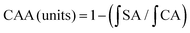The effect of bound polyphenols on the fermentation and antioxidant properties of carrot dietary fiber in vivo and in vitro
Shuai
Liu
a,
Qiang
Yu
 *a,
Hairong
Huang
b,
Kunyou
Hou
a,
Ruihong
Dong
a,
Yi
Chen
*a,
Hairong
Huang
b,
Kunyou
Hou
a,
Ruihong
Dong
a,
Yi
Chen
 a,
Jianhua
Xie
a,
Jianhua
Xie
 a,
Shaoping
Nie
a,
Shaoping
Nie
 a and
Mingyong
Xie
a and
Mingyong
Xie
 a
a
aState Key Laboratory of Food Science and Technology, China–Canada Joint Lab of Food Science and Technology (Nanchang), Nanchang University, 235 Nanjing East Road, Nanchang 330047, China. E-mail: yuqiang8612@163.com
bSchool of Food Science and Technology, Nanchang University, Nanchang 330031, China
First published on 23rd December 2019
Abstract
Growing attention has been paid to the importance of bound polyphenols in dietary fiber. This study aimed to elucidate the effect of bound polyphenols on the fermentation and antioxidant properties of carrot dietary fiber (CDF) in vivo and in vitro. Compared with CDF treatment, 16S rRNA pyrosequencing of in vivo mice feces and in vitro human fecal fermentation samples showed that dephenolized carrot dietary fiber (CDF-DF) treatment decreases operational taxonomic units (OTUs), ACE and Chao1 indexes, increases Firmicute/Bacteroidetes ratio and relative abundance (RA) of Parabacteroides at phylum, restrains RAs of typical beneficial bacteria as well as improves RAs of various harmful bacteria at genus. Meanwhile, short-chain fatty acid (SCFA) contents were lower, while the pH value was higher in the CDF-DF group than those in the CDF group. Interestingly, the combination of bound polyphenols and CDF-DF (CDDP) could recover these indexes influenced by the removal of bound polyphenols in in vitro fermentation samples. Furthermore, the CDF-DF-fed mice exhibited higher MDA content and lower SOD and GSH-Px activities in the colon. The cellular antioxidant activity (CAA) value of CDF-DF was lower than that of CDF and CDDP. These results revealed that bound polyphenols significantly contribute to the fermentation and antioxidant properties of CDF.
1. Introduction
The intestinal microbiota, a vast bacterial community that resides primarily in the lower gut, constitute a physical and immunologic barrier between the host and the environment.1–3 The major functions of the intestinal microbiota for the host are protection against gastrointestinal disorders and pathogens, nutrient processing, and modulation of the intestinal immune response.4 Moreover, beneficial bacteria predominantly exceeding harmful bacteria in the intestinal microbial communities provide further health benefits to the host and accelerates beneficial immune functions.5However, certain endogenous and environmental factors, including genetics, physiology, age, and diet, can destroy the balance of gut microbiota, and thus cause many intestinal diseases due to endotoxins and carcinogens.6,7 Among these factors, diet directly influences the composition and metabolic function of the intestinal microbiota by making substrates available in the form of undigested dietary residues.8 Dietary fiber and polyphenols, two major nutritional components in a diet, have become increasingly interesting as they can be helpful for the improvement of the intestinal microbiota.9,10 Dietary fiber (DF) can pass through the stomach and small intestine to reach the colon, where it is partially or completely fermented by the gut microbiota and produces short-chain fatty acids (SCFAs), including acetic acid, propionic acid, and butyric acid.11,12 In addition, many studies in humans have shown that the intake of dietary fiber modified gut bacterial diversity, increased satiety, and prevented some forms of cancer.13,14 Polyphenols as chemical and biologically active plant secondary metabolites have many health benefits, especially in the prevention of diseases associated with oxidative stress such as cancer, as well as cardiovascular, inflammatory and neurodegenerative diseases.15,16
The DF compounds and polyphenols were generally considered separately as two groups of food constituents in both chemical and nutritional studies. However, there is adequate scientific evidence demonstrating that DF is a carrier of a significant amount of phytochemicals linked to the complex food matrix, mainly polyphenols.9 These partial polyphenols associated with DF, namely bound polyphenols, can pass through the stomach and small intestine with negligible release to reach the colon, followed by fermentation by the intestinal microflora, and thus releasing substances into the colon.17 This finding suggests that the bound polyphenols may play an important role in the function of DF.
In the previous study, our laboratory had identified that carrot dietary fiber (CDF) was more conducive for the growth of Lactobacillus rhamnosus as compared with the dephenolized carrot dietary fiber (CDF-DF), and the antioxidant properties of CDF-DF, including superoxide anion radical, hydroxyl radical and DPPH radical scavenging activity, were significantly lower than that of CDF.18 Since the bound polyphenols released by microorganisms may have an effect on the composition of gut microbiota and intestinal ecological environment during the fermentation of DF, it was necessary to elucidate the impact of bound polyphenols on fermentation and antioxidant properties of DF.19 In this study, 16S rRNA gene sequencing, pH-metry, and gas chromatography were performed to measure the gut microbial composition, pH value and short-chain fatty acid (SCFA) content in the mouse fecal samples and in vitro fermentation samples with the aim to estimate the influences of bound polyphenols on the fermentation property of carrot dietary fiber. In addition, the determination of the MDA content and total SOD and GSH-Px activities in the colon as well as cellular antioxidant activity in vitro were applied to investigate the effect of bound polyphenols on the antioxidant property of carrot dietary fiber.
2. Materials and methods
2.1 Materials and reagents
The carrot of origin from Jiangxi province was purchased from the Rainbow supermarket in Nanchang, Jiangxi province.Heat-stable α-amylase (2100 U g−1, Aladdin Reagent Co. Ltd, Shanghai, China), protease (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 U g−1, Pangbo Biological Engineering Co., Nanning, China), amyloglucosidase from Aspergillus niger (100
000 U g−1, Pangbo Biological Engineering Co., Nanning, China), amyloglucosidase from Aspergillus niger (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 U mL−1, Aladdin Reagent Co. Ltd, Shanghai, China), 2,2′-azobis (2-methylpropionamidine) dihydrochloride (AAPH) (Sigma-Aldrich, St Louis, MO, USA), fluorescein (FL) and 2′,7′-dichlorofluorescin diacetate (DCFH-DA) (Aladdin Reagent Co., USA), lipid peroxidation (MDA) assay kit, total superoxide dismutase (SOD) (including Cu/Zn-SOD and Mn-SOD) assay kit with NBT, cellular glutathione peroxidase (GSH-Px) assay kit and BCA protein assay kit (Beyotime Biotechnology, Nantong, China) were used in the study.
