The effect of regular consumption of lupin-containing foods on glycaemic control and blood pressure in people with type 2 diabetes mellitus†‡
Natalie C.
Ward
 *ab,
Trevor A.
Mori
b,
Lawrence J.
Beilin
b,
Stuart
Johnson
c,
Carolyn
Williams
d,
Seng Khee
Gan
b,
Ian B.
Puddey
b,
Richard
Woodman
e,
Michael
Phillips
f,
Emma
Connolly
g and
Jonathan M.
Hodgson
bg
*ab,
Trevor A.
Mori
b,
Lawrence J.
Beilin
b,
Stuart
Johnson
c,
Carolyn
Williams
d,
Seng Khee
Gan
b,
Ian B.
Puddey
b,
Richard
Woodman
e,
Michael
Phillips
f,
Emma
Connolly
g and
Jonathan M.
Hodgson
bg
aSchool of Public Health, Curtin University, Perth, Australia. E-mail: Natalie.ward@curtin.edu.au; Tel: +61 8 9266 4188
bMedical School, University of Western Australia, Perth, Australia
cSchool of Molecular & Life Sciences, Curtin University, Perth, Australia
dCentre for Entrepreneurial Research & Innovation, Harry Perkins Institute for Medical Research, Perth, Australia
eSchool of Public Health, Flinders University, Adelaide, Australia
fCentre for Medical Research, University of Western Australia, Perth, Australia
gSchool of Health & Medical Sciences, Edith Cowan University, Perth, Australia
First published on 16th December 2019
Abstract
Background: Type 2 diabetes mellitus is a metabolic disorder characterized by high glucose and insulin resistance. It is strongly linked to lifestyle, including poor diet and physical inactivity. Lupin is a novel food ingredient, rich in protein and fibre with negligible sugar and starch, which can be incorporated into various foods to reduce glycaemic load. Regular consumption of lupin-enriched foods may be a novel and easily achievable means of reducing overall glycaemic load and improving glycaemic control in diabetes. Objective: To determine whether regular consumption of lupin-enriched foods can improve glycaemic control and lower blood pressure in people with type 2 diabetes mellitus. Design: Fourteen men and 8 women (mean age 58.0 ± 6.6 years and BMI 29.0 ± 3.5 kg m−2) with type 2 diabetes mellitus were recruited from the general population to take part in a double-blind, randomised, controlled cross-over study. Participants consumed lupin or control foods for breakfast and lunch every day, and for dinner at least 3 days per week during the 8-week treatment periods. Lupin-enriched foods consisted of bread, pasta, Weetbix™ cereal and crumbs, with energy-matched control products. Treatments were completed in random order with an 8-week washout period. All participants monitored their blood glucose levels pre- and post-breakfast and lunch, and their blood pressure in the morning and evening, 3 days per week for the duration of each treatment period. Results: Seventeen participants completed both treatment arms, with all 22 participants (14 males, 8 females) analysed on an intention-to-treat basis. Eight weeks consumption of lupin-enriched food had no significant effect on mean blood glucose levels (mean difference: −0.08 ± 0.06 mmol L−1, p = 0.214) or post-prandial blood glucose levels (−0.13 ± 0.10 mmol L−1, p = 0.196). There was no effect on home systolic (−0.4 ± 0.4 mmHg, p = 0.33) or diastolic (0.3 ± 0.3 mmHg, p = 0.321) blood pressure and heart rate (0.5 ± 0.3 bpm, p = 0.152), and no effect on body weight throughout the treatment periods. Conclusion: Regular consumption of lupin-enriched foods had no significant effect on glycaemic control or blood pressure in people with type 2 diabetes mellitus.
