DOI:
10.1039/C9MD00478E
(Research Article)
RSC Med. Chem., 2020,
11, 72-84
Synthesis and evaluation of novel 2,4-disubstituted arylthiazoles against T. brucei†
Received
8th October 2019
, Accepted 22nd November 2019
First published on 19th December 2019
Abstract
The design, synthesis and pharmacological evaluation of the 4-substituted-2-[3-(adamant-1-yl)-4-fluorophenyl]thiazoles 1a–j, the 4-substituted-2-[4-(adamant-1-yl)phenyl]thiazoles 2a–h, the 2-substituted-4-[4-(adamant-1-yl)phenyl]thiazoles 3a–e, the N-substituted 2-phenylthiazol-4-ethylamides 4a, b and the N-substituted 4-phenylthiazol-2-ethylamides 4c, d is described. Compounds 1a and 2a exhibit trypanocidal activity in the range of IC50 = 0.42 μM and IC50 = 0.80 μM, respectively. Both of these derivatives bear a lipophilic end, which consists of a 4-(1-adamantyl) phenyl or a 3-(1-adamantyl)phenyl moiety, a 1,3-thiazole ring and a functional end, which comprises of an alkylamine and can be considered as promising candidates for the treatment of Trypanosoma brucei infections.
Introduction
The African sleeping sickness and the Chagas disease are two of the major neglected tropical diseases (NTDs). The trypanosomiases are vector-borne parasitic infections caused by flagellated protozoa of the class Kinetoplastida.1 There are two species of human-infectious trypanosomes, Trypanosoma brucei, that causes human African trypanosomiasis (HAT), and Trypanosoma cruzi, which is responsible for the Chagas disease. HAT is prevalent in sub-Saharan Africa, transmitted by the bite of a tsetse fly infected with one of the two subspecies, Trypanosoma brucei gambiense or Trypanosoma brucei rhodesiense. The Chagas disease is spread predominantly in Latin and Central America by Triatominae bugs infected with T. cruzi.2,3 Trypanosomiases, as with other NTDs, are becoming public health problems in non-endemic countries, as a result of travel and migration. New drugs are urgently required, as those that are currently available are characterized by side-effects and treatment failures.4,5
Various initiatives6–8 have led to the discovery of promiscuous trypanocidal derivatives from phenotypic high-throughput screening of a number of compound libraries. These have been further refined and optimized to enhance drug-like properties. The Walter and Eliza Hall Institute (WEHI), in partnership with the Drugs for Neglected Diseases initiative (DNDi), and the Genomics Institute of the Novartis Research Foundation (GNF) have described the amide and urea derivatives of thiazolethylamines I, II and sulfonamides III, shown in Fig. 1, as potent trypanocidals.6,7
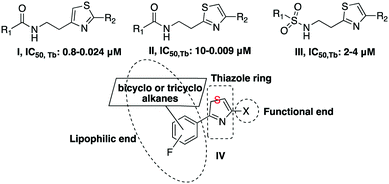 |
| | Fig. 1 General type scaffolds with trypanocidal activity. | |
Based on these findings and our involvement in the adamantane chemistry,9–20 we report herein on the chemistry and biology of thiazole derivatives of the general type scaffold IV. The thiazole moiety is an important pharmacophore in many compounds used against several tropical infectious diseases.21
Scaffold IV includes a 1,3-thiazole moiety, which is 2,4-disubstituted. One substituent is the lipophilic end of the scaffold, which consists of a phenyl ring bearing fluoro- and 1-adamantyl-functionalities. The 4-(1-adamantyl)phenyl substituent has been proven to be well tolerated and is endowed with trypanocidal properties.22 The thiazole ring bears a variety of functional groups (Fig. 2).
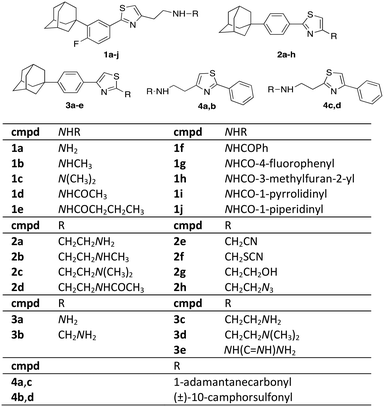 |
| | Fig. 2 Novel thiazole derivatives 1a–j, 2a–h, 3a–e, 4a–d. | |
2-Phenylthiazol-4-ethylamines 1a–d and 2a–d share the same structural features, apart from the relative position of the 1-adamantyl core and the addition of a fluoro-substituent in series 1. Fluorine alters the biophysical and chemical properties, such as lipophilicity, acidity, as well as the reactivity and conformation of the substituted derivatives.23 In 2018, 18 out of the 38 small drug molecules, that were approved by the FDA, contain a fluorine atom.24,25 Derivatives 3 differ in the thiazole moiety compared to adducts 1 and 2. The 2,4-substituents of the thiazoles 2a, c have their positions switched in derivatives 3c, d. The functionalization of the amino-end of congeners 1 involves various amide (aromatic and non-aromatic) and urea substituents. In adducts 2, the polar heads were translocated to the functional end of the general type scaffold IV. The length of the side chain of derivatives 2e, 2g and 3e was kept at the distance of three atoms (2C and 1N and vice versa), which in the derivatives I, II and III was found to be the optimal length for enhanced trypanocidal potency.6–8 The length of the R group is different in adducts 2f, h and 3a, b. 2-Aminothiazole (adduct 3a), is a frequent-hitting fragment in biophysical binding assays.26 Moreover, an analogous thiazole guanidinium system of derivative 3e has been used as a substitute for other aromatic rings improving biological activity.27
The relative position of the adamantane cage, the phenyl ring and the thiazole moiety was altered in derivatives 4a, c. Compounds 4a, c bear the same thiazole ring substituents as derivatives 2 and 3. Additionally, the adamantane core was replaced in the camphor skeleton in adducts 4b, 4. The latter molecules are sulfonamides in alignment with the scaffolds of compounds III.7
Results and discussion
Synthesis
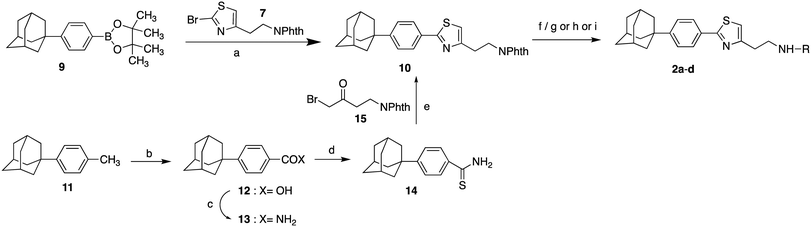
The 4-substituted-2[3-(adamant-1-yl)-4-fluorophenyl]thiazoles 1a–j were synthesized as shown in Scheme 1. As starting material, for the synthesis of thiazoles 1a–j, the (3-adamant-1-yl)-(4-fluorophenyl)boronic acid (6) was used. The reported method for the preparation of the boronic acid 6 (ref. 28) has been modified, by changing the reaction times. The synthetic route involved a Suzuki–Miyaura palladium-catalyzed coupling between the boronic acid 6 and the 2-thiazole bromide 7 (ref. 6) to provide the phthalimide protected adamantane derivative 8. The hydrazinolysis of phthalimide derivative 8 led to the deprotected parent compound 2-{2-[3-(adamant-1-yl)-4-fluorophenyl]thiazol-4-yl}ethan-1-amine (1a), which was subsequently methylated, dimethylated,29 acylated and carbamoylated to deliver adducts 1b–j, via the procedures shown in Scheme 1.
 |
| | Scheme 1 Reagents and conditions: (a) i. n-BuLi, THF, −78 °C, 20 min, ii. (i-PrO)3B, r.t. 18 h, 90%; (b) Pd(PPh3)4, Na2CO3, anh. toluene, 80 °C, 18 h, 80%; (c) H2NNH2.H2O, EtOH, reflux, 1 h; (d) i. ClCOOEt, NEt3, THF, r.t. 18 h, ii. LiAlH4, THF, reflux 4 h, iii. EtOH, H2O, NaOH 10%, 0 °C, 88% from 8; (e) MeONa, CH3COOH, HCHO 38% in H2O, NaBH3CN, MeOH, r.t. 18 h, 75% from 8. (f) Benzoyl chloride (1f: 96% from 8) or 4-fluorobenzoyl chloride (1g: 93% from 8) or 1-piperidinecarbonyl chloride (1j: 36% from 8) or 1-pyrrolidinecarbonyl chloride (1i: 46% from 8), Et3N, EtOAc, r.t. 18 h; (g) acetic anhydride (1d: 84% from 8) or butyric anhydride (1e: 92% for 8), Et3N, EtOAc, r.t. 48 h; (h) 3-methyl-furoic acid, EDCI, HOBt, DMAP, DMF/DCM, 45 °C, 18 h, 29% from 8. | |
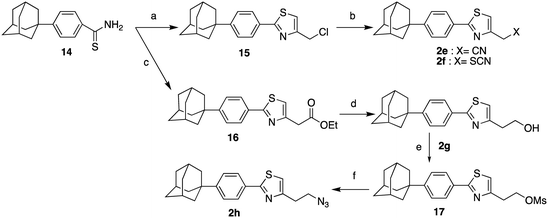
The synthesis of the 4-substituted-2[4-(adamant-1-yl)phenyl]thiazoles 2a–d was realized following two synthetic pathways, as illustrated in Scheme 2. The key-compound for the preparation of the thiazoles 2a–h, the 2-{2-[(2-(adamant-1-yl)phenyl)thiazol-4-yl]ethyl}isoindoline-1,3-dione (10) was obtained via two different synthetic routes. The first involves a Suzuki–Miyaura30 palladium-catalysed coupling between the 4,4,5,5-tetramethyl-2-[4-(adamant-1-yl)phenyl]-2H-1,3,2-dioxaborolane (9)31 and the 2-thiazole bromide 7, which led to the protected precursor 10. The second synthetic approach, towards the thiazole adduct 10, was based on the Hantzsch condensation32 of thiobenzamide 14 with the α-bromoketone 15.6 Our lab has previously published the preparation of the 4-(adamant-1-yl)benzoic acid (12),31 which was now obtained by a different transition metal ion catalyzed oxidation of 1-(4-tolyl)adamantane (11).33 The reported method of oxidation34 was modified as the reaction mixture was bubbled with oxygen gas and heated at 105 °C for 6 h. The benzoic acid 12 was subsequently converted to the corresponding benzamide 13 and the thiobenzamide 14. Comparing the two methods, the first involves 5 steps (17% total yield), while the second 6 steps (25% total yield), a more facile work-up and cheaper reagents. The parent thiazole 2a was methylated, dimethylated and acetylated to the respective congeners 2b–d, as previously shown.
 |
| | Scheme 2 Reagents and conditions: (a) Pd(PPh3)4, Na2CO3, anh. toluene, 80 °C, 18 h, 87%; (b) O2, NaBr, Mn(OAc)2, Co(OAc)2, AcOH/dioxane, 90 °C, 6 h, 89%; (c) i. SOCl2, 65 °C, 45 min, ii. aq. NH3, 25%, r.t. 1 h, 96%; (d) Lawesson's reagent, dioxane, 110 °C, 2 h, 50%; (e) i-PrOH, autoclave, 120 °C, 18 h, 65%; (f) H2NNH2·H2O, EtOH, reflux 1 h; (g) acetic anhydride, Et3N, EtOAc, r.t. 48 h, 53% from 10; (h) i. ClCOOEt, NEt3, THF, r.t. 18 h, ii. LiAlH4, THF, reflux 4 h, iii. EtOH, H2O, NaOH 10%, 0 °C, 54% from 10; (i) MeONa, CH3COOH, HCHO 38% in H2O, NaBH3CN, MeOH, r.t. 18 h, 85% from 10. | |
The functionalized thiazoles 2e–h were obtained by the route shown in Scheme 3. The thiobenzamide 14 was condensed with 1,3-dichloroacetone and 4-chloroacetoacetate, under Hantzsch reaction conditions, to give the chloromethylthiazole 15 and the thiazolethyl acetate 16, respectively. Treatment of the choromethylthiazole 15 with KCN or KSCN led to the respective cyanide 2e and the thiocyanide 2f. The thiazolethyl acetate 16 was reduced to the corresponding alcohol 2g, which was then converted to the azide 2h, via activation of the methanesulphonyl derivative 17.
 |
| | Scheme 3 Reagents and conditions: (a) 1,3-dichloroacetone, acetone, reflux, 18 h, 75%; (b) KCN, anh. DMF, 60 °C, 36 h (2e: 41%) or KSCN, EtOH, 45 °C, 18 h (2f: 67%); (c) 4-chloroacetoacetate, i-PrOH, autoclave, 120 °C, 18 h, 92%; (d) i. LiAlH4, THF, r.t. 2 h, ii. EtOH, H2O, NaOH 10%, 0 °C, 80%; (e) MsCl, Et3N, DCM, 0 °C then r.t. 18 h, 95%; (f) NaN3, anh. DMF, 60 °C, 2 h, 65%. | |
The synthesis of the 2-substituted-4-{4-(adamant-1-yl)phenyl}thiazoles 3a–d and the guanidyl derivative 3e, is shown in Scheme 4. (1-Phenyl)adamantane (18)4 was acylated under Friedel–Crafts reaction conditions36 to deliver the corresponding α-bromoketone 19, which via a Hantzsch condensation with the appropriate reagent, thiourea,37 thioamides 20,3821 (ref. 7) and guanylthiourea provided the desired thiazoles 3a–c and 3e, respectively. The dimethylthiazole 3d was prepared from the parent thiazole 3c, as shown before.
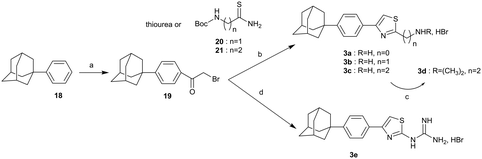 |
| | Scheme 4 Reagents and conditions: (a) BrCOCH2Br, AlCl3, DCM, −10 °C then r.t. 18 h, 57%; (b) appropriate thiobenzamide 20 (3b: 36%), 21 (3c: 85%), i-PrOH, autoclave, 120 °C 18 h or thiourea (3a: 88%), EtOH, reflux, 18 h; (c) MeONa, CH3COOH, HCHO 38% in H2O, NaBH3CN, MeOH, r.t. 18 h, 85%; (d) guanylthiourea, EtOH, reflux, 18 h, 89%. | |
The 1-adamantylcarbonylamides 4a, c and the (±)-10-camphorsulfonyl amides 4b, d were obtained upon coupling the commercially available 1-adamantylcarboxylic acid and (±)-10-camphorsulfonyl chloride with the 2-phenylthiazol-4-ethylamine (22)6 and the 4-phenylthiazol-2-ethylamine (23),7 respectively. The acid reacted in the presence of the coupling reagent HBTU, while the chlorides reacted without the aid of any activating reagent (Scheme 5).
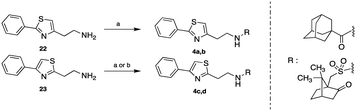 |
| | Scheme 5 Reagents and conditions: (a) appropriate chloride NEt3, DCM or THF, r.t. 18 h, (4a: 49%, 4b: 50%, 4d: 81%); (b) 1-adamantanecarboxylic acid HBTU, DIPEA, DCM/DMF, r.t. 24 h, 94%. | |
Pharmacology
The 27 new thiazole derivatives were tested for their activity against the bloodstream form Trypanosoma brucei and the results are shown in Table 1.
Table 1 Screening of the new thiazole derivatives against T. brucei
| Cmpd |
T. brucei IC50a (μM) |
T. brucei IC90a (μM) |
L6 cells IC50a (μM) |
S.I.b |
|
IC50 and IC90; concentration that inhibits growth by 50% and 90%.
S.I.; selectivity index, the ratio of IC50 values obtained with L6 cells and T. brucei respectively.
|
|
1a
|
0.42 ± 0.01 |
0.56 ± 0.01 |
1.05 ± 0.23 |
2.5 |
|
1b
|
0.90 ± 0.01 |
1.10 ± 0.01 |
2.01 ± 0.32 |
2.2 |
|
1c
|
0.79 ± 0.02 |
1.03 ± 0.01 |
1.53 ± 0.09 |
1.9 |
|
1d
|
15.3 ± 0.2 |
18.1 ± 0.2 |
— |
— |
|
1e
|
>25 |
— |
— |
— |
|
1f
|
>20 |
— |
— |
— |
|
1g
|
>20 |
— |
— |
— |
|
1h
|
>20 |
— |
— |
— |
|
1i
|
>20 |
— |
— |
— |
|
1j
|
10.7 ± 0.3 |
12.6 ± 0.2 |
<10.30 |
<1 |
|
2a
|
0.80 ± 0.03 |
1.17 ± 0.01 |
4.08 ± 0.15 |
5.1 |
|
2b
|
0.59 ± 0.02 |
0.79 ± 0.01 |
0.96 ± 0.26 |
1.6 |
|
2c
|
1.27 ± 0.07 |
1.60 ± 0.22 |
— |
— |
|
2d
|
>20 |
— |
— |
— |
|
2e
|
>20 |
— |
— |
— |
|
2f
|
∼10 |
— |
— |
— |
|
2g
|
∼10 |
— |
— |
— |
|
2h
|
>20 |
— |
— |
— |
|
3a
|
22.5 ± 0.6 |
30.5 ± 4.5 |
13.8 ± 1.6 |
<1 |
|
3b
|
9.76 ± 0.77 |
12.8 ± 0.2 |
12.6 ± 0.9 |
<1 |
|
3c
|
2.74 ± 0.29 |
4.40 ± 0.07 |
4.16 ± 0.24 |
1.5 |
|
3d
|
1.41 ± 0.09 |
3.58 ± 0.05 |
3.19 ± 0.25 |
2.3 |
|
3e
|
∼10 |
— |
— |
— |
|
4a
|
12.2 ± 0.8 |
18.7 ± 0.4 |
— |
— |
|
4b
|
20.6 ± 0.7 |
31.1 ± 0.3 |
— |
— |
|
4c
|
9.82 ± 0.22 |
13.1 ± 0.2 |
— |
— |
|
4d
|
23.8 ± 1.1 |
31.5 ± 0.6 |
— |
— |
It is apparent from the test results that the ethylamines 1a–c exhibit the highest activity among the new 2,4-disubstituted arylthiazoles. Bulkier substituents than the methyl group at the amino end have a negative impact on trypanocidal activity. Amido adducts (alkyl 1d and 1c, the aromatic 1f and 1g and the heteroaromatic 1h) and the ureido derivatives, 1i and 1j, have a non-significant activity. The same pattern is also observed in the 2 series, as compounds 2a–c are ca. 20 times more active than their acetamido congener 2d. Comparing series 1 and 2, it becomes apparent that the fluorine substitution has little positive effect on the activity. The dimethylamino isomeric thiazoles 2c and 3d present almost the same potency, while the nor-derivatives, the isomeric thiazoles 2a and 3c, show a substantial difference in potency. The 2-phenylthiazol-4-ethylamines 1a, c and 2a, c seem to be, in general, more potent than their isomeric 4-phenylthiazol-2-ethylamines 3c and 3d. The decrease of the length of the side chain does not enhance activity. Methanamine 3b bears two atoms (carbon and nitrogen) in its side chain and is twice as potent as the 2-aminothiazole 3a, which has only one nitrogen atom. The polar functionalization of the side chain did not improve the trypanocidal activity. The azido and cyano-tailored derivatives 2e and 2h are less potent, and the thiocyanate 2f, the ethanol 2g and the guanyl derivative 3e exhibit modest activity. The change in the relative position of the adamantane cage, the phenyl ring and the thiazole moiety, in adducts 4a and 4c, did not lead to activity enhancement. Last, the replacement of the adamantane skeleton by the camphorsulfonyl moiety in derivatives 4b and 4d has not led to antitrypanosomal enhancement. The 2,4-disubstituted arylthiazole adamantane derivatives, the ethylamines 1a–c and 2a–c, present a notable pharmacological profile, which merits further investigation in terms of activity and toxicity. These findings suggest that an aliphatic amine moiety at the side chain is mandatory to achieve notable trypanocidal activity. This amine group is positively charged at the cytosolic pH, which is not the case for all the other polar heads tested. The presence of this particular group might also enhance the cellular accumulation into the protozoa, as it is reported in the case of bacteria.39,40 Thus, the ethylamines 1a–c and 2a–c seem to exhibit promising trypanocidal properties, although further optimisation will be necessary to reduce their cytotoxicity and to develop a more drug-like profile.
