 2018, 54, 13006–13009 RSC.
- X. Zhang and C. Wang, Chem. Soc. Rev., 2011, 40, 94–101 RSC.
- G.-C. Yu, K.-C. Jie and F.-H. Huang, Chem. Rev., 2015, 115, 7240–7303 CrossRef CAS.
- D.-Y. Xia, Y. Li, K.-C. Jie, B.-B. Shi and Y. Yao, Org. Lett., 2016, 18, 2910–2913 CrossRef CAS.
- C. D. Gutsche, Acc. Chem. Res., 1983, 16, 161–170 CrossRef CAS.
- V. Bohmer, Angew. Chem., Int. Ed. Engl., 1995, 34, 713–745 CrossRef.
- K. Helttunen and P. Shahgaldian, New J. Chem., 2010, 34, 2704–2714 RSC.
- N. Basilio, V. Francisco and L. Garcia-Rio, Int. J. Mol. Sci., 2013, 14, 3140–3157 CrossRef CAS.
- R. V. Rodik, A. S. Klymchenko, Y. Mely and V. I. Kalchenko, J. Inclusion Phenom. Macrocyclic Chem., 2014, 80, 189–200 CrossRef CAS.
- J. Wang, X. Ding and X. Guo, Adv. Colloid Interface Sci., 2019, 269, 187–202 CrossRef CAS.
- S. Kolusheva, O. Molt, M. Herm, T. Schrader and R. Jelinek, J. Am. Chem. Soc., 2005, 127, 10000–10001 CrossRef CAS.
- T. Sun, L.-R. Qi, W.-W. Li, Y. Li, X.-M. Shuai, Z.-Q. Cai, H.-P. Chen, X.-G. Qiao and L.-F. Ma, J. Chromatogr. A, 2019, 1601, 310–318 CrossRef CAS PubMed.
- V. I. Kalchenko, Theor. Exp. Chem., 2018, 54, 74–84 CrossRef CAS.
- A. B. Mirgorodskaya, E. I. Yackevich, Y. R. Kudryashova, R. R. Kashapov, S. E. Solovieva, A. T. Gubaidullin, I. S. Antipin, L. Y. Zakharova and A. I. Konovalov, Colloids Surf., B, 2014, 117, 497–504 CrossRef CAS PubMed.
- E. J. Cho, J. K. Kang, W. S. Han and J. H. Jung, Langmuir, 2008, 24, 5229–5232 CrossRef CAS.
- J. Luo and Y.-S. Zheng, Prog. Chem., 2018, 30, 601–615 Search PubMed.
- Z. Xu, D. Gonzalez-Abradelo, J. Li, C. A. Strassert, B. J. Ravoo and D.-S. Guo, Mater. Chem. Front., 2017, 1, 1847–1852 RSC.
- Z. Zheng, W.-C. Geng, Z. Xu and D.-S. Guo, Isr. J. Chem., 2019, 59, 1–16 CrossRef.
- W.-C. Geng, Q. Huang, Z. Xu, R. Wang and D.-S. Guo, Theranostics, 2019, 9, 3094–3106 CrossRef CAS.
- E. S. Espanol and M. M. Villamil, Biomolecules, 2019, 9 DOI:10.3390/biom9030090.
- J. Gao and D.-S. Guo, Sci. Sin.: Chim., 2018, 48, 949–956 Search PubMed.
- J. L. J. Blanco, J. M. Benito, C. O. Mellet and J. M. G. Fernandez, J. Drug Delivery Sci. Technol., 2017, 42, 18–37 CrossRef.
- S. Karakurt, T. F. Kellici, T. Mavromoustakos, A. G. Tzakos and M. Yilmaz, Curr. Org. Chem., 2016, 20, 1043–1057 CrossRef CAS.
- Y. Zhou, H. Li and Y.-W. Yang, Chin. Chem. Lett., 2015, 26, 825–828 CrossRef CAS.
- X. Ma and Y. Zhao, Chem. Rev., 2015, 115, 7794–7839 CrossRef CAS.
- M. Giuliani, I. Morbioli, F. Sansone and A. Casnati, Chem. Commun., 2015, 51, 14140–14159 RSC.
- M.-F. Ma, P.-Y. Xing, S.-Y. Li, X.-X. Chu, B. Wang and A.-Y. Hao, Prog. Chem., 2014, 26, 1317–1328 CAS.
- K.-C. Jie, Y.-J. Zhou, Y. Yao and F.-H. Huang, Chem. Soc. Rev., 2015, 44, 3568–3587 RSC.
- S. Sayin and M. Yilmaz, Tetrahedron, 2016, 72, 6528–6535 CrossRef CAS.
- J. J. Michels, J. Huskens, J. F. J. Engbersen and D. N. Reinhoudt, Langmuir, 2000, 16, 4864–4870 CrossRef CAS.
- M. Moradi, L. G. Tulli, J. Nowakowski, M. Baljozovic, T. A. Jung and P. Shahgaldian, Angew. Chem., Int. Ed., 2017, 56, 14395–14399 CrossRef CAS.
- Z.-F. Xiao, W.-P. Yang, F.-Y. Yan, L.-B. Ji, W. Li and W. Wang, CrystEngComm, 2019, 21, 439–448 RSC.
- K. Suwinska, O. Shkurenko, C. Mbemba, A. Leydier, S. Jebors, A. W. Coleman, R. Matar and P. Falson, New J. Chem., 2008, 32, 1988–1998 RSC.
- M. Strobel, K. Kita-Tokarczyk, A. Taubert, C. Vebert, P. A. Heiney, M. Chami and W. Meier, Adv. Funct. Mater., 2006, 16, 252–259 CrossRef CAS.
- P. K. Eggers, T. Becker, M. K. Melvin, R. A. Boulos, E. James, N. Morellini, A. R. Harvey, S. A. Dunlop, M. Fitzgerald, K. A. Stubbs and C. L. Raston, RSC Adv., 2012, 2, 6250–6257 RSC.
- Q. Zhao, Y. Chen, S. H. Li and Y. Liu, Chem. Commun., 2018, 54, 200–203 RSC.
- S. E. Sestito, F. A. Facchini, I. Morbioli, J. M. Billod, S. Martin-Santamaria, A. Casnati, F. Sansone and F. Peri, J. Med. Chem., 2017, 60, 4882–4892 CrossRef CAS.
- Z. Xu, S. Peng, Y.-Y. Wang, J.-K. Zhang, A. I. Lazar and D.-S. Guo, Adv. Mater., 2016, 28, 7666–7671 CrossRef CAS.
- K. I. Assaf, A. Hennig, S. Peng, D.-S. Guo, D. Gabel and W. M. Nau, Chem. Commun., 2017, 53, 4616–4619 RSC.
- V. A. Burilov, G. A. Fatikhova, D. A. Mironova, S. E. Solovieva and I. S. Antipin, Macroheterocycles, 2015, 8, 409–414 CrossRef CAS.
- S. Shinkai, T. Arimura, K. Araki and H. Kawabata, J. Chem. Soc., Perkin Trans. 1, 1989, 2039–2045 RSC.
- J. Cui, V. D. Uzunova, D.-S. Guo, K. Wang, W. M. Nau and Y. Liu, Eur. J. Org. Chem., 2010, 1704–1710 CrossRef CAS.
- X.-P. Ding, D.-B. Tang, T. Li, S.-F. Wang and Y.-Y. Zhou, Spectrochim. Acta, Part A, 2011, 81, 44–47 CrossRef CAS.
- N. Basilio, V. Francisco and L. Garcia-Rio, J. Org. Chem., 2012, 77, 10764–10772 CrossRef CAS.
