Identification and physiological comparison of plant species that show positive or negative co-occurrence with selenium hyperaccumulators
R. Jason B.
Reynolds
a,
Rachel R.
Jones
a,
Gavin C.
Stonehouse
a,
Ali F.
El Mehdawi
a,
Leonardo W.
Lima
a,
Sirine C.
Fakra
b and
Elizabeth A. H.
Pilon-Smits
 *a
*a
aColorado State University, Biology Department, Fort Collins, CO 80523, USA. E-mail: epsmits@colostate.edu; Tel: +1-970-491-4991
bAdvanced Light Source, Lawrence Berkeley National Laboratory, Berkeley, CA 94720, USA
First published on 28th November 2019
Abstract
In these studies we identified and compared the properties of plant species that showed positive or negative co-occurrence with selenium (Se) hyperaccumulators in their natural habitat. The main questions addressed were: which species are most abundant directly adjacent to hyperaccumulators, and which are absent? How do Se accumulation and tolerance compare in species found to positively or negatively co-occur with hyperaccumulators? Approaches included field surveys, X-ray microprobe analysis of field samples, and a lab Se tolerance and accumulation study. When 54 hyperaccumulators across two naturally seleniferous sites were surveyed for their five nearest neighboring species, and the relative abundance of these species around hyperaccumulators compared to that in the overall vegetation, some species were identified to positively or negatively co-occur with hyperaccumulators. Several positively co-occurring species showed high Se accumulation capability (up to 900 mg Se per kg dry weight), which may reflect Se tolerance. Leaf X-ray microprobe analysis found relatively more organic forms of Se in two positively co-occurring species than in a negatively co-occurring one. There were elevated soil Se levels around Se hyperaccumulators, and neighbors of Se hyperaccumulators had a higher tissue Se concentration as compared to when the same species grew elsewhere in the area. The elevated soil Se levels around Se hyperaccumulators – likely resulting from litter deposition- may significantly affect the local plant community, facilitating Se-tolerant plant community members but lowering the fitness of Se-sensitive members.
Significance to metallomicsSpecific questions addressed in this study were: which plant species are most abundant directly adjacent to hyperaccumulators, and which are absent? How do these species that positively or negatively co-occur with hyperaccumulators compare in Se accumulation and tolerance? Several species were identified to positively or negatively co-occur with hyperaccumulators across three field sites. Several positively co-occurring species showed high Se accumulation capability and thus apparent Se tolerance, which was perhaps associated with an ability to accumulate relatively more organic forms of Se. Neighbors of Se hyperaccumulators had a higher tissue Se concentration as compared to when the same species grew elsewhere in the area, which was associated with elevated soil Se levels around Se hyperaccumulators, These elevated soil Se levels around Se hyperaccumulators – likely resulting from hyperaccumulator leaf drop- may be toxic to Se-sensitive species but offer an opportunity to Se-tolerant species. These different effects may significantly influence the species composition of the local plant community. |
Introduction
The element selenium (Se) is a micronutrient for many species, but becomes toxic at elevated levels, with a narrow concentration range between sufficiency and toxicity.1 The natural Se distribution in the earth's crust is variable, and its primary bioavailable form in soils is SeO42− (selenate).2While Se is not essential for plants, it can be taken up and assimilated due to the chemical similarity between Se and the plant macronutrient sulfur (S).3 One reason why Se is toxic is the non-specific incorporation of selenocysteine into proteins, which can disrupt protein function; the other mechanism by which Se becomes toxic to plants is due to oxidative stress from inorganic selenate and selenite.4
Interestingly, Se can benefit plant growth at low tissue levels, typically <5 mg Se per kg DW (dry weight), by providing physiological benefits related to induction of oxidative stress resistance mechanisms.5 Moreover, at higher levels, plants can enjoy ecological benefits from Se accumulation, owing to protection from fungal infections or herbivory.6 The tissue concentration where plants start to experience toxicity varies by species: some are quite Se-sensitive (toxicity <100 mg Se per kg DW), while others are moderately Se tolerant (toxicity >500 mg Se per kg DW). Remarkably, some plants show extreme Se tolerance and also hyperaccumulate Se to >1000 mg Se per kg DW while growing in naturally seleniferous habitats.7 Examples are Astragalus bisulcatus (Fabaceae) which may contain 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg Se per kg DW and Stanleya pinnata (Brassicaceae), with 4000 mg Se per kg DW.8 These hyperaccumulators also differ from other species in that they have higher Se to S ratios, accumulate relatively more organic Se (particularly methyl-selenocysteine), and sequester Se in specific tissues (leaf epidermis and margins) and organs (reproductive organs).7,9
000 mg Se per kg DW and Stanleya pinnata (Brassicaceae), with 4000 mg Se per kg DW.8 These hyperaccumulators also differ from other species in that they have higher Se to S ratios, accumulate relatively more organic Se (particularly methyl-selenocysteine), and sequester Se in specific tissues (leaf epidermis and margins) and organs (reproductive organs).7,9
Likely, Se hyperaccumulation serves as an elemental defense, protecting plants from generalist herbivores and some fungal pathogens.10–14 Furthermore, concentration of Se around hyperaccumulators may result in elemental allelopathy, inhibiting the growth of Se-sensitive other plant species.15 Supporting evidence for Se-mediated allelopathy is that soil collected adjacent to hyperaccumulators was found to be Se-enriched (∼10 fold), and inhibited germination and growth of Se-sensitive species Arabidopsis thaliana.15 This Se enrichment is thought to be due to deposition of hyperaccumulator litter, and perhaps root exudation, all in the form of highly bioavailable organic selenocompounds.16,17
Interestingly, there is also evidence that some native plants growing around Se hyperaccumulators are positively affected by the concentrated Se around hyperaccumulators.18 Their proximity to Se hyperaccumulators was associated with higher Se accumulation, higher biomass and less herbivory damage. Thus, if neighbors of hyperaccumulators are able to tolerate the associated high levels of Se, positive allelopathy may result, which may be a combination of physiological benefits (enhanced growth) as well as protection from herbivory.18,19 However, if they cannot tolerate the Se, they may suffer toxicity.15
The hypothesis that may be derived from these earlier observations is that through these allelopathic processes, hyperaccumulators may affect the fitness of other plant species, favoring Se-tolerant individuals and exerting a negative effect on Se-sensitive individuals. This may over time lead to differences in vegetation patterns relative to communities without hyperaccumulators. In an earlier study it was found that sparser vegetation cover was observed directly around hyperaccumulators, and there were some differences in species composition.17 The studies described here focused on identifying plant species in the community that showed the clearest positive or negative co-occurrence with hyperaccumulators, and characterizing their Se-related properties. The specific questions addressed were: (1) How does soil Se concentration relate to distance from Se hyperaccumulators? (2) Is Se concentration in a hyperaccumulator correlated with that in its neighboring plants? (3) Which species are found in the near vicinity of hyperaccumulators (<50 cm, under the canopy), and do they occur more frequently there, as compared to the overall landscape? (4) How do Se tolerance and accumulation properties compare between species that positively or negatively co-occur with hyperaccumulators, and what are the patterns of Se tissue distribution and chemical speciation in these species?
First, a field survey approach was used across two naturally seleniferous sites, to pinpoint species of interest. The shale soils at these sites support several Se hyperaccumulator plant species, as described in earlier studies.8,15 The survey was followed by a controlled laboratory Se tolerance and accumulation experiment using selected species. Furthermore, X-ray microprobe analysis was done to image the distribution of Se and other elements in intact frozen field-collected samples from species of interest, as well as forms of Se accumulated (Se speciation).