000 U mL−1, Aladdin Reagent Co. Ltd, Shanghai, China), 2,2′-azobis (2-methylpropionamidine) dihydrochloride (AAPH) (Sigma-Aldrich, St Louis, MO, USA), fluorescein (FL) and 2′,7′-dichlorofluorescin diacetate (DCFH-DA) (Aladdin Reagent Co., USA), lipid peroxidation (MDA) assay kit, total superoxide dismutase (SOD) (including Cu/Zn-SOD and Mn-SOD) assay kit with NBT, cellular glutathione peroxidase (GSH-Px) assay kit and BCA protein assay kit (Beyotime Biotechnology, Nantong, China) were used in the study.
2.2 Preparation of carrot dietary fiber
Carrot dietary fiber (CDF) was prepared according to the reported method.18 In short, sample (1 g) was mixed with distilled water (8.5 mL), and the mixture was subjected to sequential enzymatic digestion by the heat-stable α-amylase (50 μL, pH 7, 66 °C), protease (0.2%, pH 7, 60 °C) and amyloglucosidase (300 μL, pH 4.5, 60 °C) to remove starch and protein. After centrifuging (4800 rpm, 10 min), the substrate was washed with distilled water, ethanol (95%), and acetone (95%), and then it was vacuum filtered and stored in a refrigerator at −20 °C.2.3 Preparation of dephenolized carrot dietary fiber (CDF-DF) and bound polyphenols
CDF-DF and bound polyphenols (33.45 mg/10 g CDF) were extracted by the procedure mentioned in our previous study.18 Briefly, 200 mg of CDF was put into dark-coloured screw-cap bottles with NaOH (2 M, 5 mL). The head-space of the bottles was flushed with N2 to remove the air. The mixture was slowly stirred in the absence of light for 4 h at room temperature. Then, the liquid was acidified with HCl (6 M) to pH 2, and extracted with ethyl acetate (twice volumes). After centrifuging (4800 rpm, 10 min), the substrate (CDF-DF) was washed with distilled water, ethanol (95%), and acetone (95%), vacuum filtered and stored in a refrigerator at −20 °C, and the supernatant was dried with N2 to obtain bound polyphenols and stored at −20 °C.2.4 Animals
Male BALB/c mice (20.0 ± 2.0 g) were purchased from Hunan SJA Laboratory Animal Co. Ltd. All animal experiments were performed in the light of the Animal Ethics Procedures and Guidelines of the People's Republic of China, and the experimental protocol was approved by the Institutional Animal Care and Use Committee of Nanchang University.Mice were fed with a standard diet and allowed free access to distilled water throughout the experimental period (7 days). After the adaptation period, the animals were divided into three groups (n = 6 per group): control group (Con group), CDF group, and CDF-DF group. The CDF and CDF-DF groups daily received approximately 0.6 g (kg d)−1 in 200 μL of CDF and CDF-DF, respectively, by oral administration for 7 consecutive days. The Con group daily received 200 μL of distilled water. The mice fecal contents and tissue samples were collected and stored at −80 °C until use.
2.5 In vitro fermentation
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 (w/v)), followed by filtration through four layers of medical gauze.
5 (w/v)), followed by filtration through four layers of medical gauze.
20 mL of the fecal suspension was added into 80 mL of the basal culture medium containing 1 g CDF (CDF group), or 1 g CDF-DF (CDF-DF group) or the combination of 3.5 mg CDF-PP and 1 g CDF-DF (CDDP group).
All groups were incubated in an anaerobic bottle at 37 °C in a shaking water bath (150 rpm min−1) (Yiheng Scientific Instruments Co., Ltd, Shanghai, China). After fermentation at 0, 4, 8, and 12 h, samples were taken out (kept on ice) for analysis. Each experiment was replicated independently three times.
2.6 Determination of pH
The pH values of in vitro fermentation samples were measured according to the reported method21 with some modifications. In short, in vitro fermentation samples of different groups were collected at 0, 4, 8, and 12 h, respectively, and immediately plunged into ice water. These fermentation products were centrifuged at 4800g for 10 min to segregate the supernatants. The pH value of each supernatant was determined by a pH-meter (Mettler Toledo, Switzerland).Determination of the pH value in mice fecal samples was executed according to the reported method.22 100 mg of mice feces were mixed with 5 mL of ultrapure water, and the supernatants (4800g, 10 min) were measured by a pH-meter.
2.7 Short-chain fatty acid (SCFA) analysis
The SCFAs produced in vivo and in vitro were determined based on the previous method.23 The mice fecal samples were mixed with ultrapure water in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 (g
6 (g![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mL). Then the mixture was shocked intermittently on a vortex mixer for 2 min and executed with ultrasonic-processing continuously for 5 min. The processes of the vortex and ultrasound were repeated once. The supernatant was collected after centrifuging at 4800g for 20 min at 4 °C and then analyzed by gas chromatography.
mL). Then the mixture was shocked intermittently on a vortex mixer for 2 min and executed with ultrasonic-processing continuously for 5 min. The processes of the vortex and ultrasound were repeated once. The supernatant was collected after centrifuging at 4800g for 20 min at 4 °C and then analyzed by gas chromatography.
Chromatographic analysis was executed using an Agilent 6890 N GC system equipped with a flame ionization detector (FID) and an N10149 automatic liquid sampler (Agilent Technologies Inc., Palo Alto, California, USA). The chromatography parameters were used according to the reported method.24 Briefly, the chromatographic column was HP-INNOWAX (30 m × 0.32 mm × 0.5 μm). Nitrogen was supplied as the carrier gas at a flow rate of 19.0 mL min−1 with a split ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. The initial oven temperature was 100 °C and was kept for 0.5 min and then raised to 180 °C by 4 °C min−1. The temperatures of the FID and injection port were 240 °C. The flow rates of hydrogen and air were 30 and 300 mL min−1, respectively. The injected sample volume for GC analysis was 0.2 μL.
10. The initial oven temperature was 100 °C and was kept for 0.5 min and then raised to 180 °C by 4 °C min−1. The temperatures of the FID and injection port were 240 °C. The flow rates of hydrogen and air were 30 and 300 mL min−1, respectively. The injected sample volume for GC analysis was 0.2 μL.