- N. Basilio and L. Garcia-Rio, ChemPhysChem, 2012, 13, 2368–2376 CrossRef CAS.
- N. Basilio, L. Garcia-Rio and M. Martin-Pastor, Langmuir, 2012, 28, 2404–2414 CrossRef CAS.
- X.-Y. Hu, S. Peng, D.-S. Guo, F. Ding and Y. Liu, Supramol. Chem., 2015, 27, 336–345 CrossRef CAS.
- Y.-C. Pan, H.-W. Tian, S. Peng, X.-Y. Hu and D.-S. Guo, Chin. Chem. Lett., 2017, 28, 787–792 CrossRef CAS.
- S. Fernandez-Abad, M. Pessego, N. Basilio and L. Garcia-Rio, Chem. – Eur. J., 2016, 22, 6466–6470 CrossRef CAS.
- Y.-X. Wang, Y.-M. Zhang, Y.-L. Wang and Y. Liu, Chem. Mater., 2015, 27, 2848–2854 CrossRef CAS.
- M. Esmaielzade Rostami, B. Gorji and R. Zadmard, Tetrahedron Lett., 2018, 59, 2393–2398 CrossRef CAS.
- I. Shulov, R. V. Rodik, Y. Arntz, A. Reisch, V. I. Kalchenko and A. S. Klymchenko, Angew. Chem., Int. Ed., 2016, 55, 15884–15888 CrossRef CAS.
- S. Kolusheva, R. Zadmard, T. Schrader and R. Jelinek, J. Am. Chem. Soc., 2006, 128, 13592–13598 CrossRef CAS PubMed.
- S. Peng, A. Barba-Bon, Y.-C. Pan, W. M. Nau, D.-S. Guo and A. Hennig, Angew. Chem., Int. Ed., 2017, 56, 1–5 CrossRef.
- S. Fujii, S. Yamada, S. Matsumoto, G. Kubo, K. Yoshida, E. Tabata, R. Miyake, Y. Sanada, I. Akiba, T. Okobira, N. Yagi, E. Mylonas, N. Ohta, H. Sekiguchi and K. Sakurai, Sci. Rep., 2017, 7, 44494 CrossRef CAS.
- S. Fujii, J. H. Lee, R. Takahashi and K. Sakurai, Langmuir, 2018, 34, 5072–5078 CrossRef CAS.
- S. Fujii, R. Takahashi, J. H. Lee and K. Sakurai, Soft Matter, 2018, 14, 875–878 RSC.
- J. H. Lee, S. Fujii, R. Takahashi and K. Sakurai, Chem. Commun., 2019, 55, 1303–1305 RSC.
- D. Elend, U. Pieles and P. Shahgaldian, Chimia, 2010, 64, 45–48 CrossRef CAS.
- N. Moridi, O. Danylyuk, K. Suwinska and P. Shahgaldian, J. Colloid Interface Sci., 2012, 377, 450–455 CrossRef CAS.
- L. G. Tulli, N. Moridi, W. Wang, K. Helttunen, M. Neuburger, D. Vaknin, W. Meier and P. Shahgaldian, Chem. Commun., 2014, 50, 3938–3940 RSC.
- L. G. Tulli, W. Wang, W. R. Lindemann, I. Kuzmenko, W. Meier, D. Vaknin and P. Shahgaldian, Langmuir, 2015, 31, 2351–2359 CrossRef CAS.
- N. O. Mchedlov-Petrossyan, L. N. Vilkova, N. A. Vodolazkaya, A. G. Yakubovskaya, R. V. Rodik, V. I. Boyko and V. I. Kalchenko, Sensors, 2006, 6, 962–977 CrossRef CAS.
- R. V. Rodik, A. S. Klymchenko, N. Jain, S. I. Miroshnichenko, L. Richert, V. I. Kalchenko and Y. Mely, Chem. – Eur. J., 2011, 17, 5526–5538 CrossRef CAS.
- N. O. McHedlov-Petrossyan, N. A. Vodolazkaya, R. V. Rodik, L. N. Bogdanova, T. A. Cheipesh, O. Y. Soboleva, A. P. Kryshtal, L. V. Kutuzova and V. I. Kalchenko, J. Phys. Chem. C, 2012, 116, 10245–10259 CrossRef CAS.
- E. V. Ukhatskaya, S. V. Kurkov, M. A. Hjalmarsdottir, V. A. Karginov, S. E. Matthews, R. V. Rodik, V. I. Kalchenko and T. Loftsson, Int. J. Pharm., 2013, 458, 25–30 CrossRef CAS.
- E. V. Ukhatskaya, S. V. Kurkov, R. V. Rodik, V. I. Kalchenko, S. E. Matthews, P. Jansook and T. Loftsson, J. Inclusion Phenom. Macrocyclic Chem., 2013, 79, 473–483 CrossRef.
- I. O. Melezhyk, R. V. Rodik, N. V. Iavorska, A. S. Klymchenko, Y. Mely, V. V. Shepelevych, L. M. Skivka and V. I. Kalchenko, Anti-Infect. Agents, 2015, 13, 87–94 CrossRef CAS.
- R. V. Rodik, A.-S. Anthony, V. I. Kalchenko, Y. Mély and A. S. Klymchenko, New J. Chem., 2015, 39, 1654–1664 RSC.
- C. Weeden, K. J. Hartlieb and L. Y. Lim, J. Pharm. Pharmacol., 2012, 64, 1403–1411 CrossRef CAS.
- T. Arimura, H. Kawabata, T. Matsuda, T. Muramatsu, H. Satoh, K. Fujio, O. Manabe and S. Shinkai, J. Org. Chem., 1991, 56, 301–306 CrossRef CAS.
- D. Hardy, R. M. Bill, A. Jawhari and A. J. Rothnie, Biochem. Soc. Trans., 2016, 44, 838–844 CrossRef CAS.
- T. Sun, L.-R. Qi, W.-W. Li, Y. Li, X.-M. Shuai, Z.-Q. Cai, H.-P. Chen, X.-G. Qiao and L.-F. Ma, J. Chromatogr. A, 2019, 1601, 310–318 CrossRef CAS.
- H.-Y. Tian, H.-J. Li, Y.-J. Chen, D. Wang and C.-J. Li, Ind. Eng. Chem. Res., 2002, 41, 4523–4527 CrossRef CAS.
- T. Jin, F. Fujii, H. Sakata, M. Tamura and M. Kinjo, Chem. Commun., 2005, 4300–4302 RSC.
- N. Basilio, L. Garcia-Rio and M. Martin-Pastor, J. Phys. Chem. B, 2010, 114, 4816–4820 CrossRef CAS.
- M.-X. Chen, T. Li, S. Peng and D. Tao, New J. Chem., 2016, 40, 9923–9929 RSC.
- H.-W. Tian, Y.-C. Pan and D.-S. Guo, Supramol. Chem., 2017, 30, 562–567 CrossRef.
- A. Fraix, D. Afonso, G. M. L. Consoli and S. Sortino, Photochem. Photobiol. Sci., 2019, 18, 2216–2224 RSC.
- X.-Y. Hu, Y. Chen and Y. Liu, Chin. Chem. Lett., 2015, 26, 862–866 CrossRef CAS.
- Y.-X. Wang, D.-S. Guo, Y.-C. Duan, Y.-J. Wang and Y. Liu, Sci. Rep., 2015, 5, 9019 CrossRef CAS.
- S. Song, J.-H. Wang, H.-T. Feng, Z.-H. Zhu and Y.-S. Zheng, RSC Adv., 2014, 4, 24909–24913 RSC.
- M. S. Becherer, B. Schade, C. Bottcher and A. Hirsch, Chem. – Eur. J., 2009, 15, 1637–1648 CrossRef CAS.