Results
Fifty-four hyperaccumulator individuals from the species S. pinnata or A. bisulcatus were selected across two field sites (Pine Ridge and Coyote Ridge natural areas, Fort Collins, CO, USA). The five species growing nearest to these hyperaccumulators were recorded and counted (Table 1, “Near 5 counts”). The relative species abundance (RSA) data calculated from these counts, as a fraction of the overall RSA of the same species were used to calculate the ratios shown in Fig. 1. Plant species found relatively more frequently near hyperaccumulators than expected based on Daubenmire surveys have ratios >1, and species that were found less frequently next to hyperaccumulators compared to overall vegetation have ratios <1 (Fig. 1, left side vs. right side).| A | ||||||
|---|---|---|---|---|---|---|
| Species code | Species name | Near 5 counts | Near 5 RSA | Daub% canopy | Daub RSA | Avg. mg Se per kg DW |
| ALSI | Alyssum simplex | 4 | 9.1 | 3.1 | 5.8 | 10 ± 1 |
| AMPS | Ambrosia psilostacriya | 1 | 2.3 | 0.1 | 0.2 | 18 |
| ASBI2 | Astraqaius Oisulcatus | 0 | 0 | 0.6 | 1.1 | 335 ± 40 |
| BOCU | Bouteloua curtipendula | 3 | 6.8 | 6.9 | 13.1 | 3 ± 1 |
| BODA2 | Bouteloua dactytoides | 5 | 11.4 | 8.2 | 15.4 | 4 ± 1 |
| BOGR2 | Bouteloua gracilis | 1 | 2.3 | 0 | 0 | 12 |
| BRIN2 | Bromus inermis | 1 | 2.3 | 0.2 | 0.4 | 5 |
| COAR4 | Convolvulus arvensls | 7 | 15.9 | 2.4 | 4.5 | 10 ± 1 |
| MEOF | Mefilotus officinalis | 6 | 13.6 | 2.4 | 4.5 | 17 ± 3 |
| NAVI | Nassella viridula | 2 | 4.6 | 0.3 | 0.6 | 10 ± 0.3 |
| OESU3 | Oenothera suffrutescens | 2 | 4.6 | 0.6 | 1.2 | 9 + 5 |
| PASM | Pascopyrum smlthit | 8 | 18.2 | 11.8 | 22 2 | 9 ± 1 |
| TAOF | Taraxacum officinale | 1 | 2.3 | 0 | 0 | ND |
| TRDU | Tragopogon dubius | 3 | 6.8 | 0.3 | 0.6 | 20 ± 10 |
| B | |||||||
|---|---|---|---|---|---|---|---|
| Species code | Species name | Near 5 Counts | Near 5 RSA | Daub% Canopv | Daub RSA | Avg. mg Se per kg DW | |
| Near | Far | ||||||
| ALSI | Alyssum simplex | 3 | 4.7 | 4.1 | 2.6 | 337 ± 189 | 0 |
| ARLU | Artemisia ludoviciana | 6 | 3.5 | 2.9 | 2.3 | 183 ± 28 | 0 |
| ASBI2 | Astragalus oisulcatus | 0 | 0 | 1.5 | 2.4 | 8284 ± 992 | n/a |
| ASPU | Asdepias pumita | 1 | 0.4 | 4.0 | 6.3 | 39 ± 0 | ND |
| BRTE | Bromus tectorum | 1 | 4.7 | 2.0 | 1.5 | 452 ± 0 | ND |
| BRIN2 | Bromus inermls | 3 | 1.6 | 22.9 | 18.1 | 369 ± 73 | ND |
| ERNA10 | Ericatneria nauseosa | 1 | 0.8 | 1.7 | 1.5 | 223 ± 0 | ND |
| HEAN3 | Hettanthus annuus | 2 | 3.1 | 6.6 | 4.4 | 219 ± 106 | 3.4 |
| OESU3 | Oenotnera suffrutescens | 1 | 2.0 | 1.0 | 0.7 | 397 ± 0 | ND |
| OPPO | Opuntia polyacantfia | 1 | 0.4 | 0.2 | 0.3 | 95 ± 0 | ND |
| PASM | Pascopyrum smitfiii | 4 | 6.7 | 18.0 | 10.8 | 503 ± 78 | 13.8 |
| PSSPS | Pseudoroegnaria spicata | 2 | 6.7 | 3.0 | 3.3 | 54 ± 19 | ND |
| RHTR | Rhus trilobata | 1 | 1.2 | 2.5 | 1.7 | 41 ± 0 | 6.7 |
| SYER | Syrpphyotnchum ericoides | 3 | 1.6 | 3.0 | 2.0 | 3838 ± 309 | 0.4 |
| STPI | Stanleya pinnate | 0 | 0 | 0 | 0 | 1459 ± 196 | n/a |
| TRDU | Traqopoqon dubius | 3 | 1.6 | 1.9 | 1.5 | 300 ± 70 | 64.7 |
| YUGL | Yucca glauca | 1 | 6.7 | 2.0 | 1.2 | 3910 | ND |
| C | ||||||
|---|---|---|---|---|---|---|
| Species code | Species | OBS Counts | OBS RSA | EXP% canopy | EXP RSA | Avg. mg So per kg DW |
| ACHY | Achnatherum hymenoides | 6 | 0.024 | 1.36 | 0.010 | 28 ± 6 |
| ALSI | Aiyssum simplex | 12 | 0.047 | 4.10 | 0.026 | 4 ± 1 |
| AMPS | Ambrosia psilostachya | 2 | 0.008 | 0.10 | 0.002 | 10 |
| ARDR4 | Artemisia dracunculus | 2 | 0.011 | 0 | 0 | 30 ± 0.3 |
| ARFR4 | Artemisia frigida | 1 | 0 004 | 0.37 | 0.002 | 50 |
| ARLU | Artemisia ludoviciana | 9 | 0.035 | 2.85 | 0.023 | 20 ± 9 |
| ARPU9 | Aristida purpurea | 2 | 0.008 | 2.69 | 0.027 | 8 ± 4 |
| ASBI2 | Astragalus bisulcatus | 0 | 0 | 0.14 | 0.35 | 1458 ± 94 |
| ASMO7 | Astragalus mollisslmus | 1 | 0.006 | 0 | 0 | 219 |
| ASTE5 | Astragalus tenellus | 4 | 0.016 | 0.74 | 0.018 | 45 ± 12 |
| BOCU | Bouteloua curtipendula | 10 | 0.039 | 7.33 | 0.046 | 57 ± 10 |
| BRAR5 | Bromus arvensls | 12 | 0.047 | 2.00 | 0.015 | 43 ± 4 |
| COUM | Comandra umbellata | 2 | 0 008 | 1.01 | 0.008 | 213 ± 12 |
| ELEL5 | Elymus elymoides | 1 | 0.004 | 0.52 | 0.005 | 71 |
| ERNA10 | Ericameria nauseosa | 2 | 0.008 | 165 | 0.015 | 6 |
| EUBR | Euphorbia brachycera | 1 | 0.004 | 0.69 | 0.017 | 68 |
| EVNU | Evolvulus nullallianus | 3 | 0.012 | 1.75 | 0.012 | 152 ± 66 |
| GUSA2 | Gutierrezia sarothrae | 3 | 0.012 | 1.86 | 0.019 | 597 ± 222 |
