A 2D covalent organic framework involving strong intramolecular hydrogen bonds for advanced supercapacitors†
Tao
Li‡
a,
Xiaodong
Yan‡
 a,
Yong
Liu
a,
Wen-Da
Zhang
a,
Qiu-Ting
Fu
a,
Haiyan
Zhu
a,
Zaijun
Li
a,
Yong
Liu
a,
Wen-Da
Zhang
a,
Qiu-Ting
Fu
a,
Haiyan
Zhu
a,
Zaijun
Li
 *a and
Zhi-Guo
Gu
*a and
Zhi-Guo
Gu
 *ab
*ab
aKey Laboratory of Synthetic and Biological Colloids, Ministry of Education, School of Chemical and Material Engineering, Jiangnan University, Wuxi 214122, China. E-mail: zaijunli@263.net; zhiguogu@jiangnan.edu.cn; Fax: +86 510 85917763; Tel: +86 510 85917090
bInternational Joint Research Center for Photoresponsive Molecules and Materials, School of Chemical and Material Engineering, Jiangnan University, Wuxi 214122, China
First published on 25th November 2019
Abstract
A stable benzobisthiazole-linked covalent organic framework (PG-BBT) involving strong intramolecular hydrogen bonds coupled with ordered pore structures, redox activity and numerous heteroatoms contributes to a high capacitance of 724 F g−1 and an excellent energy density of 69 W h kg−1.
Covalent organic frameworks (COFs) are an emerging class of crystalline porous polymers that enable atomically precise spatial integration of organic building blocks into functionalized architectures with long-range ordered skeletons and nanopores.1 Therefore, COFs have attracted great attention owing to their multiple applications in gas adsorption and separation, catalysis, sensing, energy storage etc.2 In principle, COFs are ideal electrode materials for supercapacitors due to their highly porous structure, conjugated polymer skeleton and tunable functionality. Much effort has been devoted to developing novel functionalized COFs as high-capacitance electrode materials for supercapacitors.3 Despite significant progress, their relatively low electron transfer ability makes their electrochemical performance only comparable to those of porous carbonaceous materials and far from those of metal–oxide materials.4
It is highly imperative to adopt new strategies to increase electron transfer in COFs. Benzobisthiazole (BBT) polymer chains are known to be efficient electron transfer materials, and this is ascribed to the empty d-orbitals of the sulfur atom, decreased energy of the π–π* transition and improved delocalization within the π-orbitals.5 With remarkable electron transport properties and facile chemical modulation, BBT-based materials have been used in organic electronics such as transistors, photovoltaics, and nonlinear optics.6 On the other hand, intramolecular hydrogen bonds have been recently introduced into the skeleton of COFs, contributing to a high specific capacitance of 416 F g−1 at 0.5 A g−1.7 Actually, intramolecular hydrogen bonds are highly related to proton-coupled electron transfer, which offers an opportunity for the redistribution of the electronic charge of molecules, resulting in extremely fast electronic mobility.8 Therefore, the combination of intramolecular hydrogen bonds and BBT units in COFs will synergistically accelerate electron transfer.
Based on the above consideration, we focused our attention on constructing benzobisthiazole-linked COFs with intramolecular hydrogen bonds for application in high-performance supercapacitors. 2-(2-Hydroxyphenyl)benzothiazole is a typical compound with strong intramolecular hydrogen bonds that are formed between the hydrogen atom of phenolic hydroxyl and the nitrogen atom of benzothiazole with a short O–H⋯N distance of 2.16 Å and a high hydrogen-bond energy of −12.8 kcal mol−1.9 Inspired by this small molecule, phloroglucinol moieties were introduced to trigger the strong intramolecular hydrogen bonds of benzobisthiazole units, and a new 2D benzobisthiazole-linked COF (PG-BBT) was successfully synthesized in this work. The formed intramolecular hydrogen bonds and benzobisthiazole are locked into a 2D full planar structure, and they can tremendously improve electron transfer. We envisaged that PG-BBT can be used as an advanced electrode material for supercapacitors, and might show high-performance capacitance.