Conclusions
In this work, we describe the synthesis of a new series of aromatic 2,4-disubstituted 1,3-thiazole analogues with trypanocidal potency. Among their congeners, the 2-phenylthiazol-4-ethylamines 1a–c and 2a–c presented the most significant trypanocidal activity against T. brucei. Analogues 1a and 2a exhibit antitrypanosomal activity in the range of IC50 = 0.42 μM and IC50 = 0.80 μM, respectively. Primary amine 2a is less potent than its congener 1a, but exhibits higher selectivity, which is a promising perspective for designing new trypanocidals in the future. Both of these classes of derivatives bear a lipophilic end, which consists of a 4-(1-adamantyl)phenyl or a 3-(1-adamantyl)phenyl moiety, a 1,3-thiazole ring and a functional end, which comprises of an alkylamine. The addition of the adamantane ring into the scaffold of the thiazole reference compounds6,7 has not improved their pharmacological profile, in terms of activity and toxicity. On the other hand, the new congeners exhibit promising trypanocidal properties that merit further investigation. These tailored-made structural modifications will be implemented in the future in the design of trypanocidal agents.
Experimental part
Biology
Cytotoxic activity against rat skeletal myoblast L6 cells.
Cytotoxicity against mammalian cells was assessed using microtitre plates. Briefly, L6 cells (a rat skeletal muscle line) were seeded at 1 × 104 mL−1 in 200 μL of growth medium containing 7 different compound concentrations in a range previously established to encompass both the IC50 and IC90 values. The plates were incubated for 6 days at 37 °C and 20 μL Alamar Blue (Biosource UK Ltd) was then added to each well. After an additional 8 hours incubation, the fluorescence was determined using a FLUOstar Omega fluorescent plate reader (BMG Labtech). Inhibition of growth was calculated by comparison with control values and IC50 and IC90 values were determined in triplicate using linear regression analysis.
Trypanosoma brucei culturing and drug testing.
Bloodstream form T. brucei (strain 427) were cultured at 37 °C in modified Iscove's medium. Trypanocidal activity was assessed by growing parasites in microtiter plates in the presence of various drug concentrations. Parasites were seeded at 0.25 × 105 mL−1 in 200 μL of growth medium containing 7 different compound concentrations in a range previously established to encompass both the IC50 and IC90 values. The plates were incubated for 48 hours at 37 °C and 20 μL Alamar Blue was then added to each well. After an additional overnight incubation, the fluorescence was determined. Inhibition of growth was calculated by comparison with control values and IC50 and IC90 values were determined in triplicate using linear regression analysis.
Synthetic procedures.
All chemicals and solvents were obtained from commercial suppliers and used without further purification. Reactions were monitored by thin layer chromatography. Melting points were determined on a Büchi 530 apparatus and are uncorrected. Infrared (IR) spectra were recorded on a Perkin-Elmer 833 spectrophotometer. 1H-NMR spectra recorded on a Bruker DRX 400 (400 MHz) spectrometer and 13C-NMR spectra were taken at 50 MHz on Bruker AC 200 (200 MHz) spectrometer and at 150 MHz on Bruker Avance 600 spectrometer (600 MHz). All NMR spectra were taken in deuterochloroform or hexadeuterodimethyl sulfoxide and the chemical shifts are reported in ppm. Elemental analyses (C, H, N) were carried out by the Institute of Chemical Biology, NHRF, Greece and the results obtained had a maximum deviation of ±0.4% from the theoretical value.
3-{(1-Tricyclo[3.3.1.13,7]decyl)-4-fluorophenyl}boronic acid (6).
n-BuLi (4 mL,1.6 M in hexanes, 6.4 mmol) was added in one portion to a stirred solution of the bromide 5 (ref. 28) (1.25 g, 4.04 mmol) in anhydrous THF (20 mL), at −73 °C, under an argon atmosphere. The mixture was then stirred at −80 °C for 25 min prior to addition of (i-PrO)3B (3 mL, 12.1 mmol). The reaction mixture was stirred for 35 min at the same temperature and subsequently at ambient temperature overnight. Next, dilute HCl (20 mL) was added dropwise at 0 °C, the mixture was stirred for 30 min at room temperature and then extracted with EtOAc (3 × 20 mL). The combined organic layers were washed with water, dried over Na2SO4 and the solvent evaporated under reduced pressure. The residue was crystallized from n-hexane to give compound 6 (1.1 g, 90%) as a white solid, which was used directly in the next step. 1H NMR (400 MHz, CDCl3) δ 8.18 (d, J = 8.7 Hz, 1H, 2-Har), 8.03 (dd, J = 8.4, 4.2 Hz, 1H, 6-Har), 7.12 (dd, J = 8.1, 7.8 Hz, 1H, 5-Har), 2.12 (bs, 3H, 3,5,7-Had), 2.06 (bs, 6H, 2,8,9-Had), 1.79 (br.s, 6H, 4,6,10-Had).
2-{2-[2-((1-Tricyclo[3.3.1.13,7]decyl)-4-fluorophenyl)thiazol-4-yl]ethyl}isoindoline-1,3-dione (8).
Argon was bubbled for 20 min through a stirred mixture of boronic acid 6 (250 mg, 0.9 mmol), the 2-thiazole bromide 7 (ref. 6) (161 mg, 0.45 mmol), toluene (5 mL) and Na2CO3 (2 M, 5 mL). The reaction mixture was then heated to 80 °C, under an argon atmosphere, Pd(PPh3)4 (107 mg) was added and heating was continued overnight. After cooling, the reaction mixture was extracted with EtOAc (3 × 25 mL) and the combined organics were washed with water dried over Na2SO4 and the solvent removed in vacuo. The residue was purified by column chromatography. Elution with 10–20% EtOAc in hexanes afforded compound 8 as a foamy solid (175 mg, 80%). 1H NMR (400 MHz, CDCl3) δ 7.85–7.79 (m, 2H, 3′,4′-Har), 7.76 (dd, J = 7.7, 2.2 Hz, 1H, 2-Har), 7.73–7.68 (m, 2H, 2′,5′-Har), 7.64–7.58 (m, 1H, 6-Har), 6.71 (s, 1H, 5-Hth), 6.96 (dd, J = 12.7, 8.3 Hz, 1H, 5-Har), 4.11 (t, J = 7.0 Hz, 2H, CH2N), 3.23 (t, J = 7.0 Hz, 2H, CH2), 2.10 (br.s, 3H, 3,5,7-Had), 2.04 (bs, 6H, 2,8,9-Had), 1.79 (br.s, 6H, 4,6,10-Had). 13C NMR (150 MHz, DMSO-d6) δ 167.2 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 166.6 (2-Cth), 162.8 (d, J = 251.9 Hz, 4-Car), 152.8 (4-Cth), 137.5 (d, J = 11.4 Hz, 3-Car), 132.9 (1′,6′-Car), 132.8 (3′,4′-Car), 129.2 (1-Car), 126.2 (d, J = 8.4 Hz, 2-Car), 125.1 (6-Car), 122.2 (2′,5′-Car) 117.5 (d, J = 25.4, 5-Car), 116.4 (5-Cth), 40.5 (2,8,9-Cad), 38.4 (NCH2), 36.2 (1-Cad), 36.0 (4,6,10-Cad), 28.8 (CH2), 28.2 (3,5,7-Cad). Anal. calcd for C29H27FN2O2S: C, 71.58; H, 5.59; N, 5.76 found C, 71.35; H, 5.41; N, 5.54.
O), 166.6 (2-Cth), 162.8 (d, J = 251.9 Hz, 4-Car), 152.8 (4-Cth), 137.5 (d, J = 11.4 Hz, 3-Car), 132.9 (1′,6′-Car), 132.8 (3′,4′-Car), 129.2 (1-Car), 126.2 (d, J = 8.4 Hz, 2-Car), 125.1 (6-Car), 122.2 (2′,5′-Car) 117.5 (d, J = 25.4, 5-Car), 116.4 (5-Cth), 40.5 (2,8,9-Cad), 38.4 (NCH2), 36.2 (1-Cad), 36.0 (4,6,10-Cad), 28.8 (CH2), 28.2 (3,5,7-Cad). Anal. calcd for C29H27FN2O2S: C, 71.58; H, 5.59; N, 5.76 found C, 71.35; H, 5.41; N, 5.54.
2-{2-[3-(1-Tricyclo[3.3.1.13,7]decyl)-4-fluorophenyl]thiazol-4-yl}ethan-1-amine (1a).
A solution of phthalimide 8 (600 mg, 1.23 mmol) and hydrazine hydride (2 mL) in EtOH (20 mL) was refluxed for 1 h and then cooled to 0 °C. The resulting suspension was filtered and the filtrate evaporated. The residue (crude amine 1a) was used in the next steps without further purification. M.p. (dihydrochloride): 246–248 °C (EtOH/Et2O). 1H NMR (600 MHz, DMSO-d6) δ 8.35 (bs, 1H, NHth), 7.83–7.73 (m, 2H, 2.6-Har), 7.50 (s, 1H, 5-Hth), 7.18 (dd, J = 12.7, 8.4 Hz, 1H, 5-Har), 6.10 (br.s, 4H, NH4), 3.31–2.93 (m, 4H, CH2,NCH2), 2.01 (br.s, 3H, 3,5,7-Had), 1.96 (br.s, 6H, 2,8,9-Had), 1.69 (br.s, 6H, 4,6,10-Had). 13C NMR (151 MHz, DMSO-d6) δ 166.6 (s, 2-Cth), 162.8 (d, J = 251.9 Hz, 4-Car), 152.8 (4-Cth), 137.5 (d, J = 11.4 Hz, 3-Car), 129.2 (1-Car), 126.2 (d, J = 8.4 Hz, 2-Car), 125.1 (6-Car), 117.5 (d, J = 25.4 Hz, 5-Car), 116.4 (5-Cth), 40.5 (2,8,9-Cad), 38.4 (NCH2), 36.2 (1-Cad), 36.0 (4,6,10-Cad), 28.8 (CH2), 28.2 (3,5,7-Cad). Anal. calcd for C21H29FCl2N2S: C, 58.74; H, 6.34; N, 6.52 found C, 58.51; H, 6.44; N, 6.68.
2-{2-[3-(1-Tricyclo[3.3.1.13,7]decyl)-4-fluorophenyl]thiazol-4-yl}-N-methylethan-1-amine (1b).
Ethyl chloroformate (0.1 mL, 1.01 mmol) and Et3N (0.15 mL) were added to a stirred solution of the amine 1a (180 mg, 0.51 mmol) in anhydrous THF (3 mL), at 0 °C, under an argon atmosphere. The reaction mixture was stirred for 5 min at 0 °C and then at ambient temperature, overnight. Next, water was added into the mixture, which was then extracted with EtOAc. The organic phase was washed with water dried over MgSO4 and the solvent evaporated. The resulting residue was used in the next step without further purification.
A solution of the crude amide (220 mg, 0.51 mmol) in anhydrous THF (5 mL) was added dropwise to a stirred suspension of LiAlH4 (100 mg, 2.52 mmol) in anhydrous THF (5 mL), under an argon atmosphere. The mixture was stirred at ambient temperature for 25 min and then refluxed for 4 h. Next, the reaction mixture was cooled in an ice bath, and ethanol, water and a NaOH (10%) solution were sequentially added. The resulting suspension was then filtered, the filtrate was evaporated in vacuo and the resulting residue was treated with water and a HCl (5%) solution. The aqueous phase was then washed with Et2O and solid Na2CO3 was added until pH = 10. The aqueous phase was then extracted with DCM and the combined organic phase was dried over Na2CO3 and the solvent evaporated in vacuo to afford compound 1b, as a viscous oil (170 mg, 88% from compound 8). M.p. (dihydrochloride): 221–223 °C (EtOH/Et2O). 1H NMR (400 MHz, DMSO-d6) δ 9.26 (br.s, 1H, NHth), 7.97 (bs, 2H, NH2), 7.86–7.76 (m, 2H, 2,6-Har), 7.53 (s, 1H, 5-Hth), 7.24 (dd, J = 12.8, 8.3 Hz, 1H, 5-Har), 3.24 (d, J = 4.7 Hz, 2H, NCH2), 3.17 (d, J = 7.5 Hz, 2H, CH2), 2.57 (t, J = 5.4 Hz, 3H, CH3), 2.07 (s, 3H, 3,5,7-Had), 2.02 (s, 6H, 2,8,9-Had), 1.74 (s, 6H, 4,6,10-Had). 13C NMR (150 MHz, DMSO-d6) δ 166.5 (2-Cth), 162.7 (d, J = 251.7 Hz, 4-Car), 152.7 (4-Cth), 137.4 (d, J = 11.1 Hz, 3-Car), 129.3 (1-Car), 126.0 (d, J = 10.2 Hz, 2-Car), 124.9 (d, J = 7.1 Hz, 6-Car), 117.4 (d, J = 26.5 Hz, 5-Car), 116.1 (5-Cth), 47.2 (NCH2), 40.4 (2,8,9-Cad), 36.2 (4,6,10-Cad), 36.0 (1-Cad), 32.4 (CH3), 28.1 (3,5,7-Cad), 27.4 (CH2). Anal. calcd for C22H31FCl2N2S: C, 58.59; H, 6.59; N, 6.32 found C, 58.71; H, 6.25; N, 6.09.
2-{2-[3-(1-Tricyclo[3.3.1.13,7]decyl)-4-fluorophenyl]thiazol-4-yl}-N,N-dimethylethyl-1-amine (1c).
A solution of MeONa (0.1 mL, 30% in MeOH, 0.52 mmol) was added to a stirred solution of compound 1a dihydrochloride (220 mg, 0.52 mmol) in MeOH (8 mL) and the resulting mixture was stirred for 10 min in ambient temperature. Then acetic acid (0.12 mL, 2 mmol) and NaCNBH3 (65 mg, 1.01 mmol) were added into the reaction mixture. Subsequently, a solution of aq. HCHO (38%, 0.1 mL, 1.20 mmol) dissolved in MeOH (2.5 mL) was added dropwise over the course of 30 min and the reaction mixture was stirred at ambient temperature, overnight. The solvent was removed in vacuo and an aqueous solution of NaOH (4N, 5 mL) was added. The resulting mixture was then extracted with EtOAc (3 × 20 mL) and the combined organic phases were washed with brine, dried over MgSO4 and the solvent evaporated to afford compound 1c, as a yellow viscous oil (150 mg, 75% from compound 8). M.p. (dihydrochloride): 280–282 °C (EtOH/Et2O). 1H NMR (400 MHz, DMSO-d6) δ 10.89 (s, 1H, NHTh), 7.86–7.76 (m, 2H, 2,6-Har), 7.53 (s, 1H, 5-Hth), 7.25 (dd, J = 12.7, 8.2 Hz, 1H, 5-Har), 6.05 (s, 1H, NH), 3.52–3.36 (m, 2H, NCH2), 3.30–3.19 (m, 2H, CH2), 2.81 (d, J = 4.9 Hz, 6H, CH3), 2.07 (s, 3H, 3,5,7-Had), 2.02 (s, 6H, 2,8,9-Had), 1.75 (s, 6H, 4,6,10-Had). 13C NMR (150 MHz, DMSO-d6) δ 166.9 (2-Cth), 163.1 (d, J = 251.8 Hz, 4-Car), 153.0 (4-Cth), 137.9 (d, J = 11.1 Hz, 3-Car), 129.8 (d, J = 2.8 Hz, 1-Car), 126.4 (d, J = 9.8 Hz, 2-Car), 125.3 (d, J = 5.4 Hz, 6-Car), 117.8 (d, J = 25.6 Hz, 5-Car), 116.6 (5-Cth), 55.8 (NCH2), 42.5 (CH3), 40.9 (2,8,9-Cad), 36.6 (4,6,10-Cad), 36.4 (1-Cad), 28.5 (3,5,7-Cad), 26.4 (CH2). Anal. calcd for C23H33FCl2N2S: C, 60.39; H, 6.83; N, 6.12 found C, 60.50; H, 6.91; N, 6.41.
General method for the preparation of amides 1d–g, i, j and 2d.
A stirred solution of amine 1a or 2a (1 eq.) and Et3N (0.5 mL) in EtOAc (7 mL) was cooled to 0 °C and the appropriate acid chloride or anhydride (2–3 eq.) was added under an argon atmosphere. The reaction mixture was stirred at ambient temperature for 24–48 h. Water was added into the mixture, which was then extracted with EtOAc. The combined organic layers were washed with water, dried over Na2SO4, the solvent evaporated and the residue was purified by column chromatography.
N-{2-[2-(3-(1-Tricyclo[3.3.1.13,7]decyl)-4-fluorophenyl]thiazol-4-yl]ethyl}acetamide (1d).