| HEAN3 | Helianthus annuus | 8 | 0.031 | 6.64 | 0.044 | 28 ± 6 |
| HECO26 | Hesperostipa comata | 20 | 0.079 | 22.84 | 0.163 | 59 ± 4 |
| LASE | Lactuca serrtola | 9 | 0.035 | 0.05 | 0.001 | 89 ± 12 |
| LI DA | Linarla dalmatica | 2 | 0.008 | 1.26 | 0.012 | 188 ± 101 |
| LILE3 | Linum lewlsii | 5 | 0 020 | 2.09 | 0.015 | 46 ± 15 |
| MEDE | Mentzelia decapetala | 1 | 0.004 | 0.31 | 0.008 | 27 |
| NAVI | Nassella viridula | 7 | 0.028 | 4.73 | 0.026 | 48 ± 10 |
| OESU3 | Oenothera suffrutescens | 5 | 0.020 | 1.03 | 0.007 | 31 ± 10 |
| OEVI | Oenothera villosa | 2 | 0.008 | 0.84 | 0.007 | 96 ± 64 |
| PASM | Pascopyrum smitfiii | 17 | 0.067 | 18.00 | 0.108 | 70 ± 23 |
| PHBE2 | Physana bellii | 5 | 0.020 | 0.17 | 0.004 | 375 ± 69 |
| POPR | Poa pratensis | 2 | 0.008 | 0.24 | 0.004 | 156 ± 108 |
| PSSPS | Pseudoroegneria spicata | 17 | 0.067 | 3.03 | 0.033 | 62 ± 5 |
| PSTE5 | Psoratidium tenuiflorum | 3 | 0.012 | 2.63 | 0.017 | 8 ± 1 |
| RACO3 | Ratibida columnlfera | 1 | 0.004 | 0.31 | 0.008 | 16 |
| RHTR | Rhus trilobata | 4 | 0.016 | 3.01 | 0.020 | 5 ± 2 |
| ROWO | Rosa vjoodsli | 1 | 0.004 | 1.15 | 0.012 | 6 |
| STPI | Stanleya pinnata | 1 | 0.004 | 0.57 | 0.014 | 1107 ± 28 |
| SYAS3 | Symphyotrichum ascendens | 3 | 0.012 | 0.95 | 0.023 | 17 ± 8 |
| SYER | Symphyotrichum ericoides | 4 | 0.016 | 1.88 | 0.015 | 382 |
| SYFE | Symphyotrichum fendieri | 3 | 0.017 | 0 | 0 | 1129 ± 126 |
| THME | Thelesperma megapotamicum | 2 | 0.008 | 0.33 | 0.003 | 150 ± 78 |
| TOHO | Tonnsendia hookers | 3 | 0.012 | 0.24 | 0.006 | 394 ± 85 |
| TRDU | Tragopogon dubius | 17 | 0 067 | 2.01 | 0.012 | 53 ± 7 |
| TRRA5 | Tragia ramose | 5 | 0.020 | 2.79 | 0 033 | 31 ± 6 |
| VETH | Verbascum thapsus | 2 | 0.008 | 0.05 | 0.000 | 19 ± 7 |
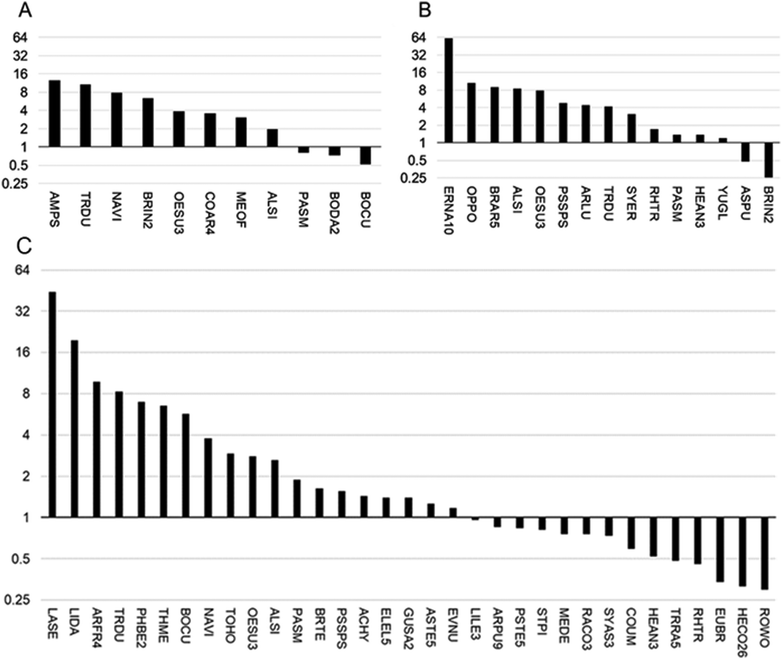 | ||
| Fig. 1 Positive and negative co-occurrence of different plant species with Se hyperaccumulator species at Pine Ridge, locations 1 (A, lower in Se) and 2 (B, higher in Se) and Coyote Ridge (C). See Table 1 for explanation of species abbreviations, and the observation data used to calculate the ratios shown here, which represent the fold difference in the frequency the species as one of the nearest five to hyperaccumulators relative to the frequency of the same species in the overall area (from Daubenmire plot data). | ||
Species found more frequently growing near hyperaccumulators at all three sites are Alyssum simplex, Oenothera suffrutescens and Tragopogon dubius, and two species were found to positively co-occur in two of the three sites: Bromus tectorum and Nasella viridula. The species with the highest Near 5/Daubenmire ratio was Ericameria nauseosa in the Pine Ridge 2 site. In regards to species less abundant near hyperaccumulators than expected, there were three that stood out: Hesperostipa comata, Euphorbia brachycera and Rosa woodsii; each of these species were at least four-fold less abundant near hyperaccumulators than expected based on Daubenmire data. It should be noted that only species that occurred in both the Daubenmire plots and as one of the nearest five species are included in Fig. 1.
The average leaf Se concentration in those plants found as one of the five nearest species to the hyperaccumulator ranged from 4.3 mg Se per kg dry weight for Bouteloua dactyloides to 2974 mg Se per kg DW for Symphyotrichum ericoides (Table 1). The hyperaccumulator S. pinnata averaged 1459 ± 196 mg Se per kg dry weight for Pine Ridge site 1 and 1107 ± 28 mg Se per kg dry weight for Coyote Ridge; there was no S. pinnata located at Pine Ridge site 2. The hyperaccumulator A. bisulcatus averaged 8284 ± 992 mg Se per kg dry weight for Pine Ridge site 1 and 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 927 ± 253 mg Se per kg dry weight for Pine Ridge site 2; there was no A. bisulcatus located at the Coyote Ridge site. After S. ericoides (hyperaccumulator co-occurrence pattern: +), the species next highest in Se were Gutierrezia sarothrae (+), Townsendia hookeri (+), Physaria bellii (+), and Bromus inermis (±, depending on the site).
927 ± 253 mg Se per kg dry weight for Pine Ridge site 2; there was no A. bisulcatus located at the Coyote Ridge site. After S. ericoides (hyperaccumulator co-occurrence pattern: +), the species next highest in Se were Gutierrezia sarothrae (+), Townsendia hookeri (+), Physaria bellii (+), and Bromus inermis (±, depending on the site).