- F. Rodler, B. Schade, C. M. Jager, S. Backes, F. Hampel, C. Bottcher, T. Clark and A. Hirsch, J. Am. Chem. Soc., 2015, 137, 3308–3317 CrossRef CAS.
- P. Shahgaldian, M. A. Sclotti and U. Pieles, Langmuir, 2008, 24, 8522–8526 CrossRef CAS PubMed.
- L. G. Tulli, W.-J. Wang, V. Rullaud, W. R. Lindemann, I. Kuzmenko, D. Vaknin and P. Shahgaldian, RSC Adv., 2016, 6, 9278–9285 RSC.
- J. H. Lee, S. Fujii, R. Takahashi and K. Sakurai, Langmuir, 2018, 34, 12109–12115 CrossRef CAS.
- M. Fricke, D. Volkmer, C. E. Krill, M. Kellermann and A. Hirsch, Cryst. Growth Des., 2006, 6, 1120–1123 CrossRef CAS.
- M. Kellermann, W. Bauer, A. Hirsch, B. Schade, K. Ludwig and C. Bottcher, Angew. Chem., Int. Ed., 2004, 43, 2959–2962 CrossRef CAS PubMed.
- C. Yihwa, M. Kellermann, M. Becherer, A. Hirsch and C. Bohne, Photochem. Photobiol. Sci., 2007, 6, 525–531 RSC.
- C. M. Jager, A. Hirsch, B. Schade, C. Bottcher and T. Clark, Chem. – Eur. J., 2009, 15, 8586–8592 CrossRef.
- I. Di Bari, A. Fraix, R. Picciotto, A. R. Blanco, S. Petralia, S. Conoci, G. Granata, G. M. L. Consoli and S. Sortino, RSC Adv., 2016, 6, 105573 RSC.
- I. Di Bari, R. Picciotto, G. Granata, A. R. Blanco, G. M. Consoli and S. Sortino, Org. Biomol. Chem., 2016, 14, 8047–8052 RSC.
- G. Granata, I. Paterniti, C. Geraci, F. Cunsolo, E. Esposito, M. Cordaro, A. R. Blanco, S. Cuzzocrea and G. M. L. Consoli, Mol. Pharmaceutics, 2017, 14, 1610–1622 CrossRef CAS.
- I. Di Bari, G. Granata, G. M. L. Consoli and S. Sortino, New J. Chem., 2018, 18096–18101 RSC.
- S. Friedman, S. Kolusheva, R. Volinsky, L. Zeiri, T. Schrader and R. Jelinek, Anal. Chem., 2008, 80, 7804–7811 CrossRef CAS.
- R. Zadmard and T. Schrader, J. Am. Chem. Soc., 2005, 127, 904–915 CrossRef CAS.
- Y.-L. Liu, L. Liu, Y.-L. Wang, Y.-C. Han, D. Wong and Y.-J. Chen, Green Chem., 2008, 10, 635–640 RSC.
- Z. Zheng, W.-C. Geng, J. Gao, Y.-J. Mu and D.-S. Guo, Org. Chem. Front., 2018, 5, 2685–2691 RSC.
- L. Gallego-Yerga, M. Lomazzi, V. Franceschi, F. Sansone, C. Ortiz Mellet, G. Donofrio, A. Casnati and J. M. Garcia Fernandez, Org. Biomol. Chem., 2015, 13, 1708–1723 RSC.
- K.-P. Wang, Y. Chen and Y. Liu, Chem. Commun., 2015, 51, 1647–1649 RSC.
- X.-M. Chen, Y. Chen, Q. Yu, B.-H. Gu and Y. Liu, Angew. Chem., Int. Ed., 2018, 57, 12519–12523 CrossRef CAS.
- G. Granata, G. M. L. Consoli, R. Lo Nigro, G. Malandrino and C. Geraci, Supramol. Chem., 2016, 28, 377–383 CrossRef CAS.
- F. Sansone, M. Dudic, G. Donofrio, C. Rivetti, L. Baldini, A. Casnati, S. Cellai and R. Ungaro, J. Am. Chem. Soc., 2006, 128, 14528–14536 CrossRef CAS.
- T. Takeuchi, V. Bagnacani, F. Sansone and S. Matile, ChemBioChem, 2009, 10, 2793–2799 CrossRef CAS.
- A. D. Martin, E. Houlihan, N. Morellini, P. K. Eggers, E. James, K. A. Stubbs, A. R. Harvey, M. Fitzgerald, C. L. Raston and S. A. Dunlop, ChemPlusChem, 2012, 77, 308–313 CrossRef CAS.
- G. M. L. Consoli, G. Granata, R. Picciotto, A. R. Blanco, C. Geraci, A. Marino and A. Nostro, MedChemComm, 2018, 9, 160–164 RSC.
- E. James, P. K. Eggers, A. R. Harvey, S. A. Dunlop, M. Fitzgerald, K. A. Stubbs and C. L. Raston, Org. Biomol. Chem., 2013, 11, 6108–6112 RSC.
- V. Rullaud, M. Siragusa, A. Cumbo, D. Gygax and P. Shahgaldian, Chem. Commun., 2012, 48, 12186–12188 RSC.
- N. Moridi, C. Wackerlin, V. Rullaud, R. Schelldorfer, T. A. Jung and P. Shahgaldian, Chem. Commun., 2013, 49, 367–369 RSC.
- V. Rullaud, N. Moridi and P. Shahgaldian, Langmuir, 2014, 30, 8675–8679 CrossRef CAS.
- A. Galukhin, A. Erokhin, I. Imatdinov and Y. Osin, RSC Adv., 2015, 5, 33351–33355 RSC.
- W.-C. Geng, Y.-C. Liu, Y.-Y. Wang, Z. Xu, Z. Zheng, C.-B. Yang and D.-S. Guo, Chem. Commun., 2016, 53, 392–395 RSC.
- S. Fujii, Y. Sanada, T. Nishimura, I. Akiba, K. Sakurai, N. Yagi and E. Mylonas, Langmuir, 2012, 28, 3092–3101 CrossRef CAS.
- S. Sakamoto, S. Fujii, K. Yoshida and K. Sakurai, Langmuir, 2016, 32, 12434–12441 CrossRef CAS.
- M. Araki, S. Fujii, J. H. Lee, R. Takahashi and K. Sakurai, Soft Matter, 2019, 3515–3519 RSC.
- E. Mylonas, N. Yagi, S. Fujii, K. Ikesue, T. Ueda, H. Moriyama, Y. Sanada, K. Uezu, K. Sakurai and T. Okobira, Sci. Rep., 2019, 9, 1982 CrossRef.
- E. Houel, A. Lazar, E. Da Silva, A. W. Coleman, A. Solovyov, S. Cherenok and V. I. Kalchenko, Langmuir, 2002, 18, 1374–1379 CrossRef CAS.
- M. Rehm, M. Frank and J. Schatz, Tetrahedron Lett., 2009, 50, 93–96 CrossRef CAS.
- J. Gasparello, A. Manicardi, A. Casnati, R. Corradini, R. Gambari, A. Finotti and F. Sansone, Sci. Rep., 2019, 9, 3036 CrossRef.
- A. D. Martin, R. A. Boulos, K. A. Stubbs and C. L. Raston, Chem. Commun., 2011, 47, 7329–7331 RSC.
- J. Mo, L. Wang, X. Huang, B. Lu, C. Zou, L. Wei, J. Chu, P. K. Eggers, S. Chen, C. L. Raston, J. Wu, L. Y. Lim and W. Zhao, Nanoscale, 2017, 9, 13142–13152 RSC.
