Anti-pseudo-allergic capacity of alkaloids screened from Uncaria rhynchophylla†
Yitong
Xie‡
,
Di
Wei‡
,
Tian
Hu
,
Yajing
Hou
,
Yuanyuan
Lin
,
Huaizhen
He
 * and
Cheng
Wang
*
* and
Cheng
Wang
*
School of Pharmacy, Xi’an Jiaotong University, Yanta Westroad, Xi’an 710061, China. E-mail: chengwang@mail.xjtu.edu.cn; hehuaizhen@mail.xjtu.edu.cn
First published on 20th November 2019
Abstract
Uncaria rhynchophylla (UR) is a traditional Chinese herb widely used for hyperpyrexia, epilepsy, preeclampsia, and hypertension. In this paper, the potential anti-pseudo-allergic components in UR were explored. Six alkaloids in UR possessed good retention properties on an established MRGPRX2/CMC model. Among them, isorhynchophylline (Isorhy) and corynoxeine (Cor) could inhibit the pseudo-allergic reaction induced by C48/80 both in vivo and in vitro. Therefore, this study provides evidence for the anti-pseudo-allergic effect of UR and for development of the biological activity of this genus.
Introduction
Pseudo-allergic reactions are similar hypersensitivity-like pathological processes produced by drugs and other antigens for the first time, which are not stimulated by IgE.1 Clinical manifestations include facial flushing, convulsions, accelerated heartbeat, headache, nausea, redness of the skin, shortness of breath, chest tightness, etc. Severe cases can lead to shock or even death.2 Mast cells are the main effector cells of immediate hypersensitivity, inflammatory response and host defensive immune response,3 mainly distributed in the skin, lymphoid tissues and respiratory tract.4 In allergic pathologies, histamine triggers acute symptoms due to its rapid effects on the vascular endothelium, bronchial and smooth muscle cells, leading to vasodilatation, increased vascular permeability, bronchoconstriction, itching, cramping, diarrhea, cutaneous wheal and flare responses.5 Anti-allergic drugs for clinical application mainly include antihistamines, allergic reaction medium release agents, and glucocorticoids at present. However, these medicines have several side effects.6Traditional Chinese medicine (TCM) has the characteristics of efficient curative effects, multiple targets and slight side effects. TCM is gradually becoming a well-known form of medication worldwide due to its considerable scientific development,7 which makes it a possible source for new drugs. Thus, screening effective anti-allergic components from TCM and studying their possible mechanisms of action are of great significance for the development of new anti-allergy drugs. Uncaria rhynchophylla (UR) is a traditional Chinese herb belonging to the family Rubiaceae.8 UR has the capacity for analgesia, anti-inflammation and acting against acute convulsion,9 and is commonly used to treat or relieve symptoms such as hypertension, headache, vertigo, vascular dementia, and stroke.10 UR is also reported for use in treating allergic diseases such as chronic urticaria, allergic rhinitis, and allergic cough. In Peru, Guyana and Brazil, decoctions of U. tomentosa and U. guianensis have been used to treat asthma and inflammatory conditions.11 However, there are no reports on the effects of UR in anaphylactoid reactions.
Cell membrane chromatography (CMC) is a biological affinity chromatography invented by He and colleagues in 1996.12 Many target components have been successfully screened from complicated TCMs by different membrane receptors.13 Mast cells or basophils are the primary effectors of pseudo-allergic reactions. Mast cell membrane chromatography model has been successfully used to screen potential anaphylactic components.14 Recently, Dong and colleagues confirmed that Mas-related G protein-coupled receptor X2 (MRGPRX2) might be a potential target for pseudo-allergy induced by small-molecule drugs.15 Hence it was of great advantage to screen anti-pseudo-allergic active compounds in TCM by MRGPRX2-CMC.16
In this study, UR was selected as the research object. Six potential active compounds were screened from UR by MRGPRX2/CMC, and their anti-pseudo-allergic activity was initially verified by in vitro and in vivo experiments.
