Prediagnostic dietary intakes of vitamin A and β-carotene are associated with hepatocellular-carcinoma survival†
Dao-ming
Zhang‡
 a,
Yun
Luo‡
a,
Dinuerguli
Yishake
a,
Zhao-yan
Liu
a,
Tong-tong
He
a,
Yan
Luo
a,
Yao-jun
Zhang
cd,
Ai-ping
Fang
*ab and
Hui-lian
Zhu
a,
Yun
Luo‡
a,
Dinuerguli
Yishake
a,
Zhao-yan
Liu
a,
Tong-tong
He
a,
Yan
Luo
a,
Yao-jun
Zhang
cd,
Ai-ping
Fang
*ab and
Hui-lian
Zhu
 *ab
*ab
aDepartment of Nutrition, School of Public Health, Sun Yat-sen University, Guangzhou 510080, People's Republic of China. E-mail: fangaip@mail.sysu.edu.cn; zhuhl@mail.sysu.edu.cn; Fax: +86-20-8733-5785; Fax: +86-020-87331811; Tel: +86-20-8733-5785 Tel: +86-020-87331811
bGuangdong Provincial Key Laboratory of Food, Nutrition and Health, School of Public Health, Sun Yat-sen University, Guangzhou, People's Republic of China
cDepartment of Hepatobiliary Oncology, Sun Yat-sen University Cancer Center, Guangzhou 510060, People's Republic of China
dState Key Laboratory of Oncology in South China, Sun Yat-sen University Cancer Center, Guangzhou, People's Republic of China
First published on 26th December 2019
Abstract
Vitamin A and its precursor (β-carotene) have been linked with cancer incidence and mortality. However, the relationship between vitamin A and the prognosis of hepatocellular-carcinoma (HCC) is still unknown. Therefore, we investigated whether dietary intakes of vitamin A, retinol, and β-carotene were associated with survival in patients with HCC who participated in the Guangdong Liver Cancer Cohort (GLCC) study. Patients aged 18–80 years with a diagnosis of incident Primary Liver Cancer (PLC) were enrolled within one month of diagnosis prior to cancer treatment at the Sun Yat-sen University Cancer Center. Dietary information one year before diagnosis of HCC was obtained using a 79-item, validated semiquantitative food frequency questionnaire (FFQ). We restricted the present analysis to 877 HCC patients enrolled in the GLCC between September, 2013 and April, 2017 who had completed FFQ. Cox proportional hazard regression models were used to estimate hazard ratios (HR) and 95% confidence intervals (CIs) for overall and HCC-specific survival. After a median follow-up of 797 days, 384 deaths were documented, 343 of which died from HCC. The multivariable-adjusted HRs (95% CI) of overall and HCC-specific survival for the highest versus the lowest quartile were 0.70 (0.53–0.94) and 0.68 (0.50–0.92) for vitamin A, and 0.72 (0.54–0.96) and 0.69 (0.51–0.94) for β-carotene, respectively. However, no significant association of dietary retinol intakes with survival outcomes was observed. Our observations suggest that higher prediagnostic dietary intakes of vitamin A and β-carotene were associated with improved overall and HCC-specific survival.
Introduction
Primary Liver Cancer (PLC), one of the most common malignant tumors, has a poor prognosis because of usually being diagnosed at advanced stages. PLC was the sixth most commonly diagnosed cancer and the fourth leading cause of cancer-related death in 2018 worldwide.1 There are approximately 50% of the total number of cases and deaths occurring in China.2 PLC comprises hepatocellular carcinoma (HCC) (75%–85% of cases) and intrahepatic cholangiocarcinoma (10%–15% of cases) as well as other rare types. A growing number of studies has indicated dietary factors not only are associated with the risk of HCC,3,4 but also play an important role in the prognosis of HCC.5Vitamin A, including preformed vitamin A (i.e., retinol and retinyl ester) from animal food and its precursor carotenoids (mainly β-carotene) from vegetables and fruits, is indispensable in numerous physiological processes.6 As is well-known, vitamin A is mainly obtained from the diet. Vitamin A in the body is mainly stored in the liver, and serum retinoids and carotenoids are carried by lipoprotein in the blood and metabolized in the liver.7 Accumulating evidence suggests that higher levels of dietary or circulating vitamin A or its precursor may reduce the risk of various cancers, including PLC.8–10 Several prospective cohort studies found that higher prediagnostic serum levels of retinol and β-carotene were associated with a lower risk of liver cancer and chronic liver disease (CLD) mortality.11–13 Consistantly, in our previous case-control study, dietary intakes of vitamin A (in RE), retinol, and β-carotene were independently and inversely associated with the risk of PLC.14 In addition, vitamin A has been linked with breast and gastrointestinal cancer survival.8,15,16 Specific to PLC, few studies have been conducted to examine the relationship between vitamin A and the prognosis of HCC.16–18
Because of the importance of vitamin A in protecting against oxidative stress and in regulation of cell proliferation and differentiation,19,20 we hypothesize that higher levels of vitamin A may prevent the progression of HCC. Therefore, the aim of this study was to evaluate whether dietary intakes of retinol, β-carotene, and total vitamin A are associated with survival among patients with HCC who participated in the Guangdong Liver Cancer Cohort (GLCC) study.