Acetamide 1d was prepared, as described in the general method, using acetic anhydride (3 eq.). Elution with EtOAc afforded compound 1d as a foamy solid (140 mg, 84% from compound 8). 1H NMR (400 MHz, CDCl3) δ 7.85 (dd, J = 7.7, 2.2 Hz, 1H, 2-Har), 7.70 (ddd, J = 8.2, 4.3, 2.2 Hz, 1H, 6-Har), 7.04 (dd, J = 12.4, 8.4 Hz, 1H, 5-Har), 6.95 (s, 1H, 5-Hth), 6.49 (br.s, 1H, NH), 3.64 (q, J = 6.3 Hz, 2H, CH2N), 2.98 (t, J = 6.3 Hz, 2H, CH2), 2.09 (br.s, J = 14.7 Hz, 9H, 3,5,7,2,8,9-Had), 1.99 (s, 3H, CH3), 1.80 (br.s, 6H, 4,6,10-Had). 13C NMR (50 MHz, CDCl3) δ 170.1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 166.1 (2-Cth), 164.5 (d, J = 349.7 Hz, 4-Car), 155.5 (4-Cth), 138.6 (d, J = 11.2 Hz, 3-Car), 129.6 (1-Car), 125.8 (d, J = 2.9 Hz, 6-Car), 125.6 (2-Car), 117.2 (d, J = 25.8 Hz, 5-Car), 114.2 (5-Cth), 41.1 (d, J = 3.5 Hz, 2,8,9-Cad), 39.1 (CH2N), 36.9 (4,6,10-Cad), 36.7 (1-Cad), 31.0 (CH2), 28.9 (3,5,7-Cad), 23.5 (CH3). M.p. (fumarate): 143-145 °C (MeOH/Et2O). Anal. calcd for C27H31FN2O5S: C, 63.03; H, 6.07; N, 5.44 found C, 62.89; H, 6.01; N, 5.21.
O), 166.1 (2-Cth), 164.5 (d, J = 349.7 Hz, 4-Car), 155.5 (4-Cth), 138.6 (d, J = 11.2 Hz, 3-Car), 129.6 (1-Car), 125.8 (d, J = 2.9 Hz, 6-Car), 125.6 (2-Car), 117.2 (d, J = 25.8 Hz, 5-Car), 114.2 (5-Cth), 41.1 (d, J = 3.5 Hz, 2,8,9-Cad), 39.1 (CH2N), 36.9 (4,6,10-Cad), 36.7 (1-Cad), 31.0 (CH2), 28.9 (3,5,7-Cad), 23.5 (CH3). M.p. (fumarate): 143-145 °C (MeOH/Et2O). Anal. calcd for C27H31FN2O5S: C, 63.03; H, 6.07; N, 5.44 found C, 62.89; H, 6.01; N, 5.21.
N-{2-[2-(3-(1-Tricyclo[3.3.1.13,7]decyl)-4-fluorophenyl)thiazol-4-yl]ethyl}butylamide (1e).
Butylamide 1e was prepared, as described in general method, using butyric anhydride (3 eq.). Elution with 50% EtOAc in hexanes afforded compound 1e as a foamy solid (165 mg, 92% from 8). 1H NMR (400 MHz, CDCl3) δ 7.85 (dd, J = 7.7, 2.2 Hz, 1H, 2-Har), 7.70 (ddd, J = 8.2, 4.3, 2.2 Hz, 1H, 6-Har), 7.04 (dd, J = 12.4, 8.4 Hz, 1H, 5-Har), 6.95 (s, 1H, 5-Hth), 6.49 (br.s, 1H, NH), 3.64 (q, J = 6.3 Hz, 2H, CH2N), 2.98 (t, J = 6.3 Hz, 2H, CH2), 2.09 (br.s, J = 14.7 Hz, 9H, 3,5,7,2,8,9-Had), 2.18 (t, J = 6.9 Hz, 2H, CH2CO), 1.80 (br.s, 6H, 4,6,10-Had), 1.67 (h, J = 6.9 Hz, 2H, CH2), 0.94 (t, J = 7.4 Hz, 3H, CH3). 13C NMR (50 MHz, CDCl3) δ 170.2 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 166.1 (2-Cth), 164.5 (d, J = 349.7 Hz, 4-Car), 155.5 (4-Cth), 138.3 (d, J = 11.2 Hz, 3-Car), 129.6 (1-Car), 125.8 (d, J = 2.9 Hz, 6-Car), 125.6 (2-Car), 117.2 (d, J = 25.8 Hz, 5-Car), 114.3 (5-Cth), 41.1 (2,8,9-Cad), 39.2 (CH2N), 36.9 (4,6,10-Cad), 36.7 (1-Cad), 31.0 (CH2), 28.9 (3,5,7-Cad), 28.9 (CH2), 19.3 (CH2), 13.9 (CH3). M.p. (fumarate): 291–293 °C (MeOH/Et2O). Anal. calcd for C29H35FN2O5S: C, 64.19; H, 6.50; N, 5.16 found C, 64.38; H, 6.61; N, 5.33.
O), 166.1 (2-Cth), 164.5 (d, J = 349.7 Hz, 4-Car), 155.5 (4-Cth), 138.3 (d, J = 11.2 Hz, 3-Car), 129.6 (1-Car), 125.8 (d, J = 2.9 Hz, 6-Car), 125.6 (2-Car), 117.2 (d, J = 25.8 Hz, 5-Car), 114.3 (5-Cth), 41.1 (2,8,9-Cad), 39.2 (CH2N), 36.9 (4,6,10-Cad), 36.7 (1-Cad), 31.0 (CH2), 28.9 (3,5,7-Cad), 28.9 (CH2), 19.3 (CH2), 13.9 (CH3). M.p. (fumarate): 291–293 °C (MeOH/Et2O). Anal. calcd for C29H35FN2O5S: C, 64.19; H, 6.50; N, 5.16 found C, 64.38; H, 6.61; N, 5.33.
N-{2-[2-(3-(1-Tricyclo[3.3.1.13,7]decyl)-4-fluorophenyl)thiazol-4-yl] ethyl}benzamide (1f).
Benzamide 1f was prepared, as described in general method, using benzoyl chloride (2 eq.). Elution with 30% EtOAc in hexanes afforded compound 1f as a foamy solid (186 mg, 96%, from 8). 1H NMR (400 MHz, CDCl3) δ 7.91–7.82 (m, 2H, 2′,6′-Har), 7.85 (dd, J = 7.7, 2.2 Hz, 1H, 2-Har), 7.70 (ddd, J = 8.2, 4.3, 2.2 Hz, 1H, 6-Har), 7.59–7.51 (m, 1H, 4′-Har), 7.46–7.36 (m, 2H, 3′,5′-Har), 7.04 (dd, J = 12.4, 8.4 Hz, 1H, 5-Har), 6.95 (s, 1H, 5-Hth), 6.49 (br.s, 1H, NH), 3.64 (q, J = 6.3 Hz, 2H, CH2N), 2.98 (t, J = 6.3 Hz, 2H, CH2), 2.09 (br.s, J = 14.7 Hz, 9H, 3,5,7-Had, 2,8,9-Had), 1.80 (bs, 6H, 4,6,10-Had). 13C NMR (50 MHz, CDCl3) δ 166.1 (2-Cth), 166.0 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 164.5 (d, J = 349.7 Hz, 4-Car), 155.5 (4-Cth), 138.3 (d, J = 11.2 Hz, 3-Car), 131.3 (4′-Car), 129.6 (1-Car), 128.5 (3′,5′-Car), 127.0 (2′,6′-Car), 125.8 (d, J = 2.9 Hz, 6-Car), 125.6 (2-Car), 117.2 (d, J = 25.8 Hz, 5-Car), 114.3 (5-Cth), 41.1 (2,8,9-Cad), 39.2 (CH2N), 36.9 (4,6,10-Cad), 36.7 (1-Cad), 31.0 (CH2), 28.9 (3,5,7-Cad). M.p. (fumarate): 305 °C (dec) (MeOH/Et2O). Anal. calcd for C32H33FN2O5S: C, 66.65; H, 5.77; N, 4.86 found C, 65.39; H, 5.90; N, 4.72.
O), 164.5 (d, J = 349.7 Hz, 4-Car), 155.5 (4-Cth), 138.3 (d, J = 11.2 Hz, 3-Car), 131.3 (4′-Car), 129.6 (1-Car), 128.5 (3′,5′-Car), 127.0 (2′,6′-Car), 125.8 (d, J = 2.9 Hz, 6-Car), 125.6 (2-Car), 117.2 (d, J = 25.8 Hz, 5-Car), 114.3 (5-Cth), 41.1 (2,8,9-Cad), 39.2 (CH2N), 36.9 (4,6,10-Cad), 36.7 (1-Cad), 31.0 (CH2), 28.9 (3,5,7-Cad). M.p. (fumarate): 305 °C (dec) (MeOH/Et2O). Anal. calcd for C32H33FN2O5S: C, 66.65; H, 5.77; N, 4.86 found C, 65.39; H, 5.90; N, 4.72.
N-{2-[2-(3-(1-Tricyclo[3.3.1.13,7]decyl)-4-fluorophenyl)thiazol-4-yl]ethyl}-4-fluorobenzamide (1g).
4-Fluorobenzamide 1g was prepared, as described in general method, using 4-fluorobenzoyl chloride (3 eq.). Elution with 50% EtOAc in hexanes afforded compound 1g as a foamy solid (185 mg, 93% from compound 8). 1H NMR (400 MHz, DMSO-d6) δ 8.05 (br.s, 1H, NH), 7.80 (m, 2H, 2′,6′-Har), 7.75 (dd, J = 7.7, 2.0 Hz, 1H, 2-Har), 7.68 (dd, J = 7.7, 8.9 Hz, 1H, 6-Har), 7.12–6.96 (m, 4H, 5-Har, 5-Hth, 3′,5′-Har), 3.70 (q, J = 5.8 Hz, 2H, CH2N), 3.05 (t, J = 6.7 Hz, 2H, CH2), 2.03 (s, 3H, 3,5,7-Had), 1.99 (s, 6H, 2,8,9-Had), 1.72 (s, 6H, 4,6,10-Had). 13C NMR (50 MHz, CDCl3) δ 166.1 (2-Cth), 166.0 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 164.5 (d, J = 349.7 Hz, 4-Car), 163.9 (d, J = 348.6 Hz, 4′-Car) 155.5 (4-Cth), 138.3 (d, J = 11.2 Hz, 3-Car), 130.1 (d, J = 3 Hz, 1′-Car), 129.6 (1-Car), 128.3 (d, J = 7 Hz, 2′,6′-Car), 125.8 (d, J = 2.9 Hz, 6-Car), 125.6 (2-Car), 117.2 (d, J = 25.8 Hz, 5-Car), 116.1 (d, J = 18 Hz, 3′,5′-Car) 114.3 (5-Cth), 41.1 (d, J = 3.5 Hz, 2,8,9-Cad), 39.2 (CH2N), 36.9 (4,6,10-Cad), 36.7 (1-Cad), 31.0 (CH2), 28.9 (3,5,7-Cad). M.p. (fumarate): 226–227 °C (MeOH/Et2O). Anal. calcd for C32H32F2N2O5S: C, 64.63; H, 5.42; N, 4.71 found C, 64.44; H, 5.73; N, 4.88.
O), 164.5 (d, J = 349.7 Hz, 4-Car), 163.9 (d, J = 348.6 Hz, 4′-Car) 155.5 (4-Cth), 138.3 (d, J = 11.2 Hz, 3-Car), 130.1 (d, J = 3 Hz, 1′-Car), 129.6 (1-Car), 128.3 (d, J = 7 Hz, 2′,6′-Car), 125.8 (d, J = 2.9 Hz, 6-Car), 125.6 (2-Car), 117.2 (d, J = 25.8 Hz, 5-Car), 116.1 (d, J = 18 Hz, 3′,5′-Car) 114.3 (5-Cth), 41.1 (d, J = 3.5 Hz, 2,8,9-Cad), 39.2 (CH2N), 36.9 (4,6,10-Cad), 36.7 (1-Cad), 31.0 (CH2), 28.9 (3,5,7-Cad). M.p. (fumarate): 226–227 °C (MeOH/Et2O). Anal. calcd for C32H32F2N2O5S: C, 64.63; H, 5.42; N, 4.71 found C, 64.44; H, 5.73; N, 4.88.
N-{2-[2-(3-(1-Tricyclo[3.3.1.13,7]decyl)-4-fluorophenyl)thiazol-4-yl] ethyl}-3-methylfuran-2-ylcarboxamide (1h).
DMAP (57 mg, 0.46 mmol), EDCI (90 mg, 0.46 mmol) and HOBt (65 mg 0.41 mmol) were added to a stirred mixture of the amine 1a (150 mg, 0.41 mmol) and 3-methylfuroic acid (52 mg, 0.41 mmol) in anhydrous DMF (2 mL) and anhydrous DCM (1 mL) at 0 °C, under an argon atmosphere. The reaction mixture was stirred at the same temperature for 1 h and then at 45 °C, overnight. Water was added into the mixture, which was then extracted with EtOAc. The organic phase was washed with water dried over Na2SO4 and the solvent evaporated in vacuo. The residue was purified by column chromatography. Elution with EtOAc afforded compound 1h as a foamy solid (50 mg, 29% from compound 8).1H NMR (400 MHz, CDCl3) δ 7.85 (dd, J = 7.7, 2.2 Hz, 1H, 2-Har), 7.70 (ddd, J = 8.2, 4.3, 2.2 Hz, 1H, 6-Har), 7.30 (d, J = 0.9 Hz, 1H, 5-Hfur) 7.04 (dd, J = 12.4, 8.4 Hz, 1H, 5-Har), 6.95 (s, 1H, 5-Hth), 6.49 (bs, 1H, NH), 6.35 (d, J = 1.0 Hz, 1H, 4-Hfur), 3.64 (q, J = 6.3 Hz, 2H, CH2N), 2.98 (t, J = 6.3 Hz, 2H, CH2), 2.40 (s, 3H, CH3), 2.09 (br.s, J = 14.7 Hz, 9H, 3,5,7,2,8,9-Had), 1.80 (br.s, 6H, 4,6,10-Had). 13C NMR (50 MHz, CDCl3) δ 166.1 (2-Cth), 164.5 (d, J = 349.7 Hz, 4-Car), 160.6 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 155.5 (4-Cth), 142.7 (4-Cfur), 141.1 (1-Cfur), 138.3 (d, J = 11.2 Hz, 3-Car), 129.6 (1-Car), 125.8 (d, J = 2.9 Hz, 6-Car), 125.6 (2-Car), 117.2 (d, J = 25.8 Hz, 5-Car), 115.6 (2-Cfur), 115.2 (5-Cth), 114.3 (3-Cfur), 41.1 (2,8,9-Cad), 39.2 (CH2N), 36.9 (4,6,10-Cad), 36.7 (1-Cad), 31.0 (CH2), 28.9 (3,5,7-Cad), 11.0 (CH3). M.p. (fumarate): 260 (dec) °C (MeOH/Et2O). Anal. Calcd for C31H32FN2O6S: C, 64.12; H, 5.73; N, 4.82 found C, 64.44; H, 5.54; N, 4.98.
O), 155.5 (4-Cth), 142.7 (4-Cfur), 141.1 (1-Cfur), 138.3 (d, J = 11.2 Hz, 3-Car), 129.6 (1-Car), 125.8 (d, J = 2.9 Hz, 6-Car), 125.6 (2-Car), 117.2 (d, J = 25.8 Hz, 5-Car), 115.6 (2-Cfur), 115.2 (5-Cth), 114.3 (3-Cfur), 41.1 (2,8,9-Cad), 39.2 (CH2N), 36.9 (4,6,10-Cad), 36.7 (1-Cad), 31.0 (CH2), 28.9 (3,5,7-Cad), 11.0 (CH3). M.p. (fumarate): 260 (dec) °C (MeOH/Et2O). Anal. Calcd for C31H32FN2O6S: C, 64.12; H, 5.73; N, 4.82 found C, 64.44; H, 5.54; N, 4.98.
N-(2-(2-(3-(1-Tricyclo[3.3.1.13,7]decyl)-4-fluorophenyl)thiazol-4-yl) ethyl)pyrrolidin-1-yl-carboxamide (1i).
Carboxamide 1i was prepared, as described in general method, using 1-pyrrolidinecarbonyl chloride (2 eq.). Elution with 75% EtOAc in hexanes afforded compound 1i as a foamy solid (80 mg, 46% from compound 8). 1H NMR (400 MHz, CDCl3) δ 7.85 (dd, J = 7.7, 2.2 Hz, 1H, 2-Har), 7.70 (ddd, J = 8.2, 4.3, 2.2 Hz, 1H, 6-Har), 7.04 (dd, J = 12.4, 8.4 Hz, 1H, 5-Har), 6.95 (s, 1H, 5-Hth), 5.24 (br.s, 1H, NH), 3.64 (q, J = 6.3 Hz, 2H, CH2N), 3.46–3.26 (m, 4H, 2,5-Hpy) 2.98 (t, J = 6.3 Hz, 2H, CH2), 2.09 (br.s, 9H, 3,5,7,2,8,9-Had), 1.94–1.85 (m, 4H, 3,4-Hpy), 1.80 (br.s, 6H, 4,6,10-Had). 13C NMR (50 MHz, CDCl3) δ 166.1 (2-Cth), 164.5 (d, J = 349.7 Hz, 4-Car), 156.9 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 155.5 (4-Cth), 138.3 (d, J = 11.2 Hz, 3-Car), 129.6 (1-Car), 125.8 (d, J = 2.9 Hz, 6-Car), 125.6 (2-Car), 117.2 (d, J = 25.8 Hz, 5-Car), 114.3 (5-Cth), 45.4 (2,5-Cpy), 41.1 (d, J = 3.5 Hz, 2,8,9-Cad), 39.2 (CH2N), 36.9 (4,6,10-Cad), 36.7 (1-Cad), 31.0 (CH2), 28.9 (3,5,7-Cad), 25.6 (3,4-Cpy). M.p. (fumarate): 301 °C (dec) (MeOH/Et2O). Anal. calcd for C30H36FN3O5S: C, 63.25; H, 6.37; N, 7.38 found C, 63.09; H, 6.14; N, 7.19.
O), 155.5 (4-Cth), 138.3 (d, J = 11.2 Hz, 3-Car), 129.6 (1-Car), 125.8 (d, J = 2.9 Hz, 6-Car), 125.6 (2-Car), 117.2 (d, J = 25.8 Hz, 5-Car), 114.3 (5-Cth), 45.4 (2,5-Cpy), 41.1 (d, J = 3.5 Hz, 2,8,9-Cad), 39.2 (CH2N), 36.9 (4,6,10-Cad), 36.7 (1-Cad), 31.0 (CH2), 28.9 (3,5,7-Cad), 25.6 (3,4-Cpy). M.p. (fumarate): 301 °C (dec) (MeOH/Et2O). Anal. calcd for C30H36FN3O5S: C, 63.25; H, 6.37; N, 7.38 found C, 63.09; H, 6.14; N, 7.19.