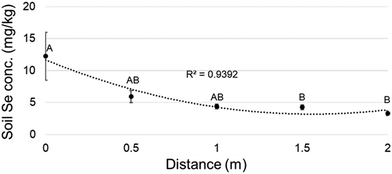
To investigate the effect of the hyperaccumulator's proximity in the field, when possible the leaf Se concentration was also determined of plants of the same species, collected at a minimum of 50 m from a hyperaccumulator. The Se concentration was lower at this longer distance for all species surveyed (Table 1B, near vs. far columns). The effect of the hyperaccumulator's proximity on soil Se concentration was also investigated: Se concentration in soil was analyzed at 0.5 meter successive intervals from the stem of five hyperaccumulator plants (0 m) to 2 m from the plant across all directions. The soil Se concentration significantly decreased with distance from the hyperaccumulator (Fig. 2).
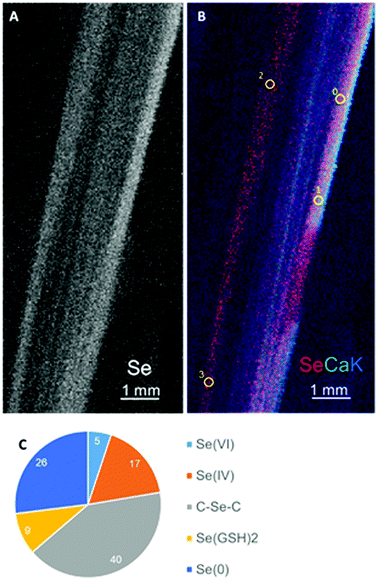
Selected species that differed in their co-occurrence properties (Table 1 and Fig. 1) were further investigated for their Se speciation and localization, as well as their Se tolerance. Bromus inermis was selected as a negatively co-occurring species, and Alyssum simplex and Artemisia frigida as positively co-occurring species. Leaves from these three species were collected in the field and analyzed by X-ray microprobe analysis for Se distribution (micro X-ray fluorescence, μXRF) and chemical speciation (micro X-ray absorption near-edge structure, μXANES). Selenium was distributed throughout the leaf of B. inermis (Fig. 3A and B), with a concentration in a pattern of straight parallel lines, likely the vasculature. The chemical speciation of Se was determined in the B. inermis leaf locations indicated (Fig. 3B) via Se K-edge XANES. Around half of the Se appeared to be organic and the other half inorganic (Fig. 3C). The organic forms made up half of the Se and were fitted as 40% C–Se–C compounds (possibly selenomethionine or methyl-selenocysteine) and 9% seleno-diglutathione (SeGSH2, C–S–Se–S–C). The other half consisted of inorganic Se and was fitted as elemental Se (26% Se(0)), selenite (17% Se(IV)) and selenate (5% Se(VI)).
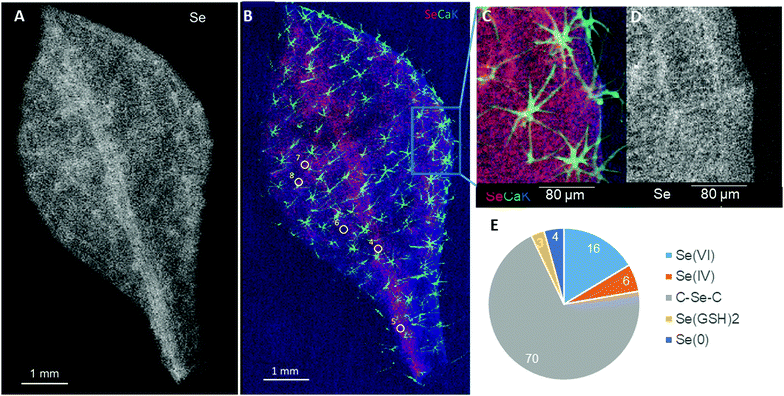
The second species tested, A. simplex, showed diffuse Se distribution throughout the leaf, with increased concentration in the central vein and slight concentration in the other vasculature and in the stellate trichomes (Fig. 4A–C). Calcium was very concentrated in the trichomes (much more so than Se), while potassium was present throughout the leaf (Fig. 4B). The form of Se was investigated in the A. simplex leaf at the positions indicated (Fig. 4B), and found to be predominantly organic (73%), consisting of 70% C–Se–C compounds and 3% SeGSH2 (Fig. 4D). The inorganic Se fraction was comprised of three forms: 16% Se(VI), 6% Se(IV) and 4% Se(0).
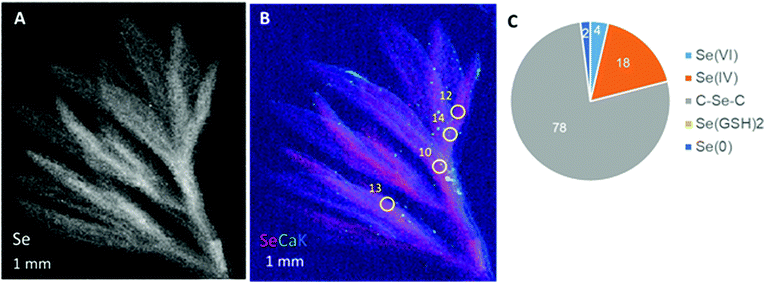
The third species tested, A. frigida, also showed diffuse Se distribution in the leaf with clearly higher levels in what appears to be the vasculature (Fig. 5A and B). XANES revealed that the Se in the A. frigida leaf at the locations indicated (Fig. 5B) consisted primarily of organic Se with C–Se–C structure (78%); the remainder was inorganic: 18% Se(IV), 4% Se(VI) and 2% Se(0) (Fig. 5C). Thus, Se localization was similar across the three species, showing diffuse distribution throughout the leaf with concentration in the vasculature. Alyssum simplex additionally appeared to store some Se in its trichomes. The Se speciation differed between negatively and positively co-occurring species in that the positively co-occurring species had relatively more organic Se (three-quarters vs. half).
A follow-up lab Se tolerance study was carried out, using seeds from species that differed in co-occurrence (+, – or neutral) with hyperaccumulators, collected from the areas where the field study was performed. Five species had sufficient germination to provide meaningful results: Artemisia ludoviciana (+)(ARLU), Bromus inermis (−) (BRIN2), Bromus tectorum (+) (BRTE), Artemisia frigida (+) (ARFR4) and Nasella viridula (∼0) (NAVI) (Fig. 1). The average biomass for plants of these five species was compared between a 20 μM selenate treatment and a control treatment (Fig. 6). For A. ludoviciana, B. inermis and A. frigida, the root and shoot biomass were on average lower for plants treated with selenate than for the control treatment, but this was only statistically significant for roots of A. frigida (Fig. 6). For N. viridula root biomass was unaffected, but shoot biomass less for plants treated with selenate (Fig. 6). Exceptionally, for B. tectorum the root biomass was significantly larger with selenate than without; shoot biomass was unaffected.
