Chelerythrine as a fluorescent light-up ligand for an i-motif DNA structure†
Hongbo
Chen
 ab,
Hongxia
Sun
*c,
Wende
Zhang
ab,
Qin
Zhang
ab,
Jun
Ma
ab,
Qian
Li
c,
Xiaomeng
Guo
c,
Kanyan
Xu
*ab and
Yalin
Tang
ab,
Hongxia
Sun
*c,
Wende
Zhang
ab,
Qin
Zhang
ab,
Jun
Ma
ab,
Qian
Li
c,
Xiaomeng
Guo
c,
Kanyan
Xu
*ab and
Yalin
Tang
 *c
*c
aShenzhou Space Biology Science and Technology Coorporation, Ltd, China Academy of Space Technology, Beijing, 100190, P. R. China. E-mail: xukanyan@cast.cn
bSpace Biology Research and Technology Center, Space Biology Research and Tecchnology of China Aerospace Science and Technology Coorporation, Ltd, Beijing, 100190, P. R. China
cNational Laboratory for Molecular Sciences, Center for Molecular Sciences, State Key Laboratory for Structural Chemistry of Unstable and Stable Species, Institute of Chemistry Chinese Academy of Sciences, Beijing, 100190, P. R. China. E-mail: tangyl@iccas.ac.cn; hongxsun@iccas.ac.cn
First published on 20th November 2020
Abstract
A fluorescent light-up ligand for an i-motif structure has been reported in this study. Chelerythrine (CHE) could bind to i-motif and show a significant increase in the fluorescence intensity, while exhibiting weak response towards single-stranded (ssDNA), double-stranded (dsDNA), and G-quadruplexes. This feature makes CHE advantageous in the label-free fluorescence sensing systems.
The excellent structural and functional features of DNA have attracted extensive research attention in biological processes, developing novel sensing systems, including various functional nanomaterials and molecular computers.1 The development of DNA structure-sensitive fluorescent probes has received considerable attention due to their numerous applications.2 The fluorescent ligands exhibit low fluorescence intensity when free in the solution but are highly fluorescent upon binding with specific DNA structures.2 In the recognition process, DNA could self-assemble into single-stranded (ssDNA), double-stranded (dsDNA), hairpins, cruciforms, i-motifs, G-quadruplexes, and other forms.3 Therefore, the fluorescence change of the ligands could be in response to the DNA structural transition.
i-Motif is a four-stranded nucleic acid secondary structure comprising of cytosine-rich sequences, which are formed by two parallel duplex hydrogen bonded together by intercalated C+–C base pairs.4 i-Motif formation is promoted by a slightly acidic pH, although some could be formed even under neutral conditions.5 i-Motif DNA structures exist in the regions of human genome including promoters and telomeric regions.6 An antibody fragment (iMab), with high selectivity and affinity towards i-motif, was used in the recognition of i-motif DNA structures in vivo.7 Numerous recent studies suggest the important roles of i-motif in gene regulation.8 Besides the significant roles in the biological systems, i-motifs have been utilized to construct various nanodevices.9 The development of new fluorescent ligands towards i-motifs is significant for research on the functions and applications of i-motifs. So far, fluorescent ligands towards the i-motifs structure is limited, such as crystal violet,10 berberine,11 thioflavin T,12 thiazole orange,13 quinaldine red,14 neutral red15 and DMSB.9a,b However, most of them are easily disturbed by ssDNA, dsDNA and G-quadruplexes. Therefore, developing i-motif fluorescent ligands is an exigent need.
Natural isoquinoline alkaloids (IQAs) are produced mostly by higher plants during metabolism.16 It is proved that IQAs possess numerous important therapeutic activities including antimicrobial, anti-inflammatory, antioxidation, and anticancer properties,17 which are speculated to play their roles by binding with nucleic acids.18 Since IQAs are almost low fluorescent and share similar molecular weight and structural frame,19 screening an aptamer that can selectively target i-motif is very important to expand the application of IQAs. In a previous study, Shao et al. found that chelerythrine (CHE), which is a stabilizer and inducer to the triplex, can sense triplex via fluorescence.20 However, there is a lack of investigation on the recognition of the i-motif structure by CHE. In this study, we selected CHE from several IQAs as a promising i-motif fluorophore. CHE could bind to i-motif and show a significant increase in the fluorescence intensity, while exhibited weak response towards other DNA structures including ssDNA, dsDNA and G-quadruplexes. Our study developed a new ligand for i-motif, which has promising applications in the construction of nanodevices (such as biosensors and logic gates) in systems without triplex.