Subjects and methods
Study population
The GLCC, an ongoing, prospective cohort study of PLC survivors, was established in 2013 at Sun Yat-sen University Cancer Center (SYSUCC) in Guangdong province, China. As previously described,21 patients aged 18–80 years with a diagnosis of incident PLC were recruited within one month of diagnosis prior to cancer treatment. Participants are mainly from Guangdong and its surrounding provinces such as Jiangxi, Hunan, Guangxi, and Hainan in southern China. PLC was diagnosed according to the National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology.22 Baseline interviews were conducted at the time of recruitment in person, including diet and lifestyle questionnaires and anthropometric measurements. A total of 1359 eligible patients were enrolled in the GLCC between September, 2013 and April, 2017. We restricted the present analysis to 1302 patients who received a diagnosis of HCC (C22.0 per ICD-10). Additionally, patients were excluded if they had incomplete dietary assessment or implausible energy intakes (i.e.,<800 kcal day−1 or >4200 kcal day−1 for men, and <500 kcal day−1 or >3500 kcal day−1 for women) (425 patients). Finally, we included 877 patients in the current study. Ethical approval was obtained from the Ethics Committee of the School of Public Health at Sun Yat-sen University. And all participants provided written informed consents at the initial registration.Data collection
Face-to-face interviews were conducted by trained medical staffs to obtain detailed information on sociodemographic characteristics (e.g., age, sex, marital status, education level, occupation, and per capita household income), lifestyles (e.g., smoking status, alcohol and tea drinking status) and physical activity, using standardized questionnaires. We also obtained the personal history of illness and medication of the patients from the SYSUCC electronic clinical and administrative databases. Participants who drank tea at least twice a week over the past year before interviews were regarded as tea drinkers. Participants who had smoked at least one cigarette per day or drank alcohol at least once a week for at least six months were considered as smokers or alcohol drinkers. Smokers or alcohol drinkers were defined as former ones if they had quit smoking or drinking for at least a year prior to diagnosis of HCC, otherwise current ones. Physical activity, including sleeping, working, transportation, housework, exercises and leisure, over the past 12 months before diagnosis of HCC was measured using a 19-item questionnaire and were eventually reported as metabolic equivalent hours per day (MET h day−1).23 Education level was divided into primary school or below, secondary school, and high school or above. We measured body height and weight at the recruitment following a standard procedure. Body mass index (BMI, in kg m−2) was then calculated by dividing weight in kilograms by height in meters squared. Information on diagnosis and treatment was obtained from the electronic medical record management system of SYSUCC, such as tumor number (single or multiple), vascular invasion (yes or no), tumor size (≥5 cm or <5 cm), Child–Pugh score (A, B, C), BCLC stage (0, A, B, C, D), and cancer treatment (surgery, other treatments).Dietary assessment
We used a validated 79-item semiquantitative food frequency questionnaire (FFQ) to get information on daily dietary intake within one year before HCC diagnosis. Participants were asked to recall the frequency (never, per year, per month, per week, per day) and amount of each food consumption. Color pictures of foods in standard portion sizes were provided to help participants to quantify their consumption. Daily dietary intakes of vitamin A, retinol, β-carotene, and other nutrients were calculated based on the 2009 China Food Composition Table.24 Dietary vitamin A (in retinol activity equivalent, RAE) was calculated as “retinol (in μg) + β-carotene (in μg)/12”. The residual method was used to adjust dietary intakes of vitamin A, β-carotene, and retinol for daily energy intake (in kcal d−1).25 The FFQ had been verified by 3-day 24-hour dietary records once every two months.26 Energy-adjusted correlation coefficients of vitamin A, β-carotene and retinol were 0.57, 0.55 and 0.56, respectively, between two FFQs. The energy-adjusted correlation coefficients of vitamin A, β-carotene, and retinol between the second FFQ and 18 d dietary records were 0.32, 0.32, and 0.31, respectively.26Follow-up and death ascertainment
Follow-up time began at the date of cancer diagnosis and continued until the date of death, or last date of follow-up (February 22, 2019), whichever came first. The survival status of participants was regularly confirmed with a combination of passive follow-up through the SYSUCC inpatient and outpatient medical records or through the death registration system from Guangdong Provincial Center for Disease Control and Prevention (CDC) and active follow-up by mail or telephone interview of patients or their relatives throughout the follow-up period. Finally, underlying cause of death was identified by physicians per ICD-10 codes. Overall mortality (deaths from any cause) and HCC-specific mortality (deaths from HCC) were primary outcomes.Statistical analysis
Because of the significant difference in vitamin A, β-carotene and retinol intakes between men and women, participants were divided into four groups on the basis of sex-specific quartiles of dietary intake of vitamin A, β-carotene, or retinol in the cohort. Quantitative variables were compared using one-way ANOVA tests and qualitative variables were compared using chi-square tests. We used Kaplan–Meier survival analysis to evaluate the overall and HCC-specific survival according to the quartiles of vitamin A, β-carotene, and retinol intakes. Differences in survival time among quartile groups were examined statistically by the log-rank test. We used Cox proportional hazard regression models to estimate hazards ratios (HRs) and 95% confidence intervals (95% CI) for the association of dietary intakes of vitamin A, retinol, and β-carotene with overall and HCC-specific mortality. The Schoenfeld residual trend test was performed to examine the proportional hazard assumption. We first adjusted for nonclinical factors and then additionally adjusted for clinical factors in the multivariable regression models. Nonclinical covariates including age, BMI, energy intake, smoking status, alcohol drinking status, educational level, household income level, and physical activity were selected by stepwise regression. Finally, the following nonclinical covariates: age, BMI, energy intake, smoking status, alcohol drinking status were included. Clinical factors including cancer treatment, tumor number, vascular invasion, tumor size, and Child–Pugh score, the well-established prognostic factors for HCC survival, were included by default. BCLC stage was not included in the regression models since we have taken tumor number, tumor size, and Child–Pugh score into account. The linear trend tests were performed by entering quartiles of the nutrients as a continuous variable into the regression models. All statistical tests except for statistical power tests were performed by SPSS 24.0 (SPSS, Chicago, IL, USA), and two-sided p < 0.05 were considered statistically significant. The statistical power tests were performed by PASS 11.0 (NCSS, LLC. Kaysville, Utah, USA).Results
Baseline characteristics
The present analysis included 778 (88.7%) men and 99 (11.3%) women. The mean (standard deviation[SD]) age at diagnosis was 51.7 (11.90) years old. The mean (SD) BMI was 22.7 (3.2) kg m−2. The mean (SD) energy, vitamin A, β-carotene, and retinol intakes were 1979.5 (608.39) kcal d−1, 764.6 (508.0) μg RAE per d, 649.8 (456.4) μg RAE per d, and 114.8 (234.9) μg RAE per d, respectively, during the past year before HCC diagnosis.Baseline characteristics of the study population according to sex-specific quartiles of dietary vitamin A intake are shown in Table 1. Overall mean (SD) dietary vitamin A (in RAE) consumption was 741.0 (476.86) μg per d among men and 950.2 (681.75) μg per d among women. Participants with higher dietary vitamin A intakes were more likely to have higher income level, to be more educated, to be never drinkers, and to be diagnosed at early stage according to the Barcelona Clinic Liver Cancer (BCLC) staging system in comparison to participants with lower intakes. ESI Tables 1 and 2† shows the distribution of baseline characteristic of the participants according to sex-specific quartiles of dietary intakes of β-carotene and retinol, respectively.
