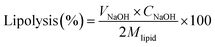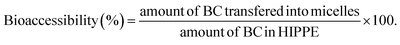Fabrication of high internal phase Pickering emulsions with calcium-crosslinked whey protein nanoparticles for β-carotene stabilization and delivery
Jiang
Yi
 *a,
Luyu
Gao
*a,
Luyu
Gao
 a,
Guitian
Zhong
a,
Guitian
Zhong
 a and
Yuting
Fan
*b
a and
Yuting
Fan
*b
aDepartment of Food Science and Engineering, College of Chemistry and Environmental Engineering, Shenzhen University, Shenzhen, Guangdong 518060, China. E-mail: yijiangjnu@gmail.com; Fax: +86-755-26536141; Tel: +86-755-26557377
bSchool of Public Health, Health Science Center, Shenzhen University, Shenzhen, Guangdong 518060, China. E-mail: fanyutingca@gmail.com
First published on 23rd December 2019
Abstract
Whey protein isolate (WPI) nanoparticles were fabricated with Ca2+ induced cross-linking and used as an effective particle stabilizer for high internal phase Pickering emulsion (HIPPE) formulation aiming to improve the chemical stability and bioaccessibility of β-carotene (BC). Ca2+ concentration dominated the characteristics of WPI nanoparticles. Spherically shaped and homogeneously dispersed WPI nanoparticles with a Z-average diameter of approximately 150.0 nm were obtained with 5.0 mM Ca2+ concentration. No cytotoxicity was observed for WPI nanoparticles even at 10.0 mg mL−1 concentration. HIPPE (oil fraction 80.0%, w/w) can be successfully prepared with WPI nanoparticles at a concentration as low as 0.2% (w/w) and was stable for at least 2 months at room temperature. A higher WPI nanoparticle concentration resulted in more solid-like HIPPEs. BC exhibited appreciably higher retention in HIPPEs than in bulk oil during 30 days of storage at 50 °C. Moreover, BC bioaccessibility was appreciably improved with the HIPPE delivery system. Both the chemical stability and bioaccessibility of BC increased with the increase of WPI nanoparticle concentrations from 0.2 to 1.0% (w/w). The results obtained in this study may facilitate the fabrication of edible and biocompatible protein-based nanoparticle stabilizers for HIPPE formulation with more innovative and tailored functionalities.
1. Introduction
High internal phase emulsions (HIPEs), with a minimal internal phase volume fraction of 0.74, have gained increasing interest over the past decade.1 HIPEs can be extensively used in stabilization and sustained release of nutraceutical, tissue engineering, and pharmaceutical and cosmetic formulations, due to their uniform features including high viscosity, gel-like structure, large specific surface area, and exceptional structural properties.2,3 Conventional HIPEs were generally stabilized by a high amount of low molecular weight surfactants (5–50%, w/w) through decreasing interfacial tension and providing steric hindrance or electrostatic repulsions to prevent coalescence and flocculation between oil droplets.2 Unfortunately, the abundant utilization of surfactants was undesirable in food formulations because of the increasing consumer and legal demands, such as hypotoxicity, biocompatibility and environmental acceptability.4Compared to traditional emulsions, rigid particle-stabilized emulsions, also known as Pickering emulsions, are even more stable.2 Pickering emulsions have been testified to possess good coalescence stability when exposed to thermal treatment, high ionic strength, and long-term storage.5,6 A layer of rigid particles irreversibly adsorbed outside the oil droplet endowed the emulsions with excellent stability against coalescence, Ostwald ripening, and creaming.7,8 Stable high internal phase Pickering emulsions (HIPPEs) (oil fraction (ϕ) over 0.90) can be prepared with synthesized microgel particles,9 silica or functionalized silica particles.10 However, the utilization of organic or inorganic particles in food systems is strictly limited due to food safety concerns. There is an urgent need for natural, edible, biocompatible, and biodegradable solid colloidal particles fabricated from edible materials in the food industry. Recently, some studies have been reported on HIPPEs stabilized with food-grade particles including polysaccharides (e.g. chitin nanocrystals,11 nanocellulose,12 and starch nanoparticles13) and proteins (e.g. gelatin,14 gliadin,15 and soybean protein16). The utilization of strong acids and alkalis in the fabrication of polysaccharide-based colloidal particles including chitin nanocrystals, nanocellulose, and starch nanoparticles is adverse to the widespread utilization in food systems. Moreover, high concentrations (at least >1.0%, w/v) of polysaccharide-based colloidal particles are needed for stable HIPPE preparation. Protein-based colloidal particles may be better HIPE stabilizers since food proteins exhibited excellent functional properties such as good deformability, great hydrophilicity/hydrophobicity balance, and high antioxidant activity.17 However, limited information is available in the literature addressing the fabrication of protein-based colloidal particles (nanoparticles) for HIPPE stabilization. Furthermore, the addition of an organic solvent and toxic glutaraldehyde in the fabrication of the aforementioned protein-based colloidal particles would also raise food safety concerns.
Whey protein isolate (WPI) is commonly used in food formulations due to a variety of nutritional values and functionalities, such as anti-aging, anticancer, foam formation or emulsification, and gelation properties. Tailored functionalities of WPI can be designed through modifications with enzymatic hydrolysis or heat-induced cross-linking for specific applications.18 Thermal treatment is likely to be one of the effective methods to fabricate a Pickering-like stabilizer with whey protein. The first work of Pickering oil-in-water emulsions stabilized by WPI microgels fabricated by a simple heat-set gel formation protocol was published in 2014.19 Zamani et al. recently prepared HIPPEs stabilized by WPI microgels (1.0%, w/w) obtained by thermal treatment with an internal phase of 0.75 (w/w).6 However, the dispersed phase (oil) fraction was relatively low, and a high protein concentration was necessary. More than 4.0% (w/w) of WPI microgels was needed for the development of stable HIPPEs with an internal oil phase volume fraction higher than 0.80 (w/w).20
A recent study reported that WPI nanoparticles can be fabricated by Ca2+ induced cross-linking at certain pH values with specific interfacial properties, which may be promising food-grade colloidal particles for HIPPE stabilization.21 The particle sizes and interfacial characteristics of WPI nanoparticles can be modulated by Ca2+ concentrations and pH values.