2.8 16S rRNA sequencing
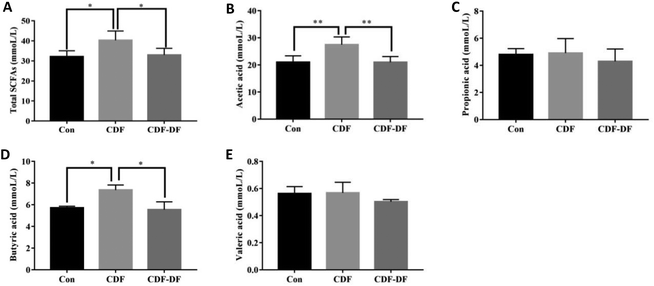
2.9 Cellular antioxidant activity (CAA) assay of different compounds
where
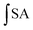 is the integrated area under the sample fluorescence versus time curve;
is the integrated area under the sample fluorescence versus time curve; 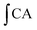 is the integrated area under the control fluorescence versus time curve.
is the integrated area under the control fluorescence versus time curve.
2.10 Determination of antioxidant indexes in the colon of mice
The antioxidant indexes and protein content of colon were determined using assay kits (Beyotime Biotechnology, Nantong, China) as described. In short, the colon samples were mixed with ice-cold PBS or homogenate (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, (w
9, (w![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v)), and the mixtures were triturated in a low-temperature homogenate machine (Servicebio, Wuhan, China) for tissue homogenate. After centrifugation (12
v)), and the mixtures were triturated in a low-temperature homogenate machine (Servicebio, Wuhan, China) for tissue homogenate. After centrifugation (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g, 15 min) of the tissue homogenate, the supernatant was collected for the measurement of MDA content, and activity of SOD and GSH-Px according to the manufacturer's instructions.
000g, 15 min) of the tissue homogenate, the supernatant was collected for the measurement of MDA content, and activity of SOD and GSH-Px according to the manufacturer's instructions.
2.11 Statistical analysis
The results were presented as means ± SD (standard deviation). SPSS Statistics V21.0 (IBM Inc., Chicago, IL, USA) was used for statistical analyses. One-way analysis of variance (ANOVA) followed by Tukey's posthoc test was used to analyse the data. The difference was considered to be statistically significant if the p-value was below 0.05.3. Results
3.1 Distribution and diversity of gut microbiota in vivo and in vitro
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 622 raw reads and an average of 33
622 raw reads and an average of 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 812.33 ± 3731.21 reads in each sample were obtained after the preliminary quality filtering. Based on a sequence similarity greater than 97%, OTUs in each group were defined (Table 1), and it was found that the number of OTUs in the CDF group were higher than that in the CDF-DF group (p = 0.063) and Con group (p < 0.05). The Chao1 and ACE indexes between the varieties of CDF and Con group were significantly different (p < 0.05). The plots of partial least squares discriminant analysis (PLS-DA) was applied to reveal a distinct clustering of gut microbiota composition in mice fecal samples based on OTUs (Fig. 1A). In the PLS-DA plots of the mice fecal microbiota, clearer boundaries among the three groups were observed. The CDF-DF group located in the lower middle of the Con group and CDF group.
812.33 ± 3731.21 reads in each sample were obtained after the preliminary quality filtering. Based on a sequence similarity greater than 97%, OTUs in each group were defined (Table 1), and it was found that the number of OTUs in the CDF group were higher than that in the CDF-DF group (p = 0.063) and Con group (p < 0.05). The Chao1 and ACE indexes between the varieties of CDF and Con group were significantly different (p < 0.05). The plots of partial least squares discriminant analysis (PLS-DA) was applied to reveal a distinct clustering of gut microbiota composition in mice fecal samples based on OTUs (Fig. 1A). In the PLS-DA plots of the mice fecal microbiota, clearer boundaries among the three groups were observed. The CDF-DF group located in the lower middle of the Con group and CDF group.
| Group | OTU numbers | Simpson | Chao1 | ACE | Shannon | |
|---|---|---|---|---|---|---|
| In vivo: Referred to the microbiota in mice fecal samples. Con: group control, mice were fed standard diet; CDF: group CDF, mice were fed a standard diet and supplemented with CDF by gavage; CDF-DF: group CDF-DF, mice were fed a standard diet and supplemented with CDF-DF by gavage. In vitro: Referred to the microbiota in vitro fermentation samples. CDF: group CDF, CDF was fermented by human feces in vitro; CDF-DF: group CDF-DF, CDF-DF was fermented by human feces in vitro; CDDP: group CDDP, the combination of CDF-PP and CDF-DF compound was fermented by human feces in vitro. Different letters denoted a significant difference that was obtained (P < 0.05). | ||||||
| In vivo | Con | 6317.40 ± 948.23a | 0.98 ± 0.02 | 1738.96 ± 446.93a | 1766.40 ± 441.74a | 8.90 ± 0.64 |
| CDF | 7310.33 ± 91.84b | 0.99 ± 0.01 | 2184.43 ± 289.75b | 2216.72 ± 306.37b | 8.95 ± 0.46 | |
| CDF-DF | 6461.67 ± 252.59ab | 0.99 ± 0.00 | 2054.65 ± 258.70ab | 2153.07 ± 304.42ab | 9.02 ± 0.22 | |
| In vitro | CDF | 4948.33 ± 324.73b | 0.89 ± 0.02 | 1033.08 ± 81.62a | 1060.51 ± 97.62a | 5.80 ± 0.11 |
| CDF-DF | 4119.00 ± 122.75a | 0.90 ± 0.01 | 885.33 ± 22.51b | 885.33 ± 27.50b | 5.76 ± 0.06 | |
| CDDP | 4750.33 ± 602.20ab | 0.90 ± 0.02 | 992.23 ± 111.87ab | 998.09 ± 114.77ab | 5.96 ± 0.26 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 484, 90
484, 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 765, and 100
765, and 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 667 sequences of microbiota were found in Group CDF, CDF-DF, and CDDP, respectively. The indexes of ACE, Chao1, and OTU numbers in the CDF group were significantly different than those in the CDF-DF group (p < 0.05) but similar to those in the CDDP group (p > 0.05; Table 1). Fig. 1B shows PLS-DA of in vitro fermentation samples. Clearer boundaries among these three groups were obtained, and the CDDP group located in the upper middle of the CDF group and CDF-DF group.