Results
Screening and structure determination of active ingredients in UR
The MRGPRX2/CMC model was constructed using the highly expressed MRGPRX2-HEK293 cells. The system suitability of the MRGPRX2/CMC model was firstly evaluated in terms of selectivity, repeatability and effectiveness. As shown in Fig. 1, only ciprofloxacin hydrochloride, a proven positive drug acting on MRGPRX2, was retained on the MRGPRX2/CMC model. While all the negative controls (gefitinib, terazosin and quercetin) were not retained, indicating the satisfactory selectivity of MRGPRX2/CMC. The MRGPRX2/CMC model also showed favorable reproducibility with RSDs of retention time of ciprofloxacin hydrochloride at 1.58% (n = 5) and 4.67% (n = 3) for different injections and columns. In addition, the biological activity of the MRGPRX2/CMC model was investigated by continuous injection of ciprofloxacin during 48 h, and there were still significant retentions on the MRGPRX2/CMC model (Fig. S1, ESI†). The results revealed the good activity of the MRGPRX2/CMC model within 48 h. Therefore, the MRGPRX2/CMC model could be applied to the screening of active components related to pseudo-allergy in UR.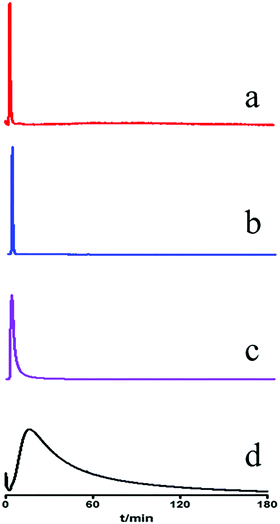 | ||
| Fig. 1 Chromatograms of gefitinib (a), terazosin (b), quercetin (c) and ciprofloxacin (d) on MRGPRX2/CMC column. | ||
The retention of different extraction sites (petroleum ether, chloroform, ethyl acetate and n-butanol) of UR was analyzed on the MRGPRX2/CMC model. The results are shown in Fig. 2. The petroleum ether, ethyl acetate and n-butanol fractions had no obvious retention behavior, while the chloroform fraction showed retention. The retained fraction was then separated by HPLC (Fig. 3a). Seven components with relatively high content (G1–G7) were enriched and tracked again with the MRGPRX2/CMC model. It was shown that G2–G7 were retained on the MRGPRX2/CMC model, but not G1 (Fig. S2, ESI†), which revealed that G2–G7 might be potential active components that interacted with MRGPRX2.
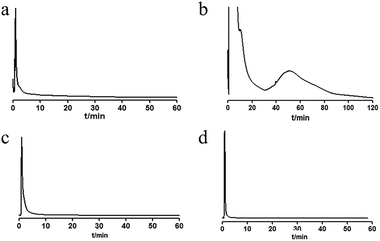 | ||
| Fig. 2 Chromatograms of petroleum ether (a), chloroform (b), ethyl acetate (c) and n-butanol (d) extracts on MRGPRX2/CMC column. | ||
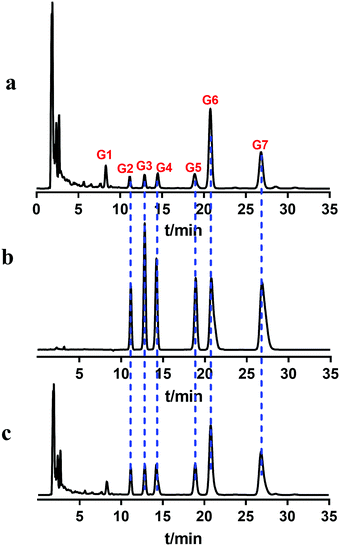 | ||
| Fig. 3 Chromatograms of chloroform extract (a), standard compounds (b) and mixed sample of extract and standards (c) using HPLC. | ||
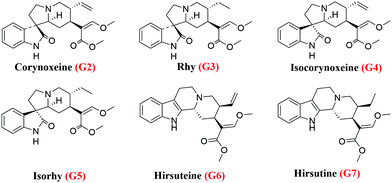
Accordingly, the retained components were assayed using the MS system (Fig. S3, ESI†). By comparing with the literature,17 it was speculated that G2 and G4 might be corynoxeine (Cor) and isocorynoxeine (Isocor), and G3 and G5 might be rhynchophylline (Rhy) and isorhynchophylline (Isorhy). G6 and G7 were identified as hirsuteine (Hie) and hirsutine (Hir) (Table S1, ESI†). Subsequently, the liquid chromatogram of the standard solution of the six alkaloids was compared with the chloroform sites of UR (Fig. 3b and c). Finally, it could be confirmed that G2–G7 were Cor, Isorhy, Isocor, Rhy, Hie and Hir, respectively, and their structures are shown in Fig. 4. In addition, six standard solutions were retained to different degrees on the MRGPRX2/CMC model (Fig. S4B, ESI†), which was also confirmatory evidence for the above results.