- K. Samanta, D. S. Ranade, A. Upadhyay, P. P. Kulkarni and C. P. Rao, ACS Appl. Mater. Interfaces, 2017, 9, 5109–5117 CrossRef CAS.
- S. Arimori, T. Nagasaki and S. Shinkai, J. Chem. Soc., Perkin Trans. 1, 1993, 887–889 RSC.
- R. Takahashi, S. Matsumoto, S. Fujii, T. Narayanan and K. Sakurai, Angew. Chem., Int. Ed., 2017, 56, 6734–6738 CrossRef CAS.
- E. V. Ukhatskaya, S. V. Kurkov, S. E. Matthews, A. El Fagui, C. Amiel, F. Dalmas and T. Loftsson, Int. J. Pharm., 2010, 402, 10–19 CrossRef CAS.
- E. V. Ukhatskaya, S. V. Kurkov, S. E. Matthews and T. Loftsson, J. Inclusion Phenom. Macrocyclic Chem., 2013, 79, 47–55 CrossRef.
- V. A. Burilov, G. A. Fatikhova, M. N. Dokuchaeva, R. I. Nugmanov, D. A. Mironova, P. V. Dorovatovskii, V. N. Khrustalev, S. E. Solovieva and I. S. Antipin, Beilstein J. Org. Chem., 2018, 14, 1980–1993 CrossRef CAS.
- R. Zadmard, M. Arendt and T. Schrader, J. Am. Chem. Soc., 2004, 126, 7752–7753 CrossRef CAS.
- Y.-X. Wang, D.-S. Guo, Y. Cao and Y. Liu, RSC Adv., 2013, 3, 8058–8063 RSC.
- S. Fujii, K. Sakurai, T. Okobira, N. Ohta and A. Takahara, Langmuir, 2013, 29, 13666–13675 CrossRef CAS.
- S. Fujii, R. Takahashi and K. Sakurai, Langmuir, 2017, 33, 4019–4027 CrossRef CAS.
- E.-H. Ryu, J. Yan, Z. Zhong and Y. Zhao, J. Org. Chem., 2006, 71, 7205–7213 CrossRef CAS.
- K. Khan, H. Huang and Y.-S. Zheng, Curr. Org. Chem., 2012, 16, 2745–2751 CrossRef CAS.
- K. Khan, S. L. Badshah, N. Ahmad, H. U. Rashid and Y. Mabkhot, Molecules, 2017, 22, 783 CrossRef.
- T. Mecca, G. M. Messina, G. Marletta and F. Cunsolo, Chem. Commun., 2013, 49, 2530–2532 RSC.
- E.-H. Ryu and Y. Zhao, Org. Lett., 2004, 6, 3187–3189 CrossRef CAS.
- H. Huang, D.-M. Li, W. Wang, Y.-C. Chen, K. Khan, S. Song and Y.-S. Zheng, Org. Biomol. Chem., 2012, 10, 729–735 RSC.
- H. Matsuoka, M. Tsurumi and N. Ise, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 38, 6279–6286 CrossRef CAS.
- H.-Y. Tian, Y.-J. Chen, D. Wang, C. C. Zeng and C. J. Li, Tetrahedron Lett., 2000, 41, 2529–2532 CrossRef CAS.
- S. Avvakumova, P. Fezzardi, L. Pandolfi, M. Colombo, F. Sansone, A. Casnati and D. Prosperi, Chem. Commun., 2014, 50, 11029–11032 RSC.
- K. Yoshida, S. Fujii, R. Takahashi, S. Matsumoto and K. Sakurai, Langmuir, 2017, 33, 9122–9128 CrossRef CAS.
- S. Shinkai, H. Koreishi, H. Mori, T. Sone and O. Manabe, Chem. Lett., 1985, 1033–1036 CrossRef CAS.
- L.-T. Zhang, L. A. Godinez, T.-B. Lu, G. W. Gokel and A. E. Kaifer, Angew. Chem., Int. Ed. Engl., 1995, 34, 235–237 CrossRef CAS.
- Y.-J. Zhang and W.-X. Cao, New J. Chem., 2001, 25, 483–486 RSC.
- I. S. Ryzhkina, Y. V. Kiseleva, L. I. Murtazina, S. E. Solovieva, N. G. Manin and A. I. Konovalov, Macroheterocycles, 2017, 10, 190–195 CrossRef CAS.
- S. Houmadi, D. Coquiere, L. Legrand, M. C. Faure, M. Goldmann, O. Reinaud and S. Remita, Langmuir, 2007, 23, 4849–4855 CrossRef CAS.
- N. Micali, V. Villari, G. M. Consoli, F. Cunsolo and C. Geraci, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2006, 73, 051904 CrossRef PubMed.
- R. Miyake, S. Fujii, J. H. Lee, R. Takahashi and K. Sakurai, J. Colloid Interface Sci., 2019, 535, 8–15 CrossRef CAS.
- Y. Tokunaga, H. Sakon, H. Kanefusa, Y. Shimomura and K. Suzuki, ARKIVOC, 2003, 135–143 Search PubMed.
- J. Dauvergne, E. M. Desuzinges, C. Faugier, S. Igonet, M. Soulié, E. Grousson, D. Cornut, F. Bonneté, G. Durand, E. Dejean and A. Jawhari, ChemistrySelect, 2019, 4, 5535–5539 Search PubMed.
- S. Shinkai, Y. Shirahama, T. Tsubaki and O. Manabe, J. Chem. Soc., Perkin Trans. 1, 1989, 1859–1860 RSC.
- J. Zhang, D.-S. Guo, L.-H. Wang, Z. Wang and Y. Liu, Soft Matter, 2011, 7, 1756–1762 RSC.
- Z. Wang, D.-S. Guo, J. Zhang and Y. Liu, Acta Chim. Sin., 2012, 70, 1709 CrossRef CAS.
- L. Gallego-Yerga, M. Lomazzi, F. Sansone, C. Ortiz Mellet, A. Casnati and J. M. Garcia Fernandez, Chem. Commun., 2014, 50, 7440–7443 RSC.
- L. Gallego-Yerga, I. Posadas, C. de la Torre, J. Ruiz-Almansa, F. Sansone, C. Ortiz Mellet, A. Casnati, J. M. Garcia Fernandez and V. Cena, Front. Pharmacol., 2017, 8, 249 CrossRef.
- X. Yan, V. Janout, J. T. Hsu and S. L. Regen, J. Am. Chem. Soc., 2003, 125, 8094–8095 CrossRef CAS.
- D. H. McCullough, V. Janout, J. W. Li, J. T. Hsu, Q. Truong, E. Wilusz and S. L. Regen, J. Am. Chem. Soc., 2004, 126, 9916–9917 CrossRef CAS.
- S. Fujii, K. Nishina, S. Yamada, S. Mochizuki, N. Ohta, A. Takahara and K. Sakurai, Soft Matter, 2014, 10, 8216–8223 RSC.
- P. Shahgaldian, A. W. Coleman, S. S. Kuduva and M. J. Zaworotko, Chem. Commun., 2005, 1968–1970 RSC.
- J. Bugler, N. A. J. M. Sommerdijk, A. J. W. G. Visser, A. van Hoek, R. J. M. Nolte, J. F. J. Engbersen and D. N. Reinhoudt, J. Am. Chem. Soc., 1999, 121, 28–33 CrossRef.
- N. Bono, C. Pennetta, A. Sganappa, E. Giupponi, F. Sansone, A. Volonterio and G. Candiani, Int. J. Pharm., 2018, 549, 436–445 CrossRef CAS.
- D. Coquiere, J. Marrot and O. Reinaud, Org. Lett., 2007, 9, 3271–3274 CrossRef CAS.