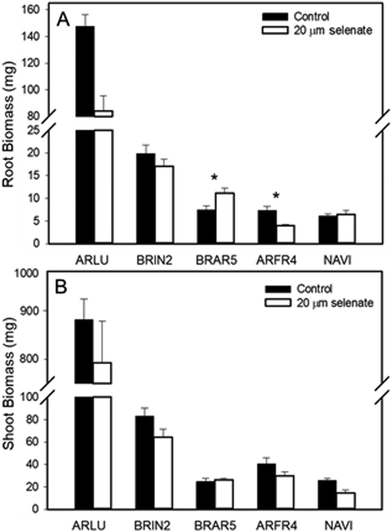 | ||
| Fig. 6 Root (A) and shoot (B) biomass of five species grown in controlled lab conditions with 20 μm sodium selenate or without selenate (control). Full names of the species can be found in Table 1. Asterisks denote significant difference between means for +/− Se treatment of a species (P < 0.05). | ||
When the selenate-supplied plants were analyzed for their tissue Se concentration, the two Artemisia species had by far the highest levels (Fig. 7). In A. ludoviciana, shoot Se was ∼800 mg per kg DW and A. frigida contained ∼700 mg Se per kg DW. When compared to the next highest shoot concentration in B. inermis, A. ludoviciana was around five-fold higher. For roots, A. ludoviciana had ∼700 mg Se per kg DW while B. inermis (the next highest) had a 10-fold lower level. The Se concentration was generally higher in shoot than in root for all species, but this was much less pronounced for A. ludoviciana and N. viridula.
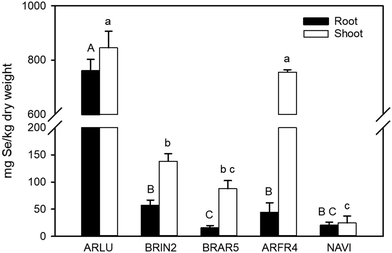 | ||
| Fig. 7 Selenium concentration in the roots and shoots of species treated with or without 20 μm sodium selenate in controlled lab conditions. Full names of the species can be found in Table 1. In cases where Se was not detectable in one or more of the replicates, half of the detection limit was used, and the resulting values thus are estimates; this is the case for BRAR5, ARFR4 and NAVI root as well as NAVI shoot. | ||
Discussion
This study addressed a significant question in regard to the ecology of hyperaccumulators: Does the presence of Se hyperaccumulators correspond with differences in their local plant communities, particularly with respect to the presence or absence of other plant species near hyperaccumulators? The hypothesis was that some species would be disproportionally more or less abundant around hyperaccumulators (A. bisulcatus and S. pinnata), depending on their Se sensitivity: less sensitive species showing positive co-occurrence, and more Se-sensitive species negative co-occurrence. Candidate species, found to occur more or less frequently near hyperaccumulators than in the overall landscape, were then further characterized by lab uptake and tolerance studies and X-ray microprobe analysis.Twenty-two plant species were found at least three-fold more frequently near hyperaccumulators than could be expected based on their overall local abundance, and thus can be said to show positive co-occurrence with Se hyperaccumulators (Fig. 1). The species of hyperaccumulator, A. bisulcatus or S. pinnata, did not matter in this respect, so the effect may be more related to a higher Se content experienced by neighbors rather than any other species-specific effect like nitrogen fixing capability (data not shown). The average Se concentrations for all of these positively co-occurring individuals exceeded levels that begin to show toxicity for most plant species20,21 and in some cases were at or near Se hyperaccumulator levels (>1000 mg Se per kg DW).10 The Se levels in plants growing near hyperaccumulators were higher than in plants of the same species growing further from hyperaccumulators. Moreover, the soil Se concentration under the canopy of hyperaccumulators was found to be elevated. Thus, it appears that hyperaccumulators are surrounded by a Se “hot spot”, and plants growing in this area experience elevated tissue Se levels. Some species that were found to be relatively more abundant in zones surrounding hyperaccumulators may benefit from these elevated Se levels while other species may experience a negative effect from growing there, perhaps due to Se toxicity, as hypothesized. Positive effects of Se on plants may be physiological or ecological. Selenium at tissue levels <5 mg Se per kg DW can benefit plant physiology by increasing antioxidant capacity.22 At higher levels (>5 mg Se per kg DW), Se increasingly protects plants from herbivory.11–14 Of course, to enjoy these positive ecological effects, plants have to tolerate the tissue Se levels they experience.
Tragopogon dubius was found in all three sites to be at least four-fold over-abundant near hyperaccumulators. Tragopogon dubius is an annual or biennial that is often found on disturbed ground (http://swbiodiversity.org). Its overabundance may be related to its ability to take advantage of bare ground, which was found to be more prevalent near hyperaccumulators in our studies; a similar trend was found in earlier study.15 The average Se levels in T. dubius were 91 ± 30 mg per kg DW (Table 1) across all three sites, with a high of 488 mg Se per kg DW at Pine Ridge 2. Thus, it appears to be fairly Se-tolerant. Interesting to note in this respect is that in the lab Se tolerance study, the single T. dubius plant obtained accumulated 882 mg Se per kg DW in its shoot when fed 20 μM Se, with no apparent toxicity symptoms (results not shown due to lack of replication).
Oenothera suffectescens was also found on all three sites to positively co-occur with hyperaccumulators. The Se levels found in this species in the field indicate a capacity to accumulate relatively high levels of Se (up to 397 mg Se per kg DW), coupled with Se tolerance. Similar to T. dubius, this species is often found in disturbed areas and has low water needs (http://swbiodiversity.org).
The third species found to positively co-occur with hyperaccumulators on all three sites was Alyssum simplex. It had high Se levels growing in the field (up to 603 mg Se per kg DW), indicating high Se tolerance. Indeed, the two seedlings obtained in the lab study each accumulated above 1000 mg Se per kg DW in the shoot after feeding with 20 μM selenate, without toxicity symptoms (results not shown for lack of sufficient replication). Similar to the two species discussed above, A. simplex is common in disturbed areas, and is an annual (http://swbiodiversity.org). As a shallow rooting winter annual, it may indeed be well suited to life near a hyperaccumulator: Se levels in the soil near hyperaccumulators have been found to be lowest in spring and highest in fall, likely due to leaching;16 competition for light is minimal, as the hyperaccumulator has not yet produced canopy; Se at the surface has been found to be lower relative to deeper horizons.15 This seasonal and spatial Se difference may have given the opportunity to develop the tolerance shown by the field and lab plants sampled. Bromus tectorum (field brome) was found to positively co-occur with hyperaccumulators on two of the three sites. It is a winter annual grass species23 that inhabits disturbed sites. Bromus tectorum had similar field levels of Se (up to 452 mg per kg DW) to the above species. When grown from seeds collected from the Pine Ridge 2 site, and treated with or without Se, the root biomass of the Se-treated plants was actually higher compared to the control treatment and the shoot biomass was nearly equal. The shoot Se levels in lab conditions reached nearly 150 mg Se per kg DW, indicating tolerance to Se and perhaps a positive growth effect. In a previous study, S. ericoides and A. ludoviciana were found to be facilitated by growing next to hyperaccumulators, showing higher Se levels and reduced herbivory.18 In the current lab study there were no herbivores, so the positive growth response must have been entirely due to physiological factors.
The list of species found less often near hyperaccumulators than expected is much smaller, because many species found in the Daubenmire plots were not at all found as one of the top five species near hyperaccumulators, and thus not counted. Only the species found in both plots could be compared and, of course, those species that negatively co-occur, by definition, should be absent more often as one of the nearest five species. Nevertheless, a few species were found with high relative abundance in the landscape overall, but infrequently near hyperaccumulators, perhaps because they are sensitive to Se. The two that stand out in this regard are B. inermis at the Pine Ridge 2 site and Hesperostipa comata at Coyote Ridge; both were ∼23% of the Daubenmire canopy cover and ∼4-fold less abundant near hyperaccumulators.