Herein, we present the rational design, syntheses and crystal structures of benzobisthiazole-linked COFs. The physical and electrochemical properties of the new porous COFs were comprehensively investigated. This work shows the flexibility of COFs in structural design for high-capacitance supercapacitors.
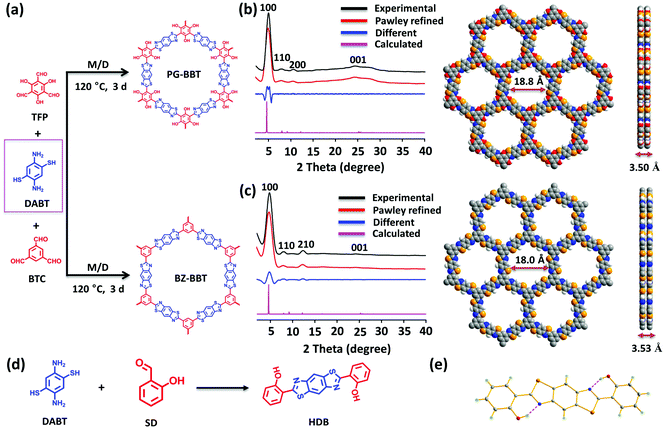
To demonstrate the role of intramolecular hydrogen bonds in supercapacitors, we first prepared a benzobisthiazole-linked COF PG-BBT by reacting 1,3,5-triformylphloroglucinol (TFP) at 120 °C for 3 days by a one-pot solvothermal reaction in a mixed solvent with 3 M aqueous acetic acid as the catalyst (Fig. 1a). For comparison, BZ-BBT without hydrogen bonds was synthesized by the reaction of 1,3,5-benzenetricarboxaldehyde (BTC) and DABT. The effect of the solvent on crystallization was evaluated (Fig. S1 and S2†). Note that air is critical for the formation of benzobisthiazole units, involving two steps: a reversible imine bond formation and a subsequent oxidation to afford the irreversible thiazole ring.10PG-BBT and BZ-BBT are insoluble in boiling water, 3 M HCl, 3 M KOH and common organic solvents (Fig. S3–S6†). TGA analysis showed that PG-BBT and BZ-BBT showed a negligible weight loss at below 300 and 220 °C, respectively (Fig. S7 and S8†), demonstrating their good thermal stability.
PG-BBT and BZ-BBT show similar XRD patterns owing to their similar structures (Fig. 1b and c). The diffraction peak at 4.8° corresponds to the (100) plane and its high intensity suggests the good crystallinity of PG-BBT and BZ-BBT. The broad peaks at 25° and 24° are ascribed to the (001) plane, indicating layer-by-layer stacking.11 Structural simulations were conducted. The simulated PXRD patterns agree well with the experimental PXRD profiles, and the structural simulations indicate that the stacking mode matches with the eclipsed stacking mode with a hexagonal P6/m space group (Fig. S9–S14†). The unit cell parameters are a = b = 23.0063 Å and c = 3.5017 Å for PG-BBT [Rwp 4.76% and Rp 3.56%], and a = b = 21.8778 Å and c = 3.5331 Å for BZ-BBT [Rwp 4.83% and Rp 3.68%]. Both PG-BBT and BZ-BBT are 2D COFs connected by benzobisthiazole units with hexagonal pores. The pore sizes of PG-BBT and BZ-BBT are 18.8 and 18.0 Å, respectively. The interlayer separation of stacking was calculated to be 3.50 and 3.53 Å for PG-BBT and BZ-BBT, respectively. As expected, the phloroglucinol moieties are retained in PG-BBT, which can be attributed to the formation of benzobisthiazole rings on the 2D hexagonal planar skeleton. In addition, the hydroxy groups on the phloroglucinol moieties form strong intramolecular hydrogen bond interactions with the nitrogen atoms of the adjacent benzobisthiazole rings with the H⋯N distance of 2.16 Å and the N–H⋯O angle of 125.46°. These intramolecular hydrogen bonds formed artificial six-membered rings, which increases the crystallinity and stability of PG-BBT (Fig. S15†). Abundant N, O and S heteroatoms are alternatively distributed on the 2D sheet of PG-BBT, resulting in high contents of nitrogen (8.53%), oxygen (14.62%), and sulfur (19.52%).
