TiO2 exposure alters transition metal ion quota in Rhodococcus ruber GIN-1†
Annastassia D.
Gallo
 a,
Mark R.
Zierden
a,
Mark R.
Zierden
 a,
Lauren A.
Profitt
a,
Kayleigh E.
Jones
a,
Christopher P.
Bonafide
a,
Lauren A.
Profitt
a,
Kayleigh E.
Jones
a,
Christopher P.
Bonafide
 bc and
Ann M.
Valentine
bc and
Ann M.
Valentine
 *a
*a
aDepartment of Chemistry, Temple University, Philadelphia, PA 19122, USA. E-mail: ann.valentine@temple.edu
bChildren's Hospital of Philadelphia, Section of Hospital Medicine, Philadelphia, PA 19104, USA
cUniversity of Pennsylvania, Perelman School of Medicine, Philadelphia, PA 19104, USA
First published on 26th December 2019
Abstract
After exposure to micron-sized TiO2 particles, anatase and/or rutile, Rhodococcus ruber GIN-1 accumulates an increased concentration (2.2 ± 0.2 mg kg−1) of mobilized Ti into its biomass with concomitant decreases in cellular biometals Fe, Zn, and possibly Mn, while levels of Cu and Al are unaffected.
Significance to metallomicsTitanium dioxide is common in the biosphere but not very soluble. It is considered very unreactive, especially when the particles are larger than nanoparticles. Certain bacteria, however, bind very tightly to this material and rather quickly liberate and incorporate titanium into their biomass. This interaction between geology and biology, in turn, impacts the cellular levels of some other important biometals, while others are not affected. Thus, the individual metal quotas within this species’ metallome can be affected by an environmentally abundant metal oxide of a metal that is not known to be essential, and not widely appreciated to be bioactive. |
Titanium is the ninth most abundant element in the Earth's crust and primarily occurs as Ti(IV) in sparingly soluble mineral oxides.1 Titanium dioxide (TiO2) makes up 0.9% and 1.4% of the continental and oceanic crusts, respectively.2 The primary crystal forms of TiO2 are anatase and rutile. Titanium also occurs in other common mineral oxides including ilmenite (FeTiO3) and titanite (CaSiTiO5).3
Titanium is not known to be essential to any organism, and its bioactivity has not been widely appreciated.4 A consideration of any role of Ti in biology is hindered by the element's reputation for inertness and extreme insolubility in aqueous environments. Yet Ti(IV) is more soluble and bioactive than has generally been recognized.5 Moreover, evolution has overcome insolubility for the similarly hydrolysis-prone metal ion Fe(III), making it bioavailable and necessary for life in nearly all species. If a small fraction of abundant environmental Ti were mobilized in a similar manner as Fe, a significant amount of the metal could be bioavailable. For example, siderophores avidly bind insoluble Fe(III) and are strong chelators of Ti(IV) in solution.6
Individual biomolecules and whole organisms do interact with solid TiO2.5 Siderophores bind to TiO2 surfaces among other metal oxides.6–11 The Gram-positive bacteria Rhodococcus ruber GIN-1 were isolated from an environmental sample by exploiting their binding of metal oxides in coal fly ash.12 Orange-colored R. ruber GIN-1 cells preferentially adsorb to TiO2 over other metal oxides.12,13 Adsorption is strong, resistant to extremes of pH and temperature, and very fast. In an early report, the authors noted qualitatively, with data not shown, that the bacteria could incorporate Ti(IV) ions into biomass after exposure to TiO2.14 This finding suggests a mobilization of titanium from apparently-inert environmental TiO2. The current work further investigates and quantitates this Ti incorporation and its dependence (or lack thereof) on TiO2 form. This work also reveals the accompanying effect on the metal ion quotas of other important biometals in the cells.
