Acid-catalyzed pyrolytic synthesis of levoglucosan through salt-mediated ring locking†
Abstract
Selectively producing chemicals from cellulosic carbohydrate pyrolysis in large quantities is challenging, especially anhydro-monosaccharides with double-ring, triple-ring, and furan/pyran structures. Formation of these sugar derivatives greatly improves when the pyranose ring opening is inhibited during pyrolysis, which is accomplished by chemically replacing the hydroxyl group at the anomeric carbon with an alkoxy group. A simpler ring-locking approach is required for scalable chemical production, however. In this work, we demonstrate that introducing Na2SO4 and H2SO4 to glucose pyrolysis significantly increases levoglucosan (LGA) formation, from a 6% yield to as high as 40% at 350 °C. With H2SO4 as the acid catalyst, Na+ acts to inhibit the ring opening. Glucose pyrolysis with different alkali metal cations (Li+, Na+, K+, Rb+ and Cs+) gives different reaction products, which can be explained largely by an ionic electronegativity effect. Weaker electronegativity promotes the formation of a ring-opened product such as 5-hydroxymethylfurfural (HMF), and stronger electronegativity increases the formation of sequential dehydration products like levoglucosenone (LGO). Sodium has the optimum ionic electronegativity for preferential association with the ring oxygen. The Na2SO4/H2SO4 combination improved LGA yields for all carbohydrate substrates tested (up to 70%), including lignocellulose. These findings highlight the potential of using alkali metal salts to produce anhydrosugars in high yields from cellulosic carbohydrate pyrolysis.
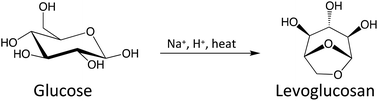


 Please wait while we load your content...
Please wait while we load your content...