667 sequences of microbiota were found in Group CDF, CDF-DF, and CDDP, respectively. The indexes of ACE, Chao1, and OTU numbers in the CDF group were significantly different than those in the CDF-DF group (p < 0.05) but similar to those in the CDDP group (p > 0.05; Table 1). Fig. 1B shows PLS-DA of in vitro fermentation samples. Clearer boundaries among these three groups were obtained, and the CDDP group located in the upper middle of the CDF group and CDF-DF group.
3.2 Microbiota composition in vivo and in vitro
| Phylum; class; order; family | Genus | Con | CDF | CDF-DF |
|---|---|---|---|---|
| Con: group Control, mice were fed standard diet; CDF: group CDF, mice were fed standard diet and supplemented with CDF by gavage; CDF-DF: group CDF-DF, mice were fed standard diet and supplemented with CDF-DF by gavage. Different letters denoted significant difference was obtained (P < 0.05). | ||||
| Firmicutes | 66.74 ± 18.75 | 50.16 ± 12.04 | 61.18 ± 15.60 | |
| Clostridia; Clostridiales; Unclassified Clostridiales | Unclassified Clostridiales | 44.78 ± 17.92b | 20.29 ± 5.66a | 43.10 ± 13.51b |
| Bacilli; Lactobacillales; Lactobacillaceae | Lactobacillus | 3.20 ± 2.29a | 19.72 ± 10.61b | 2.07 ± 2.02a |
| Clostridia; Clostridiales; Lachnospiraceae | [Ruminococcus] | 1.93 ± 0.65b | 0.43 ± 0.27a | 1.14 ± 0.75c |
| Coprococcus | 0.33 ± 0.11a | 0.15 ± 0.11b | 0.17 ± 0.07c | |
| Clostridia; Clostridiales; Ruminococcaceae | Oscillospira | 1.80 ± 0.59b | 1.20 ± 0.93a | 1.83 ± 0.60b |
| Clostridia; Clostridiales; Clostridiaceae | Clostridium | 0.11 ± 0.06b | 0.20 ± 0.20ab | 0.03 ± 0.03a |
| Clostridia; Clostridiales; Dehalobacteriaceae | Dehalobacterium | 0.21 ± 0.20b | 0.14 ± 0.11a | 0.15 ± 0.05b |
| Bacteroidetes | 31.72 ± 17.81a | 48.91 ± 12.38b | 35.46 ± 16.71ab | |
| Bacteroidia; Bacteroidales; S24-7 | Unclassified S24-7 | 26.98 ± 16.89a | 42.98 ± 15.73b | 32.91 ± 15.14ab |
| Bacteroidia; Bacteroidales; Bacteroidaceae | Bacteroides | 1.39 ± 0.99ab | 2.92 ± 2.01a | 1.10 ± 0.46b |
| Proteobacteria | 1.38 ± 1.35ab | 0.47 ± 0.42a | 3.18 ± 2.73b | |
| Epsilonproteobacteria; Campylobacterales; Helicobacteraceae | Helicobacter | 0.97 ± 0.54ab | 0.00 ± 0.00a | 1.92 ± 0.68b |
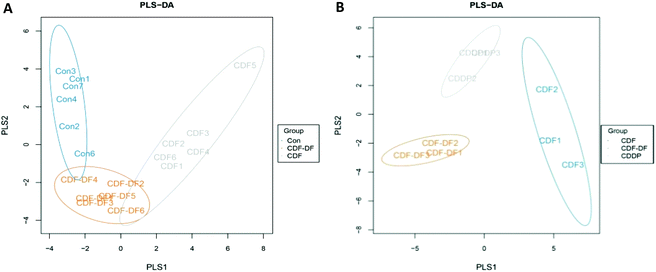
Fig. 2C shows the RA of gut microbiota at the genus, and it was found that Unclassified Clostridiales, Unclassified S24-7, Lactobacillus, and Unclassified Lachnospiraceae were predominant in the mice fecal samples. In addition, many genera in the mice fecal samples were influenced by CDF and CDF-DF, and the details are shown in Table 2. In Firmicutes, the CDF group had lower RAs in Unclassified Clostridiales, [Ruminococcus], Coprococcus, Oscillospira, and Dehalobacterium (p < 0.05) and higher RA in Lactobacillus than those in the CDF-DF and Con groups. Nevertheless, a significant difference was observed in the RA of Clostridium between the varieties of CDF-DF group and Con group (p < 0.05), but variations between the Con group and CDF group were insignificant (p > 0.05). In Bacteroides, the RA of Unclassified S24-7 was predominant in all groups. The RAs of Bacteroides and Unclassified S24-7 in the CDF group were the highest among the three groups. Among these, there was a significant difference between the Con group and CDF group in the RA of Unclassified S24-7 (p < 0.05) as well as the CDF group and CDF-DF group in the RA of Bacteroides (p < 0.05). In the Proteobacteria, the regnant RA of bacteria: Helicobacter was found to be highest in the CDF-DF group, and a significant difference was found between the CDF-DF group and CDF group (p < 0.05).