Scheme 1 shows the structure of CHE and the principle of recognization of the i-motif structure by CHE. CHE exhibits weak fluorescence intensity in buffer solutions. We tested CHE with various DNA structures including ssDNA, dsDNA, i-motif and G-quadruplex. Among these DNA structures, CHE interacts with i-motif alone and exhibits increasing fluorescence intensity, while it has no response towards other DNA structures. This feature allows CHE as a fluorescent light-up ligand to i-motif.
We first screened i-motif as the promising ligand from several IQAs. Natural IQAs exhibit weak fluorescence signal in free solutions. Chelerythrine (CHE), coptisine (COP), jatrorrhizine (JAT), sanguinarine (SAN) and stephania (STE) were investigated. We added dsDNA, G-quadruplex and the i-motif structure into IQAs. Among these IQAs, only the fluorescence intensity of CHE has an obvious increase after binding with i-motif and has weak increase towards other DNA structures, implying that CHE could recognize the i-motif structure (Fig. S1 and S2, ESI†). COP exhibits increasing fluorescent intensity towards i-motif, but respond similarly to dsDNA and G-quadruplex. This result indicates that CHE is expected to be a promising fluorescent light-up ligand to i-motif.
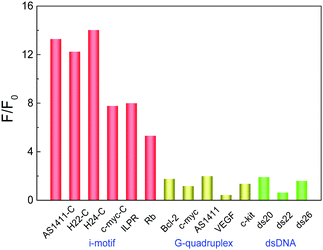
Before exploring the recognition of i-motif by CHE, the influence of pH on CHE was measured. There was no significant difference in the CHE fluorescence when CHE was placed in different pH, indicating that the CHE fluorescence is less sensitive towards pH (Fig. S3, ESI†). The interaction of CHE to various DNA structures was investigated by titration experiments. With the gradual addition of i-motifs, the fluorescence intensity of CHE increased sharply with the fluorescence peaks blue shifting from 566 nm to 558 nm, corresponding to the compact binding of CHE with i-motif (Fig. 1A and Fig. S4, ESI†). The fluorescence intensity of CHE exhibits weak variation for the titration of other nucleic acid models, implying that the combination of CHE with i-motifs is much stronger than that with other nucleic acid structures (Fig. S4, ESI†). The plots of the fluorescence intensity of CHE at 558 nm versus the increasing concentration of various DNA structures were measured (Fig. 1B). The CHE fluorescence intensity at 558 nm increases with the addition of the i-motif structure including AS1411-C,9a,b H22-C,13,14 H24-C, ILPR,21 Rb22 and c-myc-C23 sequences. However, the CHE fluorescence intensity at 558 nm has weak fluctuation with increasing G-quadruplex (AS1411, c-myc,24 VEGF,25 Bcl-226 and c-kit27) and dsDNA (ds20, ds22 and ds26). As shown in Fig. 2, the comparison of CHE fluorescence intensity (F/F0) monitored at 558 nm demonstrated that i-motif enhanced the CHE fluorescence by 5–14 folds than in the absence of i-motif. In contrast, the fluorescence enhancement of CHE by ssDNA, dsDNA and G-quadruplexes was less than 2-fold (Fig. 2). While binding with the i-motif structure, the movement of CHE was inhibited and the loss of energy decreased, leading to the increase in the fluorescence intensity. In order to validate this viewpoint, we measured the fluorescence intensity of CHE with increasing viscosity in the glycerol–water system (Fig. S5, ESI†). With the increase in viscosity, the fluorescence intensity of CHE increased, which was caused by the inhibition of the movement of CHE and the loss of energy decreased in a high viscosity environment. By a Job's Plot analysis (Fig. S6, ESI†), we know that the binding stoichiometry (n) of numerous i-motif structures withCHE is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, indicating that CHE may only bind to one i-motif molecule. Further, we computed the apparent binding equilibrium constants (Ka) of CHE to i-motifs utilizing the fluorimetric titration results. The binding stoichiometry and apparent binding equilibrium constants are listed in Table S2 (ESI†).