Introduction
Type 2 diabetes mellitus (DM), characterized by high glucose and insulin resistance, is a significant risk factor for the development of premature cardiovascular disease (CVD) and often presents with comorbidities including hypertension and dyslipidaemia.1 Whilst genetics plays a role in its development, it has been strongly linked to lifestyle. Poor diet and physical inactivity lead to body weight gain or obesity, and predispose to type 2 DM.1,2 Lifestyle modification remains the initial treatment recommendation following diagnosis of type 2 DM.There is evidence to suggest that meals with higher protein and fibre reduce post-prandial glycaemia, which may result in better long-term glycaemic control.3 Lupin, a legume that belongs to the genus Lupinus, has traditionally been used as stock feed, but may also function as a unique food ingredient for humans. L. angustifolious, also known as the Australian sweet lupin or the narrow-leaf lupin, is the largest legume crop grown in Australia. High in protein and dietary fibre, and low in carbohydrate, lupin grain also contains vitamins and antioxidants, whilst containing very little trypsin inhibitors and saponins.4 Flour and other products derived from the endosperm of the lupin kernel are novel food ingredients that contain approximately 40–42% protein, 38–40% dietary fibre and negligible glycaemic carbohydrate (sugar and starch).5 In comparison, according to the Australia Food Composition Database, whole grain wheat flour contains 11.5% protein, 11.4% dietary fibre and 58.2% glycaemic carbohydrate (primarily starch). Incorporation of lupin flour into foods that typically contain wheat flour results in significant increases in both protein and fibre content and a reduction in refined carbohydrate and thus glycaemic load.
We have previously shown that bread enriched in lupin flour reduces the 2-hour post-prandial glucose response in healthy adults, when compared to white bread, mediated by an increased insulin response.6 We further showed that in people with type 2 DM, lupin flour had a similar effect on 4-hour postprandial glucose response, again mediated by an increase in the insulin response.7 These findings suggest that the beneficial effect of lupin is likely driven by an increase in protein and fibre intake, as both lupin and white breads were matched for carbohydrate content. Other studies also support a role for a reduction in the amount of glycaemic carbohydrate, where energy-matched, but not carbohydrate-matched lupin-enriched bread reduced 3-hour postprandial glucose and insulin responses in healthy participants.8 While these acute studies support a beneficial role of lupin on glycaemic control, the impact of sustained regular consumption of lupin on glycaemic management in people with type 2 DM remains unclear. Therefore, the aim of this study was to investigate the effect of sustained (8 weeks) regular consumption of lupin-enriched foods on glycaemic control and blood pressure (BP) in people with type 2 DM.
Methods
Participants
Fourteen men and eight women, 40–70 years old, with moderate-to-well controlled type 2 DM (glycated HbAlc < 9%) were recruited from the Perth general population to the research clinic of the Medical School of the University of Western Australia at Royal Perth Hospital. Type 2 DM was confirmed via a fasting blood glucose >7 mmol L−1, current use of type 2 diabetes medication or confirmed diagnosis from their general practitioner. Exclusion criteria included: Type 1 diabetes, insulin use, type 2 DM duration > 10 years, HbA1c > 9%, current or recent (<12 months) smoking, BMI < 18 or >35 kg m−2, psychiatric illness or other major illnesses such as cancer within the previous 6 months, weight loss or gain in the last 6 months (>6% of body weight), alcohol intake >280 g per week for men and >210 g per week for women, change in prescription medication within the last 3 months, an allergy to lupin, wheat, nuts, soya or dairy, or an unwillingness to consume study foods or follow the study protocol. The study was carried out in accordance with the Declaration of Helsinki and was approved by the University of Western Australia Human Research Ethics Committee. All participants provided written informed consent before inclusion in the study. The trial was registered at http://www.anzctr.org.au as ACTRN 12615000297527.Study design and interventions
A randomised, double-blind, controlled cross-over study was performed between June 2015 and December 2017. All participants underwent screening to assess suitability to take part in the study. The screening visit consisted of a fasting blood sample (blood glucose, HbA1c, serum creatinine, liver function tests, and full blood picture), measurement of height, weight and waist-to-hip circumference, clinic BP, electrocardiogram, medical history and medical assessment. Following screening, eligible participants were randomly allocated to their treatment order by computer generated random numbers. Following a 1-week baseline run-in period, participants completed an 8-week treatment period, followed by an 8-week wash-out period where participants returned to their usual diet (inclusive of the next 1-week run-in period), before switching to the second 8-week treatment period. At the beginning and end of each treatment period, all participants provided a fasting blood sample and a spot urine sample, underwent clinic BP and weight measurement, and completed a Health & Lifestyle questionnaire detailing their medication, alcohol consumption and physical activity, as well as a bowel symptoms questionnaire.Study food products were developed and provided by commercial companies based on our previously developed recipe formulations.6,9 Foods provided were lupin-enriched or energy-matched wheat-based control foods and included breakfast cereal (Weetbix™), multigrain bread, pasta and bread crumbs. Participants were asked to replace approximately 20% of their daily energy intake with the study foods, consuming them at both breakfast and lunch daily, and at a minimum of 3 dinners per week during the treatment periods. The diets equated to an average daily intake of ∼45 g of lupin per day for the test food, which comprised ∼12 g day−1 of protein and 10 g day−1 of fibre. Normal dietary habits were maintained throughout the study, with participants reminded to replace existing foods with the study foods so as to minimise weight gain or increase energy consumption.