Effects of six alkaloids on cell viability
The effects of Rhy, Isorhy, Cor, Isocor, Hir and Hie on cell viability were investigated by CCK8 assay. The results are shown in Fig. S5 (ESI†). Hir and Hie revealed significant inhibitory effects on LAD2 cells when the concentration exceeded 100 μM. In contrast, Rhy, Isorhy, Cor and Isocor had no effect on the proliferation of LAD2 cells under the tested concentration. So, the anti-pseudo-allergic experiments were conducted at 25, 50, 100, 200 μM for the four alkaloids.The effect of four alkaloids on C48/80-induced degranulation in LAD2 cells
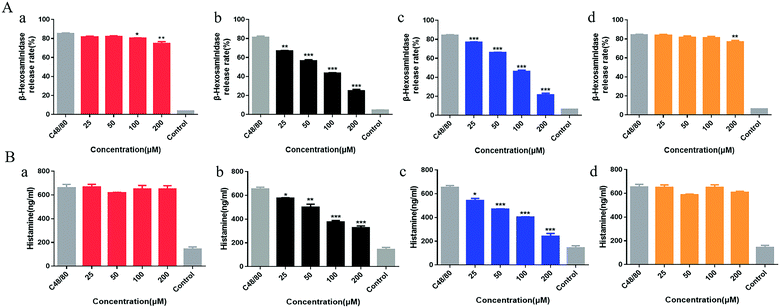
Compound 48/80 is commonly used as a model drug for studying pseudo-allergy. In this study, β-hexosaminidase was selected as one of the indicators of mast cell degranulation, which is related to allergic reaction. As shown in Fig. 5A, Rhy was statistically different from the model group at 100 μM and 200 μM as well as Isocor at 200 μM, while Cor and Isocor decreased the release rate of β-hexosaminidase of LAD2 cells in a dose-dependent manner. Cells treated with Rhy or Isocor had no significant change in histamine release compared with the model group. However, the release of histamine in LAD2 cells incubated with Isorhy and Cor decreased in a dose-dependent manner with increasing concentration (Fig. 5B). The results were similar to the change trend of β-hexosaminidase release rate. In general, the effects of Rhy and Isocor on the degranulation of mast cells are relatively weak, whereas Isorhy and Cor can significantly inhibit C48/80-induced cell degranulation in vitro. Thus, Isorhy and Cor were selected for the intracellular Ca2+ mobilization assay.Anti-pseudo-allergy effect of Isorhy and Cor may be related to MRGPRX2
Mast cell degranulation is Ca2+-dependent and C48/80-induced mast cell activation has been reported to be related to MRGPR2. Therefore, the calcium flux was tested using MRGPRX2-HEK293 cells. The results are shown in Fig. S6 (ESI†). C48/80 increased the intracellular Ca2+ concentration, while Isorhy and Cor can reduce the increase of calcium concentration induced by C48/80 in a dose-dependent manner, suggesting that Isorhy and Cor might inhibit the degranulation of mast cells by decreasing calcium influx by MRGPRX2.Isorhy and Cor suppressed C48/80-induced local anaphylaxis
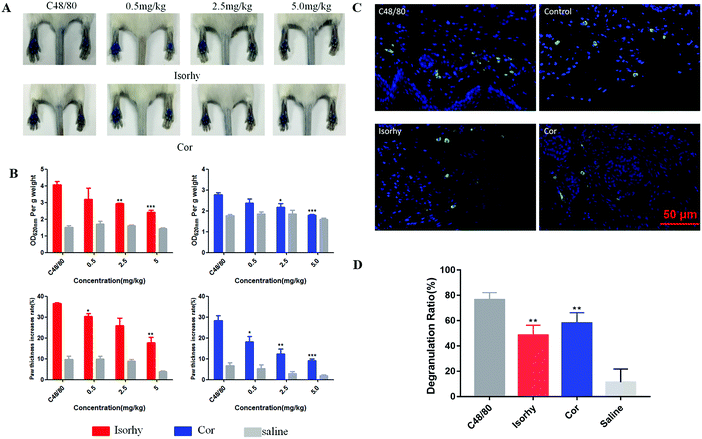
A passive cutaneous anaphylaxis mouse model was used to investigate the anti-pseudo-allergic effect of Isorhy and Cor on C48/80-induced skin inflammation in vivo. The degree of swelling and Evans blue dye exudation in paws were both suppressed (Fig. 6A and B), while the differences between saline groups were caused by individual differences in mice. Next, we marked the mast cells in the paw skin using avidin and found a significant decrease in the percentage of degranulated mast cells upon test alkaloid treatment, which clearly suggested that Isorhy and Cor suppressed degranulation of mast cells in vivo (Fig. 6C and D). Based on these results, we concluded that Isorhy and Cor could inhibit C48/80-induced local anaphylaxis.Isorhy and Cor inhibited C48/80-induced systemic anaphylaxis
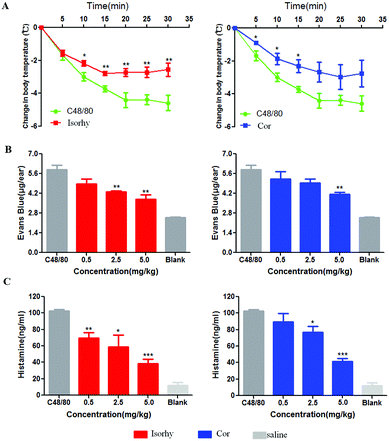
The inhibition effect of Isorhy and Cor on C48/80-induced systemic anaphylaxis was also studied. Isorhy and Cor decreased dye ear diffusion to different degrees in an ear swelling assay (Fig. 7A). Subsequently, body temperature changes in mice were monitored. C48/80 significantly reduced the body temperature of the mice, whereas Isorhy and Cor pre-treatment group showed a low degree of reduction in body temperature (Fig. 7B). This indicated that Isorhy and Cor could inhibit C48/80-induced systemic anaphylaxis.Isorhy and Cor inhibited the release of histamine in vivo
When the body is subjected to physicochemical stimulation or an allergic reaction, mast cells may be degranulated, resulting in the release of histamine, which binds to histamine receptors to produce biological effects. Therefore, we tested the effect of the alkaloids on the release of histamine in serum stimulated by C48/80. As shown in Fig. 7C, Isorhy and Cor treatment inhibited the production of histamine in a dose-dependent manner in vivo, which indicated that Isorhy and Cor may inhibit pseudo-allergic reactions by reducing histamine release induced by C48/80 in vivo.Discussion