| Phylum; class; order; family | Genus | CDF | CDF-DF | CDDP |
|---|---|---|---|---|
| CDF: group CDF, CDF was fermented by human feces in vitro; CDF-DF: group CDF-DF, CDF-DF was fermented by human feces in vitro; CDDP: group CDDP, CDF-DF + CDF-PP was fermented by human feces in vitro. Different letters denoted significant difference was obtained (P < 0.05). | ||||
| Firmicutes | 28.64 ± 6.38 | 30.09 ± 2.67 | 32.14 ± 1.84 | |
| Clostridia; Clostridiales; Veillonellaceae | Megamonas | 22.31 ± 7.20 | 26.60 ± 2.27 | 27.66 ± 1.70 |
| Phascolarctobacterium | 0.12 ± 0.00a | 0.05 ± 0.01b | 0.09 ± 0.01c | |
| Megasphaera | 0.71 ± 0.26b | 0.03 ± 0.02a | 0.40 ± 0.56ab | |
| Erysipelotrichi; Erysipelotrichales; Erysipelotrichaceae | Catenibacterium | 2.58 ± 0.52a | 1.29 ± 0.90b | 2.18 ± 1.99ab |
| Bacteroidetes | 22.17 ± 1.14a | 9.05 ± 1.30b | 17.54 ± 0.15c | |
| Bacteroidia; Bacteroidales; Prevotellaceae | Prevotella | 17.21 ± 2.37a | 0.72 ± 0.31b | 9.14 ± 1.01c |
| Bacteroidia; Bacteroidales; Bacteroidaceae | Bacteroides | 3.40 ± 0.74a | 6.39 ± 1.41b | 6.96 ± 0.95b |
| Bacteroidia; Bacteroidales; S24-7 | Unclassified S24-7 | 0.20 ± 0.01a | 0.12 ± 0.04b | 0.19 ± 0.05a |
| Proteobacteria | 48.68 ± 7.34a | 60.32 ± 14.61b | 49.75 ± 1.80ab | |
| Gammaproteobacteria; Enterobacteriales; Enterobacteriaceae | Unclassified Enterobacteriaceae | 48.15 ± 7.15 | 57.41 ± 1.05 | 48.20 ± 1.71 |
| Klebsiella | 0.19 ± 0.09a | 1.53 ± 0.35b | 0.68 ± 0.01c | |
| Erwinia | 0.22 ± 0.10a | 1.15 ± 0.09b | 0.66 ± 0.02a | |
| Enterobacter | 0 ± 0.00a | 0.05 ± 0.00b | 0.01 ± 0.00a | |
At the genus, the RA of gut microbiota in in vitro fermentation samples was displayed in Fig. 2F and Unclassified Enterobacteriaceae, Megamonas, Prevotella, and Bacteroides were dominant. The composition of intestinal microbes was affected due to the fermentation of different compounds (Table 3). In Firmicutes, the most diverse genus in Firmicutes was Megamonas, and there was no significant difference in the RA of Megamonas among the three groups (p < 0.05). In addition, the RAs of Phascolarctobacterium, Megasphaera, and Catenibacterium in the CDF-DF group were lower than those in the CDF group (p < 0.05) and a similar trend was also detected in the CDF-DF group when compared with those in the CDDP group. In Bacteroidetes, the RAs of Prevotella and Bacteroides were prevailing. The Prevotella to Bacteroides ratio in the CDF-DF group was the lowest, and hadsignificant difference among the CDF group and CDDP group (p < 0.05). Moreover, the CDF and CDDP groups, compared with the CDF-DF group, had higher RA in Unclassified S24-7 (p < 0.05). In Proteobacteria, the CDF-DF group increased the RAs in Unclassified Enterobacteriaceae, Klebsiella, Erwinia, and Enterobacter compared with the CDF and CDDP groups (p < 0.05).
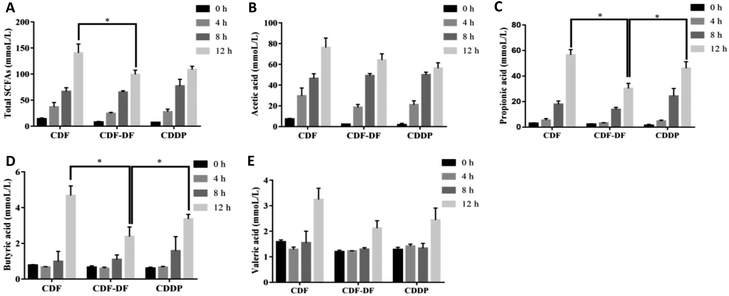
3.3 Determination of SCFAs in vivo and in vitro
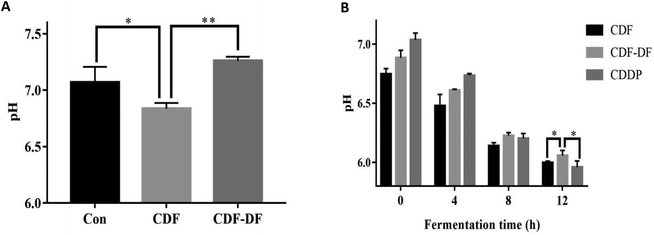
3.4 Shift of pH in vivo and in vitro
3.5 Antioxidant indexes in vivo and in vitro
| Group | MDA (nmol mg−1 protein) | SOD (U mg−1 protein) | GSH-Px (mU mg−1 protein) |
|---|---|---|---|
| * P < 0.05, CDF vs. Con group; #P < 0.05, CDF vs. CDF-DF group. | |||
| Con | 3.06 ± 0.21 | 22.39 ± 1.27 | 219.62 ± 33.76 |
| CDF | 2.40 ± 0.72* | 33.69 ± 2.89* | 382.69 ± 42.65* |
| CDF-DF | 3.94 ± 0.38# | 29.35 ± 1.05# | 272.18 ± 24.07# |
4. Discussion
In 1995, Glenn Gibson and Marcel Roberfroid defined a prebiotic as a nondigestible food ingredient that beneficially affects the host by selectively stimulating the growth and/or activity of one or a limited number of bacteria in the colon, and thus, improves the host health. However, in 2017, prebiotics was redefined as a substrate that is selectively utilized by host microorganisms conferring a health benefit by the International Scientific Association for Probiotics and Prebiotics (ISAPP).25 This definition expands the concept of prebiotics to include non-carbohydrate substances, applications to body sites other than the gastrointestinal tract, and diverse categories other than food. Growing evidence indicates that polyphenols favored the growth of beneficial gut microbes, suggesting that polyphenols are capable of acting as prebiotics. In the present study, CDF and CDF-DF were applied in vivo and in vitro to investigate the impact of bound polyphenols on dietary fiber with respect to the gut microbiota.The complexity of species diversity for samples could be analyzed by OTU numbers and alpha-diversity, including Shannon, Chao1, ACE, and Simpson indexes. The OTU numbers can be used to assess the richness of communities,26 and the alpha-diversity is a measure of species diversity.27 Our data displayed that the OTU numbers and alpha-diversity indexes of the CDF-DF group had a reduction compared with those of the CDF group in mice fecal samples and in vitro fermentation samples, indicating that CDF was conducive to increase the flora richness and diversity than CDF-DF. Furthermore, the OTU numbers and alpha-diversity indexes were recovered by the combination of CDF-PP and CDF-DF, proving that the presence of CDF-PP in CDF affected the composition of the intestinal flora. To further identify the effect of bound polyphenols on variations in the intestinal microbial composition of DF, we analyzed the microbial composition of mice fecal samples and in vitro fermentation samples.