To further confirm the formation of benzobisthiazole and the presence of the phenolic hydroxyl group, a model compound, o-hydroxy diphenylbenzobisthiazole (HDB), was synthesized under similar reaction conditions by reacting DABT with salicylaldehyde (SD) (Fig. 1d). The single-crystal structure unambiguously demonstrated that the expected benzobisthiazole unit and two phenol groups were formed in HDB (Fig. 1e). The structure of HDB makes it coplanar and flat, and two intramolecular hydrogen bonds between the nitrogen atoms of benzobisthiazole and the hydrogen atoms of phenolic hydroxyl are generated.
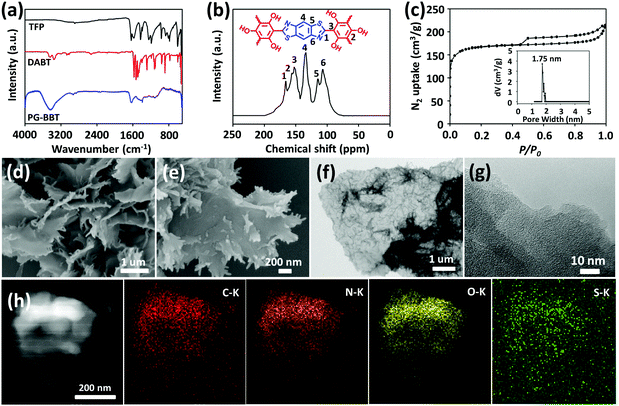
FT-IR spectra indicated the successful synthesis of PG-BBT based on the disappearance of the characteristic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching band (1642 cm−1), N–H vibration band (3351–3068 cm−1) and S–H vibration band (2533 cm−1), and the formation of the C
O stretching band (1642 cm−1), N–H vibration band (3351–3068 cm−1) and S–H vibration band (2533 cm−1), and the formation of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N characteristic signal (1628 cm−1) (Fig. 2a).12PG-BBT was further analysed by 13C CP-MAS NMR spectroscopy (Fig. 2b). The characteristic resonance signals at 165 and 156 ppm are derived from the C
N characteristic signal (1628 cm−1) (Fig. 2a).12PG-BBT was further analysed by 13C CP-MAS NMR spectroscopy (Fig. 2b). The characteristic resonance signals at 165 and 156 ppm are derived from the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and C–O groups, respectively.13 The XPS survey of PG-BBT was carried out to analyse the spectra of C, N, O and S atoms (Fig. S16†). The N 1s signal at 399 eV is ascribed to the C
N and C–O groups, respectively.13 The XPS survey of PG-BBT was carried out to analyse the spectra of C, N, O and S atoms (Fig. S16†). The N 1s signal at 399 eV is ascribed to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N group, and the S 2p signal at 164 eV represents the C–S bond.14 In addition, the O 1s peak at 532.8 eV indicates the C–OH bond.15 More information on the spectral characterization of BZ-BBT is provided in the ESI (Fig. S17–S19†).
N group, and the S 2p signal at 164 eV represents the C–S bond.14 In addition, the O 1s peak at 532.8 eV indicates the C–OH bond.15 More information on the spectral characterization of BZ-BBT is provided in the ESI (Fig. S17–S19†).