- A. V. Solovyov, S. O. Cherenok, O. I. Kalchenko, L. I. Atamas, Z. I. Kazantseva, I. A. Koshets, I. F. Tsymbal and V. I. Kalchenko, J. Mol. Liq., 2011, 159, 117–123 CrossRef CAS.
- R. Bussolati, P. Carrieri, A. Secchi, A. Arduini, A. Credi, M. Semeraro, M. Venturi, S. Silvi, D. Velluto, R. Zappacosta and A. Fontana, Org. Biomol. Chem., 2013, 11, 5944–5953 RSC.
- D. Vollhardt, J. Gloede, G. Weidemann and R. Rudert, Langmuir, 2003, 19, 4228–4234 CrossRef CAS.
- F. Mayer, S. Tiruvadi Krishnan, D. T. Schuhle, S. V. Eliseeva, S. Petoud, E. Toth and K. Djanashvili, Front. Chem., 2018, 6, 1 CrossRef.
- Y. Cakmak, T. Nalbantoglu, T. Durgut and E. U. Akkaya, Tetrahedron Lett., 2014, 55, 538–540 CrossRef CAS.
- O. Kasyan, E. R. Healey, A. Drapailo, M. Zaworotko, S. Cecillon, A. W. Coleman and V. Kalchenko, J. Inclusion Phenom. Macrocyclic Chem., 2006, 58, 127–132 CrossRef.
- N. Moridi, D. Elend, O. Danylyuk, K. Suwinska and P. Shahgaldian, Langmuir, 2011, 27, 9116–9121 CrossRef CAS.
- Z. Xu, S. Jia, W. Wang, Z. Yuan, B. Jan Ravoo and D.-S. Guo, Nat. Chem., 2019, 11, 86–93 CrossRef CAS.
- J. Gao, J. Li, W.-C. Geng, F.-Y. Chen, X.-C. Duan, Z. Zheng, D. Ding and D.-S. Guo, J. Am. Chem. Soc., 2018, 140, 4945–4953 CrossRef CAS.
- J. Gao, Z. Zheng, L. Shi, S.-Q. Wu, H. Sun and D.-S. Guo, Beilstein J. Org. Chem., 2018, 14, 1840–1845 CrossRef CAS PubMed.
- Y. Zhao and E.-H. Ryu, J. Org. Chem., 2005, 70, 7585–7591 CrossRef CAS.
- E.-H. Ryu and Y. Zhao, J. Org. Chem., 2006, 71, 9491–9494 CrossRef CAS.
- E.-H. Ryu, H. Cho and Y. Zhao, Org. Lett., 2007, 9, 5147–5150 CrossRef CAS.
- M. Lee, S.-J. Lee and L.-H. Jiang, J. Am. Chem. Soc., 2004, 126, 12724–12725 CrossRef CAS PubMed.
- Y.-Y. Xie, L.-F. Mao and L.-H. Li, J. Chem. Res., 2013, 476–479 CrossRef CAS.
- M. Mori, A. Hirayama, H. Tsue and S. Tanaka, Acta Chromatogr., 2007, 19, 73–80 CAS.
- L. S. Yakimova, L. H. Gilmanova, V. G. Evtugyn, Y. N. Osin and I. I. Stoikov, J. Nanopart. Res., 2017, 19, 173 CrossRef.
- F. Liu, G.-Y. Lu, W.-J. He, M.-H. Liu and L.-G. Zhu, Thin Solid Films, 2004, 468, 244–249 CrossRef CAS.
- P. Shahgaldian, E. Da Silva and A. W. Coleman, J. Inclusion Phenom. Macrocyclic Chem., 2003, 46, 175–177 CrossRef CAS.
- D. Momekova, D. Budurova, E. Drakalska, S. Shenkov, G. Momekov, B. Trzebicka, N. Lambov, E. Tashev and S. Rangelov, Int. J. Pharm., 2012, 436, 410–417 CrossRef CAS.
- G. Sautrey, I. Clarot, E. Rogalska and J.-B. Regnouf-de-Vains, New J. Chem., 2012, 36, 2060 RSC.
- J. Gualbert, P. Shahgaldian and A. W. Coleman, Int. J. Pharm., 2003, 257, 69–73 CrossRef CAS.
- G. M. L. Consoli, G. Granata, E. Galante, I. Di Silvestro, L. Salafia and C. Geraci, Tetrahedron, 2007, 63, 10758–10763 CrossRef CAS.
- J.-H. Yim, J. Kim, D. W. Gidley, R. S. Vallery, H.-G. Peng, D. K. An, B.-K. Choi, Y.-K. Park and J.-K. Jeon, Macromol. Mater. Eng., 2006, 291, 369–376 CrossRef CAS.
- G. Sautrey, I. Clarot, A. B. Salem, E. Rogalska and J.-B. Regnouf de Vains, New J. Chem., 2012, 36, 78–85 RSC.
- P. Shahgaldian, A. W. Coleman and V. I. Kalchenko, Tetrahedron Lett., 2001, 42, 577–579 CrossRef CAS.
- V. Bagnacani, V. Franceschi, L. Fantuzzi, A. Casnati, G. Donofrio, F. Sansone and R. Ungaro, Bioconjugate Chem., 2012, 23, 993–1002 CrossRef CAS.
- D. Volkmer, M. Fricke, M. Gleiche and L.-F. Chi, Mater. Sci. Eng., C, 2005, 25, 161–167 CrossRef.
- L. Y. Zakharova, Y. R. Kudryashova, N. M. Selivanova, M. A. Voronin, A. R. Ibragimova, S. E. Solovieva, A. T. Gubaidullin, A. I. Litvinov, I. R. Nizameev, M. K. Kadirov, Y. G. Galyametdinov, I. S. Antipin and A. I. Konovalov, J. Membr. Sci., 2010, 364, 90–101 CrossRef CAS.
- P. Shahgaldian and A. W. Coleman, Langmuir, 2003, 19, 5261–5265 CrossRef CAS.
- O. M. Martin and S. Mecozzi, Supramol. Chem., 2005, 17, 9–15 CrossRef CAS.
- O. M. Martin and S. Mecozzi, Tetrahedron, 2007, 63, 5539–5547 CrossRef CAS.
- A. B. Mirgorodskaya, E. I. Yatskevich, Y. R. Kudryashova, S. E. Solov’eva, I. S. Antipin, L. Y. Zakharova and A. I. Konovalov, Kinet. Catal., 2011, 52, 529–535 CrossRef CAS.
- Y. R. Kudryashova, F. G. Valeeva, L. Y. Zakharova, S. E. Solovieva, I. S. Antipin, A. T. Gubaidullin and A. I. Konovalov, Colloid J., 2012, 74, 67–77 CrossRef CAS.
- B. Korchowiec, A. Ben Salem, Y. Corvis, J. B. R. de Vains, J. Korchowiec and E. Rogalska, J. Phys. Chem. B, 2007, 111, 13231–13242 CrossRef CAS.
- M. Pojarova, G. S. Ananchenko, K. A. Udachin, F. Perret, A. W. Coleman and J. A. Ripmeester, New J. Chem., 2007, 31, 871–878 RSC.
- A. Mustafina, L. Zakharova, J. Elistratova, J. Kudryashova, S. Soloveva, A. Garusov, I. Antipin and A. Konovalov, J. Colloid Interface Sci., 2010, 346, 405–413 CrossRef CAS PubMed.
- A. V. Ten’kovstev, A. B. Razina and M. M. Dudkina, Polym. Sci., Ser. B, 2014, 56, 274–281 CrossRef.
- D. Volkmer and M. Fricke, Z. Anorg. Allg. Chem., 2003, 629, 2381–2390 CrossRef CAS.