β-Carotene (BC) is a carotenoid possessing a variety of health benefits including high pro-vitamin A activity, antioxidant activity, and anti-obesity property, which led it to be the subject of an increasing number of studies.22 However, the susceptibility to degradation, the insolubility in the aqueous phase, and the poor bioavailability in vivo greatly limited its utilization in food systems.23
In this work, WPI nanoparticles were fabricated by Ca2+ induced cross-linking and characterized by DLS, turbidity analysis, TEM, and AFM. The in vitro cytotoxicity of Ca2+ cross-linked WPI nanoparticles was studied. The ability of Ca2+ induced WPI nanoparticles to form stable HIPPEs is evaluated in comparison with HIPPEs stabilized by native WPI under the same conditions. The potential of HIPPEs in loading, stabilizing, and delivering BC was estimated. The chemical stability of BC in HIPPEs stabilized by various concentrations of WPI nanoparticles during 30 days of storage at 50 °C was investigated. The extent of lipolysis and BC bioaccessibility of HIPPEs were evaluated with a simulated in vitro gastrointestinal digestion model.
2. Materials and methods
2.1 Materials
Whey protein isolate (WPI) with 95% total protein was generously provided as a gift from Davisco Foods International Inc. (Le Sueur, MN, USA). CaCl2, β-carotene (BC, 97%), bile salt, pancreatin, and 1-anilino-8-naphthalene-sulfonate (ANS) were acquired from Macklin (Shanghai, China) and used without further purification. Corn oil was purchased from a local supermarket (Shenzhen, China). Ultrapure water purified to a resistivity of 18.2 MΩ with a Sartorius arium® mini water purification system (Goettingen, Germany) was used in all experiments. All other analytical grade chemicals and reagents were obtained from Sinopharm Chemical Reagent Co., Ltd.2.2 WPI nanoparticle fabrication
Ca2+ induced WPI nanoparticles were fabricated using a well-established protocol with some modification.21 WPI powder (12.0 mg mL−1) was magnetically stirred and dissolved in ultrapure water for 2 h and kept in a refrigerator overnight to ensure full hydration. Sodium azide (0.02%, w/v) was added to inhibit the growth of microorganisms. The dispersion was centrifuged (8000 rpm, 30 min, 10 °C) to remove insoluble matter. Then, the solution was heated at 85 °C for 30 min using a thermostat water bath. The solution was cooled to room temperature immediately and the pH was adjusted slightly back to 6.0 with HCl and NaOH (0.1 M). Stock CaCl2 solution was added dropwise to obtain a final concentration of 1.0, 2.5, 5.0, 7.5, and 10.0 mM under magnetic stirring, respectively. Then the solution was stirred for 5 h to induce nanoparticle formation. Lastly, the pH and protein concentration of the dispersion were adjusted to pH 7 and 10 mg mL−1, respectively.2.3 Characteristics of Ca2+ induced WPI nanoparticles
For turbidity, the values of Ca2+ induced WPI nanoparticles were measured on a UV-vis spectrophotometer (UV-2600, Shimadzu, Japan) as the absorbance at 600 nm without dilution. The Z-average size, polydispersity index (PDI), and zeta-potential were determined using a Zetasizer (Nano ZSE, Malvern Instrument Ltd, U.K.) with a He/Ne laser (= 633 nm) at a fixed scattering angle of 90° at room temperature. Samples were transferred into polystyrene cuvettes and capillary cells for Z-average particle size, PDI, and zeta-potential analysis, respectively. The refractive indexes of WPI nanoparticles and ultrapure water were 1.45 and 1.33, respectively.The surface hydrophobicity (H0) of WPI or Ca2+ induced WPI nanoparticles was determined using ANS as the fluorescent probe. The fluorescence intensity was measured using a fluorescence spectrophotometer (F-7000, Hitachi, Japan) with an excitation wavelength of 390 nm and an emission wavelength of 470 nm. The morphology and size of Ca2+ induced WPI nanoparticles (5 mM) were determined by atomic force microscopy (AFM) and transmission electron microscopy (TEM), respectively. Native WPI at the same concentration was used as a control. For AFM, a droplet (approximately 4 μL) of each diluted nanoparticle was added on a mica disk and spread and kept overnight to evaporate under ambient conditions. AFM images were obtained in the tapping mode using a Dimension 3000 microscope (CA, USA). For TEM, diluted nanoparticles were pre-treated by the conventional negative-staining method and observed with a transmission electron microscope (Hitachi H-700, Tokyo, Japan).
2.4 In vitro cytotoxicity
Caco-2 cell monolayer was obtained by incubating cells in a 96-well plate with a seeded density of 1.0 × 104 cells per well at 37 °C under 90% humidity and 5% CO2 for 3 days with one medium change. DMEM fortified with 10% FBS, 1× nonessential amino acids (NAA), and 1× penicillin and streptomycin was used as the culture medium. For in vitro cytotoxicity, Caco-2 cells were treated with Ca2+ induced WPI nanoparticles at various concentrations (1, 2, 3, 5, and 10 mg mL−1) for 24 h. After treatment, the morphology of the Caco-2 cells was observed and photographed using a Zeiss light microscope (Axio Scope A1 pol, Germany), and cell viability was investigated by the MTT approach. Caco-2 cells without WPI nanoparticle treatment were used as a control. The absorbances were measured using a microplate reader (Synergy HTX, BioTek, USA) at 570 nm. Cell viability was estimated as the percent absorbance of WPI nanoparticles relative to the control.242.5 High internal phase Pickering emulsion (HIPPE) preparation
HIPPEs were obtained by mixing edible corn oil with fabricated Ca2+ induced WPI nanoparticle solutions at various concentrations (0.1%, 0.2%, 0.5%, and 1.0%, w/w) in the oil phase: an aqueous phase mass ratio of 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (w/w) with a high-speed homogenizer (20
20 (w/w) with a high-speed homogenizer (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, T-25 digital Ultra Turrax®, IKA-Werke, Germany). The freshly obtained HIPPEs were immediately subjected to emulsification and characteristic determination. HIPPEs were stored for 60 days at room temperature for physical stability evaluation. Native WPI (0.5%, w/w) was used as a control.
000 rpm, T-25 digital Ultra Turrax®, IKA-Werke, Germany). The freshly obtained HIPPEs were immediately subjected to emulsification and characteristic determination. HIPPEs were stored for 60 days at room temperature for physical stability evaluation. Native WPI (0.5%, w/w) was used as a control.
2.6 Characteristics of HIPPE
2.7 Storage stability of BC
BC-loaded HIPPEs were obtained by mixing BC fully dispersed corn oil (0.1%, w/w) with Ca2+ induced WPI nanoparticle solutions at various concentrations (0.2%, 0.5%, and 1.0%, w/w) in the oil phase: an aqueous phase ratio of 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (w/w) with a high-speed homogenizer (20
20 (w/w) with a high-speed homogenizer (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, T-25 digital Ultra Turrax®, IKA-Werke, Germany). BC storage stability analysis was performed at 50 °C for 30 days in an incubator, and the BC contents in HIPPEs at various concentrations (0.2%, 0.5%, and 1.0%, w/w) were determined at certain intervals. Corn oil dissolved at the same concentration of BC with HIPPEs was used as a control.