1, indicating that CHE may only bind to one i-motif molecule. Further, we computed the apparent binding equilibrium constants (Ka) of CHE to i-motifs utilizing the fluorimetric titration results. The binding stoichiometry and apparent binding equilibrium constants are listed in Table S2 (ESI†).
C-rich oligonucleotides can form the i-motif structure in an acidic environment.5 To validate the conformation transition of C-rich DNA in this process, we recorded their circular dichroism (CD) spectra in the pH range of 5.8–7.2 (Fig. S7, ESI†). The CD spectra of AS1411-C in pH 5.8 show a positive peak at 285 nm and a negative peak at 253 nm, indicating the characteristic signal of the i-motif structures.28 When pH changes to 6.4, the CD spectra of AS1411-C show a positive peak at 277 nm and a negative peak at 242 nm, indicating that the i-motif structure turn into ssDNA. The changing rule of the CD spectra of c-myc-C and H22-C was similar to AS1411-C, indicating the formation of c-myc-C and H22-C change from i-motif to ssDNA in the pH range of 5.8–7.2. These results illustrate that these C-rich oligonucleotides can form the i-motif structure at pH 5.8.
Furthermore, we measured the fluorescence spectra of CHE in numerous pH values in the presence of AS1411-C (Fig. 3, Fig. S8A and B, ESI†). With pH changing from 5.0 to 7.5, the fluorescence intensity of CHE at 588 nm decreased, indicating that the formation of AS1411-C DNA changed from i-motif to ssDNA and CHE released from AS1411-C. Besides, we measured the fluorescence spectra of CHE with the increasing concentration of Ag+ in the presence of AS1411-C (Fig. 3, Fig. S8C and D, ESI†). It was found that i-motif can form with Ag+ present at neutral pH owing to the strong interaction of Ag+ with cytosine to form a C–Ag+–C complex.9a,b,29 With the addition of Ag+, the fluorescence intensity of CHE at 588 nm increased obviously, indicating AS1411-C ssDNA form into i-motif under Ag+ solution and CHE binds to the AS1411-C i-motif specially. These results further manifest the selectivity of CHE towards i-motif rather than ssDNA. In order to validate the formation of AS1411-C i-motif, we compared the response of CHE to AS1411-C and ds12 (a specific double helical structure for the whole length of the sequence). The fluorescence intensity of CHE towards AS1411-C increased sharply while exhibiting weak change in the presence of ds12 (Fig. S9A, ESI†). The plot of the fluorescence intensity at 558 nm with increasing sample showed the difference of CHE to AS1411-C and ds12 (Fig. S9B, ESI†). This experiment illustrated that the CHE can bind with the i-motif structure rather than the double helical structure.
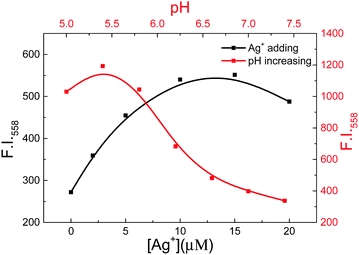 | ||
| Fig. 3 The plot of the fluorescence intensity of CHE (5 μM) at 558 nm with increasing concentrations of Ag+ (blank) and versus pH (red) in the presence of 5 μM AS1411-C in the 10 mM PB solution. | ||
The influence of CHE towards i-motif thermostability was discussed via the melting temperature experiment. In pH 5.8, H22-C DNA exhibits the characteristic signal of i-motif structures (a positive peak at 290 nm and a negative peak at 255 nm). With the increase in temperature, the CD signal of H22-C at 290 nm decreased and the melting temperature (Tm) is around 41 °C. After the addition of CHE, the CD signal of H22-C at 290 nm decreased and Tm was also around 41 °C, indicating that CHE has no influence on the thermostability of the H22-C structure (Fig. S10, ESI†). Moreover, in the melting fluorescence spectra, the fluorescence intensity of the CHE-H22-C complex at 558 nm decreased with the increase in temperature, indicating that the H22-C i-motif structure was destructed and CHE was released from H22-C. Tm in the melting fluorescence spectra is around 40 °C, which is consistent with that in the CD-melting experiment (Fig. S11, ESI†). The results of the melting temperature experiment exhibit that CHE has no influence on the i-motif thermostability.