N2 sorption isotherm of PG-BBT shows a type I isotherm with a sharp N2 uptake at a low relative pressure (P/P0 < 0.01), indicating a characteristic of microporous nature.16a The hysteresis loop indicates the presence of a mesoporous structure, which could be ascribed to the stacking of PG-BBT nanosheets.16b The BET specific surface area of PG-BBT was calculated to be 507 m2 g−1. By using the nonlocal density functional theory (NLDFT), the porosity of PG-BBT was estimated. The average pore size of PG-BBT is 1.75 nm, which is close to the simulated value based on the eclipsed stacking model (inset of Fig. 2c). The SEM image of the as-synthesized PG-BBT shows a flower-like morphology, consisting of petals with dimension in the micrometre range from 1 to 2 μm (Fig. 2d and e). TEM images indicate that PG-BBT has ultrathin, optically transparent and slightly wrinkled layered structures (Fig. 2f and g). The elemental mapping of PG-BBT sheets shows the even distribution of C, N, O and S elements (Fig. 2h). More information on the N2 sorption and desorption isotherms, and the SEM and TEM images of BZ-BBT are provided in the ESI (Fig. S20–S22†).
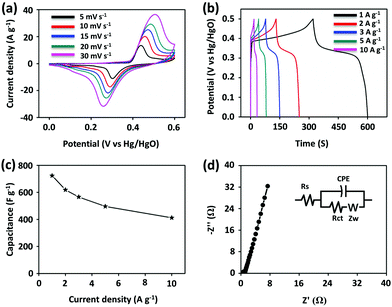
The CV curves of PG-BBT were recorded at various sweep rates from 10 to 30 mV s−1 in the potential window of 0–0.6 V vs. Hg/HgO (Fig. 3a). The shape of the CV profile was maintained well even at high scan rates, indicating good electrochemical reversibility and fast reaction kinetics.17a The peak current is proportional to the square root of the scan rate, which suggests a typical battery-type behaviour owing to the diffusion-controlled process (Fig. S23†).17b,c The GCD tests were performed at various current densities in the range of 1–10 A g−1 to further investigate the electrochemical properties of PG-BBT (Fig. 3b). The GCD profiles show a severely distorted triangular shape due to pseudocapacitive behaviour, which is in agreement with CV analyses. The discharge capacitances of PG-BBT were calculated to be 724, 619, 566, 496 and 412 F g−1 from 1 to 10 A g−1 (Fig. 3c). The highest specific capacitance is consistent with the calculated result of CV profiles (729 F g−1 at 5 mV s−1). PG-BBT showed good rate capability, retaining 56% of its capacitance at 10 A g−1 relative to that at 1 A g−1. The GCD measurement was also used to examine the cycling stability of PG-BBT at 1 A g−1. The capacitance was 96% of the initial value after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, which is indicative of outstanding cycling performance (Fig. S24†). The specific capacitance of PG-BBT is much higher than those of the reported COFs and is comparable to those of metal oxide materials (Table S1†). To confirm the electrochemical performance of PG-BBT, electrical resistance was evaluated by EIS (Fig. 3d and S25†). The Nyquist plot of PG-BBT shows a semi-circular arc in the high-frequency region and a slope in the low-frequency region. The semicircle represents the electron transfer resistance at the electrode–electrolyte interface, which was estimated to be 1.1 Ω, indicating excellent proton-coupled electron transfer behaviour. Complex capacitance studies determined the dielectric relaxation time constant (τ0), which represents the supercapacitor factor of merit.7,18 The τ0 values were 3.3 and 0.4 s after 2000 and 10
000 cycles, which is indicative of outstanding cycling performance (Fig. S24†). The specific capacitance of PG-BBT is much higher than those of the reported COFs and is comparable to those of metal oxide materials (Table S1†). To confirm the electrochemical performance of PG-BBT, electrical resistance was evaluated by EIS (Fig. 3d and S25†). The Nyquist plot of PG-BBT shows a semi-circular arc in the high-frequency region and a slope in the low-frequency region. The semicircle represents the electron transfer resistance at the electrode–electrolyte interface, which was estimated to be 1.1 Ω, indicating excellent proton-coupled electron transfer behaviour. Complex capacitance studies determined the dielectric relaxation time constant (τ0), which represents the supercapacitor factor of merit.7,18 The τ0 values were 3.3 and 0.4 s after 2000 and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, respectively (Fig. S26†). The corresponding resistances were 1.0 and 0.6 Ω after 2000 and 10
000 cycles, respectively (Fig. S26†). The corresponding resistances were 1.0 and 0.6 Ω after 2000 and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, respectively. The decreased resistances can be ascribed to the improved wettability between the electrode and the electrolyte and the activation of the electrode during the charge–discharge processes (Fig. S27†).
