Open Access Article
Open Access ArticleInterfacial engineering with carbon–graphite–CuδNi1−δO for ambient-air stable composite-based hole-conductor-free perovskite solar cells†
Yousheng
Wang
 *,
Yuzhao
Yang
*,
Yuzhao
Yang
 ,
Shaohang
Wu
,
Cuiling
Zhang
,
Zhen
Wang
,
Jinlong
Hu
,
Chong
Liu
,
Shaohang
Wu
,
Cuiling
Zhang
,
Zhen
Wang
,
Jinlong
Hu
,
Chong
Liu
 ,
Fei
Guo
,
Fei
Guo
 and
Yaohua
Mai
and
Yaohua
Mai
 *
*
Institute of New Energy Technology, College of Information Science and Technology, Jinan University, Guangzhou 510632, China. E-mail: wangys0120@jnu.edu.cn; yaohuamai@jnu.edu.cn
First published on 12th November 2020
Abstract
Ambient air atmosphere is inimical to organic–inorganic halide perovskites and organic hole transport materials, and is, thus, necessarily avoided during device fabrication. To solve this issue, it is highly desirable to design stable perovskite-based composites and device configurations. Here, fully ambient-air and antisolvent-free-processed, stable and all-inorganic metal-oxide selective contact hole-conductor-free perovskite solar cells (HCF-PSCs) based on perovskite-based composites with an interfacial engineering strategy are reported. The formation of perovskite-based composites by interfacial engineering with carbon–graphite–CuδNi1−δO not only improved interfacial contacts, charge extraction and transport but also passivated trap states of perovskite thin films and charge recombination at the interfaces. Thus, such perovskite composites with interfacial engineering-based HCF-PSCs without encapsulation showed excellent stability by sustaining 94% of initial PCE over 300 days under ambient conditions.
Introduction
Organic–inorganic halide perovskite (OIHP)-based solar cells have recently achieved an astonishing record-high power conversion efficiency (PCE) up to 25.5% just in a decade, which has endowed them with superb potential for next-generation low-cost, easy-to-fabricate, flexible, wearable and semi-transparent photovoltaics (PVs) in the near future.1–4 However, the poor stability of OIHPs and organic hole transport materials (OHTMs) owing to long time exposure to harsh environmental conditions (i.e., moisture, air, light and heat stress) has threatened to hinder their widespread applications in optoelectronics and energy harvesting. To get closer to commercialization, the long-term operational stability and ambient-air scalable fabrication of perovskite solar cells (PSCs) are of utmost importance.5–8 Thus, more stable, printable and highly recyclable carbon-based mesoscopic hole-conductor-free PSCs (HCF-PSCs) may become one of the most promising perovskite-based PVs towards rapid commercialization. However, the efficiency of HCF-PSCs still largely lags behind as compared to that of small-molecule OHTM-based PSCs. The unsatisfactory efficiency of HCF-PSCs is mainly ascribed to low hole transport efficiency, high charge recombination, undesired energy band diagram match and imperfect contacts at the interface of the perovskite/carbon electrode as well as poor carbon adhesion and insufficient incident light by carbon electrodes.9,10 In order to further improve the efficiency of HCF-PSCs, many efforts have been made for developing advanced and stable perovskite composites with organic or inorganic materials11–21 and designing novel device configurations with the interfacial engineering strategy.10,22–26The perovskite-based composition engineering with a mixed-cation/halide have significantly enhanced optical and electronic properties, electron–hole extraction and transport as well as intrinsic hybrid-perovskite structure stability.12–15,27–29 Han et al. pioneered HCF-PSCs based on mixed-cation or mixed-halide perovskites ((5-AVA)xMA1−xPbI3−y(BF4)y, 5-AVA: 5-aminovaleric acid) by controlling the ratio of two cations or halides, thus endowing the corresponding cells with an improved PCE over 12% and operational stability.12,13 Interestingly, Grancini et al. showed that ultra-stable 2-dimensional/3-dimensional (2D/3D) perovskite-based composites (i.e., (HOOC(CH2)4NH3)2PbI4/MAPbI3) could be formed via 5-AVAI composition engineering. The 2D/3D perovskite composite-based HCF-PSCs and modules have shown excellent stability over one year.15 For the development of perovskite composites with organic or inorganic materials, Wang et al.16 reported efficient and stable HCF-PSC-based perovskite-composites with a configuration of FTO/c-TiO2/mp-TiO2/MAPbI3–NiO composite/Au. The MAPbI3–NiO composites can effectively facilitate charge carrier generation by improving photo-absorption and faster extraction of holes from perovskite to the Au electrode by NiO NPs. Hou et al.18 fabricated perovskite composite-based (i.e., MAPbI3–GuCl and guanidinium chloride) HCF-PSC with a higher open-circuit voltage (Voc) of 1.02 V when compared to pristine MAPbI3-based devices with only 0.88 V. By combining experimental and theoretical studies, the perovskite composites with organic or inorganic materials can significantly enhance the intrinsic stability of perovskite materials via strong chemical interactions and bonding or cross-linking as well as using the protective effect of perovskite grains by additive molecules. More importantly, the perovskite composites prepared with cost-effective and environmentally stable inorganic semiconductors, such as NiO nanostructures16,30–34 and carbon-based nanomaterials,35–39 have the potential for future commercialization.