As a bioaffinity chromatography technique, CMC has been proven to be an important method for studying drug–receptor interactions and screening active components from medicinal herbs. Considering that MRGPRX2 is a crucial receptor in IgE-independent allergy, we established an MRGPRX2/CMC model to screen active compounds related to pseudo-allergy in UR. The chloroform site of alcohol extract of UR has good retention characteristics. Subsequently, we used HPLC to separate seven major components from the chloroform site, six of which were retained using CMC. By comparing with standard compounds by HPLC and mass spectra, six compounds were finally identified as Rhy, Isorhy, Cor, Isocor, Hir and Hie. In order to further verify the chromatographic retention of Rhy, Isorhy, Cor, Isocor, Hir and Hie, their standard solutions were injected into the MRGPRX2/CMC model. These results showed that the six compounds may be active ingredients that have an effect on MRGPRX2.LAD2 cells are skin-derived mast cells found in human connective tissue, which are widely used in allergy research. Activation of mast cells results in the release of diverse bioactive substances, such as histamine, β-hexosaminidase, cytokines, chemokines and other inflammatory mediators.18 Therefore, we used LAD2 cells to study whether the six compounds have an anti-pseudo-allergic effect in vitro. Firstly, cell viability was evaluated by CCK8 assay. LAD2 cells incubated with Rhy, Isorhy, Cor and Isocor in different doses from 0 μM to 400 μM for 24 h were observed to be almost 100% viable, indicating that LAD2 cell growth was not affected by any of the concentrations of the four alkaloids. The four concentrations below 400 μM were then selected for cell degranulation experiments to avoid the reduction of degranulation caused by cytotoxicity. C48/80 is a classical mast cell activator and canonical basic secretagogue, which has been widely used in much pseudo-allergy research.19 We found that Isorhy and Cor could significantly inhibit C48/80-induced cell degranulation, such as histamine and β-hexosaminidase, while Rhy and Isocor had hardly any influence on cell degranulation. Therefore, we concluded that the inhibition of C48/80-induced degranulation in LAD2 cells is related to the anti-pseudo-allergy effect of Isorhy and Cor. Cytosolic Ca2+ is a universal and highly versatile signal,20 and an increase of calcium concentration in mast cells causes degranulation and leading to allergic reactions.21 It was reported that the concentration of intracellular calcium can be regulated to affect cell degranulation by acting with MRGPRX2. In our study, we assessed the effect of Isorhy and Cor on intracellular calcium fluctuations of MRGPRX2-HEK293 cells. The result is consistent with previous reports, suggesting that Isorhy and Cor may inhibit cellular calcium flux through MRGPRX2, thereby inhibiting degranulation in vitro.
Mast cells located around vasculature and skin are activated; as a result, degranulation occurs on vascular endothelial cells to relax smooth muscle cells and thus promote vascular permeability.22 In our present study, Isorhy and Cor suppressed the increase in footpad swelling and blood vessel permeability induced by C48/80 in a dose-dependent manner. Subsequently, we performed avidin staining on paw skin and observed that Isorhy and Cor could inhibit the degranulation of mast cells in the skin of the foot. Body temperature change is always an obvious indicator of anaphylaxis.23 Mice had a significant attenuation of body temperature compared with the model group, likely due to changes in peripheral vasodilation.24 According to reports in the literature, this phenomenon might be associated with a reduction of histamine in serum.25 Therefore, we evaluated the effect of Isorhy and Cor on the histamine levels in the serum of mice, and the results were consistent with previous reports. All above results demonstrated that Isorhy and Cor exhibit a favorable anti-pseudo-allergy effect.