000 cycles, respectively. The decreased resistances can be ascribed to the improved wettability between the electrode and the electrolyte and the activation of the electrode during the charge–discharge processes (Fig. S27†).
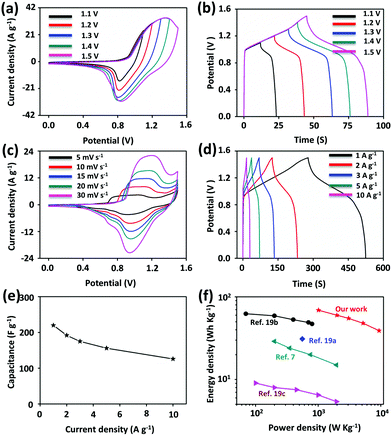
The electrode materials were further evaluated in a two-electrode configuration to demonstrate the practical electrochemical performance of PG-BBT. Asymmetric supercapacitors were assembled with activated carbon (AC) and PG-BBT. The highest operating potential of the supercapacitors was 1.5 V (Fig. 4a). With the increase of potential windows (1.1–1.5 V), no evident polarization was observed (Fig. 4b). Fig. 4c shows the CV curves of the supercapacitors at different scan rates (5–30 mV s−1). The GCD curves of the supercapacitors at different current densities (1–10 A g−1) were tested (Fig. 4d). The capacitance values of PG-BBT were calculated to be 220 and 126 F g−1 at 1 and 10 A g−1, respectively. Note that the capacitance retention is more than 57% at 10 A g−1, confirming its excellent rate performance (Fig. 4e). The energy density and power density were analysed, and the results are presented in a Ragone plot (Fig. 4f). The highest energy density of the asymmetric supercapacitor was calculated to be 69 W h kg−1 at a power density of 1010 W kg−1, which is higher than those of other reported COFs (Fig. 4f)7,19 and is comparable to those of metal–oxide materials.20 Moreover, the energy density still reached 39 W h kg−1 at a power density of 9360 W kg−1.
To further demonstrate the effect of intramolecular hydrogen bonds on electrochemical performance, the capacitive properties of non-hydroxyl functionalized BZ-BBT and partially hydroxyl-functionalized PGxBZ1−xBBT (molar ratios of [TFP]/[BTC] are 0.25, 0.5 and 0.75) were studied (Fig. S28–S31†). As expected, these materials exhibited similar CV and charge–discharge curves, which were attributed to benzobisthiazole units. The specific capacitance of BZ-BBT was 166 F g−1 at 1 A g−1 (Fig. S32†). The specific capacitance values of PG0.25BZ0.75BBT, PG0.50BZ0.50BBT and PG0.75BZ0.25BBT were 197, 248 and 366 F g−1, respectively, at 1 A g−1 (Fig. S33†). These results indicate that the specific capacitance of benzobisthiazole-linked COFs can be easily tuned by controlling the number of hydroxyl groups.