| Sex-specific quartiles of total vitamin A intake | P value | ||||
|---|---|---|---|---|---|
| Q1 (n = 218) | Q2 (n = 221) | Q3 (n = 219) | Q4 (n = 219) | ||
| Abbreviations: SD, standard deviation; FLD, fatty liver disease; BCLC stage, Barcelona clinic liver cancer stage; RAE, retinol activity equivalent; MET h, metabolic equivalent hours.a 10 individuals were missed.b Including local radiofrequency ablation, hepatic arterial intervention, radiotherapy, chemotherapy and systemic treatment. | |||||
| Dietary total vitamin A intake (μg RAE per d), range | |||||
| Men | <415.7 | 415.7–642.1 | 642.1–932.0 | ≥932.0 | — |
| Women | <526.2 | 526.2–720.4 | 720.4–1202.4 | ≥1202.4 | — |
| Men/women, n | 194/24 | 195/26 | 194/25 | 195/24 | — |
| Age (y), mean(SD) | 51.3(12.49) | 50.3(11.64) | 52.6(11.74) | 52.7(11.82) | 0.105 |
| BMI (kg m−2), mean(SD) | 22.5(3.45) | 22.7(3.22) | 22.9(3.19) | 22.6(2.92) | 0.503 |
| Education level, n(%) | 0.026 | ||||
| Primary school or below | 52(23.9) | 49(22.2) | 40(18.3) | 33(15.1) | |
| Secondary school | 138(63.3) | 133(60.2) | 126(57.5) | 139(63.5) | |
| High school or above | 28(12.8) | 39(17.6) | 53(24.2) | 47(21.4) | |
| Household income level | 0.005 | ||||
| <¥2000 per person per month | 95(43.6) | 89(40.3) | 67(30.6) | 68(31.1) | |
| ¥2000–4000 per person per month | 85(39.0) | 84(38.0) | 84(38.4) | 87(39.7) | |
| >¥4000 per person per month | 38(17.4) | 48(21.7) | 68(31.0) | 64(29.2) | |
| Smoking status, n(%) | 0.403 | ||||
| Current smoker | 64(29.4) | 71(32.1) | 61(27.9) | 63(28.8) | |
| Ever smoker | 74(33.9) | 64(29.0) | 71(32.4) | 56(25.6) | |
| Never smoker | 80(36.7) | 86(38.9) | 87(39.7) | 100(45.6) | |
| Alcohol drinking status, n(%) | 0.030 | ||||
| Current drinker | 53(24.3) | 68(30.8) | 63(28.8) | 48(21.9) | |
| Ever drinker | 45(20.6) | 29(13.1) | 41 (18.7) | 28(12.8) | |
| Never drinker | 120(55.1) | 124(56.1) | 115(52.5) | 143(65.3) | |
| Energy intake (kcal day−1), mean(SD) | 1977.9(629.38) | 2023.3(650.92) | 1952.4(569.35) | 1963.8(581.68) | 0.630 |
| FLD, n(%) | 35(16.1) | 39(17.6) | 41(18.7) | 46(21.0) | 0.596 |
| Liver cirrhosis, n(%) | 144(66.1) | 146(66.1) | 146(66.7) | 145(66.2) | 0.999 |
| BCLC stage, n(%) | 0.045 | ||||
| 0 | 18(8.3) | 21(9.5) | 26(11.9) | 29(13.2) | |
| A | 51(23.4) | 74(33.5) | 74(33.8) | 77(35.2) | |
| B | 19(8.7) | 21(9.5) | 21(9.6) | 20(9.1) | |
| C | 130(59.6) | 105(47.5) | 98(44.7) | 93(42.5) | |
| Tumor number, n(%) | 0.474 | ||||
| Single | 137(62.8) | 126(57.0) | 139(63.5) | 137(62.6) | |
| Multiple | 81(37.2) | 95(43.0) | 80(36.5) | 82(37.4) | |
| Tumor size , n(%) | 0.251 | ||||
| <5 cm | 85(39.4) | 105(47.9) | 102(47.4) | 99(45.6) | |
| ≥5 cm | 131(60.6) | 114(52.1) | 113(52.6) | 118(54.4) | |
| Vascular invasion, n(%) | 46(21.1) | 51(23.1) | 42(19.2) | 41(18.7) | 0.658 |
| Child–Pugh score, n(%) | 0.710 | ||||
| A | 214(98.2) | 219(99.1) | 215(98.2) | 217(99.1) | |
| B | 4(1.8) | 2(0.9) | 4(1.8) | 2(0.9) | |
| Cancer treatment, n(%) | 0.559 | ||||
| Surgery | 99(45.4) | 105(47.5) | 104(47.5) | 114(52.1) | |
| Other treatmentsb | 119(54.6) | 116(52.5) | 115(52.5) | 105(47.9) | |
| Dietary β-carotene intake (RAE, μg d−1), mean(SD) | 235.4(93.32) | 459.7(96.17) | 695.0(139.25) | 1208.9(524.40) | <0.001 |
| Dietary retinol intake (μg d−1), mean(SD) | 49.0(40.88) | 81.0(79.61) | 101.8(107.26) | 227.6(428.62) | <0.001 |
| Physical activity (MET h day−1) | 35.6(14.92) | 32.7(11.30) | 33.7(14.38) | 32.3(14.09) | 0.055 |
Dietary intakes of vitamin A, β-carotene, and retinol and HCC mortality
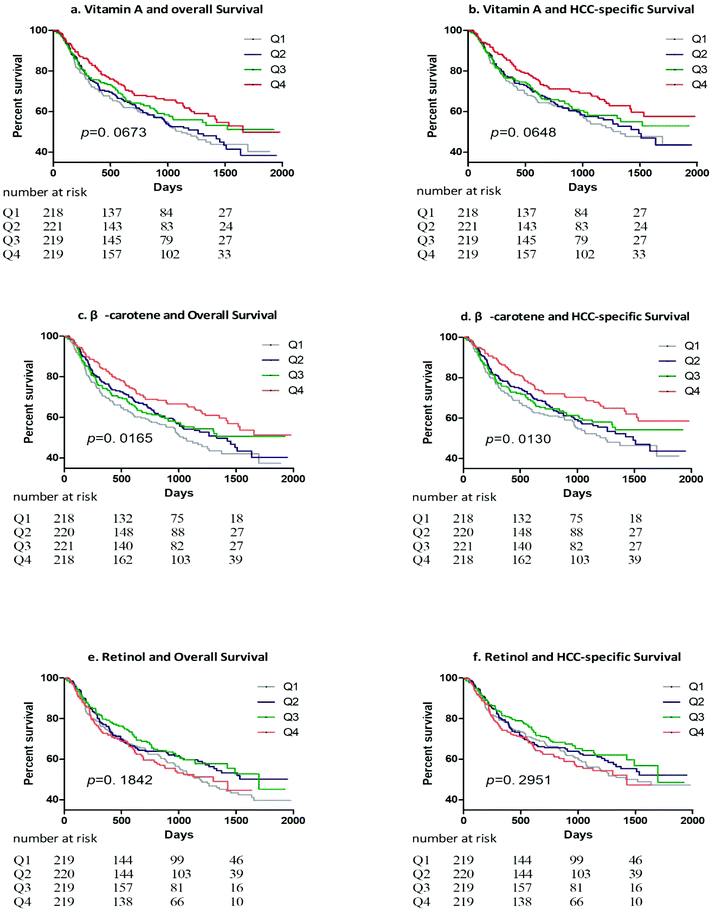
After a median follow-up of 797 days with a total of 712![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 964 person-days, we documented 384 deaths, 343 of which died from HCC. The HCC-specific and overall mortality were 4.81 and 5.38 per 10
964 person-days, we documented 384 deaths, 343 of which died from HCC. The HCC-specific and overall mortality were 4.81 and 5.38 per 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 person-days, respectively. Survival curves for overall and HCC-specific survival according to sex-specific quartiles of vitamin A, β-carotene, and retinol are shown in Fig. 1.