Experimental
Drugs and reagents
Rhy, Isorhy, Cor, Isocor, Hir, and Hie were purchased from Chengdu Pufei De Biotech Co. Ltd (Chengdu, China) and purified to ≥98%. Silica gel (ZEX-II, 5 μm, 200 Å) was obtained from Qingdao Meigao Chemical (Qingdao, China). Cell counting kit-8 (CCK-8) was supplied by 7Sea Pharmatech. Compound 48/80 (C48/80), ciprofloxacin and Evans blue were all from Sigma-Aldrich (St Louis, MO, USA). Both p-nitrophenyl-N-acetyl-β-D-glucosamide and Triton X-100 were purchased from Sigma Aldrich (Shanghai, China). Fluo-3 and pluronic F-127 were procured from Biotium (California, USA). HPLC-grade methanol was purchased from Merck (Darmstadt, Germany). Analytical-grade triethylamine was obtained from Kermel (Tianjin, China). Analytical reagents and all aqueous solutions were prepared using ultrapure water, which was produced using an MK-459 Millipore Milli-Q Plus ultra-pure water system.Ethics statement
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals from the National Institutes of Health. The animal care and the experimental protocols for the mice experiments were approved by the Animal Ethics Committee at Xi’an Jiaotong University, Xi’an, China (Permit Number: XJTU 2011-0045). All the animals were anesthetized with 3.5% chloral hydrate.Mice
Male C57BL/6 mice were purchased from the Experimental Animal Center at Xi’an Jiaotong University (Xi’an, China). Young adult mice weighing 18–22 g were used in this study. The mice were housed at the Experimental Animal Center of Xi’an Jiaotong University of SPF grade with free access to water and were supplied standard dry food twice a day before the formal experiment. All animal experiments requiring identical treatments of the animals were conducted by experimenters blinded to the conditions.Cell lines
Laboratory of Allergic Disease 2 human mast cell line (LAD2) was generously gifted by A. Kirshenbaum and D. Metcalfe (NIH, USA). HEK293-MRGPRX2 cells were kindly provided by Professor Xinzhong Dong from Johns Hopkins University (MD, USA). LAD2 cells (highly expressing MRGPRX2) were cultured in StemPro-34 medium containing penicillin–streptomycin (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100), L-glutamine (2 mmol L−1), recombinant human stem cell factor (100 ng mL−1) and StemPro nutrient supplement (10 mL L−1). LAD2 cell culture medium was hemi-replaced per week with fresh medium. Human high expression MRGPRX2 HEK293 cells (MRGPRX2-HEK293) were maintained in DMEM medium supplemented with penicillin–streptomycin (1
100), L-glutamine (2 mmol L−1), recombinant human stem cell factor (100 ng mL−1) and StemPro nutrient supplement (10 mL L−1). LAD2 cell culture medium was hemi-replaced per week with fresh medium. Human high expression MRGPRX2 HEK293 cells (MRGPRX2-HEK293) were maintained in DMEM medium supplemented with penicillin–streptomycin (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100) and fetal bovine serum (1
100) and fetal bovine serum (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10). MRGPRX2-HEK293 cell culture medium was replaced every 2 days with fresh culture medium. Both cell lines were incubated in a 95% humidified atmosphere containing 5% CO2 at 37 °C.
10). MRGPRX2-HEK293 cell culture medium was replaced every 2 days with fresh culture medium. Both cell lines were incubated in a 95% humidified atmosphere containing 5% CO2 at 37 °C.
Preparation of MRGPRX2-HEK293/CMC column
Cultured MRGPRX2-HEK293 cells (1 × 107) were collected and washed three times with physiological saline by centrifuging at 1000g, 4 °C for 5 min. The cells were resuspended in 50 mM Tris–HCl (pH 7.4) to be ruptured by ultrasonic procedure for 30 min. The resulting cell suspension was centrifuged at 1000g, 4 °C for 10 min. The precipitate was discarded and the cell membrane supernatant was centrifuged at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g, 4 °C for 20 min to afford a cell membrane pellet. The MRGPRX2-HEK293 cell membrane pellet was then resuspended in 5 mL physiological saline. To prepare the MRGPRX2-HEK293 cell membrane stationary phase (CMSP), the cell membrane suspension was added into 0.05 g activated silica gel under vacuum and agitation at 4 °C. The mixture was then agitated with a magnetic stirrer for 30 min and then left for 12 h. Finally, The MRGPRX2-HEK293 CMSP was packed into the CMC column (10 mm × 2.0 mm i.d.) by a wet packing method.
000g, 4 °C for 20 min to afford a cell membrane pellet. The MRGPRX2-HEK293 cell membrane pellet was then resuspended in 5 mL physiological saline. To prepare the MRGPRX2-HEK293 cell membrane stationary phase (CMSP), the cell membrane suspension was added into 0.05 g activated silica gel under vacuum and agitation at 4 °C. The mixture was then agitated with a magnetic stirrer for 30 min and then left for 12 h. Finally, The MRGPRX2-HEK293 CMSP was packed into the CMC column (10 mm × 2.0 mm i.d.) by a wet packing method.
Chromatography
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B = 32
B = 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 68). Flow rate: 1.0 mL min−1; column temperature: 37 °C; detection wavelength: 254 nm; injection volume: 5 μL.
68). Flow rate: 1.0 mL min−1; column temperature: 37 °C; detection wavelength: 254 nm; injection volume: 5 μL.