000 rpm, T-25 digital Ultra Turrax®, IKA-Werke, Germany). BC storage stability analysis was performed at 50 °C for 30 days in an incubator, and the BC contents in HIPPEs at various concentrations (0.2%, 0.5%, and 1.0%, w/w) were determined at certain intervals. Corn oil dissolved at the same concentration of BC with HIPPEs was used as a control.
2.8 Extent of lipolysis
The extent of lipolysis of BC-loaded HIPPEs was determined by performing a simulated in vitro digestion procedure described previously with minor modification.25 For the gastric digestion phase, 0.5 g of BC-loaded HIPPE was dispersed in 7.5 mL ultrapure water and magnetically stirred for 5 min in a water jacketed beaker. Afterwards, 10 mL simulated gastric fluid (3.2 mg mL−1 pepsin and 0.15 M NaCl) was added and the pH of the mixture was adjusted to 2.0 with 2.5 M HCl. The mixture was incubated at 37 °C for 1 h under continuous stirring at 250 rpm (Scientz, Ningbo, China). After simulated gastric digestion, the pH of the mixture was immediately adjusted to 7 with 1 M NaOH and 15 mL of simulated intestinal fluid (pH 7, 1.0 mg mL−1 pancreatin, 20.0 mg mL−1 bile extract, 5 mM CaCl2) was supplemented. During the 2 h simulated intestinal digestion phase, the pH was maintained at 7.0 by the addition of 0.25 M NaOH dropwise. The volume of NaOH added during the whole intestinal digestion was recorded. The extent of lipolysis (%) was calculated based on the volume of NaOH solution consumed using the following formula:where VNaOH represents the volume of NaOH used. CNaOH = 0.25 M (the concentration of NaOH added). Mlipid is the average molecular weight of corn oil (M).
2.9 In vitro bioaccessibility of BC
After in vitro digestion, the aqueous phase containing BC micelles was obtained by centrifuging a 10 mL aliquot of the digesta at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm at 10 °C for 60 min. Afterward, 1 mL of the aqueous phase containing BC micelles was collected, and extracted with ethanol and n-hexane (1
000 rpm at 10 °C for 60 min. Afterward, 1 mL of the aqueous phase containing BC micelles was collected, and extracted with ethanol and n-hexane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v). Lastly, the BC concentration was quantitated using a UV-vis spectrophotometer (UV-2600, Shimadzu, Japan) at 450 nm. BC bioaccessibility was defined as the amount of BC transferred from HIPPE into micelles relative to the initial amount of BC in HIPPE before in vitro digestion. BC bioaccessibility was calculated using the following formula:
3, v/v). Lastly, the BC concentration was quantitated using a UV-vis spectrophotometer (UV-2600, Shimadzu, Japan) at 450 nm. BC bioaccessibility was defined as the amount of BC transferred from HIPPE into micelles relative to the initial amount of BC in HIPPE before in vitro digestion. BC bioaccessibility was calculated using the following formula:2.10 Statistical analysis
Measurements for each sample were performed in triplicate, and the results were averaged. All data were evaluated with an analysis of variance (ANOVA) with Duncan's test and p < 0.05 was defined as statistically significant.3. Results and discussion
3.1 Characteristics of Ca2+ induced WPI nanoparticles
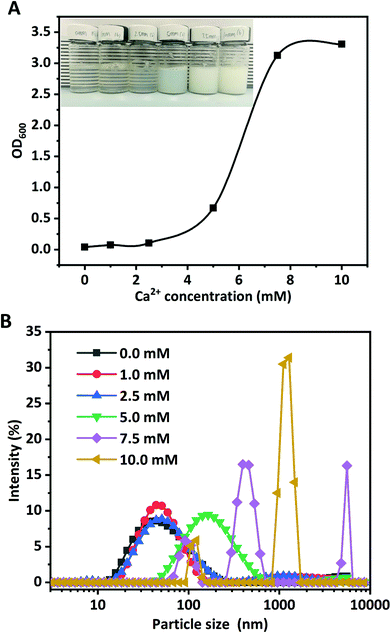
Ca2+ ions acted as a salt bridge to enable negatively charged carboxylic groups on polypeptide chains of WPI to interact with each other, and to induce the formation of WPI nanoparticles.26 The effects of Ca2+ concentrations (0.0, 1.0, 2.5, 5.0, 7.5, and 10.0 mM) on the formation of WPI nanoparticles at pH 6.0 and at 10 mg mL−1 protein concentration were evaluated in terms of turbidity, visual observation, and particle size, as depicted in Fig. 1. Increasing the Ca2+ concentration led to a remarkable increase of the turbidity, and a quick increase (more than 100%) occurred above 5.0 mM Ca2+ concentration (Fig. 1A). No pronounced difference of turbidity was observed at Ca2+ concentrations above 7.5 mM. The turbidity results evidently suggested that the Ca2+-induced aggregation of WPI was highly dependent on the added Ca2+ concentration. According to the definition by Zhang et al., protein dispersion with an increase of turbidity by at least 50.0% and without any precipitates was considered as “potential nanodispersion”.26 The Z-average diameter and particle size distribution of WPI in the presence of various concentrations of Ca2+ were measured to testify this hypothesis.As can been seen in Table 1, nanoscale WPI particles (WPI nanoparticles) can be formed with Ca2+ induced ion cross-linking. The particle size of WPI used in this study without Ca2+ cross-linking is 43.8 nm, consistent with the results in the previous work.21 No pronounced changes occurred for the Z-average diameter of WPI in the presence of 1.0 mM Ca2+, consistent with the particle size distribution shown in Fig. 1B. The increase of Ca2+ concentration from 1.0 to 2.5 mM resulted in the pronounced increase of the particle size from 45.6 nm to 51.0 nm. When the Ca2+ concentration was further increased to 5.0, the z-average diameter remarkably increased from approximately 51.0 nm to 146.9 nm; simultaneously, a significant shift of the particle size distribution towards greater sizes (Fig. 1B) occurred. The particle size was unimodally distributed. However, a sharp increase of the Z-average diameter occurred at 7.5 mM Ca2+ concentration (1529.0 nm), suggesting that large soluble WPI aggregates were formed at a higher Ca2+ concentration even though no visual precipitates were found. The particle size distribution was multimodal and a big peak close to 1500 nm was observed. The Z-average diameter (5502.3 nm) was the highest at 10 mM. The results evidently proved the formation of WPI nanoparticles and the aggregation of WPI was highly Ca2+ concentration-dependent, which was consistent with a previous report by Giroux et al.21
| pH | Ca2+ concentration (mM) | Turbidity | Z-Average diameter (nm) | PDI | Zeta-potential (mV) |
|---|---|---|---|---|---|
| Various letters (a, b, c, d, e, and f) in the same column represent a significant difference at p < 0.05 for various samples. | |||||
| 6.0 | 0 | 0.040 ± 0.002a | 43.8 ± 0.445a | 0.348 ± 0.010a | −23.5 ± 1.2f |
| 1 | 0.073 ± 0.006b | 45.6 ± 0.182b | 0.335 ± 0.014a | −20.2 ± 0.8e | |
| 2.5 | 0.105 ± 0.011c | 51.0 ± 0.516c | 0.321 ± 0.005a | −17.3 ± 0.9d | |
| 5 | 0.669 ± 0.003d | 146.9 ± 1.935d | 0.305 ± 0.043a | −13.7 ± 1.1c | |
| 7.5 | 3.125 ± 0.002e | 1529.0 ± 149.5e | 1.000 ± 0.000c | −10.1 ± 0.6b | |
| 10 | 3.306 ± 0.001f | 5502.3 ± 230.8f | 0.552 ± 0.008b | −7.3 ± 1.4a | |
The change in the zeta-potential of WPI particles as a function of Ca2+ concentration is presented in Table 1. The isoelectric point of WPI is approximately pH 4.8, and the zeta-potential of WPI particles was negative at pH 6.0 (−23.5 mV). The zeta-potential of WPI nanoparticles obtained at 1.0 mM Ca2+ (−20.2 mV) was appreciably lower than that in the absence of Ca2+ (−23.5 mV). Further increasing the Ca2+ concentration from 1.0 mM to 10.0 mM led to the continued decrease of the zeta-potential from −20.2 mV to −7.3 mV. These results suggested that adding Ca2+ ions neutralized negative charges (carboxylic groups) on the WPI particle surface.
