Biocatalytic ketone reductions using Biobeads for miniaturized high throughput experimentation†
Jia Shen
Chew
,
Thi Thanh Nha
Ho
and
Chi-Lik Ken
Lee
 *
*
Division of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, 21 Nanyang Link, 637371, Singapore. E-mail: ken.lee@ntu.edu.sg
First published on 15th December 2020
Abstract
Miniaturized reactions conducted in parallel can lead to increased productivity in laboratories without depleting high value reagents. The use of polystyrene beads to coat thin layers of solid reagents offers an elegant strategy to tackle the microscale high throughput solid dispensing conundrum. Herein we report the successful utilization of Biobeads as the first example of high throughput reaction screening with nanomole quantities of ketoreductase on polystyrene beads for asymmetric biocatalytic reactions.
High Throughput Experimentation (HTE) technologies enable vast numbers of biocatalytic reactions to be screened in parallel, this allows the best enzymes and conditions for a desired biotransformation to be determined with high speeds and efficiencies.1 Over the past three decades, enzyme engineering has enhanced the stability of wild type enzymes which led to amplifications in catalytic efficiency and stereoselectivity.2–4 More importantly, inert non-native substrates are able to undergo the desired biotransformations with enzymes to afford non-native products.3 According to Fig. 1, large scale manufacturing of (S)-licarbazepine5 and montelukast6 provides a glimpse of the efficiencies that can be achieved via engineered ketoreductases (Kred). Traditionally, enzyme-based processes were planned based on the limitations of the enzyme. Recent strategies suggested that enzyme variants can be engineered with improved properties to fit the process requirements. These approaches have allowed enzymes to be further engineered with extreme precision to carry out complex molecular processes with higher efficiency.3 Yet, designing a biocatalyst at a necessary quicker pace remains a daunting challenge since the final enzyme variant normally undergoes 10–20% alteration compared to its parent wild-type enzyme.7
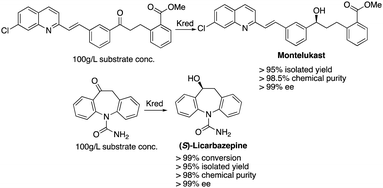 | ||
| Fig. 1 Large scale manufacturing of (S)-licarbazepine and montelukast via biocatalytic reductions of ketones. | ||
This roughly equates to 10390 different permutations of amino acid sequences for a protein that has 300 amino acids. Truppo described a fourth wave of biocatalysis where biocatalysts are engineered through a rational directed evolution in a design-make-test cycle, combining multi-disciplines in this harmonious industrialized work process.7 This cycle provided a model of how a protein's function could be impacted via each mutation. While the design phase utilized computation and informatics to generate diverse characteristics of the sequence space to be explored, the make phase exploited biology to afford the necessary enzymes for testing. Finally, the test phase capitalized on HTE to evaluate the performance of each enzyme under the right experimental conditions.7,8 Therefore, the role of HTE technologies is paramount to enable more efficient correlations of the enzyme's mutations to its activity, selectivity, expression, proper folding, and stability, amongst other parameters. This leads to a significant reduction in time for each round of protein engineering and thus increasing the performance gain across a variety of metrics.7–11 The furtherance and broad impact of high-throughput reaction screening in biocatalysis can be impeded by the scarcity of suitable technologies.12,13 Hence it is quintessential to exploit and develop technologies for reaction handling on miniaturized scales to preserve high-value biocatalysts and their co-factors. To this end, glass and polystyrene beads coated with powdered solid reagents have been demonstrated to deliver chemicals with sufficient accuracy on the nanomole scale for HTE.12,13 A summary of carrier systems and immobilization techniques for multienzyme systems can be found in a review by Hwang et al.14 By employing this universal solid dispensing procedure as mentioned by Tu et al.,12,13 we herein report the first successful utilization of Biobeads (polystyrene beads coated with Kred and the corresponding co-factors) for the convenient asymmetric biocatalytic reductions of ketones on miniaturised scales. Kred reactions (native) are typically carried out in an aqueous buffer with either the nicotinamide adenine dinucleotide phosphate (NADPH) or nicotinamide adenine dinucleotide (NADH) co-factor. This co-substrate approach has been identified as the most efficient and cost-effective co-factor recycling approach, whereby isopropanol (IPA) is often used as the hydride source for recycling the NADPH cofactor as shown in Scheme 1. In the standard native Kred reaction approach, NADPH/NADH and the necessary buffer solutions are prepared separately, before Kred and the substrate are added.