000 person-days, respectively. Survival curves for overall and HCC-specific survival according to sex-specific quartiles of vitamin A, β-carotene, and retinol are shown in Fig. 1.
Table 2 shows multivariable-adjusted HRs (95% CIs) for total and HCC-specific mortality according to sex-specific quartiles of dietary intakes of vitamin A, β-carotene, and retinol. In the multivariable analyses of mortality with adjustment for nonclinical covariates, higher intakes of vitamin A were associated with a lower risk of death from any cause (Q4 vs. Q1: HR = 0.74; 95% CI: 0.56–0.99, P-trend = 0.031) and death from HCC (Q4 vs. Q1: HR = 0.71; 95% CI: 0.52–0.97, P-trend = 0.033). Similarly, higher intakes of β-carotene were inversely associated with a lower risk of overall mortality (Q4 vs. Q1: HR = 0.68; 95% CI: 0.51–0.91, P-trend = 0.012) and HCC-specific mortality (Q4 vs. Q1: HR = 0.65; 95% CI: 0.48–0.89, P-trend = 0.009). After additional adjustment for clinical factors, the inverse associations remained significant for vitamin A and β-carotene. The participants who had dietary intakes of vitamin A in the highest quartile had a reduced risk of death from any cause (Q4 vs. Q1: HR = 0.70; 95% CI: 0.53–0.94, P-trend = 0.018) and death from HCC (Q4 vs. Q1: HR = 0.68; 95% CI: 0.50–0.92, P-trend = 0.021). Similarly, participants who had dietary intakes of β-carotene in the highest quartile have improved overall survival (Q4 vs. Q1: HR = 0.72; 95% CI: 0.54–0.96, P-trend = 0.033) and HCC-specific survival (Q4 vs. Q1: HR = 0.69; 95% CI: 0.51–0.94, P-trend = 0.025). There was no statistical evidence indicating that dietary retinol intakes were associated with overall and HCC-specific mortality whether we adjusted for covariates or not. The statistical powers of COX regression for overall survival were 1.000, 1.000 and 0.500, and for HCC-specific survival were 1.000, 1.000 and 0.049 for vitamin A, β-carotene and retinol, respectively.
| HR (95%CI) | P-Trendc | ||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | ||
| Abbreviations: HR, hazard ratio; CI, confidence interval.a Stratified by gender and adjusted for age at diagnosis (in years), BMI (in kg m−2), energy intake (in kcal d−1), smoking status (never smoker, ever smoker, current smoker), and alcohol drinking status (never drinker, ever drinker, current drinker).b Adjusted for covariates in model 1 plus tumor size (≥5 cm, <5 cm), tumor number (single, multiple), cancer treatment (surgery, other treatments), vascular invasion (yes, no), and Child–Pugh score (A, B, C).c The linear trend tests were performed by entering quartiles of the nutrients as a continuous variable into the regression models. | |||||
| Overall survival | |||||
| Vitamin A | |||||
| No. of deaths/total | 107/218 | 105/221 | 88/219 | 84/219 | |
| Model 1a | 1.00(ref) | 0.98 (0.75,1.28) | 0.87 (0.65,1.15) | 0.74 (0.56,0.99) | 0.031 |
| Model 2b | 1.00(ref) | 0.94 (0.72,1.24) | 0.91 (0.68,1.21) | 0.70 (0.53,0.94) | 0.018 |
| β-Carotene | |||||
| No. of deaths/total | 109/218 | 100/220 | 93/221 | 82/218 | |
| Model 1a | 1.00(ref) | 0.89 (0.68,1.17) | 0.86 (0.65,1.14) | 0.68 (0.51,0.91) | 0.012 |
| Model 2b | 1.00(ref) | 0.93 (0.71,1.23) | 0.94 (0.71,1.24) | 0.72 (0.54,0.96) | 0.033 |
| Retinol | |||||
| No. of deaths/total | 113/219 | 91/220 | 84/219 | 96/219 | |
| Model 1a | 1.00(ref) | 0.83 (0.63,1.09) | 0.80 (0.61,1.07) | 0.97 (0.74,1.27) | 0.706 |
| Model 2b | 1.00(ref) | 0.87 (0.65,1.15) | 0.80 (0.60,1.07) | 0.91 (0.69,1.21) | 0.353 |
| HCC-specific survival | |||||
| Vitamin A | |||||
| No. of deaths/total | 96/218 | 92/221 | 83/219 | 72/219 | |
| Model 1a | 1.00(ref) | 0.95 (0.71,1.27) | 0.92 (0.68,1.23) | 0.71 (0.52,0.97) | 0.033 |
| Model 2b | 1.00(ref) | 0.93 (0.70,1.25) | 0.96 (0.71,1.29) | 0.68 (0.50,0.92) | 0.021 |
| β-Carotene | |||||
| No. of deaths/total | 97/218 | 92/220 | 84/221 | 70/218 | |
| Model 1a | 1.00(ref) | 0.91 (0.69,1.22) | 0.88 (0.66,1.18) | 0.65 (0.48,0.89) | 0.009 |
| Model 2b | 1.00(ref) | 0.98 (0.74,1.31) | 0.95 (0.71,1.28) | 0.69 (0.51,0.94) | 0.025 |
| Retinol | |||||
| No. of deaths/total | 96/219 | 85/220 | 74/219 | 88/219 | |
| Model 1a | 1.00(ref) | 0.91 (0.68,1.22) | 0.83 (0.61,1.12) | 1.04 (0.77,1.39) | 0.993 |
| Model 2b | 1.00(ref) | 0.95 (0.70,1.27) | 0.84 (0.61,1.14) | 0.99 (0.73,1.33) | 0.742 |
Discussion