Rhodococcus ruber GIN-1 cells were grown in artificial sea water media to late log phase, the most TiO2-adhesive stage (Fig. S1, ESI†).13 Cells were split into two populations, one of which was exposed to ∼40 μm anatase and/or rutile Sachtopore beads (Fig. 1) for 1 h. Under these conditions, >90% of the cells adhere within 1 min.13 This interval is much smaller than the bacterial doubling time (10–20 h during log phase12). The second cell population was treated in an identical manner except that it was never exposed to TiO2. The cells were desorbed from the particles,14 the relatively large TiO2 particles were removed by slow centrifugation, and the cells were washed. Scanning electron microscopy (SEM) confirmed that this method removed all TiO2 particles before metal ion quantitation and recovered the cells (Fig. S2, ESI†). The gross cell morphology was unchanged. The cell samples were lyophilized and digested for metal ion quantitation by inductively coupled plasma-optical emission spectroscopy (ICP-OES), which allows detection of multiple elements in the same sample. Concentrations of six metals were determined for each sample: Al, Cu, Fe, Mn, Ti, and Zn.
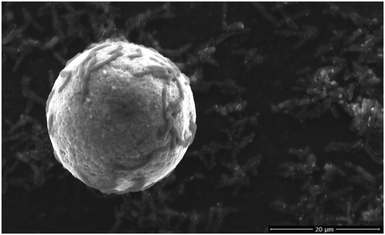 | ||
| Fig. 1 Scanning electron micrograph of R. ruber GIN-1 cells (∼5–10 μm rods) adsorbed to an anatase Sachtopore particle. | ||
Although the concentrations of Ti, both in the original artificial seawater medium and in the spent culture medium after growth, were <0.005 mg L−1, appreciable Ti was detected even in control cells that were not exposed to TiO2 particles (n = 14, [Ti] = 0.29 ± 0.05 mg kg−1) (Fig. 2 and Table S1, ESI†). The dry-matter content in these bacterial cells is ∼40%,15 so this value corresponds to ∼2.4 μM Ti. This value is well within the range commonly found in biological samples.5 The cells may obtain Ti from the dilute Ti in the growth medium and/or from the container surface during the relatively long growth time to reach late log phase. R. ruber GIN-1 cells were exposed to and then desorbed from anatase (n = 5), rutile (n = 5), or a mixed anatase/rutile sample (n = 4; data not shown). Each experiment was run alongside an unexposed control, for 14 total unexposed controls. The TiO2-exposed cells had significantly elevated Ti levels with respect to unexposed cells (Fig. 2 and Table S1, ESI†). This finding is consistent with the earlier, qualitative report.14 Titanium concentrations in exposed cells averaged 2.2 ± 0.2 mg kg−1. This value represents approximately an eightfold increase over the control cells. It remains much lower than the highest value ever reported for Ti in an organism (1500 mg kg−1 in the ascidian Eudistoma ritteri).16 There was no significant difference in Ti incorporation from anatase, rutile, or a mixture of these oxides.
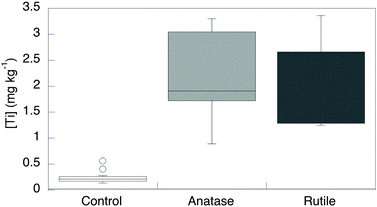 | ||
| Fig. 2 Titanium dry weight concentration in R. ruber GIN-1 with and without exposure to TiO2 particles. | ||
Once cellular uptake of Ti from purportedly-inert titanium oxides was confirmed and quantitated, we considered whether and how that uptake affected the levels of other biometals in the cells. The results were not statistically different between exposure to anatase and rutile, so the data were averaged (n = 14). The artificial seawater medium had 0.22 mg L−1 Zn, 0.045 mg L−1 Fe, 0.009 mg L−1 Mn, and Al and Cu less than 0.005 mg L−1 (Table S1, ESI†). The native levels of these metal ions in R. ruber GIN-1 vary over several orders of magnitude (Table S1, ESI† and Fig. 3, white boxes). Each of the metals was more concentrated in the cell biomass than in the growth medium. After exposure to TiO2, and concomitant with the Ti increases described above, biometal concentrations decreased significantly for Fe, Zn, and Mn (Table S1, ESI† and Fig. 3, grey boxes). Of the biometals analyzed, only Zn had any variation in concentration between TiO2 form (exposure to anatase resulted in a slightly greater decrease in Zn). There was no change in Cu or Al concentrations in cells exposed to TiO2.