In summary, we have roundly investigated the interaction of CHE with numerous DNA structures. CHE could bind to i-motif and show a significant increase in the fluorescence intensity. In contrast, CHE exhibits low fluorescence response towards ssDNA, dsDNA and G-quadruplex. We expect that our results could make a new way for research on the structures and functions of i-motif with the advantages of simplicity, label-free detection, low cost and sensitivity for future studies.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by Beijing Nova Program (No. Z201100006820048), Beijing Natural Science Foundation (7182189), the Distinguished Young Scholars Fundation of China Academy of Apace Technology and the National Natural Science Foundation of China (Grant No. 21977096, 21977099, 21675162 and 21778058).Notes and references
- (a) E. Persch, O. Dumele and F. Diederich, Angew. Chem., Int. Ed., 2015, 54, 3290–3327 CrossRef CAS; (b) K. Ariga, H. Ito, J. P. Hill and H. Tsukube, Chem. Soc. Rev., 2012, 41, 5800–5835 RSC; (c) J. Zheng, A. Jiao, R. Yang, H. Li, J. Li, M. Shi, C. Ma, Y. Jiang, L. Deng and W. Tan, J. Am. Chem. Soc., 2012, 134, 19957–19960 CrossRef CAS; (d) I. Willner, B. Shlyahovsky, M. Zayats and B. Willner, Chem. Soc. Rev., 2008, 37, 1153–1165 RSC.
- (a) W. Gai, Q. Yang, J. Xiang, W. Jiang, Q. Li, H. Sun, A. Guan, Q. Shang, H. Zhang and Y. Tang, Nucleic Acids Res., 2013, 41, 2709–2722 CrossRef CAS; (b) S. Zhang, H. Sun, L. Wang, Y. Liu, H. Chen, Q. Li, A. Guan, M. Liu and T. Tang, Nucleic Acids Res., 2018, 46, 7522–7532 CrossRef CAS; (c) H. Chen, H. Sun, S. Zhang, W. Yan, Q. Li, A. Guan, J. Xiang, M. Liu and Y. Tang, Chem. Commun., 2019, 55, 5060–5063 RSC; (d) L. Liu, Y. Shao, J. Peng, C. Huang, H. Liu and L. Zhang, Anal. Chem., 2014, 86, 1622–1631 CrossRef CAS; (e) B. Jin, X. Zhang, W. Zheng, X. Liu, C. Qi, F. Wang and D. Shangguan, Anal. Chem., 2014, 86, 943–952 CrossRef CAS.
- (a) J. Choi and T. Majima, Chem. Soc. Rev., 2011, 40, 5893–5909 RSC; (b) Y. Du and X. Zhou, Chem. Rec., 2013, 13, 371–384 CrossRef CAS.
- (a) D. Lieblein, M. Krämer, A. Dreuw, B. Fürtig and H. Schwalbe, Angew. Chem., Int. Ed., 2012, 51, 4067–4070 CrossRef; (b) H. Day, P. Pavlou and Z. Waller, Bioorg. Med. Chem., 2014, 22, 4407–4418 CrossRef CAS; (c) T. Torring, R. Toftegaard, J. Arnbjerg, P. Ogilby and K. Gothelf, Angew. Chem., Int. Ed., 2010, 49, 7923–7925 CrossRef.
