The synthesis and investigation of the reversible conversion of layered ZrS2 and ZrS3†
Abstract
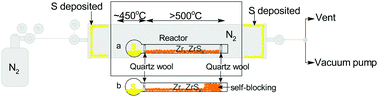
Metal sulfides, particularly zirconium sulfides, have been successfully synthesized from elemental zirconium and sulfur by a so-called diffusion method. The method allows one to control the morphology and phases of zirconium sulfides via tuning the reaction temperature and sulfur vapor pressure. At 550 °C and 650 °C, ZrS3 nanobelts with a width from 90 to 160 nm and a thickness of about 26 ± 8 nm and 6 ± 2 μm in length were experimentally synthesized. Wider (from 90 to 743 nm) and longer (19.7 ± 3.4 μm) ZrS3 nanobelts can be obtained by increasing the sulfur vapor pressure. Meanwhile, flake-like ZrS2 particles were produced at 750 °C. Experimental observation revealed that the liquid–solid mechanism, in combination with sulfur intercalation, is likely a major mechanism that is responsible for the formation of ZrS3 nanobelts. The reversible conversion between ZrS3 and ZrS2 has been observed for the first time by thermal decomposition of ZrS3 at 650 °C and sulfurization of ZrS2 in sulfur vapor at 550 °C. This result suggests a potential application of ZrS2 in the H2S splitting process for H2 recovery.


 Please wait while we load your content...
Please wait while we load your content...