The interfacial contacts and properties at the interfaces of the perovskite/electron-transport-layer (perovskite/ETL) and perovskite/electrode are critical for the photovoltaic performance and stability in HCF-PSCs. In particular, except for the degradation of OIHPs themselves, poor stability of the state-of-the-art PSCs can be attributed to interfacial collapse induced by the TiO2 photocatalysis effect and charge accumulation at the TiO2/perovskite interface,40,41 light-induced ion migration and segregation behavior,42–44 and formation of AuI2− or AgI2− because of the diffusion of Au or Ag into the perovskite.45,46 For improving the interface contact between the perovskite layer and top electrode, interfacial engineering has been proposed.22–26,47–49 Overall, the development of stable perovskite-based composites combined with proper materials and designing novel configuration with interfacial engineering are efficient strategies to achieve highly efficient and stable HCF-PSCs under ambient conditions.
In this study, we report fully-ambient-air and antisolvent-free-processed, highly efficient and stable HCF-PSCs with interfacial engineering-based perovskite composites. The perovskite composites (i.e., CuδNi1−δO–MAPbI3−xClx, carbon–graphite (C–G)–CuδNi1−δO–MAPbI3−xClx and Al2O3–MAPbI3−xClx) were self-formed via a one-step deposition of the as-prepared MAPbI3−xClx–CuδNi1−δO mixed-solution on the stacked configuration of FTO/SnO2/Al2O3/CuδNi1−δO/C–G–CuδNi1−δO, where SnO2 is the ETL and Al2O3/CuδNi1−δO/C–G–CuδNi1−δO is used for interfacial engineering between ETL and the active layer (AL). It was found that CuδNi1−δO and C–G in the perovskite composites effectively facilitated charge extraction and transport as well as passivated the trap states of perovskite thin films, recombination and ion migration at the interfaces. Furthermore, the multi-stacked SnO2/Al2O3–MAPbI3−xClx/CuδNi1−δO–MAPbI3−xClx/C–G–CuδNi1−δO–MAPbI3−xClx composite layer effectively reduced the photocurrent density loss and improved interfacial contacts. Finally, the HCF-PSCs based on perovskite composites with interfacial engineering yielded the best PCE of 14.78% with a Voc of 0.95 V, Jsc of 23.85 mA cm−2 and an FF of 65.23%. More importantly, such perovskite composites with interfacial engineering-based devices showed excellent air, photo- and thermal stability, which are attributed to moisture- and ion migration-sequestration by C–G and strong chemical interactions between CuδNi1−δO and perovskite molecules.
Results and discussion
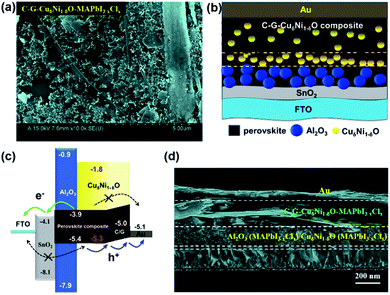
The surface morphologies of mesoporous Cu-doped NiO (CuδNi1−δO) and Al2O3, and the cross-sectional structure of FTO/SnO2/Al2O3/CuδNi1−δO were examined via field emission scanning electron microscopy (FESEM), clearly showing uniform and mesoporous structures (Fig. S1†). Such thin-film morphologies of metal oxide nanoparticles (MONs) effectively facilitate the perovskite MAPbI3−xClx solution infiltration and increase the contact areas between perovskite molecules and mesoporous MONs (i.e., CuδNi1−δO and Al2O3), leading to the formation of perovskite–MONs composites (i.e., CuδNi1−δO–MAPbI3−xClx and Al2O3–MAPbI3−xClx). Besides, the surface morphologies of the as-formed C–G–CuδNi1−δO–MAPbI3−xClx composite film are also clearly observed in the FESEM image, as shown in Fig. 1a. Fig. 1d further verifies that the perovskite (MAPbI3−xClx) solution readily infiltrated into all the mesoporous layers of C–G–CuδNi1−δO, CuδNi1−δO, and Al2O3. It can be seen that all the layers are well-defined with good adhesion to one another, exhibiting the top electrode of Au, the AL of C–G–CuδNi1−δO–MAPbI3−xClx, the interfacial layer of Al2O3/CuδNi1−δO/C–G–CuδNi1−δO infiltrated with