At phylum, F/B is considered as a biological indicator of obesity, which is higher in obesity group with a high-calorie diet.28 Furthermore, Proteobacteria is a microbial signature of dysbiosis in gut microbiota.29 The CDF-fed mice decreased the F/B ratio and the RA of Proteobacteria than normal diet mice. In comparison with CDF-fed mice, the CDF-DF-fed mice had higher F/B and RA of Proteobacteria (p < 0.05). Meanwhile, a similar trend was also observed for the F/B and the RA of Proteobacteria in vitro fermentation samples. Furthermore, the combination of CDF-PP and CDF-DF, namely the CDDP group, also verified these results that CDF-PP contributed to reduce the F/B and the RA of Proteobacteria. It was consistent with previous reports that the polyphenols could decrease the F/B ratio and the RA of Proteobacteria.30
At the genus, in mice fecal samples, the CDF-DF group had lower RAs in Lactobacillus, Unclassified S24-7, and Bactericides, which could protect the host against various types of intestinal diseases and promote the production of SCFAs31,32 and higher RAs in Unclassified Clostridiales and Helicobacter that were not conducive to human health.33 In particular, the RA of Helicobacter in CDF-DF-fed mice was 1.92 ± 0.68%, while there was no Helicobacter in CDF-fed mice fecal samples. In the in vitro fermentation samples, the RAs of beneficial gut microbiota including Phascolarctobacterium, Unclassified S24-7, and Megasphaera decreased in the CDF-DF group, while the RAs of harmful bacteria including Unclassified Enterobacteriaceae, Klebsiella, Erwinia, and Enterobacter increased. Various mechanisms for the impact of polyphenols on the gut microbiota have been proposed and investigated. Some polyphenols could be catabolized into more active and better absorbed phenolic compounds by gut microbes, thus influencing the composition of bacteria.34 In addition, polyphenols may inhibit the growth bacteria by the inhabitation of microbial enzymes such as hydrolases, and constitute on the adhesion of bacteria. Besides, polyphenols can restrain the growth of bacteria due to their antimicrobial effects.35 The results demonstrated that the presence of bound polyphenols in dietary fiber could effectively improve the growth of typical beneficial bacteria and restrain the growth of various harmful bacteria, suggesting that the bound polyphenols played an important role in the modification of microbial structure and maintained the balance of intestinal microbes of dietary fiber.
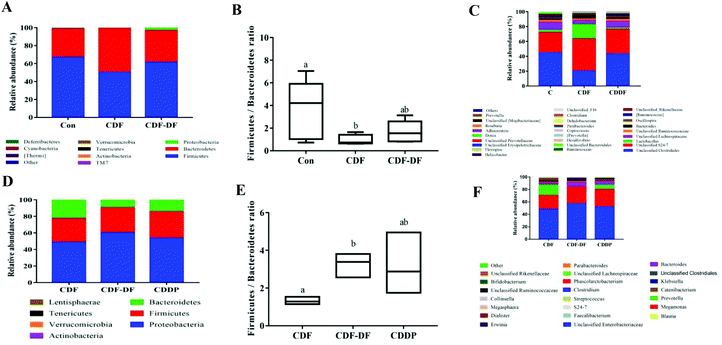
SCFAs are metabolic by-products of the intestinal flora fermentation, which play an important role in maintaining the normal function of the large intestine and the morphology of colon epithelial cells.36 Acetic, propionic and butyric acids are the primary forms of SCFA, with acetic acid being the majority of the total SCFA in feces. Propionic acid and butyric acid could accelerate differentiation and apoptosis of colon cancer cells, and thus, protect the colon from carcinogenesis.37 Earlier studies have highlighted the significance of dietary fiber in ameliorating the production of SCFAs. However, the effect of bound polyphenols in dietary fiber on the production of SCFAs has been neglected. Therefore, the contents of acetic, propionic and butyric acids after fermentation were measured to evaluate the potential CDF-PP on the production of SCFAs. In the present study in vivo, acetic acid and butyric acid contents were significantly decreased in CDF-DF-fed mice than those in CDF-fed mice, which could be explained by the reduction of RAs of Bacteroidetes and Lactobacillus,38,39 while the propionic acid content had no significant change, which may be due to the lower relative abundance of flora producing propionic acid such as Odoribacter. In vitro, the CDF-DF group had lower propionic and butyric acid content compared to the CDF group and CDDP group, which may be due to the increase of RAs of Prevotella, Unclassified S24-7 and Bacteroidetes38–40 in the CDF-DF group. Nevertheless, there was no significance in total SCFAs between the CDF-DF group and CDDP group, and this may have occurred due to the addition of CDF-PP promoting the production of lactate metabolite, and then afterward microbial activity by propionic acid producers, thus, causing the decrease of total SCFAs in the CDDP group. These results showed that bound polyphenols in CDF may affect the production of SCFAs in the intestinal fermentation of dietary fiber by changing the structure of the intestinal flora.
pH values in colon is also an important index to evaluate the health status of the colon; a lower pH can prevent the growth of pathogenic bacteria.22,41 Our results indicated that the pH value in the CDF group was lower than the CDF-DF group in mice fecal samples and in vitro fermentation samples at 12 h. In addition, the combination of bound polyphenols and CDF-DF in vitro could recover the pH value after 12 h fermentation, and this may be due to the increase in SCFA production, which could contribute to the decrease of pH. This implies that bound polyphenols in dietary fiber may lower the pH values due to the increase of SCFA production.
In recent years, the effect of bound polyphenols on the antioxidant activity of dietary fiber has attracted lots of interest. Our previous experiments have verified that CDF-PP contributed to the antioxidant properties of CDF by in vitro chemical methods including superoxide anion radical, hydroxyl radical, and DPPH radical scavenging activity,18 but no study has been done on the effects of CDF-PP on colonic antioxidant, and CAA properties of CDF. In colon tissue, GSH-Px and SOD were a part of the antioxidant system to protect membranes and essential proteins from the potentially damaging effects of reactive oxygen and lipid peroxides. Moreover, the MDA as the product of chain reaction of lipid peroxidation can be used to assess the degree of lipid oxidation.42 Therefore, the colonic antioxidant property was evaluated by measuring the MDA content and GSH-Px and SOD activities. Our experimental data showed that CDF intake compared with normal diet mice increased the activity of GSH-Px and SOD and reduced the content of MDA, which was consistent with the previous report.43,44 However, when the bound polyphenols were removed, it was found that CDF-DF-fed mice had higher MDA contents and lower GSH-Px and SOD activities than CDF-fed mice, demonstrating that the removal of bound polyphenols from DF could significantly influence the colonic antioxidant property of DF.