N-{2-[2-(3-(1-Tricyclo[3.3.1.13,7]decyl)-4-fluorophenyl)thiazol-4-yl}ethyl)piperidine-1-yl-carboxamide (1j).
Carboxamide 1j was prepared, as described in general method, using 1-piperidinecarbonyl chloride (2 eq.). Elution with 50% EtOAc in hexanes afforded compound 1j as a foamy solid (50 mg, 36% from compound 8). 1H NMR (400 MHz, CDCl3) δ 7.85 (dd, J = 7.7, 2.2 Hz, 1H, 2-Har), 7.70 (ddd, J = 8.2, 4.3, 2.2 Hz, 1H, 6-Har), 7.04 (dd, J = 12.4, 8.4 Hz, 1H, 5-Har), 6.95 (s, 1H, 5-Hth), 5.24 (br.s, 1H, NH), 3.64 (q, J = 6.3 Hz, 2H, CH2N), 3.51–3.20 (m, 4H, 2,6-Hpi) 2.98 (t, J = 6.3 Hz, 2H, CH2), 2.09 (br.s, J = 14.7 Hz, 9H, 3,5,7,2,8,9-Had), 1.80 (bs, 6H, 4,6,10-Had), 1.66–1.39 (m, 6H, 3,4,5-Hpi). 13C NMR (150 MHz, CDCl3) δ 166.6 (2-Cth), 162.8 (d, J = 251.9 Hz, 4-Car), 156.9 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 152.8 (4-Cth), 137.5 (d, J = 11.4 Hz, 3-Car), 129.2 (1-Car), 126.2 (d, J = 8.4 Hz, 2-Car), 125.1 (6-Car), 117.5 (d, J = 25.4, 5-Car), 116.4 (5-Cth), 43.9 (2,6-Cpi), 40.5 (2,8,9-Cad), 38.4 (NCH2), 36.2 (1-Cad), 36.0 (4,6,10-Cad), 28.8 (CH2), 28.2 (3,5,7-Cad), 24.8 (3,5-Cpi), 23.5 (4-Cpi). Anal. calcd for C27H34FN3OS: C, 69.35; H, 7.33; N, 8.99 found C, 69.55; H, 7.39; N, 9.12.
O), 152.8 (4-Cth), 137.5 (d, J = 11.4 Hz, 3-Car), 129.2 (1-Car), 126.2 (d, J = 8.4 Hz, 2-Car), 125.1 (6-Car), 117.5 (d, J = 25.4, 5-Car), 116.4 (5-Cth), 43.9 (2,6-Cpi), 40.5 (2,8,9-Cad), 38.4 (NCH2), 36.2 (1-Cad), 36.0 (4,6,10-Cad), 28.8 (CH2), 28.2 (3,5,7-Cad), 24.8 (3,5-Cpi), 23.5 (4-Cpi). Anal. calcd for C27H34FN3OS: C, 69.35; H, 7.33; N, 8.99 found C, 69.55; H, 7.39; N, 9.12.
4-(1-Tricyclo[3.3.1.13,7]decyl)benzoic acid (12).
Oxygen gas was bubbled into a stirred solution of 1-(4-tolyl)adamantane (11)33 (1.4 g, 6.17 mmol), Co(OAc)2 (73 mg, 0.31 mmol), Mn(OAc)2 (9 mg, 0.03 mmol) and NaBr (35 mg, 0.32 mmol) in AcOH (26 mL)/dioxane (2.8 mL)/H2O (0.7 mL) for 6 h, at 105 °C. The resulting mixture was cooled to ambient temperature and water was added into the mixture. The residue obtained was filtered and washed with water. The resulting solid was then dried in vacuo, in the presence of P2O5, overnight to afford compound 12, as a white solid (1.4 g, 89%). 1H NMR (400 MHz, CDCl3) δ 12.30 (br.s, 1H, OH) 8.04 (d, J = 8.4 Hz, 2H, 3,5-Har), 7.46 (d, J = 8.5 Hz, 2H, 2,6-Har), 2.12 (s, 3H, 3,5,7-Had), 1.94 (s, 6H, 2,8,9-Had), 1.78 (d, J = 7.1 Hz, 6H, 4,6,10-Had).
4-(1-Tricyclo[3.3.1.13,7]decyl)benzamide (13).
A solution of 4-(adamant-1-yl)benzoic acid (12) (2 g, 6.17 mmol) in SOCl2 (12 ml) was heated at 65 °C for 45 min. The excess of SOCl2 was removed under reduced pressure and subsequently by azeotropic distillation with benzene. The resulting precipitate was then dissolved in anhydrous THF (10 mL) and added to a stirred solution of NH3 (25%) in water (50 mL), dropwise, at 0 °C. The reaction mixture was stirred for 30 min at ambient temperature and extracted with EtOAc. The organic layer dried over MgSO4 and the solvent evaporated to afford compound 13 as an off white solid (1.9 g, 96%). 1H NMR (400 MHz, CDCl3) δ 7.83 (d, J = 8.5 Hz, 2H, 3,5-Har), 7.64 (br.s, 2H, NH2), 7.39 (d, J = 8.5 Hz, 2H, 2,6-Har), 2.11 (s, 3H, 3,5,7-Had), 1.90 (br.s, 6H, 2,8,9-Had), 1.77 (q, J = 12.2 Hz, 6H, 4,6,10-Had). Anal. calcd for C17H21NO: C, 79.96; H, 8.29 found C, 79.77; H, 8.57.
4-(1-Tricyclo[3.3.1.13,7]decyl)thiobenzamide (14).
Lawesson's reagent (1.2 g) was added to a stirred solution of benzamide 13 (1.5 g, 6.17 mmol) in dioxane (15 mL) and the reaction mixture was heated to 110 °C, overnight. The solvent was removed in vacuo and the crude residue was crystallised from DCM. The filtrate of the recrystallisation still contained benzamide 13, thus it was purified by column chromatography. Elution with DCM afforded compound 14 as a yellow solid (780 mg, 50%). M.p.: 201–202 °C 1H NMR (400 MHz, CDCl3) δ 7.83 (d, J = 8.5 Hz, 2H, 3,5-Har), 7.64 (br.s, 1H, NH), 7.39 (d, J = 8.5 Hz, 2H, 2,6-Har), 7.20 (s, 1H, NH), 2.11 (s, 3H, 3,5,7-Had), 1.90 (br.s, 6H, 2,8,9-Had), 1.77 (q, J = 12.2 Hz, 6H, 4,6,10-Had). 13C NMR (150 MHz, CDCl3) δ 202.6 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S), 156.0 (1-Car), 136.4 (4-Car), 126.9 (2,6-Car), 125.1 (3,5-Car), 42.9 (2,8,9-Cad), 36.7 (4,6,10-Cad), 36.6 (1-Cad), 28.8 (3,5,7-Cad). C17H21NS: C, 75.23; H, 7.80 found C, 75.07; H, 7.99.
S), 156.0 (1-Car), 136.4 (4-Car), 126.9 (2,6-Car), 125.1 (3,5-Car), 42.9 (2,8,9-Cad), 36.7 (4,6,10-Cad), 36.6 (1-Cad), 28.8 (3,5,7-Cad). C17H21NS: C, 75.23; H, 7.80 found C, 75.07; H, 7.99.
2-{2-[2-(1-Tricyclo[3.3.1.13,7]decyl)phenyl]ethyl}isoindoline-1,3-dione (10).
Method A.
Phthalimide 10 was prepared in a similar way as for compound 8 using pinacolborane 9 (ref. 31) (300 mg, 0.88 mmol) and bromothiazole 7 (ref. 6) (250 mg, 0.73 mmol) as starting materials. Elution with 10–20% EtOAc in hexanes afforded compound 10 as a white solid (300 mg, 87%).
Method B.
The bromoketone 15 (ref. 6) (200 mg, 0.66 mmol) was added to a stirred solution of the thiobenzamide 14 (180 mg, 0.66 mmol) in EtOH (4 mL) and the reaction mixture was refluxed overnight. The resulting suspension was filtered and the precipitate was washed with Et2O and dried over MgSO4 to afford compound 10, as a white solid (200 mg, 65%) M.p.: 213–214 °C. 1H NMR (600 MHz, CDCl3) δ 7.81 (dt, J = 6.8, 3.4 Hz, 2H, 2′,5′-Har), 7.73 (d, J = 8.4 Hz, 2H, 2,6-Har), 7.66 (dt, J = 6.8, 3.4 Hz, 2H, 3′,4′-Har), 7.34 (d, J = 8.4 Hz, 2H, 3,5-Har), 6.94 (s, 1H, 5-Hth), 4.10 (t, J = 7.1 Hz, 2H, NCH2), 3.21 (t, J = 7.1 Hz, 2H, CH2), 2.12 (br.s, 3H, 3,5,7-Had), 1.91 (br.s, 6H, 2,8,9-Had), 1.78 (dd, J = 25.4, 12.1 Hz, 6H, 4,6,10-Had). 13C NMR (150 MHz, CDCl3) δ 168.4 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 168.2 (2-Cth), 154.2 (4-Cth), 153.4 (1-Car), 133.9 (3′,4′-Car), 132.3 (1′,6′-Car), 131.1 (4-Car), 126.4 (2,6-Car), 125.4 (3,5-Car), 123.3 (2′,5′-Car), 114.2 (5-Cth), 43.1 (2,8,9-Cad), 37.7 (NCH2), 36.9 (4,6,10-Cad), 36.5 (1-Cad), 30.2 (CH2), 29.0 (3,5,7-Cad). C29H28N2O2S: C, 74.33; H, 6.02; N, 5.98 found C, 74.64; H, 6.12; N, 6.21.
O), 168.2 (2-Cth), 154.2 (4-Cth), 153.4 (1-Car), 133.9 (3′,4′-Car), 132.3 (1′,6′-Car), 131.1 (4-Car), 126.4 (2,6-Car), 125.4 (3,5-Car), 123.3 (2′,5′-Car), 114.2 (5-Cth), 43.1 (2,8,9-Cad), 37.7 (NCH2), 36.9 (4,6,10-Cad), 36.5 (1-Cad), 30.2 (CH2), 29.0 (3,5,7-Cad). C29H28N2O2S: C, 74.33; H, 6.02; N, 5.98 found C, 74.64; H, 6.12; N, 6.21.
2-{2-[4-(1-Tricyclo[3.3.1.13,7]decyl)phenyl]thiazol-4-yl}ethan-1-amine (2a).
The amine 2a was prepared in a similar way as the amine 1a, using phthalimide 10 as starting material to afford 2a as a green solid. M.p. (dihydrochloride): 225 °C (dec) (MeOH/Et2O). 1H NMR (400 MHz, DMSO-d6) δ 8.31 (s, 1H, NHth), 7.86 (d, J = 8.3 Hz, 2H, 2,6-Har), 7.52 – 7.44 (m, 3H, 3,5-Har, 5-Hth), 6.75 (s, 3H, NH3), 3.20–3.07 (m, 4H, CH2, NCH2), 2.06 (s, 3H, 3,5,7-Had), 1.88 (s, 6H, 2,8,9-Had), 1.74 (s, 6H, 4,6,10-Had).13C NMR (75 MHz, DMSO-d6) δ 167.5 (2-Cth), 153.6 (4-Cth), 153.4 (1-Car), 130.9 (4-Car) 126.4 (2,6-Car), 126.0 (3,5-Car), 116.1 (5-Cth), 42.8 (2,8,9-Cad), 38.6 (NCH2), 36.5 (4,6,10-Cad), 36.4 (1-Cad), 29.2 (CH2), 28.7 (3,5,7-Cad). Anal. calcd for C21H27Cl2N2S: C, 61.31; H, 6.86; N, 6.81 found C, 61.19; H, 6.95; N, 6.69.
2-{2-[4-(1-Tricyclo[3.3.1.13,7]decyl)phenyl]thiazol-4-yl}-N-methylethan-1-amine (2b).
The amine 2b was prepared in a similar way as the methylamine 1b, using the amine 2a (180 mg, 0.51 mmol) as starting material to afford compound 2b as yellow solid (95 mg, 54% from 10). M.p. (difumarate): 147–149 °C (EtOH/Et2O). 1H NMR (600 MHz, DMSO-d6) δ 7.89–7.83 (d, J = 8.2 Hz, 2H, 2,6-Har), 7.46 (m, 3H, 5-Hth, 3,5-Har), 6.57 (s, 4H, Hfum), 3.31–3.22 (m, 2H, NCH2), 3.17–3.08 (m, 2H, CH2), 2.68 (br.s, 2H, NH2), 2.60 (s, 3H, CH3), 2.06 (br.s, 3H, 3,5,7-Had), 1.87 (br.s, 6H, 2,8,9-Had), 1.75 (br.s, 6H, 4,6,10-Had). 13C NMR (150 MHz, DMSO-d6) δ 167.2 (2-Cth), 167.0 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, fum), 153.2 (1-Car), 152.9 (4-Cth), 134.6 (C-fum), 130.5 (4-Car), 126.0 (2,6-Car), 125.6 (3,5-Car), 115.7 (5-Cth), 47.4 (NCH2), 42.4 (2,8,9-Cad), 36.1 (4,6,10-Cad), 36.0 (1-Cad), 32.5 (CH3), 28.3 (3,5,7-Cad), 27.6 (CH2). Anal. calcd for C30H36N2O8S: C, 61.63; H, 6.21; N, 4.79 found C, 61.47; H, 6.08; N, 5.01.
O, fum), 153.2 (1-Car), 152.9 (4-Cth), 134.6 (C-fum), 130.5 (4-Car), 126.0 (2,6-Car), 125.6 (3,5-Car), 115.7 (5-Cth), 47.4 (NCH2), 42.4 (2,8,9-Cad), 36.1 (4,6,10-Cad), 36.0 (1-Cad), 32.5 (CH3), 28.3 (3,5,7-Cad), 27.6 (CH2). Anal. calcd for C30H36N2O8S: C, 61.63; H, 6.21; N, 4.79 found C, 61.47; H, 6.08; N, 5.01.
2-{2-[4-(1-Tricyclo[3.3.1.13,7]decyl)phenyl]thiazol-4-yl}-N,N-dimethylethan-1-amine (2c).
Dimethylamine 2c was prepared in a similar way as the dimethylamine 1c, using the amine 2a (220 mg, 0.64 mmol), as starting material to afford compound 2c as yellow solid (150 mg, 85% from 10). M.p. (difumarate): 302 °C (dec) (EtOH/Et2O). 1H NMR (400 MHz, DMSO-d6) δ 7.89–7.83 (d, J = 8.2 Hz, 2H, 2,6-Har), 7.46 (m, 3H, 5-Hth, 3,5-Har), 6.57 (s, 4H, Hfum), 3.31–3.22 (m, 2H, NCH2), 3.17–3.08 (m, 2H, CH2), 2.68 (br.s, 2H, NH), 2.46 (s, 6H, CH3), 2.06 (br.s, 3H, 3,5,7-Had), 1.87 (br.s, 6H, 2,8,9-Had), 1.75 (br.s, 6H, 4,6,10-Had). 13C NMR (150 MHz, DMSO-d6) δ 167.5 (4-Cth), 166.6 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, fum), 153.7 (4-Cth), 134.7 (1-Car), 134.6 (CH, fum), 130.9 (4-Car), 126.4 (2,6-Car), 126.0 (3,5-Car), 116.0 (5-Cth), 56.4 (CH2N), 43.0 (CH3), 42.9 (2,8,9-Cad), 36.6 (4,6,10-Cad), 36.5 (1-Cad), 28.7 (3,5,7-Cad), 27.0 (CH2). Anal. calcd for C31H38N2O8S: C, 62.19; H, 6.40; N, 4.68 found C, 62.31; H, 6.64; N, 4.14.
O, fum), 153.7 (4-Cth), 134.7 (1-Car), 134.6 (CH, fum), 130.9 (4-Car), 126.4 (2,6-Car), 126.0 (3,5-Car), 116.0 (5-Cth), 56.4 (CH2N), 43.0 (CH3), 42.9 (2,8,9-Cad), 36.6 (4,6,10-Cad), 36.5 (1-Cad), 28.7 (3,5,7-Cad), 27.0 (CH2). Anal. calcd for C31H38N2O8S: C, 62.19; H, 6.40; N, 4.68 found C, 62.31; H, 6.64; N, 4.14.
N-{2-[2-(4-(1-Tricyclo[3.3.1.13,7]decyl)phenyl)thiazol-4-yl]ethyl}acetamide (2d).
The acetamide 2d was prepared as described in the general method, using the amine 2a (150 mg, 0.42 mmol) and acetic anhydride (0.15 ml, 1.42 mmol). Elution with 10% MeOH in DCM afforded compound 2d as a white solid (100 mg, 53% from compound 10). M.p. (fumarate): 164–166 °C (MeOH/Et2O). 1H NMR (600 MHz, DMSO-d6) δ 13.12 (s, 1H, NHTh), 7.95 (s, 1H NHC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 7.84 (d, J = 8.2 Hz, 2H, 2,6-Har), 7.46 (d, J = 8.2 Hz, 2H, 3,5-Har), 7.34 (s, 1H, 5-Hth), 6.63 (s, 2H, CH-Fum), 3.39 (dt, J = 13.3, 6.7 Hz, 2H, NCH2), 2.88 (t, J = 7.2 Hz, 2H, CH2), 2.06 (s, 3H, 3,5,7-Had), 1.88 (s, 6H, 2,8,9-Had), 1.80 (s, 3H, CH3), 1.74 (br.s, 6H, 4,6,10-Had). 13C NMR (75 MHz, DMSO-d6) δ 169.1 (NC
O), 7.84 (d, J = 8.2 Hz, 2H, 2,6-Har), 7.46 (d, J = 8.2 Hz, 2H, 3,5-Har), 7.34 (s, 1H, 5-Hth), 6.63 (s, 2H, CH-Fum), 3.39 (dt, J = 13.3, 6.7 Hz, 2H, NCH2), 2.88 (t, J = 7.2 Hz, 2H, CH2), 2.06 (s, 3H, 3,5,7-Had), 1.88 (s, 6H, 2,8,9-Had), 1.80 (s, 3H, CH3), 1.74 (br.s, 6H, 4,6,10-Had). 13C NMR (75 MHz, DMSO-d6) δ 169.1 (NC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 166.5 (2-Cth), 166.0 (C
O), 166.5 (2-Cth), 166.0 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, fum), 155.2 (4-Cth), 153.0 (1-Car), 134.0 (CH-fum), 130.7 (4-Car), 125.9 (2,6-Car), 125.5 (3,5-Car), 114.6 (5-Cth), 42.4 (2,8,9-Cad), 38.2 (NCH2), 36.1 (4,6,10-Cad), 36.0 (1-Cad), 31.3 (CH2), 28.3 (3,5,7-Cad), 22.7 (CH3). Anal. calcd for C27H32N2O5S: C, 65.30; H, 6.50; N, 5.64 found C, 65.27; H, 6.33; N, 5.45.