perovskite molecules and the ETL layer of SnO2. Fig. 1b shows the device configuration of FTO/SnO2/Al2O3–MAPbI3−xClx/CuδNi1−δO–MAPbI3−xClx/C–G–CuδNi1−δO–MAPbI3−xClx/Au, where the SnO2 is the ETL and Al2O3/CuδNi1−δO/C–G–CuδNi1−δO is for interfacial engineering between ETL and AL. Such multi-layers of mesoporous configuration remarkably enhance the visible light-harvesting as well as improve Jsc (see Table 1 and Fig. 3a). Fig. 1c shows the energy band diagram and blocking properties in HCF-PSCs: (1) CuδNi1−δO and C–G in the AL have a suitable band energy alignment of the valence band (VB) (−5.3 eV) and (−5.0 eV), which is quite close to that of perovskite MAPbI3−xClx (−5.4 eV), leading to fast hole transport; (2) the formation of the C–G–CuδNi1−δO–MAPbI3−xClx-graded heterojunction also enhances hole transport but efficiently blocks the electron going to the Au electrode due to electron blocking property of CuδNi1−δO with a high conduction band (CB) (−1.8 eV); (3) the wide-bandgap Al2O3 (∼7 eV) plays an important role in hole–electron extraction and recombination suppression by avoiding direct contact between n-type SnO2 and p-type CuδNi1−δO; (4) highly conductive C–G effectively improves the conductivity and work function of C–G–CuδNi1−δO composites; (5) defect density of perovskite films are suppressed by the C–G–CuδNi1−δO interface modification and strong chemical interactions or crosslinking between CuδNi1−δO and perovskite molecules.16,30–33,50,51 Upon illumination, the HCF device configuration probably four conduction paths are available for holes: direct transport to the Au electrode, transport to CuδNi1−δO to the Au electrode, transport to C–G to the Au electrode and transport to CuδNi1−δO to C–G and finally to the Au electrode. It is believed that SnO2, Al2O3, CuδNi1−δO, and C–G can play individual roles in the effective electron and hole transport. Therefore, such a device configuration with perovskite composites and multi-layer interface engineering not only eliminates undesired interface energy disorder but also results in efficient charge separation and conduction in the AL and at the interfaces, leading to a remarkable improvement of device performance and stability.| Device configuration | V oc (V) | J sc (mA cm−2) | FF (%) | PCE (%) | R s (Ω) | R 1 (Ω) | R 2 (Ω) |
|---|---|---|---|---|---|---|---|
| FTO/c-TiO2/mp-TiO2/Al2O3/CuδNi1−δO/C–G–MAPbI3−xClx/Au | 0.88 | 20.97 | 55.30 | 10.21 | 14.5 | 346 | 748 |
| FTO/SnO2/Al2O3/CuδNi1−δO/C–G–MAPbI3−xClx/Au | 0.90 | 23.01 | 57.12 | 11.83 | 12.2 | 338 | 956 |
| FTO/SnO2/Al2O3/CuδNi1−δO/C–G–CuδNi1−δO–MAPbI3−xClx/Au | 0.95 | 23.85 | 65.23 | 14.78 | 10.8 | 245 | 1078 |
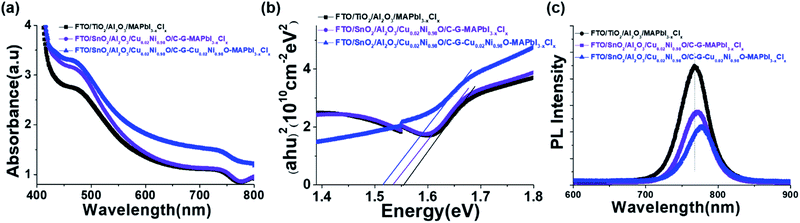
To compare the optical and electronic properties of C–G–CuδNi1−δO–MAPbI3−xClx composites, C–G–MAPbI3−xClx composites and pristine perovskite MAPbI3−xClx films, we recorded ultraviolet-visible (UV-vis) spectra and photoluminescence (PL) spectra, as shown in Fig. 2. All films exhibit remarkable light-harvesting absorption from 400 to 800 nm (Fig. 2a). Compared to the absorption of pristine perovskite MAPbI3−xClx and C–G–MAPbI3−xClx films, the C–G–CuδNi1−δO–MAPbI3−xClx composite film shows enhanced light absorption over the entire visible spectrum, resulting in the highest light-harvesting ability. More importantly, such a composite-based film shows an efficient PL quenching and red-shift toward longer wavelengths (Fig. 2c), which is attributed to a reduced band gap from 1.561 to 1.533 eV (Fig. 2b). The fast PL quenching, red-shift and reduced band gap indicate efficient carrier extraction and transport, and suppression of interfacial recombination and trap density, which can remarkably improve solar cell PV parameters (see Table 1 and Fig. 3a).