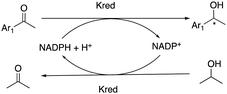 | ||
| Scheme 1 General co-substrate approach to biocatalytic ketone reduction using isopropanol to recycle NADPH. | ||
Previous studies have indicated that physical adsorption of powdered enzymes onto non-porous solid supports leads to little or no loss of enzyme activity,14–17 which is necessary for reaction screening to be characteristic of its native conditions. However, our initial efforts using glass beads did not achieve such desired results. By adopting a similar “mixed ChemBead” approach by Tu et al.12,13 and the reported co-immobilization of multienzyme complexes, mixed Biobeads were prepared by coating nanomole quantities of Kred, co-factors (β-NADP-Na2 and β-NAD-Na2; oxidized salts of NADPH/NADH) and buffer reagents onto polystyrene beads. To our delight, the initial test reactions with Biobeads afforded the desired products in relatively high conversion yields. In our standard Biobeads reaction approach, calibrated scoops were used to weigh Biobeads in a volumetric fashion and the subsequent reactions were activated simply by the addition of water, IPA and the carbonyl substrates.
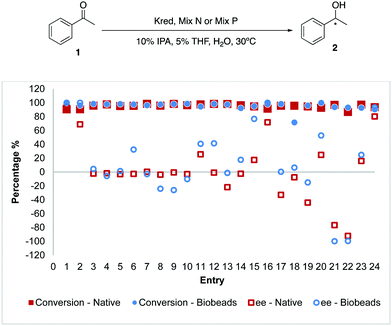
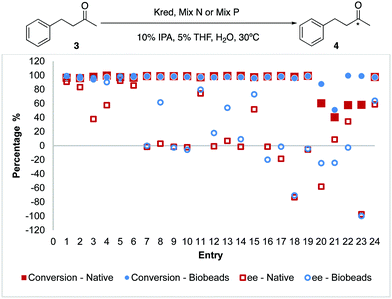
Using acetophenone 1 as the general substrate, we sought to unravel and compare the differences between the native and Biobeads reactions on a 10 mg Kred scale. For this, the panel of engineered Kred 1–24 from Codexis was tested. Reactions performed with Biobeads were comparable to the native equivalents such that in most cases, the conversions and enantiomeric excess (ee) were similar (Fig. 2). To improve the wider applicability of Biobeads, 4-phenylbutan-2-one 3 was used as the substrate and similar trends were also observed (Fig. 3).
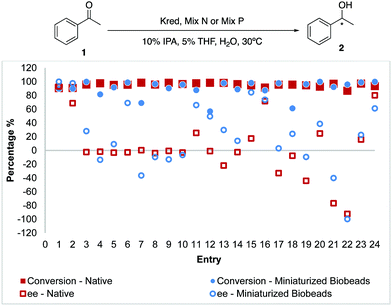
With the successful translation of Kred from its native protocols to Biobeads, we set out to conduct the Biobeads reaction on miniaturized scales. While the recommended quantity of Kred for each reaction was 10 mg, we were able to demonstrate the same reaction on a 0.036 mg scale with Biobeads (Fig. 4). Generally, similar conversions and enantioselectivities were obtained though some entries showed lower % conversion compared to native conditions at lower reaction scales. Yet, the reduction of Kred by approximately 278 times revealed the necessary tradeoff in the context of miniaturized reaction screenings and this miniaturized scale was eventually determined as the most practical method that fell within the detection limits of HPLC.