O, fum), 155.2 (4-Cth), 153.0 (1-Car), 134.0 (CH-fum), 130.7 (4-Car), 125.9 (2,6-Car), 125.5 (3,5-Car), 114.6 (5-Cth), 42.4 (2,8,9-Cad), 38.2 (NCH2), 36.1 (4,6,10-Cad), 36.0 (1-Cad), 31.3 (CH2), 28.3 (3,5,7-Cad), 22.7 (CH3). Anal. calcd for C27H32N2O5S: C, 65.30; H, 6.50; N, 5.64 found C, 65.27; H, 6.33; N, 5.45.
2-[4-(1-Tricyclo[3.3.1.13,7]decyl)phenyl]-4-(chloromethyl)thiazole (15).
Thiobenzamide 14 (200 mg, 0.74 mmol) was added to a stirred solution of 1-3-dichloroacetone (125 mg, 0.99 mmol) in acetone (4 mL) and the reaction mixture was refluxed overnight. The solvent was removed in vacuo and the resulting residue was dissolved in conc. H2SO4 (5 mL), stirred for 30 min and subsequently poured onto a mixture of water and ice. The resulting suspension was the filtered and the precipitate was washed with water to afford compound 15 as a yellow-white solid (190 mg, 75%), which was used in the next step without further purification.
2-{2-[4-(1-Tricyclo[3.3.1.13,7]decyl)phenyl]thiazol-4-yl}acetonitrile (2e).
A solution of the chloride 15 (180 mg, 0.52 mmol) and KCN (260 mg, 5.24 mmol) in anhydrous DMF (2 mL), was heated at 60 °C under an argon atmosphere for 36 h and then chilled to room temperature. Water was then added into the reaction mixture, which was extracted with EtOAc. The organic layer was washed with water and brine, dried over MgSO4, and then the solvent was evaporated in vacuo. The solid residue was then purified by column chromatography. Elution with 20% EtOAc in hexanes afforded compound 2e as a yellow crystalline solid (70 mg, 41%). M.p.: 159–160 °C. 1H NMR (400 MHz, CDCl3) δ 7.86 (d, J = 8.5 Hz, 2H, 2,6H-ar), 7.44 (d, J = 8.4 Hz, 2H, 3,5-Har), 7.26 (s, 1H, 5-Hth), 3.95 (s, 2H, CH2), 2.12 (s, 3H, 3,5,7-Had), 1.93 (d, J = 2.1 Hz, 6H, 2,8,9-Had), 1.79 (q, 6H, 4,6,10-Had). 13C NMR (150 MHz, CDCl3) δ 169.7 (2-Cth), 154.2 (1-Car), 145.6 (4-Cth), 130.4 (4-Car), 126.4 (2,6-Car), 125.6 (3,5-Car), 116.9 (CN), 115.6 (5-Cth), 43.0 (2,8,9-Cad), 36.7 (4,6,10-Cad), 36.5 (1-Cad), 28.9 (3,5,7-Cad), 20.7 (CH2). Anal. calcd for C21H22N2S: C, 75.41; H, 6.63; N, 8.38 found C, 74.34; H, 6.29; N, 8.51.
2-[4-(1-Tricyclo[3.3.1.13,7]decyl)phenyl]-4-(thiocyanatemethyl)thiazole (2f).
A solution of the chloride 15 (250 mg, 0.73 mmol) and KSCN (100 mg, 1.34 mmol) in EtOH (4 ml), was heated to 45 °C under an argon atmosphere overnight. The reaction mixture was then poured onto a mixture of ice and water and the resulting suspension was filtered. The residue obtained was crystallized from EtOH to afford compound 2f as a white solid (250 mg, 67%). M.p.: 169–170 °C. 1H NMR (600 MHz, CDCl3) δ 7.85 (d, J = 8.4 Hz, 2H, 2,6H-ar), 7.41 (d, J = 8.4 Hz, 2H, 3,5-Har), 7.23 (s, 1H, 5-Hth), 4.29 (s, 2H, CH2), 2.09 (s, 3H, 3,5,7-Had), 1.91 (d, 6H, 2,8,9-Had), 1.76 (q, 6H, 4,6,10-Had). 13C NMR (150 MHz, CDCl3) δ 169.7 (2-Cth), 154.2 (1-Car), 149.7 (4-Cth), 130.4 (4-Car), 126.5 (2,6-Car), 125.6 (3,5-Car), 117.5 (5-Cth), 112.0 (SCN), 43.0 (2,8,9-Cad), 36.7 (4,6,10-Cad), 36.50 (1-Cad), 33.9 (CH2), 28.9 (3,5,7-Cad). Anal. calcd for C21H22N2S2: C, 68.81; H, 6.05; N, 7.64 found C, 68.81; H, 6.09; N, 7.59.
Ethyl 2-{2-[4-(1-Tricyclo[3.3.1.13,7]decyl)phenyl]thiazol-4-yl}acetate (16).
4-Chloroacetoacetate (700 mg, 2.43 mmol) was added to a stirred mixture of thiobenzamide 14 (1 g, 3.68 mmol) in i-PrOH (8 mL), and the reaction mixture was stirred overnight, at 110 °C, in an autoclave. The solvent was then removed in vacuo and the resulting residue was dissolved in EtOAc and washed with water, a saturated aqueous solution of NaHCO3 and brine. The organic layer was then dried over MgSO4 and the solvent evaporated under vacuum. The resulting oil was triturated with EtOAc/hexanes to afford compound 16 as a light orange solid (1.3 g, 92%) which was used in the next step without further purification.
2-{2-[4-(1-Tricyclo[3.3.1.13,7]decyl)phenyl]thiazol-4-yl}ethan-1-ol (2g).
To a stirred suspension of LiAlH4 (85 mg, 2.23 mmol) in anhydrous THF (5 mL), was added dropwise a solution of compound 16 (170 mg, 0.44 mmol) in anhydrous THF (3 mL), under an argon atmosphere and then the reaction mixture was stirred at ambient temperature for 2 h. Next, the mixture was cooled in an ice bath and ethanol, water and a NaOH (10%) solution were added in order. The resulting suspension was then filtered, the filtrate was evaporated in vacuo and extracted with DCM. The organic layer was then washed with water, dried over MgSO4 and the solvent evaporated to afford compound 2g as an off white solid (120 mg, 80%). M.p.: 93–94 °C. 1H NMR (400 MHz, CDCl3) δ 7.86 (d, J = 8.4 Hz, 2H, 2,6H-ar), 7.42 (d, J = 8.4 Hz, 2H, 3,5-Har), 6.94 (s, 1H, 5-Hth), 3.99 (s, 2H, CH2OH), 3.68 (s, 1H, OH), 3.02 (t, J = 5.5 Hz, 2H, CH2), 2.12 (s, 3H 3,5,7-Had), 1.95 (d, J = 11.0 Hz, 6H, 2,8,9-Had), 1.83–1.73 (m, 6H, 4,6,10-Had). 13C NMR (150 MHz, CDCl3) δ 168.5 (2-Cth), 155.7 (4-Cth), 153.8 (1-Car), 130.7 (4-Car), 126.3 (2,6-Car), 125.5 (3,5-Car), 113.4 (5-Cth), 62.1 (CH2OH), 43.0 (2,8,9-Cad), 36.7 (4,6,10-Cad), 36.4 (1-Cad), 33.8 (CH2), 28.9 (3,5,7-Cad). Anal. calcd for C21H25NOS: C, 74.30; H, 7.42; N, 4.13 found C, 74.53; H, 7.31; N, 3.95.
2-{2-[4-(1-Tricyclo[3.3.1.13,7]decyl)phenyl]thiazol-4-yl)ethyl}methanesulfonate (17).
To a stirred solution of the alcohol 2g (170 mg, 0.50 mmol) and Et3N (85 μL) in DCM (2 mL), MsCl (50 μL) was added dropwise at 0 °C. The reaction mixture was stirred at ambient temperature overnight. 1 M hydrochloride solution was added, into the reaction mixture, which was then extracted with EtOAc and the organic layer was dried over MgSO4 and the solvent evaporated to afford compound 17 as a yellow solid (170 mg, 95%), which used in the next step without further purification.
2-[4-(1-Tricyclo[3.3.1.13,7]decyl)phenyl]-4-(2-azidoethyl)thiazole (2h).
To a stirred solution of the mesylate 17 (180 mg, 0.42 mmol) in anhydrous DMF (2 mL), was added NaN3 (40 mL) and the reaction mixture was heated at 60 °C, for 2 h. The reaction mixture was then extracted with EtOAc and the organic layer was dried over MgSO4 and evaporated in vacuo. The resulting residue was then purified by column chromatography. Elution with 20% EtOAc in hexanes afforded compound 2h as a white solid (100 mg, 65%). 1H NMR (400 MHz, CDCl3) δ 7.87 (d, J = 8.6 Hz, 2H, 2,6H-ar), 7.42 (d, J = 8.5 Hz, 2H, 3,5-Har), 6.99 (s, 1H, 5-Hth), 3.70 (t, J = 6.9 Hz, 2H, CH2N), 3.09 (t, J = 7.1 Hz, 2H, CH2), 2.12 (s, 3H, 3,5,7-Had), 1.93 (d, 6H, 2,8,9-Had), 1.85–1.71 (m, 6H, 4,6,10-Had). 13C NMR (75 MHz, CDCl3) δ 168.5 (2-Cth), 154.0 (4-Cth), 153.7 (1-Car) 131.1 (4-Car), 126.5 (2,6-Car), 125.6 (3,5-Car), 114.6 (5-Cth), 50.7 (CH2N3), 43.2 (2,8,9-Cad), 36.9 (4,6,10-Cad), 36.6 (1-Cad), 31.5 (CH2), 29.0 (3,5,7-Cad). Anal. calcd for C21H24N4S: C, 69.20; H, 6.64; N, 15.37 found C, 69.08; H, 6.47; N, 14.99.
2-Bromo-1-[4-(1-Tricyclo[3.3.1.13,7]decyl)phenyl]ethanone (19).
To a stirred solution of (1-phenyl)adamantane (18)35 (1.00 g, 4.71 mmol) and AlCl3 (700 mg, 5.10 mmol) in anhydrous DCM (10 mL) was added a solution of BrCOCH2Br (0.5 mL, 4.71 mmol) in anhydrous DCM (10 mL) under an argon atmosphere, at −10 °C. The reaction mixture was then heated to room temperature and stirred under an argon atmosphere overnight. Then the reaction mixture was poured onto ice-water, extracted with DCM and the organic layer was dried over MgSO4 and the solvent evaporated under vacuum. The resulting crude residue was purified by gradient column chromatography. Elution with EtOAc 3–5% in hexanes afforded compound 19 as a white solid (900 mg, 57%). M.p.: 94–96 °C (EtOAc/Hex). 1H NMR (400 MHz, CDCl3) δ 7.94 (d, J = 8.6 Hz, 2H, 2,6-Har), 7.48 (d, J = 8.6 Hz, 2H, 3,5-Har), 4.45 (s, 2H, CH2), 2.12 (s, 3H, 3,5,7-Had), 1.92 (d, J = 2.5 Hz, 6H, 2,8,9-Had), 1.83–1.75 (br.s, 6H, 4,6,8-Had). 13C NMR (75 MHz, CDCl3) δ 191.0 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 158.0 (1-Car), 129.0 (2,6-Cad), 127.5 (4-Cad), 125.6 (3,5-Car), 42.9 (2,8,9-Cad), 36.9 (1-Cad), 36.8 (3,6,10-Cad), 31.1 (CH2), 28.8 (3,5,7-Cad). Anal. calcd for C18H21BrO: C, 64.87; H, 6.35 found C, 64.63; H, 6.46.
O), 158.0 (1-Car), 129.0 (2,6-Cad), 127.5 (4-Cad), 125.6 (3,5-Car), 42.9 (2,8,9-Cad), 36.9 (1-Cad), 36.8 (3,6,10-Cad), 31.1 (CH2), 28.8 (3,5,7-Cad). Anal. calcd for C18H21BrO: C, 64.87; H, 6.35 found C, 64.63; H, 6.46.
4-[4-(1-Tricyclo[3.3.1.13,7]decyl)phenyl]thiazol-2-amine hydrobromide (3a).
A solution of the bromoketone 19 (130 mg, 0.39 mmol) and thiourea (30 mg, 0.39 mmol) in EtOH (3 mL) was heated at 80 °C, in an autoclave, overnight. The reaction mixture was then cooled to room temperature, treated with Et2O and the resulting precipitate was filtered to afford compound 3a hydrobromide as a white solid (150 mg, 88%). M.p. (hydrobromide): 345 °C (dec) (EtOH/Et2O). 1H NMR (400 MHz, DMSO-d6) δ 8.84 (br.s, 3H, NH3), 7.66 (d, J = 8.5 Hz, 2H, 2,6-Har), 7.47 (d, J = 8.5 Hz, 2H, 3,5-Har), 7.18 (d, J = 5.1 Hz, 1H, 5-Hth), 2.07 (d, J = 8.1 Hz, 3H, 3,5,7-Had), 1.87 (br.s, 6H, 2,8,9-Had), 1.74 (s, 6H, 4,6,10-Had). 13C NMR (150 MHz, DMSO-d6) δ 170.1 (4-Cth), 152.2 (2-Cth), 127.2 (1-Car), 125.7 (4-Car), 125.6 (2.6-Car), 125.3 (3,5-Car), 101.9 (5-Cth), 42.3 (2,8,9-Cad), 36.1 (4,6,10-Cad), 35.9 (1-Cad), 28.2 (3,5,7-Cad). Anal. calcd for C19H22BrN2S: C, 58.31; H, 5.92; N, 7.16 found C, 58.12; H, 5.85; N, 6.98.
2-{4-[4-(1-Tricyclo[3.3.1.13,7]decyl)phenyl]thiazol-2-yl}methanamine difumarate (3b).
A solution of the bromoketone 19 (1.10 g, 3.30 mmol) and amide 20 (ref. 38) (750 mg, 3.60 mmol) in i-PrOH (12 mL) was heated at 120 °C, in an autoclave, overnight. The reaction mixture was then cooled to room temperature, treated with Et2O and the resulting precipitate was filtered to afford compound 3b (480 mg, 36%). M.p. (difumarate): 172 °C (dec) (EtOH/Et2O). 1H NMR (400 MHz, DMSO-d6) δ 8.02 (s, 1H, 5-Hth), 7.90 (d, J = 8.5 Hz, 2H, 2,6-Har), 7.42 (d, J = 8.5 Hz, 2H, 3,5-Har), 6.57 (s, 4H, Hfum), 4.33 (s, 2H, CH2), 2.06 (s, 3H, 3,5,7-Had), 1.88 (d, J = 2.2 Hz, 6H, 2,8,9-Had), 1.74 (s, 6H, 4,6,10-Had). 13C NMR (75 MHz, DMSO-d6) δ 167.7 (2-Cth), 166.8 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, fum), 151.0 (4-Cth), 134.5 (CH2fum), 131.4 (1-Car), 127.1 (4-Car), 125.9 (2,6-Car), 125.2 (3,5-Car), 114.5 (5-Cth), 42.6 (2,8,9-Cad), 40.8 (CH2N), 36.3 (4,6,10-Cad), 35.8 (1-Cad), 28.4 (3,5,7-Cad). Anal. calcd for C28H32N2O8S: C, 60.62; H, 5.79; N, 5.03 found C, 60.33; H, 6.02; N, 4.89.
O, fum), 151.0 (4-Cth), 134.5 (CH2fum), 131.4 (1-Car), 127.1 (4-Car), 125.9 (2,6-Car), 125.2 (3,5-Car), 114.5 (5-Cth), 42.6 (2,8,9-Cad), 40.8 (CH2N), 36.3 (4,6,10-Cad), 35.8 (1-Cad), 28.4 (3,5,7-Cad). Anal. calcd for C28H32N2O8S: C, 60.62; H, 5.79; N, 5.03 found C, 60.33; H, 6.02; N, 4.89.
2-{4-[4-(1-Tricyclo[3.3.1.13,7]decyl)phenyl]thiazol-2-yl}ethanamine hydrobromide (3c).
Ethanamine 3c was prepared in a similar way as the amine 3b, using the bromoketone 19 (800 mg, 2.40 mmol) and derivative 21 (ref. 7) (550 mg, 2.69 mmol) as starting materials to afford compound 3c hydrobromide as a white solid (850 mg, 85%). M.p. (hydrobromide): 261–263 °C (EtOH/Et2O). 1H NMR (400 MHz, DMSO-d6) δ 7.98 (s, 1H, 5-Cth), 7.94–7.85 (m, 5H, NH3, 2,6-Har), 7.42 (d, J = 8.4 Hz, 2H, 3,5-Har), 3.34–3.26 (m, 4H, NCH2, CH2), 2.07 (s, 3H, 3,5,7-Had), 1.88 (s, 6H, 2,8,9-Had), 1.74 (s, 6H, 4,6,10-Had). 13C NMR (150 MHz, DMSO-d6) δ 165.5 (4-Cth), 154.1 (2-Cth), 150.8 (1-Car), 125.9 (2,6-Car), 1245.0 (3,5-Car), 113.5 (5-Cth), 42.5 (2,8,9-Cad), 38.1 (NCH2), 36.1 (4,6,10-Cad), 35.7 (1-Cad), 30.3 (CH2), 28.3 (3,5,7-Cad). Anal. calcd for C21H27BrN2S: C, 60.14; H, 6.49; N, 6.68 found C, 60.38; H, 6.59; N, 6.45.
2-{4-[4-(1-Tricyclo[3.3.1.13,7]decyl)phenyl]thiazol-2-yl}-N,N-dimethylethan-1-amine dihydrochloride (3d).