- (a) A. Stavrakoudis, I. Tsoulos, K. Uray, F. Hudecz and V. Apostolopoulos, Chem. Commun., 2012, 48, 10739–10741 RSC; (b) S. Benabou, A. Avino, R. Eritja, C. Gonzalez and R. Gargallo, RSC Adv., 2014, 4, 26956–26980 RSC.
- (a) A. Phan, M. Gueron and J. Leroy, J. Mol. Biol., 2000, 299, 123–144 CrossRef CAS; (b) S. Nonin-Lecomte and J. Leroy, J. Mol. Biol., 2001, 309, 491–506 CrossRef CAS; (c) M. Garavis, N. Escaja, V. Gabelica, A. Villasante and C. Gonzalez, Chemistry, 2015, 21, 9816–9824 CrossRef CAS; (d) T. Brooks, S. Kendrick and L. Hurley, FEBS J., 2010, 277, 3459–3469 CrossRef CAS.
- M. Zeraati, D. Langley, P. Schofield, A. Moye, R. Rouet, W. Hughes, T. Bryan, M. Dinger and D. Christ, Nat. Chem., 2018, 10, 631–637 CrossRef CAS.
- (a) B. Roy, P. Talukder, H. Kang, S. Tsuen, M. Alam, L. Hurley and S. Hecht, J. Am. Chem. Soc., 2016, 138, 10950–10962 CrossRef CAS; (b) S. Kendrick, H. Kang, M. Alam, M. Madathil, P. Agrawal, V. Gokhale, D. Yang, S. Hecht and L. Hurley, J. Am. Chem. Soc., 2014, 136, 4161–4171 CrossRef CAS; (c) S. Dhakal, J. Schonhoft, D. Koirala, Z. Yu, S. Basu and H. Mao, J. Am. Chem. Soc., 2010, 132, 8991–8997 CrossRef CAS.
- (a) Y. Shi, H. Sun, J. Xiang, H. Chen, Q. Yang, A. Guan, Q. Li, L. Yu and Y. Tang, Chem. Commun., 2014, 50, 15385–15388 RSC; (b) Y. Shi, H. Sun, J. Xiang, L. Yu, Q. Yang, Q. Li, A. Guan and Y. Tang, Anal. Chim. Acta, 2015, 857, 79–84 CrossRef CAS; (c) Y. Dong, Z. Yang and D. Liu, Acc. Chem. Res., 2014, 47, 1853–1860 CrossRef CAS; (d) L. Lu, M. Wang, L. Liu, C. Wong, C. Leung and D. Ma, Chem. Commun., 2015, 51, 9953–9956 RSC.
- D. Ma, H. Kwan, S. Chan, P. Lee, H. Yang, P. Ma, L. Bai, Z. Jiang and C. Leung, Analyst, 2011, 136, 2692–2696 RSC.
- L. Xu, S. Hong, N. Sun, K. Wang, L. Zhou, L. Ji and R. Pei, Chem. Commun., 2015, 52, 179–182 RSC.
- I. Lee, S. Patil, K. Fhayli, S. Alsaiari and N. Khashab, Chem. Commun., 2015, 51, 3747–3749 RSC.
- L. Xu, X. Shen, S. Hong, J. Wang, L. Zhou, X. Chen and R. Pei, Asian J. Org. Chem., 2015, 4, 1375–1378 CrossRef CAS.
- (a) G. Jiang, L. Xu, K. Wang, X. Chen, Z. Wang, W. Cao and R. Pei, Anal. Methods, 2017, 9, 1585–1588 RSC; (b) S. Nonin-Lecomtea and J. Leroy, J. Mol. Biol., 2001, 309, 491–506 CrossRef.
- L. Xu, J. Wang, N. Sun, M. Liu, Y. Cao, Z. Wang and R. Pei, Chem. Commun., 2016, 52, 14330–14333 RSC.