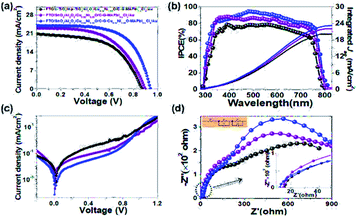
To evaluate the device performance of C–G-based HCF-PSCs based on perovskite composites with interfacial engineering, we fabricated three C–G-based devices: FTO/c-TiO2/mp-TiO2/Al2O3/CuδNi1−δO/C–G–MAPbI3−xClx/Au (device A, Fig. S2†), FTO/SnO2/Al2O3/CuδNi1−δO/C–G–MAPbI3−xClx/Au (device B, Fig. S3†), and FTO/SnO2/Al2O3/CuδNi1−δO/C–G–CuδNi1−δO–MAPbI3−xClx/Au (device C, Fig. 1d). Devices B and C incorporated SnO2 to replace TiO2 ETL and device C used the C–G–CuδNi1−δO–MAPbI3−xClx composite in AL. It was confirmed that the CuδNi1−δO optoelectronic properties greatly depend on Cu doping concentrations.17 Herein, we scrutinized the PV performance of the device A for different concentrations of Cu doping and obtained the best PCE with an optimal content of δ = 0.02 (Fig. S2c and Table S1†). Besides, the thickness of SnO2 and the concentration of C–G–CuδNi1−δO composites in HCF-PSCs were also optimized, as shown in Fig. S3c and Table S3.† In conclusion, the incorporation of the C–G–CuδNi1−δO–MAPbI3−xClx composite in AL together with SnO2, ETL and Al2O3/CuδNi1−δO/C–G–CuδNi1−δO interfacial engineering layers showed the overall best performance for the HCF devices (i.e., a PCE of 14.78% with a Voc of 0.95 V, Jsc of 23.85 mA cm−2, and FF of 65%, Table 1). Such enhancement of Voc and Jsc is ascribed to the reduced defect-related losses and trap density in materials, desirable energy-level alignment with almost no electron-transport-barrier SnO2 as ETL, efficient blocking properties of Al2O3 and CuδNi1−δO, and formation of a graded heterojunction by perovskite composites. It can be noted that the incident-photon-to-current-efficiency (IPCE) spectrum of the HCF-champion cell (device C) shows the strongest light absorption over a broad wavelength from 350 to 780 nm than that of device A and device B (Fig. 3b). Moreover, the integrated Jsc of the HCF-champion cell calculated from the IPCE spectrum was 23.21 mA cm−2, which is in good agreement with the measured J–V curve (23.85 mA cm−2). Fig. 3c clearly shows that the dark current density of the C–G–CuδNi1−δO–MAPbI3−xClx composite-based device is almost one order of magnitude lower than that of the pristine MAPbI3−xClx perovskite-based device. Note that device C shows the lowest series-resistant Rs and charge transfer resistance R1 of 10.8 and 245 Ω, and highest recombination resistance R2 of 1078 Ω, which were obtained via electronic impedance spectroscopy (EIS) measurements (Fig. 3d and Table 1). Such results indicate that the C–G–CuδNi1−δO–MAPbI3−xClx composite films can not only improve interfacial contacts, charge extraction and transport but also lower the current leakage and charge recombination as well as increase the shunt resistance and longer carrier lifetime.
It was found that compared to TiO2 ETL devices (device A, black curve), the SnO2 ETL devices (devices B (red) and C (blue) in Fig. 3a) showed the remarkable enhancement of PCE and photo-stability (Fig. S3d and S5†). Furthermore, we examined how light soaking time affects the PV performance of HCF devices and found that the TiO2-based device A increased its PV parameters for 90 s and then were gradually decreasing (Fig. S2d and Table S2†). However, the SnO2-based devices B and C resulted in the substantial improvement of photovoltaic parameters as a function of light soaking time and was stabilized after 200 s (Fig. S3d, S4b, S5, Tables S4 and S6†). Thermal stability is another challenge towards the rapid commercialization of PSCs. Compared to the pristine MAPbI3−xClx with spiro-OMeTAD device (control device), C–G–CuδNi1−δO–MAPbI3−xClx with interfacial engineering-based HCF-PSCs shows excellent thermal-stability at 85 °C under ambient conditions (45–50% humidity) (Fig. S6†). In particular, all PV parameters of the pristine MAPbI3−xClx with HTM spiro-OMeTAD-based cell showed severely deteriorated thermal stability after 120 h, i.e., original PCEs decreased from 18.02% to 7.71% (only retaining 42.8%, Table S7 and Fig. S7a†). However, C–G–CuδNi1−δO–MAPbI3−xClx based on interfacial engineering HCF-PSCs sustained its stability over 120 h by retaining ∼97.8% of its original Voc, ∼92.6% of Jsc, ∼99.1% of FF and ∼89.8% of PCE at 85 °C in an ambient environment (45–50% humidity) (Fig. S7b and Table S8†). Furthermore, we examined UV-vis spectrum performance as a function of time for FTO/SnO2/MAPbI3−xClx/spiro-OMeTAD and FTO/SnO2/Al2O3/CuδNi1−δO/C–G–CuδNi1−δO–MAPbI3−xClx films at 85 °C heating in ambient air (humidity 45–50%). The UV-vis spectrum performance clearly shows that the pristine MAPbI3−xClx with the spiro-OMeTAD film totally changed to PbI2 after 20 days. Adversely, the C–G–CuδNi1−δO–MAPbI3−xClx-based film had almost no change (Fig. S8†). To examine the long-term stability of the HCF devices based on C–G–CuδNi1−δO–MAPbI3−xClx with Al2O3/CuδNi1−δO/C–G–CuδNi1−δO interfacial engineering, the target device fabricated by the antisolvent-free strategy was stored under ambient conditions without any encapsulation. Fig. 4 shows the control (pristine MAPbI3−xClx with spiro-OMeTAD-based cell) and the target device (C–G–CuδNi1−δO–MAPbI3−xClx-based HCF-cell) of long-term stability as a function of storage days in terms of the normalized performance parameters, i.e. Voc (a), Jsc (b), FF (c) and PCE (d). Compared to the control device, it can be clearly seen that the normalized performance parameters in the target device are almost not changed in 300 d. More importantly, the target device sustained its stability over 300 days by retaining ∼94% of PCE in an ambient environment (45–50% humidity) (Fig. S9 and Table S9†).