- X. Yu, C.-L. Tu, L. He, R.-B. Wang, G.-M. Sun, D.-Y. Yan and X.-Y. Zhu, J. Macromol. Sci., Part A: Pure Appl. Chem., 2009, 46, 360–367 CrossRef CAS.
- D. Volkmer, M. Fricke, D. Vollhardt and S. Siegel, J. Chem. Soc., Dalton Trans., 2002, 4547 RSC.
- P.-F. Gou, W.-P. Zhu and Z.-Q. Shen, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 5643–5651 CrossRef CAS.
- R. Roy and J. M. Kim, Angew. Chem., Int. Ed., 1999, 38, 369–372 CrossRef CAS.
- P. Shahgaldian, M. Cesario, P. Goreloff and A. W. Coleman, Chem. Commun., 2002, 326–327 RSC.
- A. Dubes, I. L. Moudrakovski, P. Shahgaldian, A. W. Coleman, C. I. Ratcliffe and J. A. Ripmeester, J. Am. Chem. Soc., 2004, 126, 6236–6237 CrossRef CAS.
- A. Dubes, K. A. Udachin, P. Shahgaldian, A. N. Lazar, A. W. Coleman and J. A. Ripmeester, New J. Chem., 2005, 29, 1141 RSC.
- G. S. Ananchenko, M. Pojarova, K. A. Udachin, D. M. Leek, A. W. Coleman and J. A. Ripmeester, Chem. Commun., 2006, 386–388 RSC.
- G. S. Ananchenko, K. A. Udachin, M. Pojarova, A. Dubes, J. A. Ripmeester, S. Jebors and A. W. Coleman, Cryst. Growth Des., 2006, 6, 2141–2148 CrossRef CAS.
- D. N. Polovyanenko, E. G. Bagryanskaya, A. Schnegg, K. Mobius, A. W. Coleman, G. S. Ananchenko, K. A. Udachin and J. A. Ripmeester, Phys. Chem. Chem. Phys., 2008, 10, 5299–5307 RSC.
- P. Shahgaldian, A. W. Coleman, B. Rather and M. J. Zaworotko, J. Inclusion Phenom. Macrocyclic Chem., 2005, 52, 241–245 CrossRef CAS.
- X.-L. Lou, F. Cheng, P.-F. Cao, Q. Tang, H.-J. Liu and Y. Chen, Chem. – Eur. J., 2009, 15, 11566–11572 CrossRef CAS.
- A. I. Litvinov, F. G. Valeeva, L. Y. Zakharova, S. E. Solovieva, I. S. Antipin and M. K. Kadirov, Russ. Chem. Bull., 2013, 62, 1350–1353 CrossRef CAS.
- L. Li, H.-Y. Zhang and Y. Liu, Sci. China: Chem., 2012, 55, 1092–1096 CrossRef CAS.
- P. Shahgaldian, Eur. J. Pharm. Biopharm., 2003, 55, 107–113 CrossRef CAS.
- P. Shahgaldian, E. Da Silva, A. W. Coleman, B. Rather and M. J. Zaworotko, Int. J. Pharm., 2003, 253, 23–38 CrossRef CAS.
- L. Zakharova, Y. Kudryashova, A. Ibragimova, E. Vasilieva, F. Valeeva, E. Popova, S. Solovieva, I. Antipin, Y. Ganeeva, T. Yusupova and A. Konovalov, Chem. Eng. J., 2012, 185–186, 285–293 CrossRef CAS.
- N. Maulucci, F. De Riccardis, C. B. Botta, A. Casapullo, E. Cressina, M. Fregonese, P. Tecilla and I. Izzo, Chem. Commun., 2005, 1354–1356 RSC.
- G. M. L. Consoli, G. Granata, R. Lo Nigro, G. Malandrino and C. Geraci, Langmuir, 2008, 24, 6194–6200 CrossRef CAS.
- M. Mourer, R. E. Duval, P. Constant, M. Daffe and J.-B. Regnouf-de-Vains, ChemBioChem, 2019, 20, 911–921 CrossRef CAS PubMed.
- X. Guo, L. Zhang, G.-Y. Lu, M.-F. Yin, F. Liu and M.-H. Liu, Chin. Chem. Lett., 2005, 16, 1543–1546 CAS.
- L. Yakimova, P. Padnya, D. Tereshina, A. Kunafina, A. Nugmanova, Y. Osin, V. Evtugyn and I. Stoikov, J. Mol. Liq., 2019, 279, 9–17 CrossRef CAS.
- O. M. Martin, L. Yu and S. Mecozzi, Chem. Commun., 2005, 4964–4966 RSC.
- Z.-S. Wang, G.-Y. Lu, X. Guo and H.-M. Wu, Supramol. Chem., 2003, 15, 327–334 CrossRef CAS.
- E. A. Andreyko, P. L. Padnya and I. I. Stoikov, Colloids Surf., A, 2014, 454, 74–83 CrossRef CAS.
- E. A. Andreyko, P. L. Padnya and I. I. Stoikov, J. Phys. Org. Chem., 2015, 28, 527–535 CrossRef CAS.
- E. A. Andreyko, P. L. Padnya, R. R. Daminova and I. I. Stoikov, RSC Adv., 2014, 4, 3556–3565 RSC.
- F. Liu, Y.-H. Wang and G.-Y. Lu, Chem. Lett., 2005, 34, 1450–1451 CrossRef CAS.
- A. R. Esker, L.-H. Zhang, C. E. Olsen, K. No and H. Yu, Langmuir, 1999, 15, 1716–1724 CrossRef CAS.
- L. H. Zhang, A. R. Esker, K. No and H. Yu, Langmuir, 1999, 15, 1725–1730 CrossRef CAS.
- X. Guo, G.-Y. Lu and Y. Li, Thin Solid Films, 2004, 460, 264–268 CrossRef CAS.
- B. Guan, M. Jiang, X.-G. Yang, Q. Liang and Y.-Y. Chen, Soft Matter, 2008, 4, 1393 RSC.
- S. Jebors, G. S. Ananchenko, A. W. Coleman and J. A. Ripmeester, Tetrahedron Lett., 2007, 48, 5503–5506 CrossRef CAS.
- S. Jebors, F. Fache, S. Balme, F. Devoge, M. Monachino, S. Cecillon and A. W. Coleman, Org. Biomol. Chem., 2008, 6, 319–329 RSC.
- F. A. Loiseau, A. M. Hill and K. K. Hii, Tetrahedron, 2007, 63, 9947–9959 CrossRef CAS.
- S. Jebors, B. Lesniewska, O. Shkurenko, K. Suwinska and A. W. Coleman, J. Inclusion Phenom. Macrocyclic Chem., 2010, 68, 207–217 CrossRef CAS.
- P. L. Padnya, E. A. Andreyko, O. A. Mostovaya, I. K. Rizvanov and I. I. Stoikov, Org. Biomol. Chem., 2015, 13, 5894–5904 RSC.
- D. Volkmer, M. Fricke, C. Agena and J. Mattay, J. Mater. Chem., 2004, 14, 2249 RSC.
- C. Tu, G. Li, Y. Shi, X. Yu, Y. Jiang, Q. Zhu, J. Liang, Y. Gao, D. Yan, J. Sun and X. Zhu, Chem. Commun., 2009, 3211–3213 RSC.
- L. S. Yakimova, P. L. Padnya, A. F. Kunafina, A. R. Nugmanova and I. I. Stoikov, Mendeleev Commun., 2019, 29, 86–88 CrossRef CAS.