To our knowledge, this is one of the first population-based studies examining the association of prediagnostic dietary intakes of vitamin A, β-carotene and retinol with mortality in patients with HCC. In this prospective study, we observed that higher intakes of vitamin A and β-carotene, but not retinol, were associated with reduced overall and HCC-specific mortality.At present, few epidemiological studies have been conducted to examine the associations of dietary vitamin A, β-carotene, and retinol intakes with overall or HCC-specific mortality. Based on previous researches, we had anticipated that higher prediagnostic dietary intakes of vitamin A, β-carotene, and retinol would be at a lower risk of death from any cause and from HCC because of their antitumor effects.19,27 However, we finally found that only dietary vitamin A and β-carotene intakes were inversely associated with overall and HCC-specific mortality. Our results were in line with previous studies that demonstrated that higher vitamin A or carotenoids intakes can reduce overall or cancer-related mortality. A prospective cohort study using data from the British National Diet and Nutrition Survey observed that higher intakes of carotenoids were significantly associated with lower overall mortality (per SD: HR = 0.89, 95% CI: 0.83–0.96).28 A randomized double-blind trial conducted in Linxian of China found that subjects younger than 55 years old with taking retinol and zinc supplements had a 41% lower HCC-specific mortality compared to those with taking a placebo (HR = 0.59, 95% CI = 0.34–1.00).18 Acyclic retinoid, a synthetic retinoid, has a similar structure to natural retinoic acid, which also can bind to retinoid nuclear receptors. A randomized trial by Muto et al. reported that acyclic retinoid had a statistically significant effect on preventing the recurrence of second primary liver cancer and improving survival in HCC survivors (HR = 0.30, 95% CI: 0.10–0.80, compared with the placebo group).17,29
Considering that higher dietary vitamin A or carotenes intakes could lead to the higher serum vitamin A or carotenes levels, our observations were in accord with some previous studies about the association of serum vitamin A and carotenes levels with mortality. In an epidemiologic study, an inverse association of serum levels of vitamin A and β-carotene with overall mortality were observed among 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 008 adults who participated in the National Health and Nutrition Examination Survey (NHANES) III.30 Another study conducted in Japan also suggested that serum levels of carotenoids such as β-carotene, which were related to intakes of vegetables and fruits rich in carotenoids, may reduce risk of mortality from liver cancer (HR = 0.38, 95% CI: 0.14–1.00).31 In the Alpha-Tocopherol, Beta-Carotene Cancer Prevention (ATBC) study, the researchers found that higher serum retinol and β-carotene levels could reduce the risk of death from chronic liver disease (retinol: HR = 0.55, 95% CI: 0.38–0.78, P-trend = 0.0007; β-carotene: HR = 0.47, 95% CI: 0.30–0.75, P-trend = 0.001).13 In contrast, there were a few studies indicating that levels of retinol in diet or in serum had null or positive associations with overall or HCC-specific mortality.32–35
008 adults who participated in the National Health and Nutrition Examination Survey (NHANES) III.30 Another study conducted in Japan also suggested that serum levels of carotenoids such as β-carotene, which were related to intakes of vegetables and fruits rich in carotenoids, may reduce risk of mortality from liver cancer (HR = 0.38, 95% CI: 0.14–1.00).31 In the Alpha-Tocopherol, Beta-Carotene Cancer Prevention (ATBC) study, the researchers found that higher serum retinol and β-carotene levels could reduce the risk of death from chronic liver disease (retinol: HR = 0.55, 95% CI: 0.38–0.78, P-trend = 0.0007; β-carotene: HR = 0.47, 95% CI: 0.30–0.75, P-trend = 0.001).13 In contrast, there were a few studies indicating that levels of retinol in diet or in serum had null or positive associations with overall or HCC-specific mortality.32–35
The possible reasons for the discrepancy may be due to differences in study design, background of participants, exposure markers, exposure dosage and adjusted covariates. For example, many studies were conducted among community-dwelling population, but we included HCC patients as participants. Besides, different populations have different dietary patterns, which resulted in different exposure dosage among these studies. In our study, the majority of dietary vitamin A consumed by the participants was derived from β-carotene which is mainly sourced from vegetables and fruits, and retinol from animal food only accounted for a small proportion of dietary vitamin A. Such a lower retinol intake with amounts fluctuating in a relatively narrow range may preclude us from discovering a significant association between dietary retinol intake and the prognosis of HCC. Moreover, higher dietary intakes of vegetables and fruits rich in carotenoids had been shown to reduce the risk of cancer-related mortality,36,37 including lung cancer,38 stomach cancer,39 and colorectal cancer.40 These may explain why HCC survival was associated with dietary intakes of vitamin A and β-carotene, but not with dietary intakes of retinol.