Dimethylamine 3d was prepared in a similar way as the amine 1c, using the derivative 3c (200 mg, 0.48 mmol) as starting material to afford compound 3d as a white solid (150 mg, 85%). M.p. (dihydrochloride): 200–202 °C (EtOH/Et2O). 1H NMR (400 MHz, DMSO-d6) δ 10.84 (s, 1H, NHTh), 7.97 (br.s, 1H, 5-Hth), 7.88 (d, J = 8.4 Hz, 2H, 2,6-Har), 7.42 (d, J = 8.5 Hz, 2H, 3,5-Har), 5.16 (br.s, 1H, NH), 3.55 (s, 4H, CH2, NCH2), 2.83 (d, J = 4.9 Hz, 6H, 3,5,7-Had), 2.07 (s, 3H), 1.88 (d, J = 2.3 Hz, 6H, 2,8,9-Had), 1.74 (s, 6H, 4,6,10-Had). 13C NMR (150 MHz, DMSO-d6) δ 165.0 (4-Cth), 154.0 (2-Cth), 150.8 (1-Car), 131.3 (4-Car), 125.9 (2,6-Car), 125.0 (3,5-Car), 113.6 (5-Cth), 54.9 (NCH2), 42.5 (2,8,9-Cad), 42.2 (CH3), 36.2 (4,6,10-Cad), 35.7 (1-Cad), 28.3 (3,5,7-Cad), 27.6 (CH2). Anal. calcd for C23H3Cl2N2S: C, 62.86; H, 7.34; N, 6.37 found C, 62.72; H, 7.23; N, 6.11.
1-{2-[4-(1-Tricyclo[3.3.1.13,7]decyl)phenyl]thiazol-4-yl}guanidine (3e).
A stirred solution of the bromoketone 19 (200 mg, 0.60 mmol) and guanylthiourea (80 mg, 0.66 mmol) in EtOH (4 mL) was refluxed overnight. The reaction mixture was then cooled to room temperature, Et2O was added and the resulting suspension was filtered to afford compound 3e hydrobromide (200 mg, 89%). M.p. (hydrobromide): 358–359 °C. 1H NMR (400 MHz, DMSO-d6) δ 11.98 (s, 1H, NH), 8.22 (br.s, 4H, 2 × NH2), 7.85 (d, J = 8.4 Hz, 2H, 2,6-Har), 7.68 (s, 1H, 5-Hth), 7.41 (d, J = 8.4 Hz, 2H, 3,5-Har), 2.06 (s, 3H, 3,5,7-Had), 1.87 (s, 6H, 2,8,9-Had), 1.74 (s, 6H, 4,6,10-Had). 13C NMR (75 MHz, DMSO-d6) δ 160.2 (2-Cth), 154.0 (4-Cth), 151.1 (1-Car), 130.9 (4-Car), 125.8 (2,6-Car) 125.1 (3,5-Car), 107.4 (5-Cth), 42.9 (2,8,9-Cad), 36.1 (4,6,10-Cad), 35.7 (1-Cad), 28.3 (3,5,7-Cad). Anal. calcd for C20H25BrN4S: C, 55.43; H, 5.81; N, 12.93 found C, 55.31; H, 5.89; N, 13.23.
N-(2-(2-Phenylthiazol-4-yl)ethyl)(1-tricyclo[3.3.1.13,7]decane)carboxamide (4a).
A solution of 1-adamantylcarbonyl chloride (450 mg, 2.26 mmol) in anhydrous THF (8 mL) was added dropwise, at 0 °C onto a stirred solution of the 2-phenylthiazol-4-ethylamine (22)6 (308 mg, 1.51 mmol) and Et3N (0.45 mL, 3.23 mmol) in THF (8 mL) and the reaction mixture was stirred at ambient temperature under an argon atmosphere overnight. The mixture was extracted with DCM and the organic phase was then washed with water, dried over MgSO4 and the solvent evaporated under reduced pressure. The resulting residue was purified with column chromatography. Elution with 50% EtOAc in hexanes afforded compound 4a as a white solid (270 mg, 49%). M.p.: 135–136 °C. 1H NMR (400 MHz, CDCl3) δ 7.99–7.91 (m, 2H, 2,6-Har), 7.46–7.38 (m, 3H, 3,4,5-Har), 6.96 (s, 1H, 5-Cth), 6.86 (br.s, 1H, NH), 3.60 (dd, J = 12.0, 5.6 Hz, 2H, CH2NH), 2.91 (t, J = 9,51 Hz 2H, CH2), 2.05 (br.s, 3H, 3,5,7-Had), 1.88 (s, 6H, 2,8,9-Had), 1.70 (q, J = 12.1 Hz, 6H 4,6,10-Had). 13C NMR (150 MHz, CDCl3) δ 177.88 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 168.2 (2-Cth), 155.9 (4-Cth), 133.5 (1-Car), 130.0 (4-Car), 128.8 (2,6-Car), 126.3 (3,5-Car), 114.2 (5-Cth), 40.5 (CH2N), 39.3 (2,8,9-Cad), 38.8 (1-Cad), 36.5 (4,6–10-Cad), 30.7 (CH2), 28.1 (3,5,7-Cad). Anal. calcd for C22H26N2OS: C, 72.08; H, 7.15; N, 7.64 found C, 72.31; H, 7.09; N, 7.88.
O), 168.2 (2-Cth), 155.9 (4-Cth), 133.5 (1-Car), 130.0 (4-Car), 128.8 (2,6-Car), 126.3 (3,5-Car), 114.2 (5-Cth), 40.5 (CH2N), 39.3 (2,8,9-Cad), 38.8 (1-Cad), 36.5 (4,6–10-Cad), 30.7 (CH2), 28.1 (3,5,7-Cad). Anal. calcd for C22H26N2OS: C, 72.08; H, 7.15; N, 7.64 found C, 72.31; H, 7.09; N, 7.88.
1-((1R,4R)-7,7-Dimethyl-2-oxobicyclo[2.2.1]hept-1-yl)-N-(2-(2-phenylthiazol-4-yl)ethyl)methanesulfonamide (4b).
The carboxamide 4b was prepared in a similar way as the derivative 4a, using 2-phenylthiazol-4-ethylamine (22)6 (339 mg, 1.66 mmol) and (±)-10-camphorsulfonyl chloride (623 mg, 2.49 mmol) as starting materials in DCM (7 mL), to afford 4b as a viscous liquid (350 mg, 50%). M.p (hydrochloride): 144–145 °C. 1H NMR (400 MHz, DMSO-d6) δ 7.93 (dd, J = 7.3, 2.2 Hz, 2H, 2,6-Har), 7.54–7.43 (m, 4H, 3,4,5-Har, 5-Hth), 7.24 (br.s, 1H, NH), 3.45–3.32 (m, 3H, CH2NH), 3.26 (d, J = 14.9 Hz, 1H, CH2S), 2.97 (t, J = 7.2 Hz, 2H, CH2), 2.85 (d, J = 14.9 Hz, 1H, CH2S), 2.30 (m, 2H, 3-Hcamexo, 6-Hcamexo), 2.00 (t, J = 4.4 Hz, 1H, 5-Hcam), 1.95–1.81 (m, 2H, 4-Hcamexo, 6-Hcamendo), 1.50 (ddd, J = 13.7, 9.3, 4.5 Hz, 1H, 3-Hcarendo), 1.39–1.30 (m, 1H, 4-Hcarendo), 0.96 (s, 3H, CH3), 0.73 (s, 3H, CH3). 13C NMR (150 MHz, DMSO-d6) δ 215.0 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 167.2 (2-Cth), 155.0 (4-Cth), 133.4 (1-Car), 130.7 (4-Car), 129.7 (2,6-Car), 126.6 (3,5-Car), 116.3 (5-Cth), 58.3 (2-Ccam), 48.2 (CH2), 48.0 (7-Ccam), 42.7 (6-Ccam), 42.5 (5-Ccam), 42.5 (CH2N), 32.2 (CH2), 26.7 (4-Ccam), 25.0 (3-Ccam), 19.8 (CH3), 19.7 (CH3). Anal. calcd for C21H27ClN2O3S: C, 55.43; H, 5.98; N, 6.16 found C, 55.21; H, 6.11; N, 6.02.
O), 167.2 (2-Cth), 155.0 (4-Cth), 133.4 (1-Car), 130.7 (4-Car), 129.7 (2,6-Car), 126.6 (3,5-Car), 116.3 (5-Cth), 58.3 (2-Ccam), 48.2 (CH2), 48.0 (7-Ccam), 42.7 (6-Ccam), 42.5 (5-Ccam), 42.5 (CH2N), 32.2 (CH2), 26.7 (4-Ccam), 25.0 (3-Ccam), 19.8 (CH3), 19.7 (CH3). Anal. calcd for C21H27ClN2O3S: C, 55.43; H, 5.98; N, 6.16 found C, 55.21; H, 6.11; N, 6.02.
N-[2-(4-Phenylthiazol-2-yl)ethyl](1-tricyclo[3.3.1.13,7]decane)carboxamide (4c).
To a stirred solution of the 4-phenylthiazol-2-ethylamine hydrobromide (23)7 (300 mg, 1.05 mmol) in DMF/DCM 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (10 mL), was added 1-adamantanecarboxylic acid (227 mg, 1.26 mmol), HBTU (478 mg, 1.26 mmol), and DIPEA (474 mg, 3.68 mmol) and the reaction mixture was stirred at ambient temperature under an argon atmosphere, overnight. The mixture was then partitioned between DCM and an aqueous solution of citric acid (10%) and the aqueous phase was extracted with DCM. The combined organic phase was then washed with water and brine, dried over MgSO4 and the solvent evaporated under reduced pressure. The resulting residue was purified with gradient column chromatography. Elution with 10% to 50% EtOAc in hexanes afforded compound 4a as a white solid (330 mg, 94%). M.p.: 107–108 °C. 1H NMR (400 MHz, CDCl3) δ 8.01–7.84 (m, 2H, 2,6-Har), 7.54–7.28 (m, 3,4,5-Har, 5-Hth), 6.92 (s, 1H, NH), 3.69 (dd, J = 11.8, 5.7 Hz, 3H, CH2N), 3.20 (t, J = 7.4 Hz, 3H, CH2), 2.02 (s, 3H, 3,5,7-Had), 1.87 (d, J = 2.3 Hz, 6H, 2,8,9-Had), 1.70 (q, J = 12.2 Hz, 6H, 4,6,10-Had).13C NMR (150 MHz, CDCl3) δ 177.9 (C
1 (10 mL), was added 1-adamantanecarboxylic acid (227 mg, 1.26 mmol), HBTU (478 mg, 1.26 mmol), and DIPEA (474 mg, 3.68 mmol) and the reaction mixture was stirred at ambient temperature under an argon atmosphere, overnight. The mixture was then partitioned between DCM and an aqueous solution of citric acid (10%) and the aqueous phase was extracted with DCM. The combined organic phase was then washed with water and brine, dried over MgSO4 and the solvent evaporated under reduced pressure. The resulting residue was purified with gradient column chromatography. Elution with 10% to 50% EtOAc in hexanes afforded compound 4a as a white solid (330 mg, 94%). M.p.: 107–108 °C. 1H NMR (400 MHz, CDCl3) δ 8.01–7.84 (m, 2H, 2,6-Har), 7.54–7.28 (m, 3,4,5-Har, 5-Hth), 6.92 (s, 1H, NH), 3.69 (dd, J = 11.8, 5.7 Hz, 3H, CH2N), 3.20 (t, J = 7.4 Hz, 3H, CH2), 2.02 (s, 3H, 3,5,7-Had), 1.87 (d, J = 2.3 Hz, 6H, 2,8,9-Had), 1.70 (q, J = 12.2 Hz, 6H, 4,6,10-Had).13C NMR (150 MHz, CDCl3) δ 177.9 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 168.2 (2-Cth), 155.9 (4-Cth), 133.5 (1-Car), 130.0 (4-Car), 128.8 (2,6-Car), 126.3 (3,5-Car), 114.2 (5-Cth), 40.5 (CH2N), 39.3 (2,8,9-Cad), 38.8 (1-Cad), 36.5 (4,6–10-Cad), 30.7 (CH2), 28.1 (3,5,7-Cad). Anal. calcd for C22H26N2OS: C, 72.09; H, 7.15; N, 7.64 found C, 72.27; H, 7.23; N, 7.92.
O), 168.2 (2-Cth), 155.9 (4-Cth), 133.5 (1-Car), 130.0 (4-Car), 128.8 (2,6-Car), 126.3 (3,5-Car), 114.2 (5-Cth), 40.5 (CH2N), 39.3 (2,8,9-Cad), 38.8 (1-Cad), 36.5 (4,6–10-Cad), 30.7 (CH2), 28.1 (3,5,7-Cad). Anal. calcd for C22H26N2OS: C, 72.09; H, 7.15; N, 7.64 found C, 72.27; H, 7.23; N, 7.92.
1-((1R,4R)-7,7-Dimethyl-2-oxobicyclo[2.2.1]hept-1-yl)-N-(2-(4-phenylthiazol-2-yl)ethyl)methanesulfonamide hydrochloride (4d).
The sulfonamide 4d was prepared in a similar way as the derivative 4b, using 4-phenylthiazol-2-ethylamine (23)7 (210 mg, 1.03 mmol) and (±)-10-camphorsulfonyl chloride (400 mg, 1.59 mmol) as starting materials, to afford compound 4d as a viscous liquid (300 mg, 70%). M.p. (hydrochloride): 130–131 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.01 (s, 1H, 5-Hth), 7.95 (d, J = 7.5 Hz, 2H, 2,6-Har), 7.54–7.20 (m, 3H, 3,4,5-Har), 5.26 (s, 2H, NH), 3.46 (s, 2H, CH2N), 3.30 (d, J = 14.9 Hz, 1H, CH2S), 3.24 (t, J = 6.9 Hz, 2H, CH2), 2.90 (d, J = 14.9 Hz, 1H, CH2S), 2.37–2.25 (m, 2H, 3-Hcamendo, 6-Hcamexo), 2.01 (t, J = 4.4 Hz, 1H, 5-Hcam), 1.95–1.83 (m, 2H, 4-Hcamexo, 6-Hcamendo), 1.51 (ddd, J = 13.7, 9.3, 4.6 Hz, 1H, 3-Hcarendo), 1.41–1.28 (m, 1H, 4-Hcarendo), 0.97 (s, 3H, CH3), 0.74 (s, 3H, CH3). 13C NMR (150 MHz, DMSO-d6) δ 214.5 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 167.4 (2-Cth), 153.7 (4-Cth), 134.0 (1-Car), 128.7 (2,6-Car), 128.0 (4-Car), 126.0 (3,5-Car), 114.0 (5-Cth), 57.8 (2-Ccam), 47.9 (CH2S), 47.6 (7-Ccam), 42.3 (6-Ccam), 42.1 (5-Ccam), 42.0 (CH2N), 33.6 (CH2), 26.3 (4-Ccam), 24.5 (3-Ccam), 19.3 (CH3), 19.2 (CH3). Anal. calcd for C21H27ClN2O3S: C, 55.43; H, 5.98; N, 6.16 found C, 55.67; H, 5.77, N; 6.23.
O), 167.4 (2-Cth), 153.7 (4-Cth), 134.0 (1-Car), 128.7 (2,6-Car), 128.0 (4-Car), 126.0 (3,5-Car), 114.0 (5-Cth), 57.8 (2-Ccam), 47.9 (CH2S), 47.6 (7-Ccam), 42.3 (6-Ccam), 42.1 (5-Ccam), 42.0 (CH2N), 33.6 (CH2), 26.3 (4-Ccam), 24.5 (3-Ccam), 19.3 (CH3), 19.2 (CH3). Anal. calcd for C21H27ClN2O3S: C, 55.43; H, 5.98; N, 6.16 found C, 55.67; H, 5.77, N; 6.23.
Conflicts of interest
There are no conflicts to declare.
Acknowledgements
We thank Dr. Dimitra Benaki (Division of Pharmaceutical Chemistry, Department of Pharmacy, School of Health Sciences, National and Kapodistrian University of Athens) and Dr. Kyriakos C. Prousis, (Institute of Chemical Biology, National Hellenic Research Found) for NMR experiments.
Notes and references
-
M. A. Sleigh, Protozoa and other protists, ed. E. Arnold, London, 2nd edn, 1989 Search PubMed.
- S. Aksoy, P. Buscher, M. Lehane, P. Solano and J. Van Den Abbeele, PLoS Neglected Trop. Dis., 2017, 11, e0005454 CrossRef PubMed.
- I. Beltran-Hortelano, S. Perez-Silanes and S. Galiano, Curr. Med. Chem., 2017, 24, 1066 CrossRef CAS PubMed.
- P. E. Cockram and T. K. Smith, J. Nat. Prod., 2018, 81, 2138 CrossRef CAS PubMed.
- S. P. S. Rao, M. P. Barrett, G. Dranoff, C. J. Faraday, C. R. Gimpelewicz, A. Hailu, C. L. Jones, J. M. Kelly, J. K. Lazdins-Helds, P. Mäser, J. Mengel, J. C. Mottram, C. E. Mowbray, D. L. Sacks, P. Scott, G. F. Späth, R. L. Tarleton, J. M. Spector and T. T. Diagana, ACS Infect. Dis., 2019, 5, 152 CrossRef CAS PubMed.
- S. Russell, R. Rahmani, A. J. Jones, H. L. Newson, K. Neilde, I. Cotillo, M. Rahmani Khajouei, L. Ferrins, S. Qureishi, N. Nguyen, M. S. Martinez-Martinez, D. F. Weaver, M. Kaiser, J. Riley, J. Thomas, M. De Rycker, K. D. Read, G. R. Flematti, E. Ryan, S. Tanghe, A. Rodriguez, S. A. Charman, A. Kessler, V. M. Avery, J. B. Baell and M. J. Piggott, J. Med. Chem., 2016, 59, 9686 CrossRef CAS PubMed.
- D. A. Patrick, T. Wenzler, S. Yang, P. T. Weiser, M. Z. Wang, R. Brun and R. R. Tidwell, Bioorg. Med. Chem., 2016, 24, 2451 CrossRef CAS PubMed.
- A. A. Rashad, A. J. Jones, V. M. Avery, J. Baell and P. A. Keller, ACS Med. Chem. Lett., 2014, 5, 496 CrossRef CAS PubMed.
- I. Papanastasiou, G. B. Foscolos, A. Tsotinis, J. Olah, J. Ovadi, S. R. Prathalingam and J. M. Kelly, Heterocycles, 2008, 75, 2043 CrossRef CAS.
- I. Papanastasiou, A. Tsotinis, G. B. Foscolos, S. R. Prathalingam and J. M. Kelly, J. Heterocycl. Chem., 2008, 45, 1401 CrossRef CAS.
- G. B. Foscolos, I. Papanastasiou and A. Tsotinis, Lett. Org. Chem., 2008, 5, 57 CrossRef.
- I. Papanastasiou, A. Tsotinis, N. Kolocouris, S. R. Prathalingam and J. M. Kelly, J. Med. Chem., 2008, 51, 1496 CrossRef CAS PubMed.