Blood glucose levels
Home blood glucose levels were measured using the participant's own glucometer. If this was not available, a glucometer and strips were provided. Participants were instructed to record their blood glucose levels immediately on waking, 1 hour after breakfast, immediately before lunch and 1 hour after lunch, on 3 days per week for the 1-week baseline run-in periods and during each week of the 8-week treatment periods.Blood pressure
Clinic BP was measured using an automated oscillometric recorder (Dinamap V100 Vital Signs Monitor, Tampa, USA). Participants were rested for 5 min in the supine position after which four BP measurements were taken at 1 min intervals, with the first reading discarded and the remaining three readings averaged. Home BP was measured using an A&D digital BP monitor (A&D Instruments Ltd, Tokyo, Japan.) provided to each participant. Participants were rested for 5 min in the sitting position after which five BP measurements were taken at 1 min intervals, with the first reading discarded and the remaining four readings averaged. Participants were instructed to record their BP every morning and night on 3 days per week for the 1-week baseline run-in periods and during each week of the 8-week treatment periods. Readings were taken in the seated position at 1 min intervals after a 5 min rest. Morning readings were taken approximately 30 min after waking and prior to eating breakfast and evening readings were taken just before going to bed.Biochemical analysis
Fasting serum total cholesterol, low-density lipoprotein cholesterol (LDL-C), triglycerides, high-density lipoprotein cholesterol (HDL-C), glucose, insulin and C-peptide were analysed using routine methods by PathWest Laboratory at Fiona Stanley Hospital, Western Australia.Compliance
Seven-day food diaries recording daily consumption of the study foods were maintained through the 8-week treatment periods and assessed using the FoodWorks Professional Version 9 (Xyris Software, Brisbane Australia). In addition, all participants completed a validated food frequency questionnaire (FFQ) at the end of each treatment period.Statistics
Statistical analysis was performed on an intention to treat basis. Sample size was calculated with home blood glucose levels as the primary outcome. Home BP was the main secondary outcome. With α = 0.05, 20 participants provided >80% power to detect a 0.35 mmol l−1 (∼5%) difference in mean self-monitored blood glucose concentration over 8 weeks. This is based on within-group SD of 0.8 mmol L−1 and a minimum of 40 measures (before and after meals) over each 8-week period. 20 participants also provided >80% power to detect a 2.5 mm Hg difference in systolic BP. This is based on within-group SD of 15 mm Hg and a minimum of 60 measures (morning and evening) over each period of measurement. Statistical analyses were performed using SPSS 21.0 (SPSS, Chicago, IL, USA) and STATA version 15.2 (StataCorp. College Station, TX, USA). Participant characteristics are presented as means ± SD. All other results are presented as mean ± SEM, mean ± SD or mean and 95% CI. Differences in primary and secondary outcomes post-treatment were analysed by a mixed model ANOVA with additional adjustment for pre-treatment outcomes. The subject was included as a random factor in each model. Fixed effects included the treatment (lupin or control), period and week (1 to 8), day (1 to 3), and reading number (1 – pre-meal or 2 –postprandial for blood glucose). A 2-sided type 1 error rate of alpha = 0.05 was used for all hypothesis testing.Results