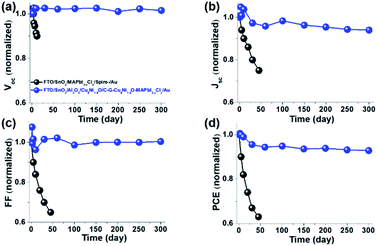 | ||
| Fig. 4 Long-term stability of FTO/SnO2/MAPbI3−xClx/spiro-OMeTAD/Au (black) and FTO/SnO2/Al2O3/CuδNi1−δO/C–G–CuδNi1−δO–MAPbI3−xClx/Au under ambient conditions (45–50% humidity and 25–30 °C). | ||
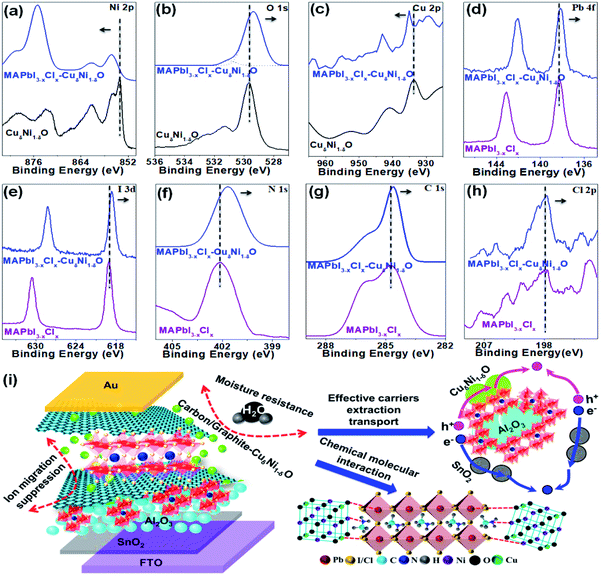
By examining XPS data (Fig. 5) on both pristine MAPbI3−xClx and composite-based CuδNi1−δO–MAPbI3−xClx films, the core levels of all components in the CuδNi1−δO–MAPbI3−xClx film (i.e., O 1s, Pb 4f, I 3d, N 1s, C 1s and Cl 2p) showed a substantial shift towards a lower binding energy compared to the pristine MAPbI3−xClx film. Interestingly, the core level of Ni 2p and Cu 2p showed a slight shift towards a higher binding energy, where the nitrogen and iodide or chloride ligands from the MA cation might serve as a ligand bridge to form Ni/Cu–N and Ni/Cu–I or Ni/Cu–Cl bonds, respectively. From the XPS data, it can be concluded that there is a presence of strong chemical interaction and chemical bonding between CuδNi1−δO and perovskite, as shown in Fig. 5. In conclusion, such improvement is mainly attributed to the effect of CuδNi1−δO and C–G in the perovskite-based composites, which can effectively facilitate charge extraction and transport and interfacial contacts as well as passivation of trap states and recombination in perovskite thin films and at interfaces (also see Fig. 5). The strong chemical interactions and bonding between perovskite and CuδNi1−δO elements as well as the presence of C–G in composites also enhance the intrinsic stability of the perovskite materials and interfacial degradation.16,30–32,52 Moreover, the lattice constant of graphite (α = 2.46 Å) is less than the ionic diameter of I/Cl (d = 4.28 Å/3.62 Å), which significantly suppresses the halide migration towards the top electrode. In addition, the multi-stacked configuration of SnO2/Al2O3/CuδNi1−δO/C–G–CuδNi1−δO–MAPbI3−xClx can effectively reduce the photocurrent density loss and improve interfacial contacts between perovskite and other layers. Such multi-layer configuration can also effectively improve device air-stability by protecting perovskite molecules from moisture resistance by C–G, and deprotonation and decomposition due to reduction with superoxide (O2−) (Fig. 5). In all, the combined perovskite-based composites and interfacial engineering can lead to substantial performance improvements and operational stability in HCF-PSCs.