No appreciable alteration was found for the PDI value (about 0.300) when the Ca2+ concentration was increased from 0.0 mM to 5.0 mM (Table 1). However, the PDI values were sharply increased to 1.000 and 0.552 at 7.5 mM and 10.0 mM of Ca2+ concentrations, suggesting that at a higher Ca2+ concentration the particle size distributions of WPI particles were multimodal, which clearly confirmed the results of the particle size distribution in Fig. 1B.
Briefly, the above findings evidently suggested that the Ca2+ concentration played dominant roles in nanoparticle formation and nanoparticle characteristics (Z-average diameter, turbidity, PDI, and zeta-potential values). The WPI nanoparticles fabricated with 5.0 mM Ca2+ at pH 6.0 and 10.0 mg mL−1 protein concentration were used in the next experiments.
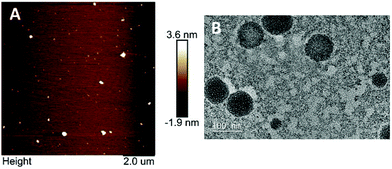
The morphology and size of WPI nanoparticles were investigated by AFM and TEM (Fig. 2). WPI nanoparticles exhibited an approximately spherical shape with a smooth surface and were dispersed uniformly, confirmed by both AFM and TEM. The Z-average diameter of WPI nanoparticles was approximately 100.0 nm, evidenced by TEM, which was consistent with the particle size result obtained from the DLS determination.
3.2 Cytotoxicity of WPI nanoparticles
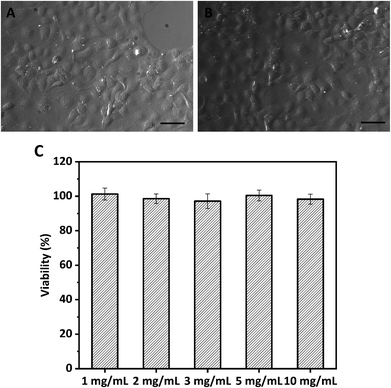
The MTT assay based on the Caco-2 cell model was used to investigate the cytotoxicity of WPI nanoparticles at various concentrations (1.0, 2.0, 3.0, 5.0, and 10.0 mg mL−1). Because the biocompatibility of protein-based nanoparticles is an important issue for their application. The morphology of Caco-2 cells exposed to media in the presence or absence (control) of 10 mg mL−1 protein nanoparticles for 24 h was evaluated and photographed using a light microscope, as depicted in Fig. 3. No profound difference or change in morphology was observed between treated Caco-2 cells and control Caco-2 cells (Fig. 3A and B), suggesting that WPI nanoparticles may be biocompatible and nontoxic. The cytotoxicity of WPI nanoparticles was further quantified by cell viability analysis (Fig. 3C). Cell viabilities of Caco-2 cells incubated with various concentrations of WPI nanoparticles were 101.3%, 98.6%, 97.2%, 100.5%, and 98.3% for 1.0, 2.0, 3.0, 5.0, and 10.0 mg mL−1, respectively. The viability of all Caco-2 cells was above 95%, regardless of the protein concentration, suggesting that WPI nanoparticles exhibit no cytotoxicity even at a high protein concentration (10 mg mL−1) and are biocompatible and safe.3.3 HIPPEs with WPI nanoparticles
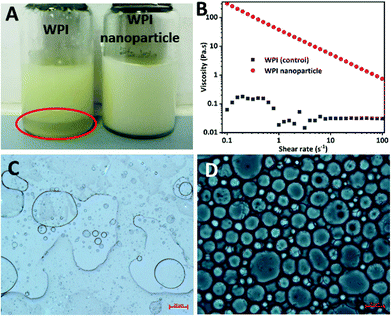
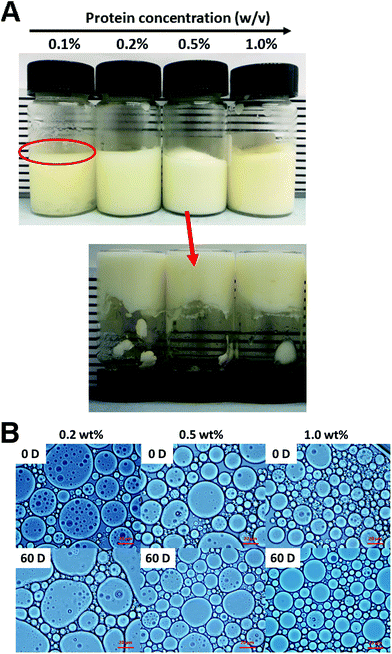
WPI nanoparticles at a concentration of 0.5% (w/w) without cytotoxicity were used as potential Pickering stabilizers in HIPPE fabrication. WPI was used as a control. As shown in Fig. 4, stable HIPPEs with 80.0% (w/w) corn oil as the internal phase cannot be obtained with WPI at a concentration of 0.5% (w/w), and obvious bulk oil (oil-phase separation) can be observed. A viscoelastic film to encapsulate oil droplets in the high internal phase cannot be formed with WPI (0.5%, w/w) primarily due to the relatively low hydrophobicity and poor emulsifying ability. Furthermore, steric hindrance provided by WPI at only 0.5% protein concentration against coalescence of oil droplets may not be enough. Because steric hindrance played a vital role in protein-based emulsion stabilization.19 The previous study showed that HIPPEs (oil fraction 75.0%, w/w) with WPI (1.0%, w/w) can be successfully formed.6 Unfortunately, the HIPPEs stabilized with WPI (1.0%, w/w) exhibited obvious creaming after 6 months of storage at 4 °C.6 Interestingly, a gel-like, stable, and homogeneous HIPPE (oil fraction 80.0%, w/w) can be formed with Ca2+ induced WPI nanoparticles at a concentration of 0.5% (w/w), suggesting the great emulsifying capacity of WPI nanoparticles. This result obviously demonstrated that HIPPEs stabilized with WPI nanoparticles possessed substantially higher stability than those stabilized with the same amount of WPI. The remarkably higher apparent viscosity of Ca2+ induced WPI nanoparticle stabilized HIPPEs, as indicated in Fig. 4B, is probably the primary mechanism driving the formation and stabilization of HIPPEs. Moreover, the formed gel-like network structure also led to the increase of stability.27 The microscopy images clearly indicated that the HIPPE oil droplets encapsulated with WPI exhibited obvious coalescence and oil-phase separation, whereas the oil droplets encapsulated with Ca2+ induced WPI nanoparticles were very stable against coalescence.The effects of WPI nanoparticle concentrations (0.1, 0.2, 0.5, and 1.0%, w/w) on the appearance and stabilization of HIPPEs were evaluated. Some unencapsulated bulk oil appeared on the top of HIPPEs with 0.1% (w/w) Ca2+ induced WPI nanoparticles, which suggested that 0.1% (w/w) of WPI nanoparticles may not be enough to fully cover the huge specific surface area of HIPPEs (oil fraction 80.0%, w/w) (Fig. 5A). Interestingly, gel-like HIPPEs can be obtained with only 0.2% (w/w) Ca2+ induced WPI nanoparticles, as confirmed by the digital photographs. The particle diameter of HIPPEs stabilized with WPI nanoparticles decreased with the increase of protein concentrations (Table 2), consistent with the results of proteins or protein-based Pickering particle stabilized emulsions.27,28 The particle diameters of HIPPEs with WPI nanoparticles were in the following order: 37.2 μm (0.2%, w/w) > 21.5 μm (0.5%, w/w) > 16.6 μm (1.0%, w/w). At a low protein concentration (0.2%, w/w), there may be insufficient WPI nanoparticles to fully cover the higher oil–water interface area and a smaller interface area is expected. The decrease of the oil–water interfacial area is primarily attributed to the increase of the oil droplet diameter. If a low droplet diameter is expected, a higher amount of WPI nanoparticles (0.5% and 1.0%, w/w) should be added to satisfy higher particle surface coverage. The value of the contact angle θw of Ca2+-crosslinked WPI nanoparticles is approximately 67°, suggesting that WPI nanoparticles are more hydrophilic. A curvature towards the oil phase will be obtained due to the large contact area of WPI nanoparticles with an aqueous phase on the interface, which will lead to the formation of an oil-in-water emulsion.3 However, some smaller droplets can be observed in large oil droplets of HIPPEs, especially at a low protein concentration (0.2%, w/w) (Fig. 5B), indicating that some water droplets were incorporated into large oil droplets during high-speed mixing even though WPI nanoparticles are more hydrophilic.
| WPI nanoparticle concentration (w/w) | Mean particle size (μm) | |
|---|---|---|
| Day 0 | Day 60 | |
| Various letters (a and b) represent a significant difference at p < 0.05 for the same sample. Various letters (i, j, and k) represent a significant difference at p < 0.05 for various samples. | ||
| 0.2% | 37.2 ± 0.7a,k | 42.3 ± 1.1b,k |
| 0.5% | 21.5 ± 0.5a,j | 23.7 ± 0.4b,j |
| 1.0% | 16.6 ± 0.3a,i | 18.3 ± 0.5b,i |
The storage stability (60 days) of HIPPEs with various concentrations of WPI nanoparticles at room temperature was evaluated by optical microscopy and the mean particle diameters were calculated (Fig. 5B and Table 2). After 2 months of storage, a little increase (2–5 μm) of the particle diameter was observed for all three HIPPEs with WPI nanoparticles, regardless of the protein concentrations. However, no significant oil droplet coalescence and oil creaming were observed, suggesting that HIPPEs with WPI nanoparticles maintain structural integrity and are highly stable. A rigid and close-packed layer around the oil droplets is formed by the irreversible adsorption of Ca2+ induced WPI nanoparticles at the oil–water interface, which results in the stabilization of HIPPEs against coalescence.29
3.4 Rheological properties
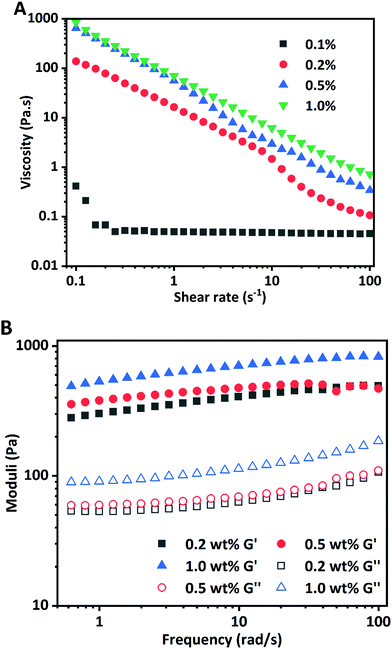
The rheological properties of HIPPEs were evaluated to study the effects of WPI nanoparticle concentration on the stability and structure of Pickering emulsions. Rheological parameters including apparent viscosity, storage modulus (G′), and loss modulus (G′′) information of HIPPEs as a function of WPI nanoparticle concentration are shown in Fig. 6. Fig. 6A shows that the viscosity of HIPPEs was dependent on the amount of Ca2+ induced WPI nanoparticles added, and the increase of the protein particle concentration resulted in higher viscosity. A strong shear-thinning effect was observed for all HIPPEs. All HIPPEs showed no significant variation in the steepness of the slope from the log–log plot, except for HIPPEs with 0.1% (w/w) WPI nanoparticles. The viscosity of HIPPEs stabilized with higher nanoparticle concentrations (0.2%, 0.5%, and 1.0%, w/w) was approximately 3–4 orders of magnitude higher than that of HIPPEs with only 0.1% WPI nanoparticles, especially at a low shear rate. This indicated that the stability of HIPPEs was poor with only 0.1% protein and the gel network could not be formed. In fact, a lumpy structure and oiling-off phenomenon can be clearly observed under these conditions (Fig. 5).The storage modulus (G′) and loss modulus (G′′) of HIPPEs from a frequency sweep as a function of the WPI nanoparticle concentration are shown in Fig. 6B. The G′ values of all HIPPEs were always significantly greater than the G′′ values in the whole frequency range (0.5–100 rad s−1) for all samples, suggesting that the viscoelastic solid behavior and the formation of the gel-like structure (gel network) of HIPPEs stabilized with WPI nanoparticles. Both G′ and G′′ exhibited slight increases with the increase of nanoparticle concentrations in the emulsion, in accordance with HIPPEs stabilized by soy β-conglycinin (0.2%–1.0%, w/w).27
3.5 BC stability
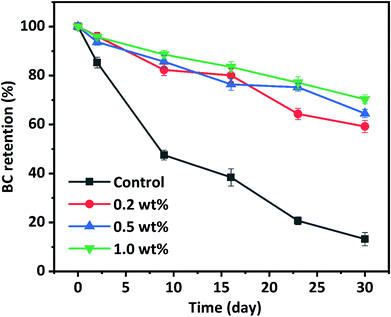