As far as we know, the liver is the most important target organ for vitamin A.6 Retinoids are a set of structural and functional derivatives of vitamin A. Retinoids and carotenoids also play an essential role in intracellular signaling pathways, having essential effects on cellular growth, differentiation, apoptosis as well as morphology.41 Retinoic acid (RA) modulates the rates of transcription of many target genes to regulate cellular proliferation and differentiation.20,42 The retinoic acid receptor (RAR) and retinoid X receptor (RXR) located in the nucleus can mediate these activities. It was shown that RA inhibits HCC cell proliferation by preventing phosphorylation of RXR via down-regulating the Ras/Erk system.43 Some in vitro and in vivo studies also reported derangement of retinoid storage, retinoid binding protein, retinoid nuclear receptors, RXR, and retinoids signaling pathways occurred during the progression of liver cancer.44–46 Recently, it was proved that all-trans retinoic acid can reverse epithelial–mesenchymal transition to inhibit proliferation, migration, invasion of heap1–6 cells in vitro.47 In addition, β-carotene not only is an important and efficient antioxidant, but also can enhance immunity related to protecting against carcinogenesis.48,49 Therefore it can protect against oxidative such as damage of cytomembrane and DNA caused by free radicals and activated oxygen species.49 The above-mentioned mechanisms may provide plausible explanations for our observations.
Our findings firstly report that dietary intakes of vitamin A and β-carotene may be a modifiable factor that influences HCC prognosis. The prospective cohort design with a large sample size is among the strengths of this study. Besides, we only included patients with newly-diagnosed HCC who had not receive any anticancer therapy to eliminate potential confounding of disease progression and cancer treatment. In addition, we adjusted for substantial covariates, including socioeconomic status, lifestyle, liver function, tumor characteristics (numbers, sizes and vascular invasion) and cancer therapy, to minimize the possibility of residual confounding.
There are several limitations that cannot be ignored. First, dietary information before HCC diagnosis was collected using FFQ at the time of diagnosis. Although we provided food pictures with portion sizes to help participants quantify their consumption, recall bias and misreporting dietary intakes would be unavoidable. Second, participants might have changed their diet and lifestyles after diagnosis and treatment. Third, most of HCC patients had a history of chronic liver disease, such as hepatitis B or C infection, liver cirrhosis, and fatty liver disease and might have suffered from HCC-related symptoms for a long time prior to the diagnosis of HCC. Dietary nutrient intakes may reflect hepatic function and disease severity. Thus there is a concern of reverse causality. Last, all participants were Chinese and cation should be taken when generalizing our results to racially diverse patient populations.
Conclusions
In conclusion, prediagnostic dietary intakes of vitamin A and β-carotene, rather than retinol, were independently and inversely associated with overall and HCC-specific mortality among patients with newly diagnosed HCC in the GLCC study. Our study provides observational evidence that we may improve HCC prognosis by appropriately increasing dietary intakes of vitamin A and β-carotene. Even though, further studies need to be conducted to confirm these findings.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank the patients who consented to participation in this study, and we also thank the staff of SYSUCC for their contributions in the data collection, processing and preparation. This work was supported by the National Natural Science Foundation of China (81803219, 81973016); and the Natural Science Foundation of Guangdong Province, China (2018A030310335).References
- F. Bray, J. Ferlay, I. Soerjomataram, R. L. Siegel, L. A. Torre and A. Jemal, Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries, CA Cancer J. Clin., 2018, 6, 394–424 CrossRef PubMed.
- L. A. Torre, F. Bray, R. L. Siegel, J. Ferlay, J. Lortet-Tieulent and A. Jemal, Global cancer statistics, 2012, CA Cancer J. Clin., 2015, 65, 87–108 CrossRef PubMed.
- C. Bamia, P. Lagiou, M. Jenab, K. Aleksandrova, V. Fedirko, D. Trichopoulos, K. Overvad, A. Tjonneland, A. Olsen, F. Clavel-Chapelon, M. C. Boutron-Ruault, M. Kvaskoff, V. A. Katzke, T. Kuhn, H. Boeing, U. Nothlings, D. Palli, S. Sieri, S. Panico, R. Tumino, A. Naccarati, H. B. Bueno-de-Mesquita, P. H. Peeters, E. Weiderpass, G. Skeie, J. R. Quiros, A. Agudo, M. D. Chirlaque, M. J. Sanchez, E. Ardanaz, M. Dorronsoro, U. Ericson, L. M. Nilsson, M. Wennberg, K. T. Khaw, N. Wareham, T. J. Key, R. C. Travis, P. Ferrari, M. Stepien, T. Duarte-Salles, T. Norat, N. Murphy, E. Riboli and A. Trichopoulou, Fruit and vegetable consumption in relation to hepatocellular carcinoma in a multi-centre, European cohort study, Br. J. Cancer, 2015, 112, 1273–1282 CrossRef CAS PubMed.
- V. Fedirko, A. Trichopolou, C. Bamia, T. Duarte-Salles, E. Trepo, K. Aleksandrova, U. Nothlings, A. Lukanova, P. Lagiou, P. Boffetta, D. Trichopoulos, V. A. Katzke, K. Overvad, A. Tjonneland, L. Hansen, M. C. Boutron-Ruault, G. Fagherazzi, N. Bastide, S. Panico, S. Grioni, P. Vineis, D. Palli, R. Tumino, H. B. Bueno-de-Mesquita, P. H. Peeters, G. Skeie, D. Engeset, C. L. Parr, P. Jakszyn, M. J. Sanchez, A. Barricarte, P. Amiano, M. Chirlaque, J. R. Quiros, M. Sund, M. Werner, E. Sonestedt, U. Ericson, T. J. Key, K. T. Khaw, P. Ferrari, I. Romieu, E. Riboli and M. Jenab, Consumption of fish and meats and risk of hepatocellular carcinoma: the European Prospective Investigation into Cancer and Nutrition (EPIC), Ann. Oncol., 2013, 24, 2166–2173 CrossRef CAS PubMed.
- K. Schutte, C. Schulz and P. Malfertheiner, Nutrition and Hepatocellular Cancer, Gastrointest. Tumors, 2016, 2, 188–194 CrossRef PubMed.
- A. Saeed, R. Dullaart, T. Schreuder, H. Blokzijl and K. Faber, Disturbed Vitamin A Metabolism in Non-Alcoholic Fatty Liver Disease (NAFLD), Nutrients, 2018, 10, E29 CrossRef PubMed.
- C. J. Bates, Vitamin A, Lancet, 1995, 345, 31–35 CrossRef CAS.
- D. Bouriez, J. Giraud, C. Gronnier and C. Varon, Efficiency of All-Trans Retinoic Acid on Gastric Cancer: A Narrative Literature Review, Int. J. Mol. Sci., 2018, 19, E3388 CrossRef PubMed.