Conclusions
In summary, we have achieved fully-ambient-air and antisolvent-free-processed stable hole-conductor-free perovskite solar cells (HCF-PSCs) based on perovskite composites with carbon–graphite (C–G)–CuδNi1−δO interfacial engineering. With the substantial improvement in the perovskite properties, energy-level alignment at the p-type interface between perovskite and C–G–CuδNi1−δO and their interfacial contacts, the perovskite composites with interfacial engineering-based HCF-PSCs yielded a PCE of 14.78% and excellent operational stability. We believe that metal oxides and carbon-based perovskite composites and interfacial engineering can pave the way for designing efficient, large-scale and stable PSCs.Experimental section
Material preparation
The synthesis of CH3NH3I (methylammonium iodide, MAI) was described in our previous works.16 The preparation of the SnO2 precursor, Al2O3 and CuδNi1−δO NP solutions can be found in our previous works.30–33 To prepare a solution of MAPbI3−xClx–CuδNi1−δO composite, 100 μL 1.5 wt% CuδNi1−δO (2 at% Cu doping) dispersion was added to the perovskite MAPbI3−xClx solution contained by mixing 475 mg MAI and 222 mg lead(II) chloride (PbCl2, 99.999%, Sigma-Aldrich) and 76 mg lead(II) acetate trihydrate (Pb(CH3CO2)2·3H2O, 99.999%, Sigma-Aldrich) in 1 mL dimethylformamide, followed by ultrasonication for 30 min and continuously stirring for 24 h under ambient conditions (45–50% humidity and 25–30 °C).Device fabrication
All the fabrication processes of PSCs were carried out via antisolvent-free and ambient-air processes (45–50% humidity and 25–30 °C). The SnO2 precursor, Al2O3 and CuδNi1−δO NP solutions were spin-coated sequentially on a cleaned FTO substrate at 4000 rpm for 40 s; next, the C–G–CuδNi1−δO composite solution was drop-casted at 6000 rpm on FTO/SnO2/Al2O3/CuδNi1−δO substrates; after that, each step was followed by annealing at 250 °C for 10 min. The pre-heated MAPbI3−xClx–CuδNi1−δO mixed solution at 130 °C was then spin-coated on the FTO/SnO2/Al2O3/CuδNi1−δO/(C–G–CuδNi1−δO composite) at 1500 rpm for 30 s, followed by annealing at 130 °C for 1.5 h. Finally, Au electrodes were deposited through a shadow mask in a thermal evaporator (QHV-R53) under a high vacuum.Characterization
The absorption properties and elemental compositions and chemical and electron states of perovskite composites were analyzed with ultraviolet-visible (UV-vis, DektakXT) spectroscopy and X-ray photoelectron spectroscopy (XPS). The perovskite device configuration was examined via field emission scanning electron microscopy (FE-SEM, FEI Apreo LoVac). Perovskite composite bonds were analyzed via Fourier transform infrared (IR) transmittance measurements. The performance parameters of PSCs were measured with a solar simulator with a source meter (Keithley series 2400) at 100 mW cm−2 under AM 1.5 illumination, which was calibrated by a silicon reference cell. The dimension of the tested solar cells was 3 cm × 3 cm. The device performances were examined with a 0.16 cm2 mask/aperture. To understand the electrical characteristics of the devices, electronic impedance spectroscopy (EIS) was performed at 250 mV applied-bias under one sunlight intensity in the range from 1 Hz to 300 kHz.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Fundamental Research Funds for the Central Universities (No. 21620347) and startup fund for young talented person program (No. 88016105) from Jinan University.References
- NREL, https://www.nrel.gov/pv/assets/pdfs/best-research-cell-efficiencies.20200925.pdf, accessed on September 25, 2020.
- B. Cai, Y. Xing, Z. Yang, W.-H. Zhang and J. Qiu, Energy Environ. Sci., 2013, 6, 1480–1485 RSC.
- E. H. Jung, N. J. Jeon, E. Y. Park, C. S. Moon, T. J. Shin, T.-Y. Yang, J. H. Noh and J. Seo, Nature, 2019, 567, 511–515 CrossRef CAS.
- X. Meng, Z. Cai, Y. Zhang, X. Hu, Z. Xing, Z. Huang, Z. Huang, Y. Cui, T. Hu, M. Su, X. Liao, L. Zhang, F. Wang, Y. Song and Y. Chen, Nat. Commun., 2020, 11, 3016 CrossRef CAS.
- S. S. Mai, H. Kim, S. E. Shim and C. K. Hong, Nanoscale, 2016, 8, 19189–19194 RSC.
- P. Wang, Y. Wu, B. Cai, Q. Ma, X. Zheng and W.-H. Zhang, Adv. Funct. Mater., 2019, 29, 1807661 CrossRef CAS.
- J. Hu, C. Wang, S. Qiu, Y. Zhao, E. Gu, L. Zeng, Y. Yang, C. Li, X. Liu, K. Forberich, C. J. Brabec, M. K. Nazeeruddin, Y. Mai and F. Guo, Adv. Energy Mater., 2020, 10, 2000173 CrossRef CAS.
- M. He, B. Li, X. Cui, B. Jiang, Y. He, Y. Chen, D. O'Neil, P. Szymanski, M. A. EI-Sayed, J. Huang and Z. Lin, Nat. Commun., 2017, 8, 16045 CrossRef CAS.
- H. Chen and S. Yang, Adv. Mater., 2017, 29, 1603994 CrossRef.