The susceptibility of BC to thermal, chemical, and oxidative degradation under exposure to heat, light, oxygen, and pro-oxidants during processing and storage is currently limiting its application in various food formulations, which is due to its highly polyunsaturated structure. The chemical stability of BC stabilized in HIPPEs during 30 days of storage at 50 °C was investigated through BC concentration analysis at certain intervals. BC dissolved in corn oil was used as a control. As depicted in Fig. 7, a rapid loss was observed for BC in corn oil (control) and less than 50% BC was left after 9 days of storage. BC retention in corn oil (control) is only 13.2% after 30 days of storage. As expected, remarkable enhancements of BC retention occurred in all WPI nanoparticle-encapsulated HIPPEs, suggesting that BC stability can be notably improved with the HIPPE-based encapsulation system. This was attributed primarily to the formation of the dense and thick WPI nanoparticle layer outside the BC-loaded oil droplets, which could impede the diffusion of free radicals, oxygen, and pro-oxidants and restrict the interaction between BC and free radicals, oxygen, and pro-oxidants. Moreover, BC retention in HIPPE encapsulation with various concentrations of WPI nanoparticles was in the following order: 1.0% (70.3%) > 0.5% (64.5%) > 0.2% (59.2%). A higher WPI nanoparticle concentration led to the formation of much denser and thicker protein layers around BC-loaded oil droplets, which were more effective in protecting BC from oxidation and degradation. In addition, WPI is a natural antioxidant which can effectively scavenge free radicals and chelate metal ions (pro-oxidants). A higher WPI concentration led to stronger antioxidant activity.Interestingly, BC retention in WPI nanoparticle-stabilized HIPPEs was more than twice higher than that (28.6%) in the WPI-stabilized emulsion with 10.0% (w/v) corn oil after 30 days of storage under almost the same conditions, regardless of the WPI nanoparticle concentrations.30 This improvement of BC retention was highly associated with the concentrated dispersed phase. The viscosity of the conventional emulsion significantly increases with the increase of the dispersed oil phase volume fraction. In addition to the thickly packed particle layers, the concentrated dispersed phase also plays a vital role in the protection of BC. BC stability in WPI nanoparticle encapsulated HIPPEs was appreciably higher than that in conventional emulsions or nanoemulsions encapsulated with nonionic surfactants, polysaccharides, and proteins after 30 days of storage under almost the same storage conditions. BC in gelatin particle encapsulated HIPPEs also showed higher retention than other conventional emulsions.14
In brief, protein nanoparticle stabilized HIPPEs may be better and more effective delivery systems for stabilizing hydrophobic nutraceuticals and protecting them from degradation.
3.6 In vitro digestion
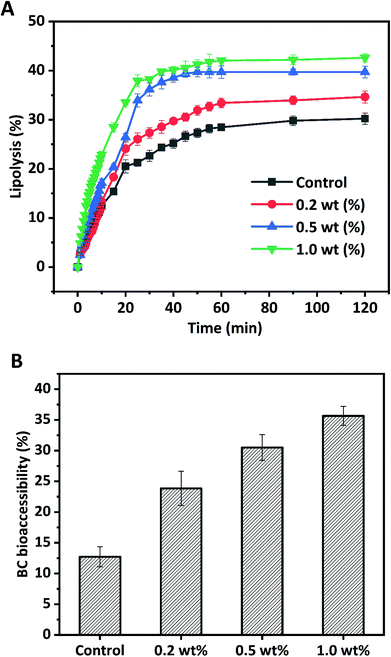
Due to the release of free fatty acids (FFAs), monoacylglycerols (MGs), and hydrogen ions (H+) during lipolysis of HIPPEs by pancreatic lipase, NaOH was added to the intestinal phase to maintain the pH at 7.0. Therefore, the amount of NaOH added dropwise measured by pH-Stat titration was used to evaluate the extent of lipolysis and the amount of free fatty acid generated. Fig. 8 shows the extent of lipolysis and FFA release measured by pH-Stat titration for BC-loaded HIPPEs stabilized with various WPI nanoparticle concentrations (0.2%, 0.5%, and 1.0%, w/v). BC dissolved bulk corn oil was used as a control. An initial rapid increase of lipolysis was observed during the first 30 min for all samples, suggesting that free fatty acids were rapidly released by pancreatic lipase digestion. A gradual increase of lipolysis followed in the next 30 min and a slower lipolysis rate occurred for all samples in the last 60 min. All HIPPEs stabilized with WPI nanoparticles showed profoundly higher lipolysis rates in the initial digestion stage and final extent of lipolysis than bulk oil (control), suggesting that the HIPPE-based delivery system promoted the digestion of encapsulated oil droplets, regardless of the protein concentration. The final lipolysis rate and extent of lipolysis were in the following order: 1.0% (42.62%) > 0.5% (39.71%) > 0.2% (34.65%). Based on light microscopy observation and analysis, the mean particle diameter of HIPPEs was in the following order: 0.2% (37.2 μm) > 0.5% (21.5 μm) > 1.0% (16.6 μm). These results evidently indicated that the decrease of the mean droplet diameter facilitated the digestion of oil droplets. Previous reports also confirmed that the lipolysis rate and extent of lipolysis tended to increase with the decrease of the droplet diameter, attributed primarily to the increased specific surface area of the lipid phase exposed to pancreatic lipase and bile salts.31,32 However, the lipolysis extent of HIPPEs was substantially less than that of conventional emulsions or nanoemulsions.30 The formation of the solid-like gel network and the high viscosity decreases the accessibility of lipase to oil droplets and retards lipolysis.14 Furthermore, the rigidity of protein nanoparticles is another possible reason. These protein nanoparticles located on the oil–water interface are capable of resisting competitive displacement by bile salts and delay the transportation of lipase to the oil droplets.333.7 BC bioaccessibility