Participant characteristics
Twenty-two participants were randomised to the study with 17 completing both treatment periods. Participant drop-out included; medication changes during the washout or treatment periods (n = 3), other illness (n = 1) and an inability to make contact (n = 1). All participants had moderate to well-controlled type 2 DM (HbA1c 5.8–8.6%, mean 7.0%). Baseline characteristics, including medication use, are present in Table 1.| Characteristic | Mean (SD) |
|---|---|
| Mean (standard deviation). ACE, angiotensin II converting enzyme; ARBs, angiotensin II receptor blocker; Ca2+, calcium; BMI, body mass index; BP, blood pressure; DM, diabetes mellitus; HbA1c, glycated haemoglobin; HDL-C, high-density lipoprotein cholesterol; HOMA-IR, homeostatic model assessment of insulin resistance; LDL-C, low-density lipoprotein cholesterol. | |
| Age (years) | 58.0 (6.6) |
| BMI (kg m−2) | 29.9 (3.5) |
| Fasting glucose (mmol L−1) | 5.56 (1.78) |
| HbA1c (%) | 7.02 (0.92) |
| HOMA-IR | 2.48 (1.46) |
| Total cholesterol (mmol L−1) | 2.97 (1.16) |
| LDL-C (mmol L−1) | 1.68 (0.80) |
| HDL-C (mmol L−1) | 0.75 (0.21) |
| Triglycerides (mmol L−1) | 1.24 (0.85) |
| Clinic BP (mm Hg) | 135/76 (15/9) |
| Type 2 DM duration (years) | 4.7 (2.4) |
| Treatment for type 2 DM (Yes/No) | 20/2 |
| Sulfonylureas | 5 |
| Metformin | 19 |
| Other | 6 |
| Diet | 2 |
| Treatment for hypertension (Yes/No) | 11/11 |
| ACE inhibitors | 6 |
| ARBs | 5 |
| Beta blockers | 4 |
| Ca2+ channel blockers | 2 |
| Lipid-lowering treatment (Yes/No) | 9/13 |
Dietary intakes
Compliance with the control and lupin foods provided was assessed using self-reported 7-day food diaries, which were maintained throughout the treatment periods. All participants reported regular consumption of the study foods. On average both the lupin and control foods were consumed at breakfast and lunch on 6–7 days per week. Lupin foods were consumed on average at 3 dinners per week, while control foods were consumed on average at 3–4 dinners per week. Average energy and nutrient intakes from the control and lupin foods were estimated from the food diaries. Overall, energy (control: 1298 ± 476 kJ d−1; lupin: 1309 ± 365 kJ d−1, p = 0.935) intake was not different between foods. Fat (control: 3 ± 1 g d−1; lupin: 4 ± 1 g d−1, p < 0.001), protein (control: 12 ± 4 g d−1; lupin: 23 ± 6 g d−1, p < 0.0001) and fibre (control: 6 ± 2 g d−1; lupin: 17 ± 5 g d−1, p < 0.0001) intakes were higher for lupin foods; and carbohydrate intake (control: 55 ± 21 g d−1; lupin: 37 ± 13 g d−1, p = 0.003) was lower for lupin foods. Participants also completed food frequency questionnaires (FFQs) at the end of each treatment period to assess intakes of foods, other than the control and lupin foods provided. Data from the FFQ was combined with data from the food diaries to provide an estimate of total energy and macronutrient intakes during each of the 8-week treatment periods, (Table 2). Palatability questionnaires indicated that overall, the energy-matched control food was preferable to the lupin-enriched food. Some participants reported mild gastrointestinal upset (bloating, flatulence, abdominal pain) in the first 1–2 weeks of the lupin treatment period, however, this generally settled over the remaining treatment period.Effect of lupin-enriched foods on fasting lipid, glucose and insulin levels