- K. Lee, J. Kim, H. Yu, J. W. Lee, C.-M. Yoon, S. K. Kim and J. Jang, J. Mater. Chem. A, 2018, 6, 24560–24568 RSC.
- S. He, L. Qiu, D.-Y. Son, Z. Liu, E. J. Juarez-Perez, L. K. Ono, C. Stecker and Y. Qi, ACS Energy Lett., 2019, 4, 2032–2039 CrossRef CAS.
- Y. Sheng, A. Mei, S. Liu, M. Duan, P. Jiang, C. Tian, Y. Xiong, Y. Rong, H. Han and Y. Hu, J. Mater. Chem. A, 2018, 6, 2360–2364 RSC.
- A. Mei, X. Li, L. Liu, Z. Ku, T. Liu, Y. Rong, M. Xu, M. Hu, J. Chen, Y. Yang, M. Grätzel and H. Han, Science, 2014, 345, 295–298 CrossRef CAS.
- P. Liu, Y. Gong, Y. Xiao, M. Su, S. Kong, F. Qi, H. Zhang, S. Wang, X. Sun, C. Wang and X.-Z. Zhao, Chem. Commun., 2019, 5, 218–221 RSC.
- G. Grancini, C. Roldàn-Carmona, I. Zimmermann, E. Mosconi, X. Lee, D. Martineau, S. Narbey, F. Oswald, F. De Angelis, M. Gräetzel and M. K. Nazeeruddin, Nat. Commun., 2017, 8, 15684 CrossRef CAS.
- Y. Wang, W.-Y. Rho, H.-Y. Yang, T. Mahmoudi, S. Seo, D.-H. Lee and Y.-B. Hahn, Nano Energy, 2016, 27, 535–544 CrossRef CAS.
- Y. Sheng, Y. Hu, A. Mei, P. Jiang, X. Hou, M. Duan, L. Hong, Y. Guan, Y. Rong, Y. Xiong and H. Han, J. Mater. Chem. A, 2016, 4, 16731–16736 RSC.
- X. Hou, Y. Hu, H. Liu, A. Mei, X. Li, M. Duan, G. Zhang, Y. Rong and H. Han, J. Mater. Chem. A, 2017, 5, 73–78 RSC.
- I. Zimmermann, P. Gratia, D. Martineau, G. Grancini, J.-N. Audinot, T. Wirtz and M. K. Nazeeruddin, J. Mater. Chem. A, 2019, 7, 8073–8077 RSC.
- H. Zhang, H. Wang, S. T. Williams, D. Xiong, W. Zhang, C.-C. Chueh, W. Chen and A. K.-Y. Jen, Adv. Mater., 2017, 29, 1606608 CrossRef.
- W.-Q. Wu, Q. Wang, Y. Fang, Y. Shao, S. Tang, Y. Deng, H. Lu, Y. Liu, T. Li, Z. Yang, A. Gruverman and J. Huang, Nat. Commun., 2018, 9, 1625 CrossRef.
- Y. Xiong, X. Zhu, A. Mei, F. Qin, S. Liu, S. Zhang, Y. Jiang, Y. Zhou and H. Han, Sol. RRL, 2018, 2, 1800002 CrossRef.
- J. Liu, Q. Zhou, N. K. K. Thein, L. Tian, D. Jia, E. M. J. Johansson and X. Zhang, J. Mater. Chem. A, 2019, 7, 13777–13786 RSC.
- E. Avigad and L. Etgar, ACS Energy Lett., 2018, 3, 2240–2245 CrossRef CAS.
- B. Li, Y. Zhang, L. Zhang and L. Yin, Adv. Mater., 2017, 29, 1701221 CrossRef.
- X. Zheng, H. Chen, Q. Li, Y. Yang, Z. Wei, Y. Bai, Y. Qiu, D. Zhou, K. S. Wong and S. Yang, Nano Lett., 2017, 17, 2496–2505 CrossRef CAS.
- M. Saliba, T. Matsui, K. Domanski, J.-Y. Seo, A. Ummadisingu, S. M. Zakeeruddin, J.-P. Correa-Baena, W. R. Tress, A. Abate, A. Hagfeldt and M. Grätzel, Science, 2016, 354, 206–209 CrossRef CAS.
- Q. Tai, P. You, H. Sang, Z. Liu, C. Hu, H. L. W. Chan and F. Yan, Nat. Commun., 2016, 7, 11105 CrossRef CAS.
- A. D. Jodlowski, C. Roldan-Carmona, G. Grancini, M. Salado, M. Ralaiarisoa, S. Ahmad, N. Koch, L. Camacho, G. de Miguel and M. K. Nazeeruddin, Nat. Energy, 2017, 2, 972–979 CrossRef CAS.
- Y. Wang, T. Mahmoudi, W.-Y. Rho, H.-Y. Yang, S. Seo, K. S. Bhat, R. Ahmad and Y.-B. Hahn, Nano Energy, 2017, 40, 408–417 CrossRef CAS.