Cell viability assay
The viability of LAD2 cells was determined with CCK-8. Briefly, 5 × 103 LAD2 cells were placed in 96-well plates and then different concentrations of alkaloid (0, 12.5, 25, 50, 100, 200 and 400 μM) were added for 24 h. After 1 day, the cell counting kit solution was added to each well and incubated for another 2 h at 37 °C. Afterwards, the optical density (OD) of each well at 450 nm was read with a microplate reader.β-Hexosaminidase assay
The β-hexosaminidase assay was carried out in a 96-well plate. 3 × 105 LAD2 cells were incubated with 50 μL drug substances at the indicated concentrations for 30 min at 37 °C under 5% CO2 and then with 50 μL C48/80 (60 μg mL−1) for 30 min. Next, the plate was centrifuged for 5 min at 25 °C. 50 μL of supernatant was transferred into a new well and mixed with 50 μL β-hexosamine. The mixture was incubated for 90 min at 37 °C. For analysis of the total amount of β-hexosaminidase released, the negative control cells were lysed by treatment with Triton X-100 (1% v/v) before incubation with β-hexosamine. The reaction was halted by addition of 0.1 μM Na2CO3/NaHCO3 and the OD at 405 nm was measured using a microplate reader.Histamine release assay
3 × 105 LAD2 cells were seeded into a round bottom 96-well plate. Cells were incubated with 50 μL drug substances at different doses for 30 min at 37 °C with 5% CO2 and then added with 50 μL C48/80 (60 μg mL−1) for 30 min. Next, the plate was centrifuged for 5 min at 25 °C. 50 μL of supernatant was obtained and mixed 100 μL 5 ng mL−1 d4-histamine under cold conditions (d4-histamine was used as an internal standard solution). The mixture was vortexed and centrifuged at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g, 4 °C for 20 min, and the supernatants were then collected for HA analysis according to a UHPLC-ESI-MS/MS method using a previously reported procedure.26
000g, 4 °C for 20 min, and the supernatants were then collected for HA analysis according to a UHPLC-ESI-MS/MS method using a previously reported procedure.26
Intracellular Ca2+ mobilization assay
MRGPRX2-HEK293 cells were placed in 96-well plates and incubated overnight at 37 °C with 5% CO2. After removing the culture media, the cells were washed twice with CIB. Different doses of drug substances diluted in the incubation solution (1 μL of Flu-3, 1 μL of Pluronic F-127 and 998 μL of CIB) were added to each well, followed by incubation at 37 °C for 30 min. Subsequently, the cells were washed again and used immediately for imaging. The cells were imaged and captured after injecting 30 μg mL−1 C48/80 under blue light.Paw swell and extravasation in C48/80-induced mice
Adult mice weighing 18–22 g were divided randomly into a control group and three experimental groups. Firstly, the experimental groups (n = 5 in each group) were administered with an intraperitoneal injection of 0.02 mL test drug (0.05, 0.25, 0.5 mg mL−1) dissolved in 0.3% CMC-Na solution and the control group were injected with 0.3% CMC-Na solution of the same volume. One hour later, all the animals were anesthetized with an intraperitoneal injection of 0.2 mL 4% chloral hydrate, followed by injection intravenously with 0.2 mL 0.4% Evans blue in saline. In addition, paw thickness was measured using a Vernier caliper before any injection. After 10 min, 5 μL C48/80 (30 μg mL−1) was injected into the left paw and 5 μL saline was injected into the right paw with a micro-injector. 15 min after injection, the thickness was measured and recorded again. The mice were subjected to euthanasia by cervical dislocation, and the hind paw tissues were harvested, dried at 50 °C and weighed. After allowing intravascular dye to drain, paw tissue was soaked in 0.4 mL acetone–saline (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) mixture, minced and disrupted for 30 min. 100 μL of the supernatant was removed into a 96-well plate and the OD at 620 nm was read with a microplate reader.
3) mixture, minced and disrupted for 30 min. 100 μL of the supernatant was removed into a 96-well plate and the OD at 620 nm was read with a microplate reader.
Skin avidin stain assay in mice
Mice were treated with test drug except the control group, which was administered intraperitoneally for 60 min. The treated group and the control group were injected with 5 μL of 30 μg mL−1 C48/80 and saline in the left paw respectively. Fifteen minutes later, the injection site of the paw skin was isolated, washed with PBS and fixed with 4% formaldehyde for 48 h and subjected to avidin staining. The tissues were then cryoprotected in 20% (w/v) sucrose for more than 24 h and sectioned (20 μm thickness) using a cryostat. Next, the slides were dried at 37 °C for 30 min and preincubated in blocking solution (10% (v/v) normal goat serum, 0.2% (v/v) Triton X-100 in PBS, pH 7.4) for 2 h at room temperature, followed by incubation with 1/500 FITC-avidin for 45 min. The sections were washed 3 times with PBS, and a drop of Fluoro-mount G (Southern Biotech, AL, USA) was added. Images were taken immediately using a confocal scanning laser microscope (Nikon, Tokyo, Japan).C48/80-induced active general anaphylaxis in mice
Adult male mice were classified randomly into a control group and three experimental groups. The experimental groups (n = 5 in each group) were injected intraperitoneally with 0.02 mL test drug at the previously indicated concentrations and the control group were injected with 0.2 mL of 0.3% CMC-Na solution. One hour later, a mixture of 90 μg mL−1 C48/80 and 4% Evans blue was intravenously injected by the caudal vein. One hour after C48/80 challenge, the mice were subjected to euthanasia, and the ears were collected to measure dye extravasation. The total Evans blue content was extracted by adding 400 μL acetone–saline (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), followed by sonicating for 20 min and centrifuging for 20 min at 37 °C. 100 μl of the supernatant was aliquoted equally into 96-well plates and the OD at 620 nm was read with a microplate reader.