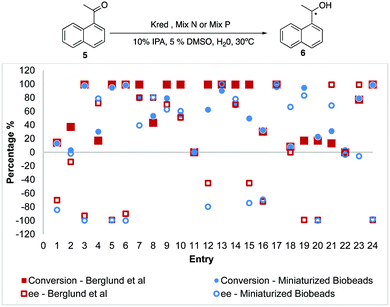
With the miniaturized scale, we extended Biobeads to another substrate 1-(naphthalen-1-yl)ethan-1-one 5, akin to the reports by Berglund et al. who performed their reaction on a 2 mg Kred scale.15 Gratifyingly, we were able to achieve representative data using much smaller quantities at 0.036 mg of Kred whereby most entries preserved the % conversions and enantioselectivities, though slightly poorer % conversions were observed for entries 7, 9, 12, 13, 14 and 15 (Fig. 5). For instance, entry 9 shows 98% conversion and 26% ee (S-isomer) for the native reaction as compared to 90% conversion and 13% ee (S-isomer) on miniaturized Biobeads.
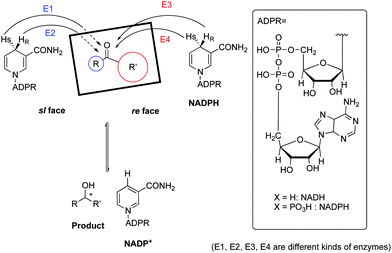
Generally, enhanced stereoselectivities were observed when reactions are performed using Biobeads. We postulate that this is due to some portions of the macromolecular Kred remaining attached on the beads and is substantiated with previous studies demonstrating the various extents to which powdered enzymes can be physically adsorbed onto solid supports,16–18 even in the presence of solvents.19–23Scheme 2 shows the mechanism of NADP(H)-dependent ketoreductases.24 There are four possible stereochemical transfers of a hydride from the co-enzyme, NADPH, to the substrate. The hydride attacks either on the si- or re-face of the carbonyl moiety depending on the orientation of the substrate bound to the enzyme. Based on the type of ketoreductase, either a pro-(R)-hydride or pro-(S)-hydride of the NADPH/NADH cofactor would be transferred, leading to the formation of the R and S secondary alcohols, respectively.24 Interactions of the Kred with the polystyrene surfaces could favour or inhibit certain orientations of the substrate towards the enzyme and thus effected a greater enantioselective profile.25
To conclude, we have successfully demonstrated the first examples of biocatalytic ketoreduction using nanomole quantities of enzyme and co-factors coated onto polystyrene beads for the context of HTS. This extension of work from previous reports has shown that the use of polystyrene beads to coat solid powdered enzymes can preclude the need to tediously weigh solids over an analytical balance or to prepare stock solutions, while achieving comparable and in some cases, enhanced conversion yields and enantioselectivities. Biobeads will continue to furnish more effective HTE strategies in biocatalytic reactions and allow larger numbers of reactions to be screened, increasing the pace to determine the best enzymes and conditions for a desired transformation. The demonstration of miniaturized Biobeads reaction array formats can offer the following advantages in HTE and protein engineering: (i) greatly reduces the time and manual labor needed to carry out solid enzyme reagent handling and weighing for large arrays of small-scale reactions; (ii) enables as little as 0.036 mg Kred to be dispensed for each reaction screening with sufficient accuracy; (iii) allows multiple reagents to be coated onto the same bead for an efficient co-factor recycling; (iv) facilitates wider applicability of polystyrene beads as a screening tool towards other biocatalytic reactions. This method is particularly useful for microscale HTE since it allows for any solid, soluble or not in the reaction solvent, to be dispensed easily at nanomolar quantities. Ongoing HTE efforts using Biobeads are currently being explored and will be reported in due course.
Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This research was supported by the Ministry of Education (MOE) AcRF Tier 1 funding (Grant Number 2019-T1-002-114) and Nanyang Technological University (SUG). Jia Shen Chew is grateful to the Agency for Science Technology and Research (A*STAR) for an undergraduate scholarship.Notes and references
- S. M. Mennen, C. Alhambra, C. L. Allen, M. Barberis, S. Berritt, T. A. Brandt, A. D. Campbell, J. Castañón, A. H. Cherney, M. Christensen, D. B. Damon, J. Eugenio de Diego, S. García-Cerrada, P. García-Losada, R. Haro, J. Janey, D. C. Leitch, L. Li, F. Liu, P. C. Lobben, D. W. C. MacMillan, J. Magano, E. McInturff, S. Monfette, R. J. Post, D. Schultz, B. J. Sitter, J. M. Stevens, I. I. Strambeanu, J. Twilton, K. Wang and M. A. Zajac, The Evolution of High-Throughput Experimentation in Pharmaceutical Development and Perspectives on the Future, Org. Process Res. Dev., 2019, 23(6), 1213–1242 CrossRef CAS.
- J. Lalonde, Highly engineered biocatalysts for efficient small molecule pharmaceutical synthesis, Curr. Opin. Biotechnol., 2016, 42, 152–158 CrossRef CAS PubMed.
- U. T. Bornscheuer, G. W. Huisman, R. J. Kazlauskas, S. Lutz, J. C. Moore and K. Robins, Engineering the third wave of biocatalysis, Nature, 2012, 485, 185 CrossRef CAS PubMed.
- T. S. Moody and J. D. Rozzell, Modern Biocatalytic Ketone Reduction. In Organic Synthesis Using Biocatalysis, ed. A. Goswami and J. D. Stewart, Academic Press, 2016, ch. 6, pp. 149–185 Search PubMed.
- N. K. Modukuru, J. Sukumaran, S. J. Collier, A. S. Chan, A. Gohel, G. W. Huisman, R. Keledjian, K. Narayanaswamy, S. J. Novick, S. M. Palanivel, D. Smith, Z. Wei, B. Wong, W. L. Yeo and D. A. Entwistle, Development of a Practical, Biocatalytic Reduction for the Manufacture of (S)-Licarbazepine Using an Evolved Ketoreductase, Org. Process Res. Dev., 2014, 18(6), 810–815 CrossRef CAS.
- J. Liang, J. Lalonde, B. Borup, V. Mitchell, E. Mundorff, N. Trinh, D. A. Kochrekar, R. Nair Cherat and G. G. Pai, Development of a Biocatalytic Process as an Alternative to the (−)-DIP-Cl-Mediated Asymmetric Reduction of a Key Intermediate of Montelukast, Org. Process Res. Dev., 2010, 14(1), 193–198 CrossRef CAS.
- M. D. Truppo, Biocatalysis in the Pharmaceutical Industry: The Need for Speed, ACS Med. Chem. Lett., 2017, 8(5), 476–480 CrossRef CAS PubMed.
- N. J. Turner and M. D. Truppo, Biocatalysis enters a new era, Curr. Opin. Chem. Biol., 2013, 17, 212 CrossRef CAS PubMed.
- D. L. Hughes, Biocatalysis in Drug Development—Highlights of the Recent Patent Literature, Org. Process Res. Dev., 2018, 22(9), 1063–1080 CrossRef CAS.
- G. W. Huisman, J. Liang and A. Krebber, Practical chiral alcohol manufacture using ketoreductases, Curr. Opin. Chem. Biol., 2010, 14(2), 122–129 CrossRef CAS PubMed.
- M. Bilal, H. M. N. Iqbal, S. Guo, H. Hu, W. Wang and X. Zhang, State-of-the-art protein engineering approaches using biological macromolecules: A review from immobilization to implementation view point, Int. J. Biol. Macromol., 2018, 108, 893–901 CrossRef CAS PubMed.