- Y. Wang, T. Mahmoudi, H.-Y. Yang, K. S. Bhat, J.-Y. Yoo and Y.-B. Hahn, Nano Energy, 2018, 49, 59–66 CrossRef CAS.
- Y. Wang, T. Mahmoudi, W.-Y. Rho and Y.-B. Hahn, Nano Energy, 2019, 64, 103964 CrossRef CAS.
- Y. Wang, T. Mahmoudi and Y.-B. Hahn, Adv. Energy Mater., 2020, 10, 2000967 CrossRef CAS.
- S. S. Mali, H. Kim, H. H. Kim, S. E. Shim and C. K. Hong, Mater. Today, 2018, 21, 483–500 CrossRef CAS.
- H.-L. Hsu, H.-T. Hsiao, T.-Y. Juang, B.-H. Jiang, S.-C. Chen, R.-J. Jeng and C.-P. Chen, Adv. Energy Mater., 2018, 8, 1802323 CrossRef.
- T. Mahmoudi, Y. Wang and Y.-B. Hahn, ACS Energy Lett., 2019, 4, 235–241 CrossRef CAS.
- T. Mahmoudi, Y. Wang and Y.-B. Hahn, Nano Energy, 2018, 47, 51–65 CrossRef CAS.
- T. Mahmoudi, S. Seo, H.-Y. Yang, W.-Y. Rho, Y. Wang and Y.-B. Hahn, Nano Energy, 2016, 28, 179–187 CrossRef CAS.
- Y. Liang, Y. Wang, C. Mu, S. Wang, X. Wang, D. Xu and L. Sun, Adv. Energy Mater., 2018, 8, 1701159 CrossRef.
- W. Li, W. Zhang, S. V. Reenen, R. J. Sutton, J. Fan, A. A. Haghighirad, M. B. Johnston, L. Wang and H. J. Snaith, Energy Environ. Sci., 2016, 9, 490–498 RSC.
- T. Leijtens, G. E. Eperon, S. Pathak, A. Abate, M. M. Lee and H. J. Snaith, Nat. Commun., 2013, 4, 2885 CrossRef.
- P. Calado, A. M. Telford, D. Bryant, X. Li, J. Nelson, B. C. O'Regan and P. R. F. Barnes, Nat. Commun., 2016, 7, 13831 CrossRef CAS.
- Z. Li, C. Xiao, Y. Yang, S. P. Harvey, D. H. Kim, J. A. Christians, M. Yang, P. Schulz, S. U. Nanayakkara, C.-S. Jiang, J. M. Luther, J. J. Berry, M. C. Beard, M. M. Al-Jassim and K. Zhu, Energy Environ. Sci., 2017, 10, 1234–1242 RSC.
- S. G. Motti, D. Meggiolaro, A. J. Barker, E. Mosconi, C. A. R. Perini, J. M. Ball, M. Gandini, M. Kim, F. D. Angelis and A. Petrozza, Nat. Photonics, 2019, 13, 532–539 CrossRef CAS.
- L. Liang, Y. Cai, X. Li, M. K. Nazeeruddin and P. Gao, Nano Energy, 2018, 52, 211–238 CrossRef CAS.
- H. Back, G. Kim, J. Kim, J. Kong, T. K. Kim, H. Kang, H. Kim, J. Lee, S. Lee and K. Lee, Energy Environ. Sci., 2016, 9, 1258–1263 RSC.
- M. Duan, C. Tian, Y. Hu, A. Mei, Y. Rong, Y. Xiong, M. Xu, Y. Sheng, P. Jiang, X. Hou, X. Zhu, F. Qin and H. Han, ACS Appl. Mater. Interfaces, 2017, 9, 31721–31727 CrossRef CAS.
- Z. Wu, Z. Liu, Z. Hu, Z. Hawash, L. Qiu, Y. Jiang, L. K. Ono and Y. Qi, Adv. Mater., 2019, 31, 1804284 CrossRef.
- Q.-Q. Chu, Z. Sun, B. Ding, K.-S. Moon, G.-J. Yang and C.-P. Wong, Nano Energy, 2020, 77, 105110 CrossRef CAS.
- G. D. Tainter, T. Horantner, L. M. Pazos-Outon, R. D. Lamboll, H. Abolins, T. Leijtens, S. Mahesh, R. H. Friend, H. J. Snaith, H. J. Joyce and F. Deschler, Joule, 2019, 3, 1301–1313 CrossRef CAS.
- P. Jiang, Y. Xiong, M. Xu, A. Mei, Y. Sheng, L. Hong, T. W. Jones, G. J. Wilson, S. Xiong, D. Li, Y. Hu, Y. Rong and H. Han, J. Phys. Chem. C, 2018, 122, 16481–16487 CrossRef CAS.
- T. Mahmoudi, Y. Wang and Y.-B. Hahn, Adv. Energy Mater., 2020, 10, 1903369 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: FESEM images of Al2O3 and CuδNi1−δO NPs, device performance parameters in tables, J–V plots, EIS spectra, J–V plots of stability test and UV-vis absorption. See DOI: 10.1039/d0na00852d |
| This journal is © The Royal Society of Chemistry 2020 |