Regular daily consumption of lupin-enriched foods for 8 weeks had no significant effect on body weight (ESI Fig. 1†), fasting lipids, glucose, insulin, HOMA-IR or C-peptide levels. There was a decrease in triglyceride levels (−0.31 mmol L−1, P = 0.059) with lupin-enriched foods which was of borderline significance (Table 3).| Control food | Lupin food | Between-group differenceb | P valueb | |||
|---|---|---|---|---|---|---|
| Prea | Posta | Prea | Posta | |||
| a Mean ± SEM. b Baseline (Pre)-adjusted between group difference – lupin versus control. c Low-density lipoprotein cholesterol. d High-density lipoprotein cholesterol. e Homeostatic model assessment for insulin resistance. | ||||||
| Body weight (kg) | 88.50 ± 4.01 | 88.80 ± 4.02 | 85.95 ± 3.80 | 86.04 ± 4.02 | −0.17 | 0.565 |
| Total cholesterol (mmol L−1) | 3.04 ± 0.27 | 2.95 ± 0.27 | 2.90 ± 0.26 | 2.63 ± 0.26 | −0.23 | 0.443 |
| LDL-Cc (mmol L−1) | 1.78 ± 0.21 | 1.70 ± 0.19 | 1.58 ± 0.15 | 1.55 ± 0.18 | 0.01 | 0.954 |
| Triglycerides (mmol L−1) | 1.23 ± 0.16 | 1.33 ± 0.20 | 1.20 ± 0.23 | 1.02 ± 0.16 | −0.31 | 0.059 |
| HDL-Cd (mmol L−1) | 0.76 ± 0.05 | 0.74 ± 0.23 | 0.74 ± 0.05 | 0.66 ± 0.06 | −0.07 | 0.358 |
| Glucose (mmol L−1) | 5.79 ± 0.46 | 5.55 ± 0.46 | 5.31 ± 0.33 | 5.07 ± 0.46 | −0.14 | 0.789 |
| Insulin (μU mL−1) | 10.67 ± 1.26 | 10.15 ± 1.23 | 9.91 ± 1.28 | 8.71 ± 1.13 | −0.86 | 0.359 |
| HOMA-IRe | 2.66 ± 0.35 | 2.38 ± 0.30 | 2.28 ± 0.31 | 2.03 ± 0.34 | −0.09 | 0.778 |
| C-peptide (nmol L−1) | 0.87 ± 0.07 | 0.85 ± 0.07 | 0.79 ± 0.07 | 1.46 ± 0.7 | 0.74 | 0.287 |
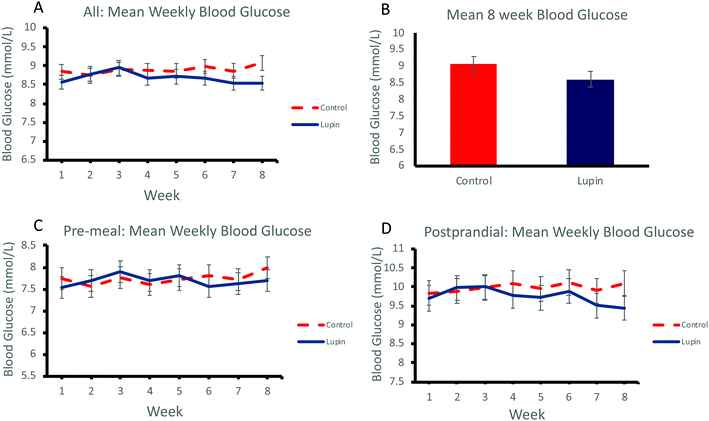
Effect of lupin-enriched foods on glycaemic control
Regular daily consumption of lupin-enriched foods had no significant effect on glycaemic control over 8 weeks as measured by mean weekly home blood glucose (Fig. 1A) and mean home blood glucose at 8 weeks (Fig. 1B). There was no significant effect on pre-meal home blood glucose levels (Fig. 1C) or postprandial home blood glucose levels (Fig. 1D) (Table 4).| Control food | Lupin food | Between-group differenceb | P valueb | |||
|---|---|---|---|---|---|---|
| Prea | Posta | Prea | Posta | |||
| a Mean ± SEM. b Baseline (Pre)-adjusted between group difference – lupin versus control. | ||||||
| All glucose (mmol L−1) | 8.68 ± 0.47 | 8.79 ± 0.47 | 9.04 ± 0.44 | 8.67 ± 0.40 | −0.08 | 0.214 |
| Pre-meal glucose (mmol L−1) | 7.60 ± 0.41 | 7.90 ± 0.40 | 7.51 ± 0.33 | 7.73 ± 0.32 | 0.03 | 0.688 |
| Postprandial glucose (mmol L−1) | 9.77 ± 0.56 | 9.67 ± 0.62 | 10.58 ± 0.60 | 9.62 ± 0.53 | 0.13 | 0.196 |
| All home SBP (mmHg) | 128 ± 3 | 124 ± 3 | 127 ± 4 | 127 ± 4 | −0.41 | 0.33 |
| All home DBP (mmHg) | 80 ± 2 | 75 ± 2 | 77 ± 2 | 74 ± 2 | 0.29 | 0.321 |
| All home HR (mmHg) | 71 ± 2 | 72 ± 2 | 74 ± 3 | 72 ± 3 | −0.47 | 0.152 |
| Morning home SBP (mmHg) | 127 ± 3 | 124 ± 3 | 125 ± 3 | 125 ± 4 | −0.21 | 0.691 |
| Morning home DBP (mmHg) | 78 ± 2 | 76 ± 2 | 78 ± 2 | 75 ± 2 | 0.48 | 0.166 |