- M. C. Martin, G. M. Goshu, J. R. Hartnell, C. D. Morris, Y. Wang and N. P. Tu, Versatile Methods to Dispense Submilligram Quantities of Solids Using Chemical-Coated Beads for High-Throughput Experimentation, Org. Process Res. Dev., 2019, 23(9), 1900–1907 CrossRef CAS.
- N. P. Tu, A. W. Dombrowski, G. M. Goshu, A. Vasudevan, S. W. Djuric and Y. Wang, High-Throughput Reaction Screening with Nanomoles of Solid Reagents Coated on Glass Beads, Angew. Chem., Int. Ed., 2019, 58(24), 7987–7991 CrossRef CAS PubMed.
- E. T. Hwang and S. Lee, Multienzymatic Cascade Reactions via Enzyme Complex by Immobilization, ACS Catal., 2019, 9(5), 4402–4425 CrossRef CAS.
- K. Nagayama, A. C. Spiess and J. Büchs, Gas phase enantioselective reduction catalyzed by immobilized ketoreductase: Effects of water activity and reaction temperature, Biochem. Eng. J., 2010, 52(2), 301–303 CrossRef CAS.
- L. Marx, N. Rios-Lombardia, J. F. Farnberger, W. Kroutil, A. I. Benitez-Mateos, F. Lopez-Gallego, F. Moris, J. Gonzalez-Sabin and P. Berglund, Chemoenzymatic Approaches to the Synthesis of the Calcimimetic Agent Cinacalcet Employing Transaminases and Ketoreductases, Adv. Synth. Catal., 2018, 360(11), 2157–2165 CrossRef CAS PubMed.
- H. B. S. Bento, H. F. de Castro, P. C. de Oliveira and L. Freitas, Magnetized poly(STY-co-DVB) as a matrix for immobilizing microbial lipase to be used in biotransformation, J. Magn. Magn. Mater., 2017, 426, 95–101 CrossRef CAS.
- E. T. Hwang and S. Lee, Multienzymatic Cascade Reactions via Enzyme Complex by Immobilization, ACS Catal., 2019, 9(5), 4402–4425 CrossRef CAS.
- K. Nagayama, A. C. Spiess and J. Buchs, Immobilization conditions of ketoreductase on enantioselective reduction in a gas-solid bioreactor, Biotechnol. J., 2010, 5(5), 520–555 CrossRef CAS PubMed.
- A. Trivedi, M. Heinemann, A. C. Spiess, T. Daussmann and J. Büchs, Optimization of adsorptive immobilization of alcohol dehydrogenases, J. Biosci. Bioeng., 2005, 99(4), 340–347 CrossRef CAS PubMed.
- C. Ferloni, M. Heinemann, W. Hummel, T. Daussmann and J. Büchs, Optimization of Enzymatic Gas-Phase Reactions by Increasing the Long-Term Stability of the Catalyst, Biotechnol. Prog., 2004, 20(3), 975–978 CrossRef CAS PubMed.
- W. Schöpp and M. Grunow, Immobilization of yeast ADH by adsorption onto polyaminomethylstyrene, Appl. Microbiol. Biotechnol., 1986, 24(4), 271–276 CrossRef.
- S. Cantone, V. Ferrario, L. Corici, C. Ebert, D. Fattor, P. Spizzo and L. Gardossi, Efficient immobilisation of industrial biocatalysts: criteria and constraints for the selection of organic polymeric carriers and immobilisation methods, Chem. Soc. Rev., 2013, 42(15), 6262–6276 RSC.
- T. Matsuda, R. Yamanaka and K. Nakamura, Recent progress in biocatalysis for asymmetric oxidation and reduction, Tetrahedron: Asymmetry, 2009, 20(5), 513–557 CrossRef CAS.
- S. M. A. De Wildeman, T. Sonke, H. E. Schoemaker and O. May, Biocatalytic Reductions: From Lab Curiosity to “First Choice”, Acc. Chem. Res., 2007, 40(12), 1260–1266 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, characterization of data and copies of NMR spectra. See DOI: 10.1039/d0nj04889e |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2021 |