Generally, the transfer of hydrophobic nutraceuticals including BC from triglycerides into bioaccessible micelles during in vitro digestion is essential for uptake by enterocytes into the body. Micelles are mainly made up of surface-active substances from lipid digestion products including FFA and monoacylglycerol BC and the interior gastrointestinal tract such as bile salts and phospholipids. The digestion of triglycerides may facilitate the transfer of water insoluble BC from HIPPE oil droplets into micelles. In this study, BC bioaccessibility was determined by measuring the amount of BC in micelles after in vitro intestinal lipid digestion. The bioaccessibility of BC for bulk oil was only 12.72%, which was much lower than that of HIPPE delivery systems with various protein concentrations (Fig. 8B). This was primarily due to the poor access of lipase to the bulk oil surface since bulk oil was simply dispersed in the digestion fluid. BC bioaccessibility was appreciably improved with HIPPE delivery systems. This was attributed primarily to the formed solid-like gel network, which inhibited the coalescence and flocculation of the lipid droplets, and therefore increased the opportunities of lipase and bile salt to access the oil droplet surfaces and increased the amount of BC transferred from the oil phase into the micelles. Furthermore, rigid and thick WPI nanoparticle layers outside oil droplets protected BC from degradation and oxidation during 6 h of gastrointestinal digestion, which may increase the bioaccessibility of BC.31 BC bioaccessibility in HIPPEs with various WPI nanoparticle concentrations was in the following order: 0.2% (23.85%) < 0.5% (30.51%) < 1.0% (35.64%). Increase of protein concentrations led to the decrease of the droplet size and increase of the specific surface area, which possibly facilitated the digestion of oil droplets and BC transfer from oil droplets into micelles. A positive correlation between BC bioaccessibility and the extent of lipolysis was observed, in agreement with previous reports.25,32,34 Increasing the extent of lipolysis resulted in the increase of the concentration of FFAs, which are one of the surface-active substances for micelles that could facilitate the micellization of encapsulated hydrophobic nutraceuticals and enhance their bioaccessibility.4. Conclusion
In this study, spherical WPI nanoparticles (approximately 150 nm) with no cytotoxicity were fabricated based on Ca2+-induced aggregation (a cold gelation method) for BC-loaded HIPPE stabilization and delivery. HIPPEs with a high oil loading amount (oil fraction 80.0%, w/w) and stability can be successfully prepared with Ca2+ induced WPI nanoparticles as low as 0.2% (w/w). Both the chemical stability and in vitro bioaccessibility of BC have been appreciably improved with WPI nanoparticle stabilized HIPPEs. A positive correlation between the extent of lipolysis and BC bioaccessibility was observed. The information obtained would be greatly beneficial for the fabrication and designing of effective HIPPE stabilizers based on edible whey protein with improved emulsifying activity and tailored lipid digestion and nutraceutical release properties. Because WPI nanoparticles themselves could be an effective carrier for nutraceuticals. The co-delivery of multiple bioactive nutraceuticals with potential human-health benefits based on HIPPEs stabilized with WPI nanoparticles will be feasible. The possible cooperative effects between two bioactive nutraceuticals on physicochemical stability, in vitro bioaccessibility, and biological activities will be investigated in a future study.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
The National Natural Science Foundation of China (Grant No. 31601512), the Guangdong Basic and Applied Basic Research Foundation (Grant No. 2019A1515011690), and the Science and Technology Innovation Commission of Shenzhen (Grant No. JCYJ20180305125358023, JCYJ20170818143102818, and KQJSCX20180328095421750) are greatly acknowledged for the financial support.References
- M. S. Silverstein, PolyHIPEs: Recent advances in emulsion-templated porous polymers, Prog. Polym. Sci., 2014, 39, 199–234 CrossRef CAS.
- C. C. Berton-Carabin and K. Schroën, Pickering Emulsions for Food Applications: Background, Trends, and Challenges, Annu. Rev. Food Sci. Technol., 2015, 6, 263–297 CrossRef CAS PubMed.
- C. Linke and S. Drusch, Pickering emulsions in foods - opportunities and limitations, Crit. Rev. Food Sci. Nutr., 2018, 58, 1971–1985 CrossRef CAS PubMed.
- S. Lam, K. P. Velikov and O. D. Velev, Pickering stabilization of foams and emulsions with particles of biological origin, Curr. Opin. Colloid Interface Sci., 2014, 19, 490–500 CrossRef CAS.
- B. Jiao, A. Shi, Q. Wang and B. P. Binks, High-Internal-Phase Pickering Emulsions Stabilized Solely by Peanut-Protein-Isolate Microgel Particles with Multiple Potential Applications, Angew. Chem., Int. Ed., 2018, 57, 9274–9278 CrossRef CAS PubMed.
- S. Zamani, N. Malchione, M. J. Selig and A. Abbaspourrad, Formation of shelf stable Pickering high internal phase emulsions (HIPE) through the inclusion of whey protein microgels, Food Funct., 2018, 9, 982–990 RSC.
- E. Dickinson, Biopolymer-based particles as stabilizing agents for emulsions and foams, Food Hydrocolloids, 2017, 68, 219–231 CrossRef CAS.
- J. Wu and G.-H. Ma, Recent Studies of Pickering Emulsions: Particles Make the Difference, Small, 2016, 12, 4633–4648 CrossRef CAS PubMed.
- Z. Li, T. Ming, J. Wang and T. Ngai, High Internal Phase Emulsions Stabilized Solely by Microgel Particles, Angew. Chem., Int. Ed., 2009, 48, 8490–8493 CrossRef CAS PubMed.
- V. O. Ikem, A. Menner and A. Bismarck, High Internal Phase Emulsions Stabilized Solely by Functionalized Silica Particles, Angew. Chem., Int. Ed., 2008, 47, 8277–8279 CrossRef CAS PubMed.