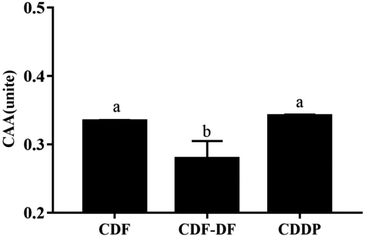
CAA assay simulated the HepG2 cellular biochemical processes, including bio-accessibility, uptake, distribution, and metabolism of samples, and can exhaustively quantify the antioxidant capacity of different samples in cell cultures.45 The CAA value of the CDF-DF group was lower than that of the CDF group (p < 0.05), and the CDDP group recovered the CAA value, which suggests the decline of the CAA value in CDF-DF may be due to the removal of CDF-PP from CDF. Overall, these results revealed that the bound polyphenols significantly contributed to the colonic antioxidant and CAA capacities of DF.
In summary, the present study suggested that bound polyphenols could significantly contribute to the fermentation and antioxidant properties of DF in vivo and in vitro in the following ways: (1) microbial structure and balance of intestinal microbes; (2) production of SCFA contents and pH value; and (3) colonic antioxidant and CAA properties. These findings could promote the understanding of the effect of bound polyphenols on DF function, and provide a theoretical foundation for the development of functional food based on dietary fiber.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (31701603, 31972066), Key Research and Development Program of Jiangxi Province of China (20171BBF60041), and Jiangxi Graduate Innovation Project (YC2018-S009).References
- F. Guarner and J. R. Malagelada, Gut flora in health and disease, Lancet, 2003, 361, 512–519 CrossRef.
- J. L. Round and S. K. Mazmanian, The gut microbiota shapes intestinal immune responses during health and disease, Nat. Rev. Immunol., 2009, 9, 313–323 CrossRef CAS PubMed.
- J. M. R. Tingirikari, Microbiota-accessible pectic poly- and oligosaccharides in gut health, Food Funct., 2018, 9, 5059–5073 RSC.
- J. Chow, S. M. Lee, Y. Shen, A. Khosravi and S. K. Mazmanian, Host-Bacterial Symbiosis in Health and Disease-Chapter 8, Adv. Immunol., 2010, 107, 243–274 CAS.
- J. Slavin, Fiber and Prebiotics: Mechanisms and Health Benefits, Nutrients, 2013, 5, 1417–1435 CrossRef CAS PubMed.
- J. K. Goodrich, J. L. Waters and A. C. Poole, et al., Human Genetics Shape the Gut Microbiome, Cell, 2014, 159, 789–799 CrossRef CAS PubMed.
- M. Conlon and A. Bird, The Impact of Diet and Lifestyle on Gut Microbiota and Human Health, Nutrients, 2015, 7, 17–44 CrossRef PubMed.
- J. Qin, Y. Li and Z. Cai, et al., A metagenome-wide association study of gut microbiota in type 2 diabetes, Nature, 2012, 490, 55–60 CrossRef CAS PubMed.
- F. Saura-Calixto, Dietary Fiber as a Carrier of Dietary Antioxidants: An Essential Physiological Function, J. Agric. Food Chem., 2011, 59, 43–49 CrossRef CAS PubMed.
- M. Jia, Q. Yu and J. Chen, et al., Physical quality and in vitro starch digestibility of biscuits as affected by the addition of soluble dietary fiber from defatted rice bran, Food Hydrocolloids, 2020, 99, 105349 CrossRef.
- H. D. Holscher, Dietary fiber and prebiotics and the gastrointestinal microbiota, Gut Microbes, 2017, 8, 172–184 CrossRef CAS PubMed.
- A. Tamargo, C. Cueva, M. D. Alvarez, B. Herranz, M. V. Moreno-Arribas and L. Laguna, Physical effects of dietary fiber on simulated luminal flow, studied by in vitro dynamic gastrointestinal digestion and fermentation, Food Funct., 2019, 10, 3452–3465 RSC.
- L. Montagne, J. R. Pluske and D. J. Hampson, A review of interactions between dietary fibre and the intestinal mucosa, and their consequences on digestive health in young non-ruminant animals, Anim. Feed Sci. Technol., 2003, 108, 95–117 CrossRef.
- M. Jia, J. Chen and X. Liu, et al., Structural characteristics and functional properties of soluble dietary fiber from defatted rice bran obtained through Trichoderma viride fermentation, Food Hydrocolloids, 2019, 94, 468–474 CrossRef CAS.
- S. Hättenschwiler and P. M. Vitousek, The role of polyphenols in terrestrial ecosystem nutrient cycling, Trends Ecol. Evol., 2000, 15, 238–243 CrossRef.
- B. Collins, J. Hoffman and K. Martinez, et al., A polyphenol-rrich Fraction Obtained from table grapes decreases adiposity, insulin resistance, and markers of inflammation and impacts gut microbiota in high-fat-fed mice, J. Nutr. Biochem., 2016, 31, 150–165 CrossRef CAS PubMed.
- A. González-Sarrías, J. C. Espín and F. A. Tomás-Barberán, Non-extractable polyphenols produce gut microbiota metabolites that persist in circulation and show anti-inflammatory and free radical-scavenging effects, Trends Food Sci. Technol., 2017, 69, 281–288 CrossRef.
- S. Liu, M. Jia and J. Chen, et al., Removal of bound polyphenols and its effect on antioxidant and prebiotic properties of carrot dietary fiber, Food Hydrocolloids, 2019, 93, 284–292 CrossRef CAS.
- H. Palafox-Carlos, J. F. Ayala-Zavala and G. A. Gonzalez-Aguilar, The role of dietary fiber in the bioaccessibility and bioavailability of fruit and vegetable antioxidants, J. Food Sci., 2011, 76, R6–R15 CrossRef CAS PubMed.
- L. Zhou, W. Wang and J. Huang, et al., In vitro extraction and fermentation of polyphenols from grape seeds (Vitis vinifera) by human intestinal microbiota, Food Funct., 2016, 7, 1959–1967 RSC.
- X. Fu, C. Cao, B. Ren, B. Zhang, Q. Huang and C. Li, Structural characterization and in vitro fermentation of a novel polysaccharide from Sargassum thunbergii and its impact on gut microbiota, Carbohydr. Polym., 2017, 183, 230–239 CrossRef PubMed.