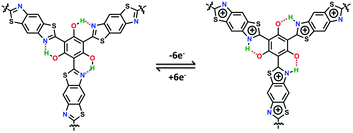
It is proposed that the improved capacitance of PG-BBT should be ascribed to four main factors: (i) intramolecular hydrogen bonds and the benzobisthiazole unit are extensively distributed in the polymer skeleton and play an important role in facilitating the electron transfer. In the redox process, each proton of –OH was transferred to the adjacent N atom to form O⋯H–N with a positive charge, indicating 6e− proton-coupled electron transfer for each unit (Fig. 5). The redox mechanism was further investigated by in situ Raman spectroscopy. The two typical bands at 1329 and 1645 cm−1 are ascribed to radical semiquinone and –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-stretching vibration in the discharge process, respectively (Fig. S34†).21a After charging, the –C
N-stretching vibration in the discharge process, respectively (Fig. S34†).21a After charging, the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-stretching vibration signal at 1616 cm−1 weakened and a signal with increased delocalization at 1522 cm−1 appeared, indicating the oxidation process.21b The efficient proton-coupled electron transfer can increase electrochemical performance. (ii) The redox-active benzobisthiazole unit can promote electrochemical reaction kinetics, resulting in pseudocapacitive effects.22 (iii) The ordered crystalline porous 2D structure can accelerate the mass transfer rate of ions. (iv) The doped N, O and S heteroatoms affect the electron distribution and enhance the wettability of electrode materials, which is beneficial for further increasing the capacitance.23 The synergistic contribution of the intramolecular hydrogen bonds, redox-active benzobisthiazole unit, ordered porous structure, and abundant heteroatoms makes PG-BBT a high-performance electrode material for supercapacitors.
N-stretching vibration signal at 1616 cm−1 weakened and a signal with increased delocalization at 1522 cm−1 appeared, indicating the oxidation process.21b The efficient proton-coupled electron transfer can increase electrochemical performance. (ii) The redox-active benzobisthiazole unit can promote electrochemical reaction kinetics, resulting in pseudocapacitive effects.22 (iii) The ordered crystalline porous 2D structure can accelerate the mass transfer rate of ions. (iv) The doped N, O and S heteroatoms affect the electron distribution and enhance the wettability of electrode materials, which is beneficial for further increasing the capacitance.23 The synergistic contribution of the intramolecular hydrogen bonds, redox-active benzobisthiazole unit, ordered porous structure, and abundant heteroatoms makes PG-BBT a high-performance electrode material for supercapacitors.
In summary, a new benzobisthiazole-linked COF with abundant intramolecular hydrogen bonds has been prepared by a simple one-pot synthetic process. The synthesized PG-BBT as an electrode material for supercapacitors showed high capacitance, good rate capability, robust long-term cycling durability, wide potential window, and high energy density. The design concept for increasing electron transfer on a porous COF skeleton through strong intramolecular hydrogen bonds sheds light on constructing advanced supercapacitors.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (21771089), the Fundamental Research Funds for the Central Universities (JUSRP21936 and JUSRP51725B), the Project for Jiangsu Scientific and Technological Innovation Team, and MOE & SAFEA for the 111 Project (B13025).Notes and references
- (a) H. Wang, Z. T. Zeng, P. Xu, L. S. Li, G. M. Zeng, R. Xiao, Z. Y. Tang, D. L. Huang, L. Tang, C. Lai, D. N. Jiang, Y. Liu, H. Yi, L. Qin, S. J. Ye, X. Y. Ren and W. W. Tang, Chem. Soc. Rev., 2019, 48, 488–516 RSC; (b) S. Kandambeth, K. Dey and R. Banerjee, J. Am. Chem. Soc., 2019, 141, 1807–1822 CrossRef CAS PubMed.