- E. Perrin, H. Bizot, B. Cathala and I. Capron, Chitin Nanocrystals for Pickering High Internal Phase Emulsions, Biomacromolecules, 2014, 15, 3766–3771 CrossRef CAS PubMed.
- I. Kalashnikova, H. Bizot, B. Cathala and I. Capron, New Pickering Emulsions Stabilized by Bacterial Cellulose Nanocrystals, Langmuir, 2011, 27, 7471–7479 CrossRef CAS PubMed.
- C. Li, P. Sun and C. Yang, Emulsion stabilized by starch nanocrystals, Starch/Staerke, 2012, 64, 497–502 CrossRef CAS.
- H. Tan, L. Zhao, S. Tian, H. Wen, X. Gou and T. Ngai, Gelatin Particle-Stabilized High-Internal Phase Emulsions for Use in Oral Delivery Systems: Protection Effect and in Vitro Digestion Study, J. Agric. Food Chem., 2017, 65, 900–907 CrossRef CAS PubMed.
- Y.-Q. Hu, S.-W. Yin, J.-H. Zhu, J.-R. Qi, J. Guo, L.-Y. Wu, C.-H. Tang and X.-Q. Yang, Fabrication and characterization of novel Pickering emulsions and Pickering high internal emulsions stabilized by gliadin colloidal particles, Food Hydrocolloids, 2016, 61, 300–310 CrossRef CAS.
- F. Liu and C.-H. Tang, Soy Protein Nanoparticle Aggregates as Pickering Stabilizers for Oil-in-Water Emulsions, J. Agric. Food Chem., 2013, 61, 8888–8898 CrossRef CAS PubMed.
- X.-N. Huang, J.-J. Zhu, Y.-K. Xi, S.-W. Yin, T. Ngai and X.-Q. Yang, Protein-Based Pickering High Internal Phase Emulsions as Nutraceutical Vehicles of and the Template for Advanced Materials: A Perspective Paper, J. Agric. Food Chem., 2019, 67, 9719–9726 CrossRef CAS PubMed.
- W. Wijaya, Q.-Q. Sun, L. Vermeir, K. Dewettinck, A. R. Patel and P. Van der Meeren, pH and protein to polysaccharide ratio control the structural properties and viscoelastic network of HIPE-templated biopolymeric oleogels, Food Struct., 2019, 21, 100112 CrossRef.
- M. Destribats, M. Rouvet, C. Gehin-Delval, C. Schmitt and B. P. Binks, Emulsions stabilised by whey protein microgel particles: towards food-grade Pickering emulsions, Soft Matter, 2014, 10, 6941–6954 RSC.
- J. Su, X. Wang, W. Li, L. Chen, X. Zeng, Q. Huang and B. Hu, Enhancing the Viability of Lactobacillus plantarum as Probiotics through Encapsulation with High Internal Phase Emulsions Stabilized with Whey Protein Isolate Microgels, J. Agric. Food Chem., 2018, 66, 12335–12343 CrossRef CAS PubMed.
- H. J. Giroux, J. Houde and M. Britten, Preparation of nanoparticles from denatured whey protein by pH-cycling treatment, Food Hydrocolloids, 2010, 24, 341–346 CrossRef CAS.
- A. V. Rao and L. G. Rao, Carotenoids and human health, Pharmacol. Res., 2007, 55, 207–216 CrossRef CAS PubMed.
- K. Gul, A. Tak, A. K. Singh, P. Singh, B. Yousuf and A. A. Wani, Chemistry, encapsulation, and health benefits of β-carotene - A review, Cogent Food Agric., 2015, 1, 1018696 Search PubMed.
- Y. Fan, J. Yi, Y. Zhang and W. Yokoyama, Improved Chemical Stability and Antiproliferative Activities of Curcumin-Loaded Nanoparticles with a Chitosan Chlorogenic Acid Conjugate, J. Agric. Food Chem., 2017, 65, 10812–10819 CrossRef CAS PubMed.
- Y. Fan, J. Yi, Y. Zhang, Z. Wen and L. Zhao, Physicochemical stability and in vitro bioaccessibility of β-carotene nanoemulsions stabilized with whey protein-dextran conjugates, Food Hydrocolloids, 2017, 63, 256–264 CrossRef CAS.
- J. Zhang, L. Liang, Z. Tian, L. Chen and M. Subirade, Preparation and in vitro evaluation of calcium-induced soy protein isolate nanoparticles and their formation mechanism study, Food Chem., 2012, 133, 390–399 CrossRef CAS PubMed.
- Y.-T. Xu, T.-X. Liu and C.-H. Tang, Novel pickering high internal phase emulsion gels stabilized solely by soy β-conglycinin, Food Hydrocolloids, 2019, 88, 21–30 CrossRef CAS.
- R. J. B. M. Delahaije, H. Gruppen, M. L. F. Giuseppin and P. A. Wierenga, Towards predicting the stability of protein-stabilized emulsions, Adv. Colloid Interface Sci., 2015, 219, 1–9 CrossRef CAS PubMed.
- M.-H. Kwok, G. Sun and T. Ngai, Microgel Particles at Interfaces: Phenomena, Principles, and Opportunities in Food Sciences, Langmuir, 2019, 35, 4205–4217 CrossRef CAS PubMed.
- J. Yi, Y. Liu, Y. Zhang and L. Gao, Fabrication of Resveratrol-Loaded Whey Protein–Dextran Colloidal Complex for the Stabilization and Delivery of β-Carotene Emulsions, J. Agric. Food Chem., 2018, 66, 9481–9489 CrossRef CAS PubMed.
- W. Liu, H. Gao, D. J. McClements, L. Zhou, J. Wu and L. Zou, Stability, rheology, and β-carotene bioaccessibility of high internal phase emulsion gels, Food Hydrocolloids, 2019, 88, 210–217 CrossRef CAS.
- J. Yi, Y. Li, F. Zhong and W. Yokoyama, The physicochemical stability and in vitro bioaccessibility of beta-carotene in oil-in-water sodium caseinate emulsions, Food Hydrocolloids, 2014, 35, 19–27 CrossRef CAS.
- A. Sarkar, S. Zhang, M. Holmes and R. Ettelaie, Colloidal aspects of digestion of Pickering emulsions: Experiments and theoretical models of lipid digestion kinetics, Adv. Colloid Interface Sci., 2019, 263, 195–211 CrossRef CAS PubMed.
- J. Yi, F. Zhong, Y. Zhang, W. Yokoyama and L. Zhao, Effects of Lipids on in Vitro Release and Cellular Uptake of β-Carotene in Nanoemulsion-Based Delivery Systems, J. Agric. Food Chem., 2015, 63, 10831–10837 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2020 |