- L. H. Maurer, C. B. B. Cazarin and A. Quatrin, et al., Grape peel powder promotes intestinal barrier homeostasis in acute TNBS-colitis: A major role for dietary fiber and fiber-bound polyphenols, Food Res. Int., 2019, 123, 425–439 CrossRef CAS PubMed.
- S. G. Sáyago-Ayerdi, A. Sara, S. José and G. I. Isabel, Dietary fiber content and associated antioxidant compounds in Roselle flower (Hibiscus sabdariffa L.) beverage, J. Agric. Food Chem., 2007, 55, 7886–7890 CrossRef PubMed.
- J. Wang, S. Hu, S. Nie, Q. Yu and M. Xie, Reviews on Mechanisms of In Vitro Antioxidant Activity of Polysaccharides, Oxid. Med. Cell. Longevity, 2015, 2016, 5692852 Search PubMed.
- G. R. Gibson, R. Hutkins and M. E. Sanders, et al., Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics, Nat. Rev. Gastroenterol. Hepatol., 2017, 14, 491 CrossRef PubMed.
- J. T. Yu, H. Guo and J. L. Xie, et al., The Alternate Consumption of Quercetin and Alliin in the Traditional Asian Diet Reshaped Microbiota and Altered Gene Expression of Colonic Epithelial Cells in Rats, J. Food Sci., 2019, 84, 678–686 CrossRef CAS PubMed.
- D. Stanley, R. J. Hughes, M. S. Geier and R. J. Moore, Bacteria within the Gastrointestinal Tract Microbiota Correlated with Improved Growth and Feed Conversion: Challenges Presented for the Identification of Performance Enhancing Probiotic Bacteria, Front. Microbiol., 2016, 7, 187 Search PubMed.
- C. De Filippo, D. Cavalieri and M. Di Paola, et al., Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 14691–14696 CrossRef PubMed.
- N.-R. Shin, T. W. Whon and J.-W. Bae, Proteobacteria: microbial signature of dysbiosis in gut microbiota, Trends Biotechnol., 2015, 33, 496–503 CrossRef CAS PubMed.
- D. E. Roopchand, R. N. Carmody, P. Kuhn and K. Moskal, et al., Dietary polyphenols promote the growth of the gut bacterium Akkermansia muciniphila and attenuate high-fat diet-induced metabolic syndrome, Diabetes, 2015, 64, 2847–2858 CrossRef CAS PubMed.
- K. Aagaard, K. Riehle and J. Ma, et al., A metagenomic approach to characterization of the vaginal microbiome signature in pregnancy, PLoS One, 2012, 7, e36466 CrossRef CAS PubMed.
- I. Khan, E. I. Azhar and A. T. Abbas, et al., Metagenomic Analysis of Antibiotic-Induced Changes in Gut Microbiota in a Pregnant Rat Model, Front. Pharmacol., 2016, 7, 104 Search PubMed.
- Z. Angelo, H. Cesare and M. Sergio, Helicobacter pylori infection and the development of gastric cancer, N. Engl. J. Med., 2002, 346, 65–67 CrossRef PubMed.
- J. C. Espín, A. González-Sarrías and F. A. Tomás-Barberán, The gut microbiota: A key factor in the therapeutic effects of (poly) phenols, Biochem. Pharmacol., 2017, 139, 82–93 CrossRef PubMed.
- A. Duda-Chodak, The inhibitory effect of polyphenols on human gut microbiota, J. Physiol. Pharmacol., 2012, 63, 497–503 CAS.
- D. Ríos-Covián, P. Ruas-Madiedo and A. Margolles, et al., Intestinal short-chain fatty acids and their link with diet and human health, Front. Microbiol., 2016, 7, 185 Search PubMed.
- T. Di, G. Chen and Y. Sun, et al., In vitro digestion by saliva, simulated gastric and small intestinal juices and fermentation by human fecal microbiota of sulfated polysaccharides from Gracilaria rubra, J. Funct. Foods, 2018, 40, 18–27 CrossRef CAS.
- Z. Q. Wang, E. M. Ammar, A. Zhang and L. Wang, et al., Engineering Propionibacterium freudenreichii subsp. shermanii for enhanced propionic acid fermentation: effects of overexpressing propionyl-CoA: Succinate CoA transferase, Metab. Eng., 2015, 27, 46–56 CrossRef CAS PubMed.
- Y. S. Jang, J. A. Im, S. Y. Choi, J. I. Lee and S. Y. Lee, Metabolic engineering of Clostridium acetobutylicum for butyric acid production with high butyric acid selectivity, Metab. Eng., 2014, 23, 165–174 CrossRef CAS PubMed.
- R. A. Gonzalez-Garcia, T. McCubbin and L. Navone, et al., Microbial Propionic Acid Production, Fermentation, 2017, 3, 21 CrossRef.
- D. Ríos-Covián, P. Ruas-Madiedo, A. Margolles, M. Gueimonde, C. G. de los Reyes-Gavilán and N. Salazar, Intestinal Short Chain Fatty Acids and their Link with Diet and Human Health, Front. Microbiol., 2016, 7, 185 Search PubMed.
- Y. Rui-Li, S. Yong-Hui, H. Gang, L. Wu and L. Guo-Wei, Increasing Oxidative Stress with Progressive Hyperlipidemia in Human: Relation between Malondialdehyde and Atherogenic Index, J. Clin. Biochem. Nutr., 2008, 43, 154–158 CrossRef PubMed.
- B. S. Thampi, G. Manoj, S. Leelamma and V. P. Menon, Dietary fiber and lipid peroxidation: effect of dietary fiber on levels of lipids and lipid peroxides in a high-fat diet, Indian J. Exp. Biol., 1991, 29, 563–567 CAS.
- I. Giannenas, E. Tsalie, E. Chronis, S. Mavridis, D. Tontis and I. Kyriazakis, Consumption of Agaricus bisporus mushroom affects the performance, intestinal microbiota composition and morphology, and antioxidant status of turkey poults, Anim. Feed Sci. Technol., 2011, 165, 218–229 CrossRef CAS.
- K. L. Wolfe and L. R. Hai, Cellular antioxidant activity (CAA) assay for assessing antioxidants, foods, and dietary supplements, J. Agric. Food Chem., 2007, 55, 8896 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2020 |