- (a) Y. P. Song, Q. Sun, B. Aguila and S. Q. Ma, Adv. Sci., 2019, 6, 1801410 CrossRef PubMed; (b) H. V. Babu, M. G. M. Bai and M. R. Rao, ACS Appl. Mater. Interfaces, 2019, 11, 11029–11060 CrossRef CAS PubMed; (c) K. Wang, D. D. Qi, Y. L. Li, T. Y. Wang, H. B. Liu and J. Z. Jiang, Coord. Chem. Rev., 2019, 378, 188–206 CrossRef CAS.
- (a) J. W. Zhou and B. Wang, Chem. Soc. Rev., 2017, 46, 6927–6945 RSC; (b) F. X. Wang, X. W. Wu, X. H. Yuan, Z. C. Liu, Y. Zhang, L. J. Fu, Y. S. Zhu, Q. M. Zhou, Y. P. Wu and W. Huang, Chem. Soc. Rev., 2017, 46, 6816–6854 RSC; (c) M. Y. Wang, H. Guo, R. Xue, Q. Li, H. Liu, N. Wu, W. Q. Yao and W. Yang, ChemElectroChem, 2019, 6, 2984–2997 CrossRef CAS.
- K. C. Ho and L. Y. Lin, J. Mater. Chem. A, 2019, 7, 3516–3530 RSC.
- A. Bhuwalka, J. F. Mike, M. He, J. J. Intemann, T. Nelson, M. D. Ewan, R. A. Roggers, Z. Q. Lin and M. Jeffries-EL, Macromolecules, 2011, 44, 9611–9617 CrossRef CAS.
- Y. Y. Qiu, J. C. Worch, D. N. Chirdon, A. Kaur, A. B. Maurer, S. Amsterdam, C. R. Collins, T. Pintauer, D. Yaron, S. Bernhard and K. J. T. Noonan, Chem. – Eur. J., 2014, 20, 7746–7751 CrossRef CAS PubMed.
- S. Chandra, D. R. Chowdhury, M. Addicoat, T. Heine, A. Paul and R. Banerjee, Chem. Mater., 2017, 29, 2074–2080 CrossRef CAS.
- T. Cheng, D. X. Shen, M. Meng, S. M. Mallick, L. J. Cao, N. J. Patmore, H. L. Zhang, S. F. Zou, H. W. Chen, Y. Qin, Y. Y. Wu and C. Y. Liu, Nat. Commun., 2019, 10, 1531 CrossRef PubMed.
- (a) S. Jenkins, K. I. Jacob and S. Kumar, J. Polym. Sci., Polm. Phys., 2000, 38, 3053–3061 CrossRef CAS; (b) H. H. Song, T. Y. Cho, D. P. Heberer, T. D. Dang, F. E. Arnold and L. S. Tan, J. Polym. Sci., Polym. Phys., 2001, 39, 559–565 CrossRef CAS; (c) A. Kovács, A. Szabó and I. Hargittai, Acc. Chem. Res., 2002, 35, 887–894 CrossRef PubMed.
- Y. H. Cho, C. Y. Lee, D. C. Ha and C. H. Cheon, Adv. Synth. Catal., 2012, 354, 2992–2996 CrossRef CAS.
- S. Karak, S. Kandambeth, B. P. Biswal, H. S. Sasmal, S. Kumar, P. Pachfule and R. Banerjee, J. Am. Chem. Soc., 2017, 139, 1856–1862 CrossRef CAS PubMed.
- (a) K. Dey, M. Pal, K. C. Rout, H. S. Kunjattu, A. Das, R. Mukherjee, U. K. Kharul and R. Banerjee, J. Am. Chem. Soc., 2017, 139, 13083–13091 CrossRef CAS PubMed; (b) G. Q. Lin, H. M. Ding, R. F. Chen, Z. K. Peng, B. S. Wang and C. Wang, J. Am. Chem. Soc., 2017, 139, 8705–8709 CrossRef CAS PubMed.