If Se hyperaccumulators, via the Se in their tissues and surrounding soil, affect neighboring species positively or negatively, is this effect Se concentration-dependent, and how large is their sphere of influence? When looking at correlation of Se in the hyperaccumulator and the Se content in the surrounding plants, a significant correlation was found for quite a few positively co-occurring species (with enough replication), both as individual species and as a group (notably, T. dubius, A. simplex, O. suffrutescens and B. tectorum), suggesting the Se enhancement effect in neighbors is proportional to the hyperaccumulator's Se concentration. However, a correlation between surface soil Se levels at the base of the hyperaccumulator and the leaf levels in the nearest five plants was not found; this may be due to fluctuating soil Se levels and forms, the limited differences in distance (most were under the canopy) and variation in other properties in the soil such as sulfur forms and levels (S inhibits Se uptake). In addition, no correlation was found between the surface soil Se concentration beneath the hyperaccumulator directly next to the stem and the leaf Se levels in the hyperaccumulator. Still, neighboring plant species contained higher Se levels when growing next to hyperaccumulators than when growing far away (Table 1B).
There was a pronounced local increase in Se concentration in soil around hyperaccumulators close to the plant, but beyond the canopy edge (>50 cm), soil Se decreased substantially (Fig. 2) Therefore, the sphere of influence of hyperaccumulators on surrounding vegetation due to higher local soil Se concentration is likely highest under their canopy. The reason for the higher Se under the canopy is likely leaf litter deposition and subsequent decomposition, but may also be root exudation.16,17 The forms of Se in hyperaccumulator species has been found to be organic, in the form of non-proteinogenic amino acids. This is hypothesized to be a possible tolerance mechanism, as it prevents oxidative stress from inorganic Se forms as well as toxic effects of non-specific incorporation of seleno-amino acids into proteins.9,17,24 Thus, hyperaccumulator litter decomposition and root deposition processes locally release organic Se into the soil, which is more readily accumulated by plants than inorganic Se.25 Therefore, even slight increases in local soil Se concentration, in organic forms, likely already elevate Se levels in neighboring vegetation, and the Se accumulated might be expected to be enriched in organic forms. In this context, it is interesting to note that the negatively co-occurring species B. inermis had relatively more inorganic and less organic Se (Fig. 3), compared to the two positively co-occurring species A. simplex (Fig. 4) and A. frigida (Fig. 5).
The observed differences in the fraction of organic Se between positive and negative co-occurring species is also interesting because it may reflect Se tolerance differences. Species with lower Se tolerance typically accumulate relatively more inorganic Se; the negative co-occurrence of B. inermis may be related to lower Se tolerance to the elevated soil Se levels around hyperaccumulators. In the lab Se tolerance study, B. inermis did not stand out for being more Se sensitive than positively co-occurring species. However, it is noteworthy that its tissue Se levels were much lower than that of the positively co-occurring species in the lab experiment. Also interesting to note is that the field leaf Se levels for B. inermis in the area where it showed negative co-occurrence (Pine Ridge area 2) were over 2.5-fold higher than those obtained in the controlled study (370 vs. 140 mg Se per kg DW, respectively). Thus, it may well be that B. inermis would show more negative effects at higher tissue Se levels. Interestingly, in Pine Ridge area 1, where overall Se levels in the soils and vegetation were much lower (only 5 mg per kg for B. inermis), B. inermis actually showed positive co-occurrence with hyperaccumulators. Thus, it is possible that the mode of interaction between a certain neighboring plant species and a hyperaccumulator can be both positive and negative, depending on the experienced Se concentration in the neighbor. As stated above, low tissue Se levels often confer positive growth effects and higher anti-oxidant production. The threshold tissue Se levels between Se benefit and Se toxicity will be species-dependent.
In the lab Se tolerance and accumulation study, the two positively co-occurring species A. ludoviciana and A. frigida accumulated much higher Se levels than B. inermis and the other species tested (Fig. 7). In the field they also contained relatively high Se levels compared to other species, but not as high as they did in the lab study. The levels obtained in the lab (∼800 mg per kg DW) appeared to be toxic, considering the reduction in biomass, albeit only significant for ARFR root. However, at the Se levels experienced in the field (183 and 50 mg per kg DW), they may have experienced benefits, considering their positive co-occurrence with hyperaccumulators. Leaves from A. frigida and A. simplex collected in the field had a relative large fraction (∼75%) of organic Se, relative to B. inermis (50%), suggestive of higher Se tolerance. Incidentally, in B. inermis a large fraction of elemental Se was found; this may be due to endophytes or soil microbes: many bacterial and fungal endophytes from hyperaccumulators growing at the Pine Ridge site have been found to produce elemental Se.26–28 In this context it is also interesting to note that there is evidence that Se hyperaccumulator rhizobiomes are more similar to each other than to related non-hyperaccumulator plant species on the same site.29 Some microbial taxa were more prevalent around hyperaccumulator roots; it is possible that these exert an effect on growth or Se accumulation in neighboring plant species. Of further interest to note is that A. simplex showed some Se accumulation in its trichomes, similar to the Se hyperaccumulator A. bisulcatus.9 Sequestration of Se in trichomes may provide Se tolerance and also may be effective in protection from herbivory.
The specific questions for this study can now be re-addressed: (1) How does soil Se concentration relate to distance from Se hyperaccumulators? It was found that soil Se concentration is higher adjacent to hyperaccumulators, but quickly diminishes after 50 cm, around the average canopy diameter (56 cm) we found for hyperaccumulators in this study. This supports the hypothesis that hyperaccumulators enrich their surrounding soil with Se; they likely influence the soil Se speciation as well, into more organic froms.18 The average distance of the nearest five species to hyperaccumulators was ∼20 cm, so well within the area of influence where hyperaccumulators affect Se form and concentration. The concentration in the hyperaccumulator correlated with the Se concentration in multiple species that showed positive co-occurrence. Therefore, it is possible that Se hyperaccumulators are providing selection pressures to neighboring plants by enriching them with Se, which may be positive or negative depending on the resulting Se concentration in the neighbour, and its tolerance. How this local influence of hyperaccumulators is projected into the greater landscape and to higher and lower trophic levels, and the possible cumulative effects over time will be interesting questions for further study.
Experimental
Field sampling and surveys
Field data collection started late June in Pine Ridge Natural Area Fort Collins, CO (latitude: 40.545496, longitude: −105.133213) and Coyote Ridge Natural Area, Fort Collins, CO (latitude: 40.480898, longitude: −105.125547). Both are naturally seleniferous sites located on shale soil, and harbor hyperaccumulator populations as described before8,15 A total of 54 hyperaccumulator plants (A. bisulcatus and S. pinnata) were located, and the five plant species growing nearest to the hyperaccumulator were identified and recorded. In addition, a soil sample was taken at the base of the stem for each hyperaccumulator as well as at the base of each of the five nearest plant species. Soil samples were taken by brushing aside all organic matter at the surface and sampling the top layer (0–5 cm depth). In addition to soil samples, the youngest mature leaf was collected from each hyperaccumulator and each of the five nearest species. For the reference a 50 m baseline was used and six 25 m transects were located along it, three East and three West, alternating. Along each of these east and west transects a Daubenmire quadrat was used at each meter alternating sides (north and south) each time.29 The data from the Daubenmire plots were then used to estimate canopy cover.30Lab studies
A follow-up lab study was performed using seeds collected in the areas described above. A total of 15 species were sown on Pro-Mix brand potting soil in the lab. Of these 15 species, five species, Tragopogon dubius, Bromus inermis, Bromus tectorum, Artemisia ludoviciana, and Artemisia frigida, produced at least six seedlings. The seedlings from these five species were split into two groups, with at least 3 replicates each: a control group, given only 1/4 strength Hoagland's solution,31 and a Se treatment group given the same solution with 20 μM sodium selenate added. All species were treated for five weeks at a 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 light
10 light![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dark photoperiod. They were then harvested, washed, roots and shoots separated and dried in a 50 °C drying oven (24 hours). After drying, they were weighed and total biomass recorded for root and shoot separately. The Se accumulated in the root and shoot material was analyzed as described below.
dark photoperiod. They were then harvested, washed, roots and shoots separated and dried in a 50 °C drying oven (24 hours). After drying, they were weighed and total biomass recorded for root and shoot separately. The Se accumulated in the root and shoot material was analyzed as described below.