- (a) S. Yang, J. Xiang, Q. Yang, Q. Li, Q. Zhou, X. Zhang, Y. Tang and G. Xu, Chin. J. Chem., 2010, 28, 771–780 CrossRef CAS; (b) S. Yang, J. Xiang, Q. Yang, Q. Zhou, X. Zhang, Q. Li, Y. Tang and G. Xu, Fitoterapia, 2010, 81, 1026–1032 CrossRef CAS.
- (a) K. Bhadra and G. Kumar, Med. Res. Rev., 2011, 31, 821–862 CrossRef CAS; (b) I. Slaninov, K. Pencikova, J. Urbanova, J. Slanina and E. Taborska, Phytochem. Rev., 2014, 13, 51–68 CrossRef; (c) Y. Sun, K. Xun, Y. Wang and X. Chen, Anti-Cancer Drugs, 2009, 20, 757–769 CrossRef CAS.
- (a) M. Ferraroni, C. Bazzicalupi, A. Bilia and P. Gratteri, Chem. Commun., 2011, 47, 4917–4919 RSC; (b) I. Bessi, C. Bazzicalupi, C. Richter, H. Jonker, K. Saxena, C. Sissi, M. Chioccioli, S. Bianco, A. Bilia, H. Schwalbe and P. Gratteri, ACS Chem. Biol., 2012, 7, 1109–1119 CrossRef CAS; (c) C. Bazzicalupi, M. Ferraroni, A. Bilia, F. Scheggi and P. Gratteri, Nucleic Acids Res., 2013, 41, 632–638 CrossRef CAS; (d) R. Sinha, I. Saha and G. Kumar, Chem. Biodiversity, 2011, 8, 1512–1528 CrossRef CAS.
- L. Zhang, H. Liu, Y. Shao, C. Lin, H. Jia, G. Chen, D. Yang and Y. Wang, Anal. Chem., 2015, 87, 730–737 CrossRef CAS.
- Y. Hu, F. Lin, T. Wu, Y. Wang, X. Zhou and Y. Shao, Chem. – Asian J., 2016, 11, 2041–2048 CrossRef CAS.
- (a) S. Dhakal, J. Schonhoft, D. Koirala, Z. Yu, S. Basu and H. Mao, J. Am. Chem. Soc., 2010, 132, 8991–8997 CrossRef CAS; (b) S. Dhakal, Z. Yu, R. Konik, Y. Cui, D. Koirala and H. Mao, Biophys. J., 2012, 102, 2575–2584 CrossRef CAS.
- (a) Y. Xu and H. Sugiyama, Nucleic Acids Res., 2006, 34, 949–954 CrossRef CAS; (b) K. Fhayli, S. Alsaiaria and N. Khashab, Chem. Commun., 2015, 51, 3747–3749 RSC; (c) D. Sun and L. Hurley, J. Med. Chem., 2009, 52, 2863–2874 CrossRef CAS.
- Y. Xu and H. Sugiyama, Nucleic Acids Res., 2006, 34(3), 949–954 CrossRef CAS.
- (a) A. Ambrus, D. Chen, J. Dai, R. Jones and D. Yang, Biochemistry, 2005, 44, 2048–2058 CrossRef CAS; (b) R. Mathad, E. Hatzakis, J. Dai and D. Yang, Nucleic Acids Res., 2011, 39(20), 9023–9033 CrossRef CAS.
- P. Agrawal, E. Hatzakis, K. Guo, M. Carver and D. Yang, Nucleic Acids Res., 2013, 41(22), 10584–10592 CrossRef CAS.
- J. Dai, D. Chen, R. Jones, L. Hurley and D. Yang, Nucleic Acids Res., 2006, 34(18), 5133–5144 CrossRef CAS.
- A. Phan, V. Kuryavyi, S. Burge, S. Neidle and D. Patel, J. Am. Chem. Soc., 2007, 129, 4386–4392 CrossRef CAS.
- J. Kypr, I. Kejnovská, D. Renčiuk and M. Vorlíčková, Nucleic Acids Res., 2009, 37, 1713–1725 CrossRef CAS.
- H. Day, C. Huguin and Z. Waller, Chem. Commun., 2013, 49, 7696–7698 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0nj04863a |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2021 |