- A. H. Davis-Yadley and M. P. Malafa, Vitamins in pancreatic cancer: a review of underlying mechanisms and future applications, Adv. Nutr., 2015, 6, 774–802 CrossRef CAS PubMed.
- T. Zhang, H. Chen, S. Qin, M. Wang, X. Wang, X. Zhang, F. Liu and S. Zhang, The association between dietary vitamin A intake and pancreatic cancer risk: a meta-analysis of 11 studies, Biosci. Rep., 2016, 36, e414 Search PubMed.
- J. M. Yuan, Y. T. Gao, C. N. Ong, R. K. Ross and M. C. Yu, Prediagnostic level of serum retinol in relation to reduced risk of hepatocellular carcinoma, J. Natl. Cancer Inst., 2006, 98, 482–490 CrossRef CAS PubMed.
- M. W. Yu, H. H. Hsieh, W. H. Pan, C. S. Yang and C. J. CHen, Vegetable consumption, serum retinol level, and risk of hepatocellular carcinoma, Cancer Res., 1995, 55, 1301–1305 CAS.
- G. Y. Lai, S. J. Weinstein, D. Albanes, P. R. Taylor, J. Virtamo, K. A. McGlynn and N. D. Freedman, Association of serum α-tocopherol, β-carotene and retinol with liver cancer incidence and chronic liver disease mortality, Br. J. Cancer, 2014, 111, 2163–2171 CrossRef CAS PubMed.
- Q. Lan, Y. Zhang, G. Liao, R. Zhou, Z. Zhou, Y. Chen and H. Zhu, The Association between Dietary Vitamin A and Carotenes and the Risk of Primary Liver Cancer: A Case–Control Study, Nutrients, 2016, 8, E624 CrossRef PubMed.
- J. He, Y. Gu and S. Zhang, Vitamin A and Breast Cancer Survival: A Systematic Review and Meta-analysis, Clin. Breast Cancer, 2018, 18, e1389–e1400 CrossRef CAS PubMed.
- G. Bjelakovic, D. Nikolova, R. G. Simonetti and C. Gluud, Antioxidant supplements for prevention of gastrointestinal cancers: a systematic review and meta-analysis, Lancet, 2004, 364, 1219–1228 CrossRef CAS.
- Y. Muto, H. Moriwaki and A. Saito, Prevention of second primary tumors by an acyclic retinoid in patients with hepatocellular carcinoma, N. Engl. J. Med., 1999, 340, 1046–1047 CrossRef CAS PubMed.
- C. X. Qu, F. Kamangar, J. H. Fan, B. Yu, X. D. Sun, P. R. Taylor, B. E. Chen, C. C. Abnet, Y. L. Qiao, S. D. Mark and S. M. Dawsey, Chemoprevention of primary liver cancer: a randomized, double-blind trial in Linxian, China, J. Natl. Cancer Inst., 2007, 99, 1240–1247 CrossRef PubMed.
- G. V. Chaves, W. A. Peres, J. C. Goncalves and A. Ramalho, Vitamin A and retinol-binding protein deficiency among chronic liver disease patients, Nutrition, 2015, 31, 664–668 CrossRef CAS PubMed.
- J. E. Balmer and R. Blomhoff, Gene expression regulation by retinoic acid, J. Lipid Res., 2002, 43, 1773–1808 CrossRef CAS PubMed.
- A. P. Fang, P. Y. Chen, X. Y. Wang, Z. Y. Liu, D. M. Zhang, Y. Luo, G. C. Liao, J. A. Long, R. H. Zhong, Z. G. Zhou, Y. J. Xu, X. J. Xu, W. H. Ling, M. S. Chen, Y. J. Zhang and H. L. Zhu, Serum copper and zinc levels at diagnosis and hepatocellular carcinoma survival in the Guangdong Liver Cancer Cohort, Int. J. Cancer, 2019, 144, 2823–2832 CrossRef CAS PubMed.
- A. R. Benson, T. A. Abrams, E. Ben-Josef, P. M. Bloomston, J. F. Botha, B. M. Clary, A. Covey, S. A. Curley, M. I. D'Angelica, R. Davila, W. D. Ensminger, J. F. Gibbs, D. Laheru, M. P. Malafa, J. Marrero, S. G. Meranze, S. J. Mulvihill, J. O. Park, J. A. Posey, J. Sachdev, R. Salem, E. R. Sigurdson, C. Sofocleous, J. N. Vauthey, A. P. Venook, L. W. Goff, Y. Yen and A. X. Zhu, NCCN clinical practice guidelines in oncology: hepatobiliary cancers, J. Natl. Compr. Cancer Network, 2009, 7, 350–391 CAS.
- B. E. Ainsworth, W. L. Haskell, S. D. Herrmann, N. Meckes, D. J. Bassett, C. Tudor-Locke, J. L. Greer, J. Vezina, M. C. Whitt-Glover and A. S. Leon, 2011 Compendium of Physical Activities: a second update of codes and MET values, Med. Sci. Sports Exercise, 2011, 43, 1575–1581 CrossRef PubMed.
- Y. X. Yang, G. Y. Wang and X. Q. Pan, China Food Composition, Peking University Medical Press, Beijing, China, 2nd edn, 2009 Search PubMed.
- W. Willett and M. J. Stampfer, Total energy intake: implications for epidemiologic analyses, Am. J. Epidemiol., 1986, 124, 17–27 CrossRef CAS PubMed.
- C. X. Zhang and S. C. Ho, Validity and reproducibility of a food frequency Questionnaire among Chinese women in Guangdong province, Asia Pac. J. Clin. Nutr., 2009, 18, 240–250 CAS.
- M. S. Cooke, M. D. Evans, M. Dizdaroglu and J. Lunec, Oxidative DNA damage: mechanisms, mutation, and disease, FASEB J., 2003, 17, 1195–1214 CrossRef CAS PubMed.
- C. J. Bates, M. Hamer and G. D. Mishra, Redox-modulatory vitamins and minerals that prospectively predict mortality in older British people: the National Diet and Nutrition Survey of people aged 65 years and over, Br. J. Nutr., 2011, 105, 123–132 CrossRef CAS PubMed.