- G. Gattuso, A. Notti, A. Pappalardo, S. Pappalardo, M. F. Parisi and F. Puntoriero, Tetrahedron Lett., 2013, 54, 188–191 CrossRef CAS.
- G. Gattuso, A. Notti, S. Pappalardo, M. F. Parisi, I. Pisagatti and S. Patanè, New J. Chem., 2014, 38, 5983–5990 RSC.
- G. Arena, A. Pappalardo, S. Pappalardo, G. Gattuso, A. Notti, M. F. Parisi, I. Pisagatti and C. Sgarlata, J. Therm. Anal. Calorim., 2015, 121, 1073–1079 CrossRef CAS.
- L. Barbera, G. Gattuso, F. H. Kohnke, A. Notti, S. Pappalardo, M. F. Parisi, I. Pisagatti, S. Patane, N. Micali and V. Villari, Org. Biomol. Chem., 2015, 13, 6468–6473 RSC.
- W.-P. Zhu, J. Ling and Z.-Q. Shen, Macromol. Chem. Phys., 2006, 207, 844–849 CrossRef CAS.
- A. V. Ten'kovtsev and A. B. Razina, Russ. J. Appl. Chem., 2009, 82, 1615–1619 CrossRef.
- I. Pisagatti, L. Barbera, G. Gattuso, S. Patanè, M. F. Parisi and A. Notti, Supramol. Chem., 2018, 30, 658–663 CrossRef CAS.
- A. V. Tenkovtsev, M. M. Dudkina, L. I. Scherbinskaya, V. Aseyev and H. Tenhu, Polymer, 2010, 51, 3108–3115 CrossRef CAS.
- Z.-M. Zhao, Y. Wang, J. Han, H.-D. Zhu and L. An, Chem. Pharm. Bull., 2015, 63, 180–186 CrossRef CAS PubMed.
- G. de Miguel, M. T. Martin-Romero, J. M. Pedrosa, E. Munoz, M. Perez-Morales, T. H. Richardson and L. Camacho, Phys. Chem. Chem. Phys., 2008, 10, 1569–1576 RSC.
- G. de Miguel, M. T. Martin-Romero, M. Perez-Morales, E. Munoz and L. Camacho, J. Porphyrins Phthalocyanines, 2009, 13, 597–605 CrossRef CAS.
- S. Jebors, A. Leydier, Q. Wu, B. Bertino Ghera, M. Malbouyre and A. W. Coleman, J. Microencapsulation, 2010, 27, 561–571 CrossRef CAS PubMed.
- A. M. Gonzalez-Delgado, J. J. Giner-Casares, G. Brezesinski, J. B. Regnouf-de-Vains and L. Camacho, Langmuir, 2012, 28, 12114–12121 CrossRef CAS PubMed.
- U. Darbost, M. Giorgi, N. Hucher, I. Jabin and O. Reinaud, Supramol. Chem., 2005, 17, 243–250 CrossRef CAS.
- Z. Rafiee, A. Kakanejadifard, R. Hosseinzadeh, M. Nemati and M. Adeli, RSC Adv., 2016, 6, 17470–17473 RSC.
- V. A. Burilov, D. A. Mironova, R. R. Ibragimova, R. I. Nugmanov, S. E. Solovieva and I. S. Antipin, Colloids Surf., A, 2017, 515, 41–49 CrossRef CAS.
- R. R. Ibragimova, V. A. Burilov, A. R. Aimetdinov, D. A. Mironova, V. G. Evtugyn, Y. N. Osin, S. E. Solovieva and I. S. Antipin, Macroheterocycles, 2016, 9, 433–441 CrossRef CAS.
- V. Burilov, A. Valiyakhmetova, D. Mironova, E. Sultanova, V. Evtugyn, Y. Osin, S. Katsyuba, T. Burganov, S. Solovieva and I. Antipin, New J. Chem., 2018, 42, 2942–2951 RSC.
- V. A. Burilov, R. I. Nugmanov, R. R. Ibragimova, S. E. Solovieva and I. S. Antipin, Mendeleev Commun., 2015, 25, 177–179 CrossRef CAS.
- A. A. Vavilova, V. V. Gorbachuk, D. N. Shurpik, A. V. Gerasimov, L. S. Yakimova, P. L. Padnya and I. I. Stoikov, J. Mol. Liq., 2019, 281, 243–251 CrossRef CAS.
- V. A. Burilov, R. R. Ibragimova, B. H. Gafiatullin, R. I. Nugmanov, S. E. Solovieva and I. S. Antipin, Macroheterocycles, 2017, 10, 215–220 CrossRef CAS.
- V. A. Burilov, D. A. Mironova, R. R. Ibragimova, S. E. Solovieva, B. König and I. S. Antipin, RSC Adv., 2015, 5, 101177 RSC.
- Q. Liang, G.-S. Chen, B. Guan and M. Jiang, J. Mater. Chem., 2011, 21, 13262 RSC.
- S. Aleandri, A. Casnati, L. Fantuzzi, G. Mancini, G. Rispoli and F. Sansone, Org. Biomol. Chem., 2013, 11, 4811–4817 RSC.
- B. Guan, Q. Liang, Y. Zhu, M.-H. Qiao, J. Zou and M. Jiang, J. Mater. Chem., 2009, 19, 7610–7613 RSC.
- Q. Liang, B. Guan and M. Jiang, J. Mater. Chem., 2010, 20, 8236 RSC.
- Q. Liang, C. Li, G. Chen and M. Jiang, J. Colloid Interface Sci., 2012, 383, 82–88 CrossRef CAS PubMed.
- D. T. Schuhle, J. Schatz, S. Laurent, L. Vander Elst, R. N. Muller, M. C. Stuart and J. A. Peters, Chem. – Eur. J., 2009, 15, 3290–3296 CrossRef PubMed.
- J. W. Steed, D. R. Turner and K. J. Wallace, Core concepts in supramolecular chemistry and nanochemistry, Wiley, Chichester, West Succex, England, 2007 Search PubMed.
- C. Lau, R. Bitton, H. Bianco-Peled, D. G. Schultz, D. J. Cookson, S. T. Grosser and J. W. Schneider, J. Phys. Chem. B, 2006, 110, 9027–9033 CrossRef CAS PubMed.
- M. Miyake, K. Yamada and N. Oyama, Langmuir, 2008, 24, 8527 CrossRef CAS PubMed.
- J. N. Israelachvli, D. J. Mitchell and B. W. Ninham, J. Chem. Soc., Faraday Trans. 2, 1976, 72, 1525–1568 RSC.
- Q. Liang, B. Guan and M. Jiang, Prog. Chem., 2010, 22, 388–399 CAS.
- H.-T. Cui, Y.-M. Feng, W.-Z. Ren, T. Zeng, H.-Y. Lv and Y.-F. Pan, Recent Pat. Nanotechnol., 2009, 3, 32–41 CrossRef CAS PubMed.
- N.-M. Hwang, J.-S. Jung and D.-K. Lee, Thermodynamics – Fundamentals and its application in science, InTech, Rijeka, Croatia, 2012 Search PubMed.
- E. Mattia and S. Otto, Nat. Nanotechnol., 2015, 10, 111–119 CrossRef CAS PubMed.
- C. Wang, Z.-Q. Wang and X. Zhang, Acc. Chem. Res., 2012, 45, 608–618 CrossRef CAS PubMed.
- Y. Kang, K. Liu and X. Zhang, Langmuir, 2014, 30, 5989–6001 CrossRef CAS PubMed.
- P. Xing, T. Sun and A. Hao, RSC Adv., 2013, 3, 24776–24793 RSC.
- C. Wang, Z. Wang and X. Zhang, Small, 2011, 7, 1379–1383 CrossRef CAS PubMed.