Elemental analysis
Soil samples were dried at 50 °C for 2 days. Each sample was homogenized and sieved through a 1 mm screen. Approximately 400 mg of each sample was weighed and placed in a 25 mm × 200 mm glass acid digestion tube. Two ml of ultra-trace grade concentrated (70%) nitric acid was added to each sample. After addition of acid, each sample was heated to 60 °C for two hours and then 125 °C for 6 hours on a digestion block. After cooling, each sample was diluted to 10 ml with distilled water. This digest was subjected to elemental analysis via inductively coupled plasma mass spectrometry (ICP-MS) using a PerkinElmer Elan DRCII instrument with a detection limit for Se of approximately 0.01 ppb in the digest. Appropriate standards and controls were included in each analysis.After drying, leaf samples were crushed to homogenize, and approximately 100 mg was weighed, placed in a 25 mm × 200 mm glass acid digestion tube and 1 ml of ultra-trace grade 70% nitric acid was added to each. The tubes were then placed on a heating block and heated to 60 °C for 2 hours and then 125 °C for 6 hours. After cooling, the samples were diluted to 10 ml with distilled water. All leaf samples were then analyzed using Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES), model PerkinElmer 7300 DV, according to the manufacturer's instructions, including appropriate standards and controls.
X-ray microprobe analyses
Selenium, calcium (Ca) and potassium (K) distribution and Se speciation were analyzed using X-ray microprobe analysis (XRF mapping and Se K-edge XANES). Analyses were performed at beamline 10.3.2 (X-ray Fluorescence Microprobe) of the Advanced Light Source (ALS), at Lawrence Berkeley National Lab (Berkeley, CA, USA) using a Peltier cooling stage (−25 °C). Localization of Se, Ca and K was determined on intact leaves collected in the field, frozen immediately and kept frozen during shipping and analysis. Micro-focused X-ray fluorescence (μXRF) maps were recorded at 13 keV incident energy, using 20 μm × 20 μm pixel size, a beam spot size of 7 μm × 7 μm, using 50 ms dwell time. Maps were then deadtime-corrected and decontaminated. Selenium K-edge micro X-ray absorption near-edge structure (μXANES) spectroscopy (in the range 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500–13
500–13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 070 eV) was used to determine Se speciation on various spots as indicated on the μXRF maps, close to areas showing high Se concentration. Spectra were energy calibrated using a red amorphous Se standard, with the main peak set at 12
070 eV) was used to determine Se speciation on various spots as indicated on the μXRF maps, close to areas showing high Se concentration. Spectra were energy calibrated using a red amorphous Se standard, with the main peak set at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 660 eV. Least-square linear combination fitting of the μXANES data was performed in the range of 12
660 eV. Least-square linear combination fitting of the μXANES data was performed in the range of 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 630 to 12
630 to 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 850 eV using a library of 52 standard selenocompounds following procedures described in Fakra et al..32 All data were recorded in fluorescence mode using a 7-elements Ge solid state detector (Canberra, ON) and processed using custom LabVIEW programs available at the beamline.
850 eV using a library of 52 standard selenocompounds following procedures described in Fakra et al..32 All data were recorded in fluorescence mode using a 7-elements Ge solid state detector (Canberra, ON) and processed using custom LabVIEW programs available at the beamline.
Statistical analyses
Statistical analyses were done using R (ver. X64 3.32). T-Tests were performed comparing the root and shoot biomass for the lab study (α = 0.05). One-way Anova was used to compare all of the different species in the lab experiment (α = 0.05).Conclusions
Overall, the results from these studies support the hypothesis that hyperaccumulators affect local Se distribution and, with that, the composition and properties of surrounding vegetation. Plant species composition around hyperaccumulators is different. Some plant species show positive, co-occurrence with hyperaccumulators, and others negative co-occurrence with hyperaccumulators. Positive or negative co-occurrence may depend on Se tolerance, which is related to the forms of Se accumulator. Neighbors of Se hyperaccumulators had a higher tissue Se concentration as compared to when the same species grew elsewhere in the area, which was related with elevated soil Se levels around Se hyperaccumulators. These “Se hot spots” may derive from hyperaccumulator concentration and deposition of Se. By concentrating Se in and around them in highly bioavailable organic forms, hyperaccumulators may have a large effect on their local plant community, facilitating Se-tolerant plant community members but lowering the fitness of Se-sensitive members. These effects on the local vegetation is in turn expected to affect other trophic levels, as well as overall Se cycling in the local ecosystem.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was funded by National Science Foundation, grant number IOS-1456361 to E. A. H. P.-S. This research used resources of the Advanced Light Source, which is a DOE Office of Science User Facility under contract no. DE-AC02-05CH11231.References
- F. M. Fordyce, Selenium deficiency and toxicity in the environment, in Essentials of medical geology, revised edn, ed. O. Selinus, B. Alloway, J. A. Centeno, et al., Dordrecht, Springer, 2013, pp. 375–416 Search PubMed.
- A. Kabata-Pendias, Trace elements in soils and plants, Boca Raton, FL, USA, CRC Press/Taylor & Francis Group, 2010, p. 548 Search PubMed.
- T. Sors, D. Ellis and D. Salt, Selenium uptake, translocation, assimilation and metabolic fate in plants, Photosynth. Res., 2005, 86, 373 CrossRef CAS PubMed.
- D. van Hoewyk, A tale of two toxicities: Malformed selenoproteins and oxidative stress both contribute to selenium stress in plants, Ann. Bot., 2013, 112, 965 CrossRef CAS PubMed.
- R. Feng, C. Wei and S. Tu, The roles of selenium in protecting plants against abiotic stresses, Environ. Exp. Bot., 2013, 87, 58 CrossRef CAS.
- M. Schiavon and E. A. H. Pilon-Smits, The fascinating facets of plant selenium accumulation – biochemistry, physiology, evolution and ecology, New Phytol., 2017, 213, 1582 CrossRef CAS.
- P. J. White, Selenium accumulation by plants, Ann. Bot., 2016, 117, 217 CAS.
- M. L. Galeas, L. H. Zhang, J. L. Freeman, M. Wegner and E. A. H. Pilon-Smits, Seasonal fluctuations of selenium and sulfur accumulation in selenium hyperaccumulators and related nonaccumulators, New Phytol., 2007, 173, 517 CrossRef CAS.