- Y. Muto, H. Moriwaki, M. Ninomiya, S. Adachi, A. Saito, K. T. Takasaki, T. Tanaka, K. Tsurumi, M. Okuno, E. Tomita, T. Nakamura and T. Kojima, Prevention of second primary tumors by an acyclic retinoid, polyprenoic acid, in patients with hepatocellular carcinoma. Hepatoma Prevention Study Group, N. Engl. J. Med., 1996, 334, 1561–1567 CrossRef CAS PubMed.
- A. Goyal, M. B. Terry and A. B. Siegel, Serum antioxidant nutrients, vitamin A, and mortality in U.S. Adults, Cancer Epidemiol. Biomarkers Prev., 2013, 22, 2202–2211 CrossRef CAS PubMed.
- Y. Ito, K. Suzuki, J. Ishii, H. Hishida, A. Tamakoshi, N. Hamajima and K. Aoki, A population-based follow-up study on mortality from cancer or cardiovascular disease and serum carotenoids, retinol and tocopherols in Japanese inhabitants, Asian Pac. J. Cancer Prev., 2006, 7, 533–546 Search PubMed.
- G. Bjelakovic, D. Nikolova, L. L. Gluud, R. G. Simonetti and C. Gluud, Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases, Sao Paulo Med. J., 2015, 133, 164–165 CrossRef PubMed.
- J. Virtamo, P. Pietinen, J. K. Huttunen, P. Korhonen, N. Malila, M. J. Virtanen, D. Albanes, P. R. Taylor and P. Albert, Incidence of cancer and mortality following alpha-tocopherol and beta-carotene supplementation: a postintervention follow-up, J. Am. Med. Assoc., 2003, 290, 476–485 CrossRef CAS PubMed.
- A. Paganini-Hill, C. H. Kawas and M. M. Corrada, Antioxidant vitamin intake and mortality: the Leisure World Cohort Study, Am. J. Epidemiol., 2015, 181, 120–126 CrossRef PubMed.
- K. J. West, P. Christian, A. B. Labrique, M. Rashid, A. A. Shamim, R. D. Klemm, A. B. Massie, S. Mehra, K. J. Schulze, H. Ali, B. Ullah, L. S. Wu, J. Katz, H. Banu, H. H. Akhter and A. Sommer, Effects of vitamin A or beta carotene supplementation on pregnancy-related mortality and infant mortality in rural Bangladesh: a cluster randomized trial, J. Am. Med. Assoc., 2011, 305, 1986–1995 CrossRef CAS PubMed.
- M. S. Donaldson, Nutrition and cancer: a review of the evidence for an anti-cancer diet, Nutr. J., 2004, 3, 19 CrossRef PubMed.
- D. Aune, E. Giovannucci, P. Boffetta, L. T. Fadnes, N. Keum, T. Norat, D. C. Greenwood, E. Riboli, L. J. Vatten and S. Tonstad, Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality-a systematic review and dose-response meta-analysis of prospective studies, Int. J. Epidemiol., 2017, 46, 1029–1056 CrossRef PubMed.
- M. L. Neuhouser, R. E. Patterson, M. D. Thornquist, G. S. Omenn, I. B. King and G. E. Goodman, Fruits and vegetables are associated with lower lung cancer risk only in the placebo arm of the beta-carotene and retinol efficacy trial (CARET), Cancer Epidemiol. Biomarkers Prev., 2003, 12, 350–358 CAS.
- N. Lunet, A. Lacerda-Vieira and H. Barros, Fruit and vegetables consumption and gastric cancer: a systematic review and meta-analysis of cohort studies, Nutr. Cancer, 2005, 53, 1–10 CrossRef PubMed.
- J. Satia-Abouta, J. A. Galanko, C. F. Martin, A. Ammerman and R. S. Sandler, Food groups and colon cancer risk in African-Americans and Caucasians, Int. J. Cancer, 2004, 109, 728–736 CrossRef CAS PubMed.
- C. Li, M. Imai, T. Matsuura, S. Hasegawa, M. Yamasaki and N. Takahashi, Inhibitory Effects of Retinol Are Greater than Retinoic Acid on the Growth and Adhesion of Human Refractory Cancer Cells, Biol. Pharm. Bull., 2016, 39, 636–640 CrossRef CAS PubMed.
- E. Doldo, G. Costanza, S. Agostinelli, C. Tarquini, A. Ferlosio, G. Arcuri, D. Passeri, M. G. Scioli and A. Orlandi, Vitamin A, cancer treatment and prevention: the new role of cellular retinol binding proteins, BioMed Res. Int., 2015, 2015, 624627 Search PubMed.
- R. Matsushima-Nishiwaki, M. Okuno, Y. Takano, S. Kojima, S. L. Friedman and H. Moriwaki, Molecular mechanism for growth suppression of human hepatocellular carcinoma cells by acyclic retinoid, Carcinogenesis, 2003, 24, 1353–1359 CrossRef CAS PubMed.
- N. Bushue and Y. J. Wan, Retinoid pathway and cancer therapeutics, Adv. Drug Delivery Rev., 2010, 62, 1285–1298 CrossRef CAS PubMed.
- A. di Masi, L. Leboffe, E. De Marinis, F. Pagano, L. Cicconi, C. Rochette-Egly, F. Lo-Coco, P. Ascenzi and C. Nervi, Retinoic acid receptors: from molecular mechanisms to cancer therapy, Mol. Aspects Med., 2015, 41, 1–115 CrossRef CAS PubMed.
- Y. Shirakami, S. A. Lee, R. D. Clugston and W. S. Blaner, Hepatic metabolism of retinoids and disease associations, Biochim. Biophys. Acta, 2012, 1821, 124–136 CrossRef CAS PubMed.
- J. Cui, M. Gong, Y. He, Q. Li, T. He and Y. Bi, All-trans retinoic acid inhibits proliferation, migration, invasion and induces differentiation of hepa1–6 cells through reversing EMT in vitro, Int. J. Oncol., 2016, 48, 349–357 CrossRef CAS PubMed.
- W. Stahl and H. Sies, Antioxidant activity of carotenoids, Mol. Aspects Med., 2003, 24, 345–351 CrossRef CAS PubMed.
- A. Bendich, From 1989 to 2001: what have we learned about the “biological actions of beta-carotene”?, J. Nutr., 2004, 134, 225S–230S CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9fo02468a |
| ‡ These authors contributed equally to this study. |
| This journal is © The Royal Society of Chemistry 2020 |