- I. Papanastasiou, A. Tsotinis, G. Zoidis, N. Kolocouris, S. R. Prathalingam and J. M. Kelly, ChemMedChem, 2009, 4, 1059 CrossRef CAS PubMed.
- I. Papanastasiou, K. C. Prousis, K. Georgikopoulou, T. Pavlidis, E. Scoulica, N. Kolocouris and T. Calogeropoulou, Bioorg. Med. Chem. Lett., 2010, 20, 5484 CrossRef CAS PubMed.
- A. Koperniku, I. Papanastasiou, G. B. Foscolos, A. Tsotinis, M. C. Taylor and J. M. Kelly, MedChemComm, 2013, 4, 856 RSC.
- A.-S. Foscolos, I. Papanastasiou, G. B. Foscolos, A. Tsotinis, T. F. Kellici, T. Mavromoustakos, M. C. Taylor and J. M. Kelly, MedChemComm, 2016, 7, 1229 RSC.
-
I. Papanastasiou, G. Foscolos, B. A. Tsotinis and J. M. Kelly, in African Trypanosomiasis: Clinical Symptoms, Diagnosis and Treatment, ed. G. T. Hughes, Nova Science Publishers, NY, 2016, p. 63 Search PubMed.
- M.-O. Georgiadis, V. Kourbeli, V. Ioannidou, E. Karakitsios, I. Papanastasiou, A. Tsotinis, D. Komiotis, A. Vocat, S. T. Cole, M. C. Taylor and J. M. Kelly, Bioorg. Med. Chem. Lett., 2019, 29, 1278 CrossRef CAS PubMed.
- O. Karoutzou, D. Benaki, I. Papanastasiou, A. Vocat and S. T. Cole, ChemistrySelect, 2019, 4, 8727 CrossRef CAS.
- A. Papageorgiou, A.-S. Foscolos, I. P. Papanastasiou, M. Vlachou, A. Siamidi, A. Vocat, S. T. Cole, T. F. Kellici, T. Mavromoustakos and A. Tsotinis, Future Med. Chem., 2019, 11, 2779 CrossRef CAS.
- C. B. Scarim, D. H. Jornada, M. G. M. Machado, C. M. R. Ferreira, J. L. dos Santos and M. C. Chung, Eur. J. Med. Chem., 2019, 162, 378 CrossRef CAS PubMed.
- A. Foscolos, I. Papanastasiou, A. Tsotinis, M. C. Taylor and J. M. Kelly, ChemMedChem, 2019, 14, 1227 CrossRef CAS PubMed.
-
S. Vukelić, J. Moschner, S. Huhmann, R. Fernandes, A. A. Berger and B. Koksch, in Modern Synthesis Processes and Reactivity of Fluorinated Compounds, ed. H. Groult, F. Leroux and A. Tressaud, Elsevier, 2017, p. 427 Search PubMed.
- H. Mei, J. Han, S. Fustero, M. Medio-Simon, D. M. Sedgwick, C. Santi, R. Ruzziconi and V. A. Soloshonok, Chem. – Eur. J., 2019, 25, 1 CrossRef.
- B. G. de la Torre and F. Albericio, Molecules, 2019, 24, 809 CrossRef PubMed.
- S. M. Devine, M. D. Mulcair, C. O. Debono, E. W. W. Leung, J. W. M. Nissink, S. S. Lim, I. R. Chandrashekaran, M. Vazirani, B. Mohanty, J. S. Simpson, J. B. Baell, P. J. Scammells, R. S. Norton and M. J. Scanlon, J. Med. Chem., 2015, 58, 1205 CrossRef CAS PubMed.
- A. Flood, C. Trujillo, G. Sanchez-Sanz, B. Kelly, C. Muguruza, L. F. Callado and I. Rozas, Eur. J. Med. Chem., 2017, 138, 38 CrossRef CAS PubMed.
- M. I. Dawson, Z. Xia, T. Jiang, M. Ye, J. A. Fontana, L. Farhana, B. Patel, L. P. Xue, M. Bhuiyan, R. Pellicciari, A. Macchiarulo, R. Nuti, X.-K. Zhang, Y.-H. Han, L. Tautz, P. D. Hobbs, L. Jong, N. Waleh, W. Chao, G.-S. Feng, Y. Pang and Y. Su, J. Med. Chem., 2008, 51, 5650 CrossRef CAS PubMed.
- S. H. Pine and B. L. Sanchez, J. Org. Chem., 1971, 36, 829 CrossRef CAS.
- N. Miyaura, T. Yanagi and A. Suzuki, Synth. Commun., 1981, 11, 513 CrossRef CAS.
- A. Koperniku, A.-S. Foscolos, I. Papanastasiou, G. B. Foscolos, A. Tsotinis and D. Schols, Lett. Org. Chem., 2016, 13, 171 CrossRef CAS.
-
Thiazole and its derivatives, ed. J. V. Metzger, Wiley, NY, 1979 Search PubMed.
- Z. I. Dehimat, A. Paşahan, D. Tebbani, S. Yaşar and İ. Özdemir, Tetrahedron, 2017, 73, 5940 CrossRef CAS.
- S. V. Krasnikov, T. A. Obuchova, O. A. Yasinskii and K. V. Balakin, Tetrahedron Lett., 2004, 45, 711 CrossRef CAS.
- P. Mosset and R. Grée, Synlett, 2013, 24, 1142 CrossRef CAS.
- F. W. Foss, T. P. Mathews, Y. Kharel, P. C. Kennedy, A. H. Snyder, M. D. Davis, K. R. Lynch and T. L. Macdonald, Bioorg. Med. Chem., 2009, 17, 6123 CrossRef CAS PubMed.
- O. Kouatly, Ph. Eleftheriou, A. Petrou, D. Hadjipavlou-Litina and A. Geronikaki, SAR QSAR Environ. Res., 2018, 29, 83 CrossRef CAS PubMed.
- K. Aihara, Y. Kano, S. Shiokawa, T. Sasaki, F. Setsu, Y. Sambongi, M. Ishii, K. Tohyama, T. Ida, A. Tamura, K. Atsumi and K. Iwamatsu, Bioorg. Med. Chem., 2003, 11, 3475 CrossRef CAS PubMed.
- M. F. Richter, B. S. Drown, A. P. Riley, A. Garcia, T. Shirai, R. L. Svec and P. J. Hergenrother, Nature, 2017, 545, 299 CrossRef CAS PubMed.
- M. F. Richter and P. J. Hergenrother, Ann. N. Y. Acad. Sci., 2019, 1435, 18 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9md00478e |
|
| This journal is © The Royal Society of Chemistry 2020 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article a,
Violeta
Kourbeli
a,
Violeta
Kourbeli
 a,
Ioannis P.
Papanastasiou
a,
Ioannis P.
Papanastasiou
 *a,
Andrew
Tsotinis
*a,
Andrew
Tsotinis
 a,
Martin C.
Taylor
a,
Martin C.
Taylor
 b and
John M.
Kelly
b
b and
John M.
Kelly
b

![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 166.6 (2-Cth), 162.8 (d, J = 251.9 Hz, 4-Car), 152.8 (4-Cth), 137.5 (d, J = 11.4 Hz, 3-Car), 132.9 (1′,6′-Car), 132.8 (3′,4′-Car), 129.2 (1-Car), 126.2 (d, J = 8.4 Hz, 2-Car), 125.1 (6-Car), 122.2 (2′,5′-Car) 117.5 (d, J = 25.4, 5-Car), 116.4 (5-Cth), 40.5 (2,8,9-Cad), 38.4 (NCH2), 36.2 (1-Cad), 36.0 (4,6,10-Cad), 28.8 (CH2), 28.2 (3,5,7-Cad). Anal. calcd for C29H27FN2O2S: C, 71.58; H, 5.59; N, 5.76 found C, 71.35; H, 5.41; N, 5.54.
O), 166.6 (2-Cth), 162.8 (d, J = 251.9 Hz, 4-Car), 152.8 (4-Cth), 137.5 (d, J = 11.4 Hz, 3-Car), 132.9 (1′,6′-Car), 132.8 (3′,4′-Car), 129.2 (1-Car), 126.2 (d, J = 8.4 Hz, 2-Car), 125.1 (6-Car), 122.2 (2′,5′-Car) 117.5 (d, J = 25.4, 5-Car), 116.4 (5-Cth), 40.5 (2,8,9-Cad), 38.4 (NCH2), 36.2 (1-Cad), 36.0 (4,6,10-Cad), 28.8 (CH2), 28.2 (3,5,7-Cad). Anal. calcd for C29H27FN2O2S: C, 71.58; H, 5.59; N, 5.76 found C, 71.35; H, 5.41; N, 5.54.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 166.1 (2-Cth), 164.5 (d, J = 349.7 Hz, 4-Car), 155.5 (4-Cth), 138.6 (d, J = 11.2 Hz, 3-Car), 129.6 (1-Car), 125.8 (d, J = 2.9 Hz, 6-Car), 125.6 (2-Car), 117.2 (d, J = 25.8 Hz, 5-Car), 114.2 (5-Cth), 41.1 (d, J = 3.5 Hz, 2,8,9-Cad), 39.1 (CH2N), 36.9 (4,6,10-Cad), 36.7 (1-Cad), 31.0 (CH2), 28.9 (3,5,7-Cad), 23.5 (CH3). M.p. (fumarate): 143-145 °C (MeOH/Et2O). Anal. calcd for C27H31FN2O5S: C, 63.03; H, 6.07; N, 5.44 found C, 62.89; H, 6.01; N, 5.21.
O), 166.1 (2-Cth), 164.5 (d, J = 349.7 Hz, 4-Car), 155.5 (4-Cth), 138.6 (d, J = 11.2 Hz, 3-Car), 129.6 (1-Car), 125.8 (d, J = 2.9 Hz, 6-Car), 125.6 (2-Car), 117.2 (d, J = 25.8 Hz, 5-Car), 114.2 (5-Cth), 41.1 (d, J = 3.5 Hz, 2,8,9-Cad), 39.1 (CH2N), 36.9 (4,6,10-Cad), 36.7 (1-Cad), 31.0 (CH2), 28.9 (3,5,7-Cad), 23.5 (CH3). M.p. (fumarate): 143-145 °C (MeOH/Et2O). Anal. calcd for C27H31FN2O5S: C, 63.03; H, 6.07; N, 5.44 found C, 62.89; H, 6.01; N, 5.21.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 166.1 (2-Cth), 164.5 (d, J = 349.7 Hz, 4-Car), 155.5 (4-Cth), 138.3 (d, J = 11.2 Hz, 3-Car), 129.6 (1-Car), 125.8 (d, J = 2.9 Hz, 6-Car), 125.6 (2-Car), 117.2 (d, J = 25.8 Hz, 5-Car), 114.3 (5-Cth), 41.1 (2,8,9-Cad), 39.2 (CH2N), 36.9 (4,6,10-Cad), 36.7 (1-Cad), 31.0 (CH2), 28.9 (3,5,7-Cad), 28.9 (CH2), 19.3 (CH2), 13.9 (CH3). M.p. (fumarate): 291–293 °C (MeOH/Et2O). Anal. calcd for C29H35FN2O5S: C, 64.19; H, 6.50; N, 5.16 found C, 64.38; H, 6.61; N, 5.33.
O), 166.1 (2-Cth), 164.5 (d, J = 349.7 Hz, 4-Car), 155.5 (4-Cth), 138.3 (d, J = 11.2 Hz, 3-Car), 129.6 (1-Car), 125.8 (d, J = 2.9 Hz, 6-Car), 125.6 (2-Car), 117.2 (d, J = 25.8 Hz, 5-Car), 114.3 (5-Cth), 41.1 (2,8,9-Cad), 39.2 (CH2N), 36.9 (4,6,10-Cad), 36.7 (1-Cad), 31.0 (CH2), 28.9 (3,5,7-Cad), 28.9 (CH2), 19.3 (CH2), 13.9 (CH3). M.p. (fumarate): 291–293 °C (MeOH/Et2O). Anal. calcd for C29H35FN2O5S: C, 64.19; H, 6.50; N, 5.16 found C, 64.38; H, 6.61; N, 5.33.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 164.5 (d, J = 349.7 Hz, 4-Car), 155.5 (4-Cth), 138.3 (d, J = 11.2 Hz, 3-Car), 131.3 (4′-Car), 129.6 (1-Car), 128.5 (3′,5′-Car), 127.0 (2′,6′-Car), 125.8 (d, J = 2.9 Hz, 6-Car), 125.6 (2-Car), 117.2 (d, J = 25.8 Hz, 5-Car), 114.3 (5-Cth), 41.1 (2,8,9-Cad), 39.2 (CH2N), 36.9 (4,6,10-Cad), 36.7 (1-Cad), 31.0 (CH2), 28.9 (3,5,7-Cad). M.p. (fumarate): 305 °C (dec) (MeOH/Et2O). Anal. calcd for C32H33FN2O5S: C, 66.65; H, 5.77; N, 4.86 found C, 65.39; H, 5.90; N, 4.72.
O), 164.5 (d, J = 349.7 Hz, 4-Car), 155.5 (4-Cth), 138.3 (d, J = 11.2 Hz, 3-Car), 131.3 (4′-Car), 129.6 (1-Car), 128.5 (3′,5′-Car), 127.0 (2′,6′-Car), 125.8 (d, J = 2.9 Hz, 6-Car), 125.6 (2-Car), 117.2 (d, J = 25.8 Hz, 5-Car), 114.3 (5-Cth), 41.1 (2,8,9-Cad), 39.2 (CH2N), 36.9 (4,6,10-Cad), 36.7 (1-Cad), 31.0 (CH2), 28.9 (3,5,7-Cad). M.p. (fumarate): 305 °C (dec) (MeOH/Et2O). Anal. calcd for C32H33FN2O5S: C, 66.65; H, 5.77; N, 4.86 found C, 65.39; H, 5.90; N, 4.72.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 164.5 (d, J = 349.7 Hz, 4-Car), 163.9 (d, J = 348.6 Hz, 4′-Car) 155.5 (4-Cth), 138.3 (d, J = 11.2 Hz, 3-Car), 130.1 (d, J = 3 Hz, 1′-Car), 129.6 (1-Car), 128.3 (d, J = 7 Hz, 2′,6′-Car), 125.8 (d, J = 2.9 Hz, 6-Car), 125.6 (2-Car), 117.2 (d, J = 25.8 Hz, 5-Car), 116.1 (d, J = 18 Hz, 3′,5′-Car) 114.3 (5-Cth), 41.1 (d, J = 3.5 Hz, 2,8,9-Cad), 39.2 (CH2N), 36.9 (4,6,10-Cad), 36.7 (1-Cad), 31.0 (CH2), 28.9 (3,5,7-Cad). M.p. (fumarate): 226–227 °C (MeOH/Et2O). Anal. calcd for C32H32F2N2O5S: C, 64.63; H, 5.42; N, 4.71 found C, 64.44; H, 5.73; N, 4.88.
O), 164.5 (d, J = 349.7 Hz, 4-Car), 163.9 (d, J = 348.6 Hz, 4′-Car) 155.5 (4-Cth), 138.3 (d, J = 11.2 Hz, 3-Car), 130.1 (d, J = 3 Hz, 1′-Car), 129.6 (1-Car), 128.3 (d, J = 7 Hz, 2′,6′-Car), 125.8 (d, J = 2.9 Hz, 6-Car), 125.6 (2-Car), 117.2 (d, J = 25.8 Hz, 5-Car), 116.1 (d, J = 18 Hz, 3′,5′-Car) 114.3 (5-Cth), 41.1 (d, J = 3.5 Hz, 2,8,9-Cad), 39.2 (CH2N), 36.9 (4,6,10-Cad), 36.7 (1-Cad), 31.0 (CH2), 28.9 (3,5,7-Cad). M.p. (fumarate): 226–227 °C (MeOH/Et2O). Anal. calcd for C32H32F2N2O5S: C, 64.63; H, 5.42; N, 4.71 found C, 64.44; H, 5.73; N, 4.88.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 155.5 (4-Cth), 142.7 (4-Cfur), 141.1 (1-Cfur), 138.3 (d, J = 11.2 Hz, 3-Car), 129.6 (1-Car), 125.8 (d, J = 2.9 Hz, 6-Car), 125.6 (2-Car), 117.2 (d, J = 25.8 Hz, 5-Car), 115.6 (2-Cfur), 115.2 (5-Cth), 114.3 (3-Cfur), 41.1 (2,8,9-Cad), 39.2 (CH2N), 36.9 (4,6,10-Cad), 36.7 (1-Cad), 31.0 (CH2), 28.9 (3,5,7-Cad), 11.0 (CH3). M.p. (fumarate): 260 (dec) °C (MeOH/Et2O). Anal. Calcd for C31H32FN2O6S: C, 64.12; H, 5.73; N, 4.82 found C, 64.44; H, 5.54; N, 4.98.
O), 155.5 (4-Cth), 142.7 (4-Cfur), 141.1 (1-Cfur), 138.3 (d, J = 11.2 Hz, 3-Car), 129.6 (1-Car), 125.8 (d, J = 2.9 Hz, 6-Car), 125.6 (2-Car), 117.2 (d, J = 25.8 Hz, 5-Car), 115.6 (2-Cfur), 115.2 (5-Cth), 114.3 (3-Cfur), 41.1 (2,8,9-Cad), 39.2 (CH2N), 36.9 (4,6,10-Cad), 36.7 (1-Cad), 31.0 (CH2), 28.9 (3,5,7-Cad), 11.0 (CH3). M.p. (fumarate): 260 (dec) °C (MeOH/Et2O). Anal. Calcd for C31H32FN2O6S: C, 64.12; H, 5.73; N, 4.82 found C, 64.44; H, 5.54; N, 4.98.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 155.5 (4-Cth), 138.3 (d, J = 11.2 Hz, 3-Car), 129.6 (1-Car), 125.8 (d, J = 2.9 Hz, 6-Car), 125.6 (2-Car), 117.2 (d, J = 25.8 Hz, 5-Car), 114.3 (5-Cth), 45.4 (2,5-Cpy), 41.1 (d, J = 3.5 Hz, 2,8,9-Cad), 39.2 (CH2N), 36.9 (4,6,10-Cad), 36.7 (1-Cad), 31.0 (CH2), 28.9 (3,5,7-Cad), 25.6 (3,4-Cpy). M.p. (fumarate): 301 °C (dec) (MeOH/Et2O). Anal. calcd for C30H36FN3O5S: C, 63.25; H, 6.37; N, 7.38 found C, 63.09; H, 6.14; N, 7.19.
