Haber-independent, diversity-oriented synthesis of nitrogen compounds from biorenewable chitin†
Abstract
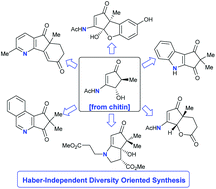
During drug discovery programmes, medicinal chemists rely on compounds derived from Haber ammonia as the source of this clinically important nitrogen heteroatom and as a result, the development of new pharmaceuticals is very energy intensive. Herein we report that biologically fixed nitrogen sourced from the biopolymer chitin can be readily incorporated into the diversity-oriented synthesis (DOS) of N-compounds relevant to drug discovery and development. The furfuryl alcohol derived from the chitin-sourced platform 3-acetamido-5-acetylfuran (3A5AF) undergoes a Piancatelli rearrangement to give a 3-aminocyclopentenone that is readily converted into a variety 4-aminocyclopentanones and 4-aminocyclopentene-1,3-diones. From two of these functionally rich bio-based platforms, structurally diverse, medicinally relevant N-scaffolds are attainable, including aminated Hajos–Parrish ketone (HPK) derivatives, aminated bicyclic ethers, bicyclic pyrrolidines, pyridines, isoquinolines and indoles. Thus, the DOS of compound libraries relevant to modern drug discovery programmes can be assembled independent of Haber ammonia.


 Please wait while we load your content...
Please wait while we load your content...