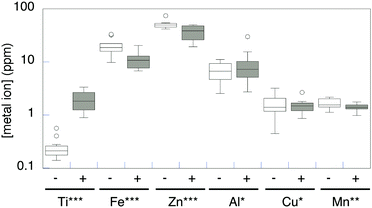 | ||
| Fig. 3 Metal concentrations (dry weight) in R. ruber GIN-1 cells (n = 14). Analysis by a Wilcoxon signed-rank test (see ESI†) supports a significant difference in metal quota between control cells (white) and cells exposed to TiO2 (grey) for Ti (increase, P = 0.007), Fe (decrease, P = 0.007), Zn (decrease, P = 0.01), and Mn (decrease, P = 0.03), but not for Cu or Al (P > 0.99). | ||
As further controls, samples having media without R. ruber GIN-1 cells were subjected to the same washing and transfer steps in the presence or absence of TiO2 particles. The samples not exposed to TiO2 exhibit 0.005 mg L−1 Fe, 0.025 mg L−1 Mn, and <0.005 mg L−1 Al, Cu, Ti, and Zn. The TiO2-exposed controls have 0.005 mg L−1 Fe, 0.011 mg L−1 Mn, 0.006 mg L−1 Al, and <0.005 mg L−1 Cu, Ti, and Zn. Thus, the metal concentrations reported in Fig. 3 were associated with the cellular biomass and did not come from the washing or manipulation steps, or from abiological dissolution of TiO2.
The genus Rhodococcus is known to degrade environmental pollutants and accumulate metal ions.17,18 There are four complete (and eleven total) annotated genome sequences available for Rhodococcus ruber species, although none are for the GIN-1 strain.19 As would be expected, there are >100 predicted metalloprotein sequences in each genome, including numerous apparent Fe, Zn, and Cu proteins. Thus, the presence of appreciable amounts of these metals in the cells is unsurprising. Although Al is not believed to be an essential biometal, it is, like Ti, very abundant and thus commonly found in organisms.1Rhodococcus ruber has gene clusters for apparent siderophore biosynthesis and siderophore uptake.19 Siderophores may help facilitate Ti uptake,6 and may be related to the interference of Ti with Fe metal ion quotas. Fleminger and coworkers noted cellular extensions between the adherent R. ruber GIN-1 and the TiO2 surface and identified a cell surface dihydrolipoamide dehydrogenase as a titanium oxide binding protein,20,21 but it is not clear whether these extensions or this protein are involved in Ti uptake.
These data suggest that the uptake of Ti from TiO2, whether adventitious or not, can interfere with the levels of some (Fe, Zn, and to a lesser degree Mn) but not all (Cu or Al) metals in R. ruber GIN-1 cells. Because Ti(IV) is similar in size and hard character to (but even more Lewis acidic than) Fe(III), it can bind tightly to at least some Fe proteins and might directly interfere with Fe uptake and function.22–24 Other properties, like reduction potential in the same biological coordination environment, diverge between Fe(III) and Ti(IV).25 Titanium binding to native Zn or Mn proteins has not been demonstrated, and a direct replacement is less chemically likely.
These data were collected at a single time point, after 1 h exposure to TiO2. For the Fe concentration to have decreased by nearly a factor of two, on average, in about one tenth of the bacterial doubling time suggests not only that iron uptake was inhibited but that metal efflux might be activated.26,27 We note that titanium uptake and disruption of biometal quotas might further vary as a function of time. Even if these changes are the result of a generalized stress response, the changes would imply that TiO2 is not, at least for this one bacterial species, an inert, non-bioactive material. Instead, exposure to TiO2 causes measurable changes in the levels of some other essential metal ions.
Conclusions
Contrary to its reputation as an inert material, titanium oxide can be bound by Rhodococcus ruber GIN-1 cells. After cell/mineral binding, titanium is liberated and incorporated into cellular biomass. Titanium levels, already appreciable in unexposed cells, increase by nearly an order of magnitude after this exposure. There was no significant difference in uptake between anatase and rutile crystal forms of TiO2. Furthermore, the metal ion quotas for some (Fe, Zn, Mn) but not all (Cu, Al) (bio)metals decrease concomitantly with this Ti incorporation. This work suggests interference between the biogeochemical cycles of Ti with those of other metals, and adds new support for that metal's biological relevance.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank Huntsman Venator Corp for the gift of the Sachtopore beads. ADG thanks Temple University for First Summers Research Initiative support and for a Dissertation Completion Grant. This work was supported by the National Science Foundation Grant CHE-1412373 and CHE-1708793 to AMV.Notes and references
- J. Emsley, The Elements, Clarendon Press, Oxford, UK, 3rd edn, 1998 Search PubMed.