| Morning home HR (mmHg) | 69 ± 2 | 69 ± 2 | 74 ± 3 | 71 ± 3 | −0.19 | 0.637 |
| Evening home SBP (mmHg) | 130 ± 4 | 125 ± 3 | 128 ± 5 | 130 ± 6 | −0.61 | 0.288 |
| Evening home DBP (mmHg) | 80 ± 2 | 74 ± 2 | 76 ± 2 | 73 ± 2 | 0.07 | 0.868 |
| Evening home HR (mmHg) | 73 ± 3 | 74 ± 3 | 75 ± 3 | 73 ± 3 | −0.73 | 0.133 |
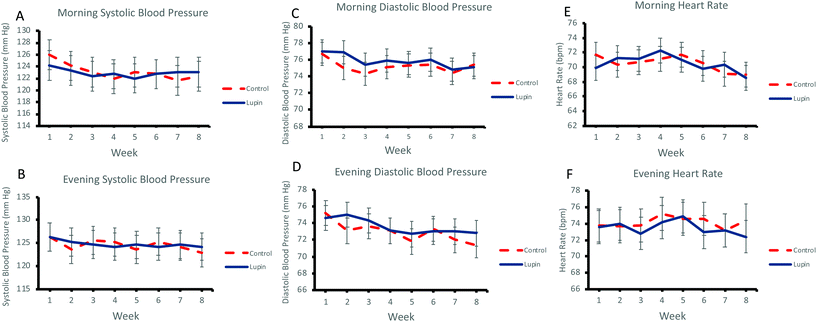
Effect of lupin-enriched foods on blood pressure
Regular daily consumption of lupin-enriched foods for 8 weeks had no significant effect on morning (Fig. 2A and C) or evening (Fig. 2B and D) systolic and diastolic BP. Similarly, there were no effects on morning or evening heart rate (Fig. 2E and F) (Table 4).Discussion
The present study has demonstrated that regular consumption of lupin-enriched foods, compared to control foods results in significantly higher protein and fibre intakes and lower carbohydrate intakes. However, there was no significant effect on glycaemic control or blood pressure in people with moderate-to-well controlled type 2 DM. In addition, regular consumption of lupin foods had no significant impact on circulating fasting glucose or insulin levels or serum lipids.Our previous acute studies have demonstrated a significant beneficial effect of lupin flour on postprandial glucose and insulin response in both healthy people and those with type 2 DM.6–8 Furthermore, we have previously shown that in overweight individuals, regular consumption of lupin-containing foods compared to carbohydrate-matched foods significantly reduced fasting insulin and HOMA-IR scores (an indicator of insulin resistance) at 4 and 12 months.10 Others have shown that high protein and fibre diets have beneficial effects on long-term glycaemic management in people with type 2 DM.3,11 In contrast, our current study in individuals with type 2 DM shows no improvements in glycaemic control, either overall or postprandial with sustained regular consumption of lupin-enriched foods. This was further evidenced by no improvements in fasting glucose, insulin or C-peptide (an indicator of insulin production). Our findings may be partly due to the inclusion of participants with relatively well-controlled type 2 DM. At baseline, fasting glucose levels averaged 5.56 mmol L−1 (range: 1.9 to 10.7 mmol L−1), and HbA1c levels were 7.02% (range: 5.8 to 8.6%). Therefore, our ability to improve glycaemic control that was already within an acceptable range and managed with existing pharmacotherapy or diet, may have been limited. It is also worth considering that any acute benefits, as previously seen in both healthy people and those with type 2 DM,6–8 are not sustained over a longer treatment period, although our previous study in overweight individuals10 does not support this. Additional differences may be due to the specific participant population studied.
Evidence suggests that both dietary protein and dietary fibre can lower BP.12,13 We have previously shown that regular consumption of lupin-containing foods can result in small but significantly lower systolic BP (∼1–2 mmHg) in overweight individuals, which appeared additive to the effects of weight loss.10,14 In the present study, however, we saw no effect of regular lupin consumption on home BP. This may be partly due to participants being recruited on the basis of having type 2 DM and not necessarily high BP. Consequently, 50% of the study participants were hypertensive with substantial variation in both BP treatment (which was maintained throughout the study period) and BP control, likely limiting our capacity to show significant improvements in BP.