3), followed by sonicating for 20 min and centrifuging for 20 min at 37 °C. 100 μl of the supernatant was aliquoted equally into 96-well plates and the OD at 620 nm was read with a microplate reader.
Body temperature
Mice were divided randomly into two groups. 0.2 mL test drug (0.25, 0.5 mg mL−1) was injected intraperitoneally in one group, while an equal volume of 0.3% CMC-Na solution was injected in the other group. One hour later, all mice were given 0.2 mL C48/80 in saline with an intravenous injection. Subsequently, body core temperature was monitored by a BL-410 biological function system and recorded every 5 min within 30 min.Histamine release during systemic anaphylaxis reactions
Mice were treated with test drug except the control group, which was administered intraperitoneally 60 min before the administration intravenously of 90 μg mL−1 C48/80. 60 min after C48/80 challenge, blood samples were collected into heparinized tubes after removing the eye, and the sample was centrifuged for 10 min at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm at 4 °C to obtain 50 μL serum. Next, 400 μL of d4-HA was added to the serum sample and centrifuged for 20 min at 14
000 rpm at 4 °C to obtain 50 μL serum. Next, 400 μL of d4-HA was added to the serum sample and centrifuged for 20 min at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm at 4 °C after vortexing for 3 min. The supernatant was stored at −80 °C until analysis according to the previously reported procedure.
000 rpm at 4 °C after vortexing for 3 min. The supernatant was stored at −80 °C until analysis according to the previously reported procedure.
Statistical analysis
The group data are expressed as the mean ± SEM and analyzed using two-tailed unpaired student's t-test. Differences were considered statistically significant at P < 0.05.Conclusions
In conclusion, an MRGPRX2/CMC model was established and applied to screen potential anti-pseudo-allergy components in UR. Six distinct components from UR were identified as Cor, Isorhy, Isocor, Rhy, Hir and Hie, which may be ligands of MRGPRX2 with potent activity. Preliminary experiments indicated that Isorhy and Cor might inhibit mast cell degranulation by inhibiting the increase of intracellular calcium ion concentration in vitro. In addition, Isorhy and Cor were further found to reduce the effect of anti-pseudo-allergy in vivo. The present study firstly demonstrated the anti-pseudo-allergy effect of Isorhy and Cor from UR. Our findings may provide a better insight for utilizing and developing UR for the treatment of pseudo-allergy in future.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant numbers: 81230079 and 81227802) and China Postdoctoral Science Foundation (grant number: 2018M643682).Notes and references
- X. Meng, A. Ariza, J. Waddington, K. Park and D. Naisbitt, Curr. Pharm. Des., 2016, 22, 6734–6747 CrossRef CAS PubMed; W. J. Pichler and O. Hausmann, Int. Arch. Allergy Immunol., 2016, 171, 166–179 CrossRef PubMed.
- S. Markus, H. Andrea and H. Martin, Front. Pharmacol., 2016, 7, 171 CAS; H. Selye, Can. Med. Assoc. J., 1949, 61, 553–556 Search PubMed.
- A. Alvarez-Perea, L. K. Tanno and M. L. Baeza, Clin. Transl. Allergy, 2017, 7, 45 CrossRef PubMed.
- H. Subramanian, K. Gupta and H. Ali, J. Allergy Clin. Immunol., 2016, 138, 700–710 CrossRef CAS PubMed.
- W. R. Su, Q. Wan, L. H. Han, J. W. Huang, X. Q. Chen, G. H. Chen, S. G. Zheng and D. Liang, Biochem. Pharmacol., 2014, 91, 359–368 CrossRef CAS.
- S. W. Han, L. Sun, F. He and H. L. Che, Sci. Rep., 2017, 7, 7222 CrossRef.
- E. S. Ong, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2004, 812, 23–33 CrossRef CAS; W. Wang, C. M. Ma and M. Hattori, Biol. Pharm. Bull., 2010, 33, 669–676 CrossRef.
- I. Sakakibara, H. Takahashi, S. Terabayashi, M. Yuzurihara, M. Kubo and A. Ishige, Phytomedicine, 1998, 5, 83–86 CrossRef CAS; T. S. Wu and Y. Y. Chan, J. Chin. Chem. Soc., 1994, 41, 209–212 CrossRef.
- M. E. Heitzman, C. C. Neto, E. Winiarz, A. J. Vaisberg and G. B. Hammond, Phytochemistry, 2005, 66, 5–29 CrossRef CAS PubMed; Y. Mimaki, N. Toshimizu, K. Yamada and Y. Sashida, Yakugaku Zasshi, 1997, 117, 1011–1021 CrossRef PubMed; C. L. Hsieh, M. F. Chen, T. C. Li, S. C. Li, N. Y. Tang and C. T. Hsieh, Am. J. Chin. Med., 1999, 27, 257–264 CrossRef PubMed.