- A. A. Yaroshevsky, Geochem. Int., 2006, 44, 48–55 CrossRef.
- S. A. Skrabal and C. M. Terry, Mar. Chem., 2002, 77, 109–122 CrossRef CAS.
- M. R. Zierden and A. M. Valentine, Metallomics, 2016, 8, 9–16 RSC.
- K. M. Buettner and A. M. Valentine, Chem. Rev., 2012, 112, 1863–1881 CrossRef CAS PubMed.
- K. E. Jones, K. L. Batchler, C. Zalouk and A. M. Valentine, Inorg. Chem., 2017, 56, 1264–1272 CrossRef CAS PubMed.
- O. W. Duckworth, J. R. Bargar and G. Sposito, Biometals, 2009, 22, 605–613 CrossRef CAS PubMed.
- O. W. Duckworth, J. R. Bargar, A. A. Jarzecki, O. Oyerinde, T. G. Spiro and G. Sposito, Mar. Chem., 2009, 113, 114–122 CrossRef CAS.
- M. J. McWhirter, P. J. Bremer, I. L. Lamont and A. J. McQuillan, Langmuir, 2003, 19, 3575–3577 CrossRef CAS.
- J. Yang, P. J. Bremer, I. L. Lamont and A. J. McQuillan, Langmuir, 2006, 22, 10109–10117 CrossRef CAS PubMed.
- H. G. Upritchard, J. Yang, P. J. Bremer, I. L. Lamont and A. J. McQuillan, Langmuir, 2011, 27, 10587–10596 CrossRef CAS PubMed.
- J. Shabtai, G. Fleminger and J. Fleming, US Pat., 5290697, 1994.
- Y. Shabtai and G. Fleminger, Appl. Environ. Microbiol., 1994, 60, 3079–3088 CrossRef CAS.
- G. Gertler, I. Brudo, R. Kenig and G. Fleminger, Materialwiss. Werkstofftech., 2003, 34, 1138–1144 CrossRef CAS.
- G. Bratbak and I. Dundas, Appl. Environ. Microbiol., 1984, 48, 755–757 CrossRef CAS.
- E. P. Levine, Science, 1961, 133, 1352–1353 CrossRef CAS PubMed.
- K. S. Bell, J. C. Philp, D. W. J. Aw and N. Christofi, J. Appl. Microbiol., 1998, 85, 195–210 CrossRef CAS PubMed.
- N. Tomioka, H. Uchiyama and O. Yagi, Appl. Environ. Microbiol., 1994, 60, 2227–2231 CrossRef CAS PubMed.
- NCBI Genome page for Rhodocuccus ruber species, https://www.ncbi.nlm.nih.gov/genome/genomes/11562, accessed 2019.
- A. Siegmann, A. Komarska, Y. Betzalel, I. Brudo, S. Jindou, G. Mor and G. Fleminger, J. Mol. Recognit., 2009, 22, 138–145 CrossRef CAS PubMed.
- A. Dayan, G. Babin, A. Ganoth, N. S. Kayouf, N. N. Eliaz, S. Mukkala, Y. Tsfadia and G. Fleminger, J. Mol. Recognit., 2017, 30, e2617 CrossRef PubMed.
- A. D. Tinoco and A. M. Valentine, J. Am. Chem. Soc., 2005, 127, 11218–11219 CrossRef CAS PubMed.
- A. D. Tinoco, C. D. Incarvito and A. M. Valentine, J. Am. Chem. Soc., 2007, 129, 3444–3454 CrossRef CAS PubMed.
- A. D. Tinoco, E. V. Eames and A. M. Valentine, J. Am. Chem. Soc., 2008, 130, 2262–2270 CrossRef CAS PubMed.
- C. J. P. Siburt, E. M. Lin, S. J. Brandt, A. D. Tinoco, A. M. Valentine and A. L. Crumbliss, J. Inorg. Biochem., 2010, 104, 1006–1009 CrossRef PubMed.
- D. H. Nies and S. Silver, J. Ind. Microbiol., 1995, 14, 186–199 CrossRef CAS PubMed.
- H. L. Pi and J. D. Helmann, Metallomics, 2017, 9, 840–851 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9mt00305c |
| This journal is © The Royal Society of Chemistry 2020 |