O), 155.5 (4-Cth), 138.3 (d, J = 11.2 Hz, 3-Car), 129.6 (1-Car), 125.8 (d, J = 2.9 Hz, 6-Car), 125.6 (2-Car), 117.2 (d, J = 25.8 Hz, 5-Car), 114.3 (5-Cth), 45.4 (2,5-Cpy), 41.1 (d, J = 3.5 Hz, 2,8,9-Cad), 39.2 (CH2N), 36.9 (4,6,10-Cad), 36.7 (1-Cad), 31.0 (CH2), 28.9 (3,5,7-Cad), 25.6 (3,4-Cpy). M.p. (fumarate): 301 °C (dec) (MeOH/Et2O). Anal. calcd for C30H36FN3O5S: C, 63.25; H, 6.37; N, 7.38 found C, 63.09; H, 6.14; N, 7.19.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 152.8 (4-Cth), 137.5 (d, J = 11.4 Hz, 3-Car), 129.2 (1-Car), 126.2 (d, J = 8.4 Hz, 2-Car), 125.1 (6-Car), 117.5 (d, J = 25.4, 5-Car), 116.4 (5-Cth), 43.9 (2,6-Cpi), 40.5 (2,8,9-Cad), 38.4 (NCH2), 36.2 (1-Cad), 36.0 (4,6,10-Cad), 28.8 (CH2), 28.2 (3,5,7-Cad), 24.8 (3,5-Cpi), 23.5 (4-Cpi). Anal. calcd for C27H34FN3OS: C, 69.35; H, 7.33; N, 8.99 found C, 69.55; H, 7.39; N, 9.12.
O), 152.8 (4-Cth), 137.5 (d, J = 11.4 Hz, 3-Car), 129.2 (1-Car), 126.2 (d, J = 8.4 Hz, 2-Car), 125.1 (6-Car), 117.5 (d, J = 25.4, 5-Car), 116.4 (5-Cth), 43.9 (2,6-Cpi), 40.5 (2,8,9-Cad), 38.4 (NCH2), 36.2 (1-Cad), 36.0 (4,6,10-Cad), 28.8 (CH2), 28.2 (3,5,7-Cad), 24.8 (3,5-Cpi), 23.5 (4-Cpi). Anal. calcd for C27H34FN3OS: C, 69.35; H, 7.33; N, 8.99 found C, 69.55; H, 7.39; N, 9.12.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S), 156.0 (1-Car), 136.4 (4-Car), 126.9 (2,6-Car), 125.1 (3,5-Car), 42.9 (2,8,9-Cad), 36.7 (4,6,10-Cad), 36.6 (1-Cad), 28.8 (3,5,7-Cad). C17H21NS: C, 75.23; H, 7.80 found C, 75.07; H, 7.99.
S), 156.0 (1-Car), 136.4 (4-Car), 126.9 (2,6-Car), 125.1 (3,5-Car), 42.9 (2,8,9-Cad), 36.7 (4,6,10-Cad), 36.6 (1-Cad), 28.8 (3,5,7-Cad). C17H21NS: C, 75.23; H, 7.80 found C, 75.07; H, 7.99.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 168.2 (2-Cth), 154.2 (4-Cth), 153.4 (1-Car), 133.9 (3′,4′-Car), 132.3 (1′,6′-Car), 131.1 (4-Car), 126.4 (2,6-Car), 125.4 (3,5-Car), 123.3 (2′,5′-Car), 114.2 (5-Cth), 43.1 (2,8,9-Cad), 37.7 (NCH2), 36.9 (4,6,10-Cad), 36.5 (1-Cad), 30.2 (CH2), 29.0 (3,5,7-Cad). C29H28N2O2S: C, 74.33; H, 6.02; N, 5.98 found C, 74.64; H, 6.12; N, 6.21.
O), 168.2 (2-Cth), 154.2 (4-Cth), 153.4 (1-Car), 133.9 (3′,4′-Car), 132.3 (1′,6′-Car), 131.1 (4-Car), 126.4 (2,6-Car), 125.4 (3,5-Car), 123.3 (2′,5′-Car), 114.2 (5-Cth), 43.1 (2,8,9-Cad), 37.7 (NCH2), 36.9 (4,6,10-Cad), 36.5 (1-Cad), 30.2 (CH2), 29.0 (3,5,7-Cad). C29H28N2O2S: C, 74.33; H, 6.02; N, 5.98 found C, 74.64; H, 6.12; N, 6.21.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, fum), 153.2 (1-Car), 152.9 (4-Cth), 134.6 (C-fum), 130.5 (4-Car), 126.0 (2,6-Car), 125.6 (3,5-Car), 115.7 (5-Cth), 47.4 (NCH2), 42.4 (2,8,9-Cad), 36.1 (4,6,10-Cad), 36.0 (1-Cad), 32.5 (CH3), 28.3 (3,5,7-Cad), 27.6 (CH2). Anal. calcd for C30H36N2O8S: C, 61.63; H, 6.21; N, 4.79 found C, 61.47; H, 6.08; N, 5.01.
O, fum), 153.2 (1-Car), 152.9 (4-Cth), 134.6 (C-fum), 130.5 (4-Car), 126.0 (2,6-Car), 125.6 (3,5-Car), 115.7 (5-Cth), 47.4 (NCH2), 42.4 (2,8,9-Cad), 36.1 (4,6,10-Cad), 36.0 (1-Cad), 32.5 (CH3), 28.3 (3,5,7-Cad), 27.6 (CH2). Anal. calcd for C30H36N2O8S: C, 61.63; H, 6.21; N, 4.79 found C, 61.47; H, 6.08; N, 5.01.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, fum), 153.7 (4-Cth), 134.7 (1-Car), 134.6 (CH, fum), 130.9 (4-Car), 126.4 (2,6-Car), 126.0 (3,5-Car), 116.0 (5-Cth), 56.4 (CH2N), 43.0 (CH3), 42.9 (2,8,9-Cad), 36.6 (4,6,10-Cad), 36.5 (1-Cad), 28.7 (3,5,7-Cad), 27.0 (CH2). Anal. calcd for C31H38N2O8S: C, 62.19; H, 6.40; N, 4.68 found C, 62.31; H, 6.64; N, 4.14.
O, fum), 153.7 (4-Cth), 134.7 (1-Car), 134.6 (CH, fum), 130.9 (4-Car), 126.4 (2,6-Car), 126.0 (3,5-Car), 116.0 (5-Cth), 56.4 (CH2N), 43.0 (CH3), 42.9 (2,8,9-Cad), 36.6 (4,6,10-Cad), 36.5 (1-Cad), 28.7 (3,5,7-Cad), 27.0 (CH2). Anal. calcd for C31H38N2O8S: C, 62.19; H, 6.40; N, 4.68 found C, 62.31; H, 6.64; N, 4.14.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 7.84 (d, J = 8.2 Hz, 2H, 2,6-Har), 7.46 (d, J = 8.2 Hz, 2H, 3,5-Har), 7.34 (s, 1H, 5-Hth), 6.63 (s, 2H, CH-Fum), 3.39 (dt, J = 13.3, 6.7 Hz, 2H, NCH2), 2.88 (t, J = 7.2 Hz, 2H, CH2), 2.06 (s, 3H, 3,5,7-Had), 1.88 (s, 6H, 2,8,9-Had), 1.80 (s, 3H, CH3), 1.74 (br.s, 6H, 4,6,10-Had). 13C NMR (75 MHz, DMSO-d6) δ 169.1 (NC
O), 7.84 (d, J = 8.2 Hz, 2H, 2,6-Har), 7.46 (d, J = 8.2 Hz, 2H, 3,5-Har), 7.34 (s, 1H, 5-Hth), 6.63 (s, 2H, CH-Fum), 3.39 (dt, J = 13.3, 6.7 Hz, 2H, NCH2), 2.88 (t, J = 7.2 Hz, 2H, CH2), 2.06 (s, 3H, 3,5,7-Had), 1.88 (s, 6H, 2,8,9-Had), 1.80 (s, 3H, CH3), 1.74 (br.s, 6H, 4,6,10-Had). 13C NMR (75 MHz, DMSO-d6) δ 169.1 (NC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 166.5 (2-Cth), 166.0 (C
O), 166.5 (2-Cth), 166.0 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, fum), 155.2 (4-Cth), 153.0 (1-Car), 134.0 (CH-fum), 130.7 (4-Car), 125.9 (2,6-Car), 125.5 (3,5-Car), 114.6 (5-Cth), 42.4 (2,8,9-Cad), 38.2 (NCH2), 36.1 (4,6,10-Cad), 36.0 (1-Cad), 31.3 (CH2), 28.3 (3,5,7-Cad), 22.7 (CH3). Anal. calcd for C27H32N2O5S: C, 65.30; H, 6.50; N, 5.64 found C, 65.27; H, 6.33; N, 5.45.
O, fum), 155.2 (4-Cth), 153.0 (1-Car), 134.0 (CH-fum), 130.7 (4-Car), 125.9 (2,6-Car), 125.5 (3,5-Car), 114.6 (5-Cth), 42.4 (2,8,9-Cad), 38.2 (NCH2), 36.1 (4,6,10-Cad), 36.0 (1-Cad), 31.3 (CH2), 28.3 (3,5,7-Cad), 22.7 (CH3). Anal. calcd for C27H32N2O5S: C, 65.30; H, 6.50; N, 5.64 found C, 65.27; H, 6.33; N, 5.45.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 158.0 (1-Car), 129.0 (2,6-Cad), 127.5 (4-Cad), 125.6 (3,5-Car), 42.9 (2,8,9-Cad), 36.9 (1-Cad), 36.8 (3,6,10-Cad), 31.1 (CH2), 28.8 (3,5,7-Cad). Anal. calcd for C18H21BrO: C, 64.87; H, 6.35 found C, 64.63; H, 6.46.
O), 158.0 (1-Car), 129.0 (2,6-Cad), 127.5 (4-Cad), 125.6 (3,5-Car), 42.9 (2,8,9-Cad), 36.9 (1-Cad), 36.8 (3,6,10-Cad), 31.1 (CH2), 28.8 (3,5,7-Cad). Anal. calcd for C18H21BrO: C, 64.87; H, 6.35 found C, 64.63; H, 6.46.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, fum), 151.0 (4-Cth), 134.5 (CH2fum), 131.4 (1-Car), 127.1 (4-Car), 125.9 (2,6-Car), 125.2 (3,5-Car), 114.5 (5-Cth), 42.6 (2,8,9-Cad), 40.8 (CH2N), 36.3 (4,6,10-Cad), 35.8 (1-Cad), 28.4 (3,5,7-Cad). Anal. calcd for C28H32N2O8S: C, 60.62; H, 5.79; N, 5.03 found C, 60.33; H, 6.02; N, 4.89.
O, fum), 151.0 (4-Cth), 134.5 (CH2fum), 131.4 (1-Car), 127.1 (4-Car), 125.9 (2,6-Car), 125.2 (3,5-Car), 114.5 (5-Cth), 42.6 (2,8,9-Cad), 40.8 (CH2N), 36.3 (4,6,10-Cad), 35.8 (1-Cad), 28.4 (3,5,7-Cad). Anal. calcd for C28H32N2O8S: C, 60.62; H, 5.79; N, 5.03 found C, 60.33; H, 6.02; N, 4.89.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 168.2 (2-Cth), 155.9 (4-Cth), 133.5 (1-Car), 130.0 (4-Car), 128.8 (2,6-Car), 126.3 (3,5-Car), 114.2 (5-Cth), 40.5 (CH2N), 39.3 (2,8,9-Cad), 38.8 (1-Cad), 36.5 (4,6–10-Cad), 30.7 (CH2), 28.1 (3,5,7-Cad). Anal. calcd for C22H26N2OS: C, 72.08; H, 7.15; N, 7.64 found C, 72.31; H, 7.09; N, 7.88.
O), 168.2 (2-Cth), 155.9 (4-Cth), 133.5 (1-Car), 130.0 (4-Car), 128.8 (2,6-Car), 126.3 (3,5-Car), 114.2 (5-Cth), 40.5 (CH2N), 39.3 (2,8,9-Cad), 38.8 (1-Cad), 36.5 (4,6–10-Cad), 30.7 (CH2), 28.1 (3,5,7-Cad). Anal. calcd for C22H26N2OS: C, 72.08; H, 7.15; N, 7.64 found C, 72.31; H, 7.09; N, 7.88.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 167.2 (2-Cth), 155.0 (4-Cth), 133.4 (1-Car), 130.7 (4-Car), 129.7 (2,6-Car), 126.6 (3,5-Car), 116.3 (5-Cth), 58.3 (2-Ccam), 48.2 (CH2), 48.0 (7-Ccam), 42.7 (6-Ccam), 42.5 (5-Ccam), 42.5 (CH2N), 32.2 (CH2), 26.7 (4-Ccam), 25.0 (3-Ccam), 19.8 (CH3), 19.7 (CH3). Anal. calcd for C21H27ClN2O3S: C, 55.43; H, 5.98; N, 6.16 found C, 55.21; H, 6.11; N, 6.02.
O), 167.2 (2-Cth), 155.0 (4-Cth), 133.4 (1-Car), 130.7 (4-Car), 129.7 (2,6-Car), 126.6 (3,5-Car), 116.3 (5-Cth), 58.3 (2-Ccam), 48.2 (CH2), 48.0 (7-Ccam), 42.7 (6-Ccam), 42.5 (5-Ccam), 42.5 (CH2N), 32.2 (CH2), 26.7 (4-Ccam), 25.0 (3-Ccam), 19.8 (CH3), 19.7 (CH3). Anal. calcd for C21H27ClN2O3S: C, 55.43; H, 5.98; N, 6.16 found C, 55.21; H, 6.11; N, 6.02.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (10 mL), was added 1-adamantanecarboxylic acid (227 mg, 1.26 mmol), HBTU (478 mg, 1.26 mmol), and DIPEA (474 mg, 3.68 mmol) and the reaction mixture was stirred at ambient temperature under an argon atmosphere, overnight. The mixture was then partitioned between DCM and an aqueous solution of citric acid (10%) and the aqueous phase was extracted with DCM. The combined organic phase was then washed with water and brine, dried over MgSO4 and the solvent evaporated under reduced pressure. The resulting residue was purified with gradient column chromatography. Elution with 10% to 50% EtOAc in hexanes afforded compound 4a as a white solid (330 mg, 94%). M.p.: 107–108 °C. 1H NMR (400 MHz, CDCl3) δ 8.01–7.84 (m, 2H, 2,6-Har), 7.54–7.28 (m, 3,4,5-Har, 5-Hth), 6.92 (s, 1H, NH), 3.69 (dd, J = 11.8, 5.7 Hz, 3H, CH2N), 3.20 (t, J = 7.4 Hz, 3H, CH2), 2.02 (s, 3H, 3,5,7-Had), 1.87 (d, J = 2.3 Hz, 6H, 2,8,9-Had), 1.70 (q, J = 12.2 Hz, 6H, 4,6,10-Had).13C NMR (150 MHz, CDCl3) δ 177.9 (C
1 (10 mL), was added 1-adamantanecarboxylic acid (227 mg, 1.26 mmol), HBTU (478 mg, 1.26 mmol), and DIPEA (474 mg, 3.68 mmol) and the reaction mixture was stirred at ambient temperature under an argon atmosphere, overnight. The mixture was then partitioned between DCM and an aqueous solution of citric acid (10%) and the aqueous phase was extracted with DCM. The combined organic phase was then washed with water and brine, dried over MgSO4 and the solvent evaporated under reduced pressure. The resulting residue was purified with gradient column chromatography. Elution with 10% to 50% EtOAc in hexanes afforded compound 4a as a white solid (330 mg, 94%). M.p.: 107–108 °C. 1H NMR (400 MHz, CDCl3) δ 8.01–7.84 (m, 2H, 2,6-Har), 7.54–7.28 (m, 3,4,5-Har, 5-Hth), 6.92 (s, 1H, NH), 3.69 (dd, J = 11.8, 5.7 Hz, 3H, CH2N), 3.20 (t, J = 7.4 Hz, 3H, CH2), 2.02 (s, 3H, 3,5,7-Had), 1.87 (d, J = 2.3 Hz, 6H, 2,8,9-Had), 1.70 (q, J = 12.2 Hz, 6H, 4,6,10-Had).13C NMR (150 MHz, CDCl3) δ 177.9 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 168.2 (2-Cth), 155.9 (4-Cth), 133.5 (1-Car), 130.0 (4-Car), 128.8 (2,6-Car), 126.3 (3,5-Car), 114.2 (5-Cth), 40.5 (CH2N), 39.3 (2,8,9-Cad), 38.8 (1-Cad), 36.5 (4,6–10-Cad), 30.7 (CH2), 28.1 (3,5,7-Cad). Anal. calcd for C22H26N2OS: C, 72.09; H, 7.15; N, 7.64 found C, 72.27; H, 7.23; N, 7.92.
O), 168.2 (2-Cth), 155.9 (4-Cth), 133.5 (1-Car), 130.0 (4-Car), 128.8 (2,6-Car), 126.3 (3,5-Car), 114.2 (5-Cth), 40.5 (CH2N), 39.3 (2,8,9-Cad), 38.8 (1-Cad), 36.5 (4,6–10-Cad), 30.7 (CH2), 28.1 (3,5,7-Cad). Anal. calcd for C22H26N2OS: C, 72.09; H, 7.15; N, 7.64 found C, 72.27; H, 7.23; N, 7.92.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 167.4 (2-Cth), 153.7 (4-Cth), 134.0 (1-Car), 128.7 (2,6-Car), 128.0 (4-Car), 126.0 (3,5-Car), 114.0 (5-Cth), 57.8 (2-Ccam), 47.9 (CH2S), 47.6 (7-Ccam), 42.3 (6-Ccam), 42.1 (5-Ccam), 42.0 (CH2N), 33.6 (CH2), 26.3 (4-Ccam), 24.5 (3-Ccam), 19.3 (CH3), 19.2 (CH3). Anal. calcd for C21H27ClN2O3S: C, 55.43; H, 5.98; N, 6.16 found C, 55.67; H, 5.77, N; 6.23.
O), 167.4 (2-Cth), 153.7 (4-Cth), 134.0 (1-Car), 128.7 (2,6-Car), 128.0 (4-Car), 126.0 (3,5-Car), 114.0 (5-Cth), 57.8 (2-Ccam), 47.9 (CH2S), 47.6 (7-Ccam), 42.3 (6-Ccam), 42.1 (5-Ccam), 42.0 (CH2N), 33.6 (CH2), 26.3 (4-Ccam), 24.5 (3-Ccam), 19.3 (CH3), 19.2 (CH3). Anal. calcd for C21H27ClN2O3S: C, 55.43; H, 5.98; N, 6.16 found C, 55.67; H, 5.77, N; 6.23.