- A. Ndagijimana, X. M. Wang, G. X. Pan, F. Zhang, H. Feng and O. Olaleye, Fitoterapia, 2013, 86, 35–47 CrossRef CAS PubMed.
- P. Montoro, V. Carbone, J. D. Quiroz, S. F. De and C. Pizza, Phytochem. Anal., 2004, 15, 55–64 CrossRef CAS PubMed; J. S. Lee, M. Y. Yang, H. Yeo, J. Kim, H. S. Lee and J. S. Ahn, Bioorg. Med. Chem. Lett., 1999, 9, 1429–1432 CrossRef PubMed.
- L. C. He and X. D. Geng, New Prog. Biomed. Chromatogr., 1996, 3, 8–9 Search PubMed.
- H. Zhang, X. Wang, L. Yang, S. Zhang, Y. Zhang, C. He, Wei Ma and J. Hou, J. Pharm. Biomed. Anal., 2014, 101, 141–150 CrossRef PubMed.
- Y. Y. Lin, C. Wang, Y. J. Hou, H. Z. He, L. M. Huang, L. Yang and M. Sun, Biomed. Chromatogr., 2017, 31, e4015 CrossRef CAS; S. L. Han, Y. N. Lv, W. T. Xue, J. Cao, R. H. Cui and T. Zhang, J. Sep. Sci., 2016, 39, 466–472 CrossRef PubMed.
- B. D. McNeil, P. Pundir, S. Meeker, L. Han, B. J. Undem, M. Kulka and X. Dong, Nature, 2015, 519, 237–241 CrossRef CAS PubMed.
- Y. Y. Lin, C. Wang, Y. J. Hou, W. Sun, D. L. Che, L. Yang, T. Zhang, M. Sun, H. Z. He and L. C. He, J. Sep. Sci., 2018, 41, 2488–2497 CrossRef CAS.
- G. Laus, Phytother. Res., 2004, 18, 259–274 CrossRef CAS PubMed; G. Laus and H. Teppner, Phyton, 1996, 36, 185–196 Search PubMed.
- H. Subramanian, K. Gupta, Q. Guo, R. Price and H. Ali, J. Biol. Chem., 2011, 286, 44739–44749 CrossRef CAS PubMed; T. Karhu, K. Akiyama, O. Vuolteenaho, U. Bergmann, T. Naito, K. Tatemoto and K. H. Herzig, Biochim. Biophys. Acta, Gen. Subj., 2017, 1861, 2530–2534 CrossRef PubMed; E. Espinosa and S. Valitutti, Curr. Opin. Immunol., 2018, 50, 39–47 CrossRef PubMed; K. Tatemoto, Y. Nozaki, R. Tsuda, S. Konno, K. Tomura, M. Furuno, H. Ogasawara, K. Edamura, H. Takagi, H. Iwamura, M. Noguchi and T. Naito, Biochem. Biophys. Res. Commun., 2006, 349, 1322–1328 CrossRef PubMed.
- J. Ring and H. Behrendt, Clin. Rev. Allergy Immunol., 1999, 17, 387–399 CrossRef CAS PubMed; A. Chahdi, P. F. Fraundorfer and M. A. Beaven, J. Pharmacol. Exp. Ther., 2000, 292, 122–130 Search PubMed.
- A. B. Parekh, Nat. Rev. Drug Discovery, 2010, 9, 399–410 CrossRef CAS PubMed.
- L. Huang, T. Li, H. Zhou, P. Qiu, J. Wu and L. Liu, Int. Immunopharmacol., 2015, 28, 945–951 CrossRef CAS PubMed.
- F. Takano, T. Takata, A. Yoshihara, Y. Nakamura, Y. Arima and T. Ohta, Biol. Pharm. Bull., 2007, 30, 922–927 CrossRef CAS PubMed.
- N. Wang, D. L. Che, T. Zhang, R. Liu, J. Cao, J. Wang, T. T. Zhao, P. Y. Ma, X. Z. Dong and L. C. He, Biochem. Pharmacol., 2017, 148, 147–154 CrossRef PubMed.
- J. M. Galand, Y. Leyva-Castillo, A. Juhan, M. S. Han, A. N. J. Lee, M. McKenzie, M. K. Stassen, F. D. Oyoshi and R. S. Finkelman, J. Allergy Clin. Immunol., 2016, 138, 1356–1366 CrossRef.
- E. Doyle, J. Trosien and M. Metz, Methods Mol. Biol., 2013, 1032, 133–138 CrossRef CAS.
- Y. N. Lv, Y. M. Sun, J. Fu, L. Y. Kong and S. L. Han, Biomed. Chromatogr., 2017, 31, e3806 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9nj04551a |
| ‡ These two authors contribute equally. |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2020 |