- Y. Kang, X. Tang, Z. Cai and X. Zhang, Adv. Funct. Mater., 2016, 26, 8920–8931 CrossRef CAS.
- X. Zhang, Supramolecular amphiphiles, The Royal Society of Chemistry, London, UK, 2017 Search PubMed.
- O. Varga, M. Kubinyi, T. Vidóczy, P. Baranyai, I. Bitter and M. Kállay, J. Photochem. Photobiol., A, 2009, 207, 167–172 CrossRef CAS.
- V. Francisco, N. Basilio, L. Garcia-Rio, J. R. Leis, E. F. Maques and C. Vazquez-Vazquez, Chem. Commun., 2010, 46, 6551–6553 RSC.
- V. Lau and B. Heyne, Chem. Commun., 2010, 46, 3595–3597 RSC.
- M. Megyesi and L. Biczok, J. Phys. Chem. B, 2010, 114, 2814–2819 CrossRef CAS PubMed.
- K. Wang, D.-S. Guo, X. Wang and Y. Liu, ACS Nano, 2011, 5, 2880–2894 CrossRef CAS PubMed.
- D.-S. Guo, B.-P. Jiang, X. Wang and Y. Liu, Org. Biomol. Chem., 2012, 10, 720–723 RSC.
- D.-S. Guo, K. Wang, Y.-X. Wang and Y. Liu, J. Am. Chem. Soc., 2012, 134, 10244–10250 CrossRef CAS PubMed.
- Z.-Q. Li, C.-X. Hu, Y.-Q. Cheng, H. Xu, X.-L. Cao, X.-W. Song, H.-Y. Zhang and Y. Liu, Sci. China: Chem., 2012, 55, 2063–2068 CrossRef CAS.
- N. Basilio, A. Pineiro, J. P. Da Silva and L. Garcia-Rio, J. Org. Chem., 2013, 78, 9113–9119 CrossRef CAS PubMed.
- Y. Cao, Y.-X. Wang, D.-S. Guo and Y. Liu, Sci. China: Chem., 2013, 57, 371–378 CrossRef.
- B.-P. Jiang, D.-S. Guo, Y.-C. Liu, K.-P. Wang and Y. Liu, ACS Nano, 2014, 8, 1609–1618 CrossRef CAS.
- Z.-B. Qin, D.-S. Guo, X.-N. Gao and Y. Liu, Soft Matter, 2014, 10, 2253–2263 RSC.
- C. Costa, V. Francisco, S. G. Silva, M. L. C. do Vale, L. García-Río and E. F. Marques, Colloids Surf., A, 2015, 480, 71–78 CrossRef CAS.
- Y.-X. Wang, Y.-M. Zhang and Y. Liu, J. Am. Chem. Soc., 2015, 137, 4543–4549 CrossRef CAS PubMed.
- J. G. Harangozo, V. Wintgens, Z. Miskolczy, J. M. Guigner, C. Amiel and L. Biczok, Langmuir, 2016, 32, 10651–10658 CrossRef CAS PubMed.
- Y.-C. Liu, Y.-Y. Wang, H.-W. Tian, Y. Liu and D.-S. Guo, Org. Chem. Front., 2016, 3, 53–61 RSC.
- D.-S. Guo, K. Chen, H.-Q. Zhang and Y. Liu, Chem. – Asian J., 2009, 4, 436–445 CrossRef CAS PubMed.
- K. Wang, D.-S. Guo and Y. Liu, Chem. – Eur. J., 2010, 16, 8006–8011 CrossRef CAS PubMed.
- N. Basilio and L. Garcia-Rio, Chem. – Eur. J., 2009, 15, 9315–9319 CrossRef CAS PubMed.
- N. Basilio, M. Martin-Pastor and L. Garcia-Rio, Langmuir, 2012, 28, 6561–6568 CrossRef CAS PubMed.
- N. Basilio, B. Gomez, L. Garcia-Rio and V. Francisco, Chem. – Eur. J., 2013, 19, 4570–4576 CrossRef CAS.
- N. Basilio, D. A. Spudeit, J. Bastos, L. Scorsin, H. D. Fiedler, F. Nome and L. Garcia-Rio, Phys. Chem. Chem. Phys., 2015, 17, 26378–26385 RSC.
- N. Basilio, B. Gomez and L. Garcia-Rio, Langmuir, 2017, 33, 13008–13013 CrossRef CAS PubMed.
- C. Bize, J. C. Garrigues, M. Blanzat, I. Rico-Lattes, O. Bistri, B. Colasson and O. Reinaud, Chem. Commun., 2010, 46, 586–588 RSC.
- V. Wintgens, C. Le Coeur, C. Amiel, J. M. Guigner, J. G. Harangozo, Z. Miskolczy and L. Biczok, Langmuir, 2013, 29, 7682–7688 CrossRef CAS PubMed.
- V. Wintgens, Z. Miskolczy, J. M. Guigner, C. Amiel, J. G. Harangozo and L. Biczok, Langmuir, 2015, 31, 6655–6662 CrossRef CAS PubMed.
- V. Wintgens, J. G. Harangozo, Z. Miskolczy, J. M. Guigner, C. Amiel and L. Biczok, Langmuir, 2017, 33, 8052–8061 CrossRef CAS PubMed.
- X. Yao, X. Wang, T. Jiang, X. Ma and H. Tian, Langmuir, 2015, 31, 13647–13654 CrossRef CAS.
- L. Di Costanzo, S. Geremia, L. Randaccio, R. Purrello, R. Lauceri, D. Sciotto, F. G. Gulino and V. Pavone, Angew. Chem., Int. Ed., 2001, 40, 4245–4247 CrossRef CAS.
- H. Huang, D.-M. Li, W. Wang, Y.-C. Chen, K. Khan, S. Song and Y.-S. Zheng, Org. Biomol. Chem., 2012, 10, 729–735 RSC.
- D.-S. Guo and Y. Liu, Acc. Chem. Res., 2014, 47, 1925–1934 CrossRef CAS PubMed.
- I. S. Ryzhkina, Y. V. Kiseleva, L. I. Murtazina, Y. N. Valitova, S. E. Solov'eva, L. M. Pilishkina and A. I. Konovalov, Russ. Chem. Bull., 2010, 59, 1327–1335 CrossRef CAS.
- C.-L. Tu, L.-J. Zhu, P.-P. Li, Y. Chen, Y. Su, D.-Y. Yan, X.-Y. Zhu and G.-Y. Zhou, Chem. Commun., 2011, 47, 6063–6065 RSC.
- B. C. M. A. Ashwin, C. Saravanan, M. Senthilkumaran, R. Sumathi, P. Suresh and P. M. Mareeswaran, Supramol. Chem., 2017, 30, 32–41 CrossRef.
- R. V. Rodik, A. S. Klymchenko, N. Jain, S. I. Miroshnichenko, L. Richert, V. I. Kalchenko and Y. Mely, Chem. – Eur. J., 2011, 17, 5526–5538 CrossRef CAS.
- S. Peng, K. Wang, D.-S. Guo and Y. Liu, Soft Matter, 2015, 11, 290–296 RSC.
- J. G. Harangozó, V. Wintgens, Z. Miskolczy, C. Amiel and L. Biczók, Colloid Polym. Sci., 2016, 294, 1807–1814 CrossRef.
- K. Wang, D.-S. Guo, M.-Y. Zhao and Y. Liu, Chem. – Eur. J., 2016, 22, 1475–1483 CrossRef CAS.
- S. Peng, J. Gao, Y. Liu and D.-S. Guo, Chem. Commun., 2015, 51, 16557–16560 RSC.
| This journal is © the Partner Organisations 2020 |