- X. Chen, M. Addicoat, E. Q. Jin, L. P. Zhai, H. Xu, N. Huang, Z. Q. Guo, L. L. Liu, S. Irle and D. L. Jiang, J. Am. Chem. Soc., 2015, 137, 3241–3247 CrossRef CAS.
- Y. N. Chang, G. X. Zhang, B. Han, H. Y. Li, C. J. Hu, Y. C. Pang, Z. Chang and X. M. Sun, ACS Appl. Mater. Interfaces, 2017, 9, 29753–29759 CrossRef CAS.
- Y. Z. Liao, H. G. Wang, M. F. Zhu and A. Thomas, Adv. Mater., 2018, 30, 1705710 CrossRef.
- (a) Q. R. Fang, J. H. Wang, S. Gu, R. B. Kaspar, Z. B. Zhuang, J. Zheng, H. X. Guo, S. L. Qiu and Y. S. Yan, J. Am. Chem. Soc., 2015, 137, 8352–8355 CrossRef CAS; (b) H. N. Kwon, G. D. Park, Y. C. Kang and K. C. Roh, Carbon, 2019, 144, 591–600 CrossRef CAS.
- (a) F. P. Zhao, Y. Y. Wang, X. N. Xu, Y. L. Liu, R. Song, G. Lu and Y. G. Li, ACS Appl. Mater. Interfaces, 2014, 6, 11007–11012 CrossRef CAS PubMed; (b) T. S. Mathis, N. Kurra, X. H. Wang, D. Pinto, P. Simon and Y. Gogotsi, Adv. Energy Mater., 2019, 9, 1902007 CrossRef CAS; (c) B. Talluri, S. Ghosh, G. R. Rao and T. Thomas, Electrochim. Acta, 2019, 321, 134709 CrossRef CAS.
- S. Bandyopadhyay, C. Singh, P. Jash, M. W. Hussain, A. Paul and A. Patra, Chem. Commun., 2018, 54, 6796–6799 RSC.
- (a) C. R. DeBlase, K. E. Silberstein, T. T. Truong, H. D. Abruña and W. R. Dichtel, J. Am. Chem. Soc., 2013, 135, 16821–16824 CrossRef CAS; (b) H. Yang, S. L. Zhang, L. H. Han, Z. Zhang, Z. Xue, J. Gao, Y. J. Li, C. S. Huang, Y. P. Yi, H. B. Liu and Y. L. Li, ACS Appl. Mater. Interfaces, 2016, 8, 5366–5375 CrossRef CAS PubMed; (c) A. M. Khattak, Z. A. Ghazi, B. Liang, N. A. Khan, A. Iqbal, L. S. Li and Z. Y. Tang, J. Mater. Chem. A, 2016, 4, 16312–16317 RSC.
- (a) Y. Li, Y. X. Xu, W. P. Yang, W. X. Shen, H. G. Xue and H. Pang, Small, 2018, 14, 1704435 CrossRef PubMed; (b) P. B. Liu, Y. D. Zhu, X. G. Gao, Y. Huang, Y. Wang, S. Y. Qin and Y. Q. Zhang, Chem. Eng. J., 2018, 350, 79–88 CrossRef CAS.
- (a) H. J. Salavagione, J. Arias-Pardilla, J. M. Pérez, J. L. Vazquez, E. Morallón, M. C. Miras and C. Barbero, J. Electroanal. Chem., 2005, 576, 139–145 CrossRef CAS; (b) M. Mostefai, M. C. Pham, J. P. Marsault, J. Aubard and P. C. Lacaze, J. Electrochem. Soc., 1996, 143, 2116–2119 CrossRef CAS.
- P. A. DePra, J. G. Gaudiello and T. J. Marks, Macromolecules, 1988, 21, 2295–2297 CrossRef CAS.
- M. Y. Liu, L. P. Guo, S. B. Jin and B. E. Tan, J. Mater. Chem. A, 2019, 7, 5153–5172 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 1860921. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9py01623f |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2020 |