- J. L. Freeman, L. H. Zhang, M. A. Marcus, S. C. Fakra, S. P. McGrath and E. A. H. Pilon-Smits, Spatial imaging, speciation, and quantification of selenium in the hyperaccumulator plants Astragalus bisulcatus and Stanleya pinnata, Plant Physiol., 2006, 142, 124 CrossRef CAS PubMed.
- R. S. Boyd and S. N. Martens, The raison d’être for metal hyperaccumulation by plants, in The Vegetation of Ultra-mafic (Serpentine) Soils, ed. A. J. M. Baker, J. Proctor and R. D. Reeves, Intercept Limited, Andover, 1992, pp. 279–289 Search PubMed.
- B. Hanson, G. F. Garifullina, S. D. Lindblom, A. L. Wangeline, A. Ackley, K. Kramer, A. P. Norton, C. B. Lawrence and E. A. H. Pilon-Smits, Selenium accumulation protects Brassica juncea from invertebrate herbivory and fungal infection, New Phytol., 2003, 159, 461 CrossRef CAS.
- J. L. Freeman, S. D. Lindblom, C. F. Quinn, S. C. Fakra, M. A. Marcus and E. A. H. Pilon-Smits, Selenium accumulation protects plants from herbivory by Orthoptera via toxicity and deterrence, New Phytol., 2007, 175, 490 CrossRef CAS.
- J. L. Freeman, C. F. Quinn, S. D. Lindblom, E. M. Klamper and E. A. H. Pilon-Smits, Selenium protects the hyperaccumulator Stanleya pinnata against black-tailed prairie dog herbivory in native seleniferous habitats, Am. J. Bot., 2009, 96, 1075 CrossRef CAS.
- C. F. Quinn, J. L. Freeman, R. J. B. Reynolds, J. J. Cappa, S. C. Fakra, M. A. Marcus, S. D. Lindblom, E. K. Quinn, L. E. Bennett and E. A. H. Pilon-Smits, Selenium hyperaccumulation offers protection from cell disruptor herbivores, BMC Ecol., 2010, 10, 19 CrossRef.
- A. F. El Mehdawi, C. F. Quinn and E. A. H. Pilon-Smits, Effects of selenium hyperaccumulation on plant-plant interactions: Evidence for elemental allelopathy?, New Phytol., 2011, 191, 120 CrossRef CAS.
- C. F. Quinn, K. A. Wyant, A. L. Wangeline, J. Shulman, M. L. Galeas, J. R. Valdez, J. R. Self, M. W. Paschke and E. A. H. Pilon-Smits, Enhanced decomposition of selenium hyperaccumulator litter in a seleniferous habitat - evidence for specialist decomposers?, Plant Soil, 2010, 341, 51 CrossRef.
- A. F. El Mehdawi, S. D. Lindblom, J. J. Cappa, S. C. Fakra and E. A. H. Pilon-Smits, Do selenium hyperaccumulators affect selenium speciation in neighboring plants and soil? An X-ray microprobe analysis, Int. J. Phytorem., 2015, 17, 753 CrossRef CAS.
- A. F. El Mehdawi, C. F. Quinn and E. A. H. Pilon-Smits, Selenium hyperaccumulators facilitate selenium-tolerant neighbors via phytoenrichment and reduced herbivory, Curr. Biol., 2011, 21, 1440 CrossRef CAS.
- A. F. El Mehdawi, R. J. B. Reynolds, C. N. Prins, S. D. Lindblom, J. J. Cappa, S. C. Fakra and E. A. H. Pilon-Smits, Analysis of selenium accumulation, speciation and tolerance of potential selenium hyperaccumulator Symphyotrichum ericoides, Physiol. Plant., 2014, 152, 70 CrossRef CAS.
- T. A. Brown and A. Shrift, Selenium: Toxicity and tolerance in higher plants, Bacteriol. Rev., 1982, 57, 59 CAS.
- S. Kaur, N. Kaur, K. H. M. Siddique and H. Nayyar, Beneficial elements for agricultural crops and their functional relevance in defence against stresses, Arch. Agron. Soil Sci., 2016, 62, 905 CrossRef.
- N. Rani, K. S. Dhillon and S. K. Dhillon, Critical levels of selenium in different crops grown in an alkaline silty loam soil treated with selenite-Se, Plant Soil, 2005, 277, 367 CrossRef CAS.
- K. G. Beck, Downy brome (Bromus tectorum) and Japanese brome (Bromus japonicus) biology, ecology, and management, 2009, accessed August 26 2019, http://mining.state.co.us/SiteCollectionDocuments/DownybromeandJapanesebromeliteraturereviewColoradoDRMSDec09.pdf.
- J. L. Freeman, M. Tamaoki, C. Stushnoff, C. F. Quinn, J. J. Cappa, J. Devonshire, S. C. Fakra, M. A. Marcus, S. P. McGrath, D. Van Hoewyk and E. A. H. Pilon-Smits, Molecular mechanisms of selenium tolerance and hyperaccumulation in Stanleya pinnata, Plant Physiol., 2010, 153, 1630 CrossRef CAS.
- A. Zayed, C. M. Lytle and N. Terry, Accumulation and volatilization of different chemical species of selenium by plants, Planta, 1998, 206, 284 CrossRef CAS.
- S. D. Lindblom, J. R. Valdez-Barillas, S. C. Fakra, M. A. Marcus, A. L. Wangeline and E. A. H. Pilon-Smits, Influence of microbial associations on selenium localization and speciation in roots of Astragalus and Stanleya hyperaccumulators, Environ. Exp. Bot., 2013, 88, 33 CrossRef CAS.
- S. D. Lindblom, A. L. Wangeline, J. R. Valdez-Barillas, B. deVilbiss, S. C. Fakra and E. A. H. Pilon-Smits, Fungal endophyte Alternaria tenuissima can affect growth and selenium accumulation in its hyperaccumulator host Astragalus bisulcatus, Front. Plant Sci., 2018, 9, 1213 CrossRef.
- M. S.-D. Jong, R. J. B. Reynolds, K. Richterova, L. Musilova, L. C. Staicu, I. Chocholata, J. J. Cappa, S. Taghavi, D. van der Lelie, T. Frantik, I. Dolinova, M. Strejcek, A. T. Cochran, P. Lovecka and E. A. H. Pilon-Smits, Selenium hyperaccumulators harbor a diverse endophytic bacterial community characterized by high selenium resistance and plant growth promoting properties, Front. Plant Sci., 2015, 6, 113 Search PubMed.
- A. T. Cochran, J. Bauer, J. L. Metcalf, P. Lovecka, M. S.-D. Jong, S. Warris, P. J. W. Mooijman, I. van der Meer, R. Knight and E. A. H. Pilon-Smits, Plant selenium hyperaccumulation affects rhizosphere: Enhanced species richness and altered species composition, Phytobiomes, 2018, 2, 82 CrossRef.
- B. Coulloudon, K. Eshelman and J. Gianola, Sampling vegetation attributes, BLM Technical Reference, BLM Business center, Denver, CO, 1999 Search PubMed.
- D. R. Hoagland and D. I. Arnon, The water culture method for growing plants without soil, Circ. – Calif. Agric. Exp. Stn., 1938, 52, 347 Search PubMed.
- S. C. Fakra, B. Luef, C. J. Castelle, S. W. Mullin, K. H. Williams, M. A. Marcus, D. Schichnes and J. F. Banfield, Correlative cryogenic spectromicroscopy to investigate selenium bioreduction products, Environ. Sci. Technol., 2018, 52, 503 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2020 |