We observed no significant changes in fasting lipids, other than a small reduction in triglycerides following the lupin-diet. This is unexpected given the known beneficial effects of fibre on cholesterol and triglyceride levels in people with dyslipidaemia and the metabolic syndrome.15 However, again this may be due to the heterogeneity in the participants studied, with 40% taking lipid-lowering medications. We have also previously found no effect of an ad libitum diet enriched in lupin flour on fasting blood lipids in overweight participants with mildly elevated total cholesterol concentrations, which may have been due to the relatively modest differences in protein and carbohydrate content between the lupin and control foods, against a background high protein diet.16
There are several other important limitations to consider when interpreting the findings of this study. Firstly, our population had significant variation in medication use between study participants, although each individual maintained their medication throughout the trial. There was also variation in diabetes duration, diabetes control and presence of co-morbidities (hypertension or dyslipidaemia). Although this represents the ‘real world’ it may have impacted the response to the study intervention, limiting the magnitude of change observed for both glycaemic control and BP. In addition, the length of the study (8-week intervention periods) could have contributed to differences in the response due to seasonal and dietary variation within the study period. Participants were asked to replace approximately 20% of their usual energy intake with the foods supplied. We have estimated that the resulting differences in protein and fibre were 12 g and 10 g respectively, which is a modest and achievable shift in protein and fibre intakes. However, it is possible that larger sustained shifts in both protein and fibre intakes are required in order to alter glycaemic control and BP. In addition, although lupin-enriched and matching control foods were provided to the participants and they were asked to maintain daily food records, compliance may have influenced the findings. Self-reported intakes indicated good compliance, but this is particularly relevant given the length of the study and the requirement to make daily changes to their diet. Lastly, the small sample size also needs to be considered, although this was minimised by the cross-over design of the study and the large number of blood glucose and BP measurements recorded by the participants during the treatment periods.
In conclusion, regular consumption of lupin-enriched foods does not significantly affect glycaemic control or BP in people with moderate-to-well controlled type 2 DM. Future studies should focus on patients whose glycaemic control requires additional management.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The study was funded by a Royal Perth Hospital Medical Research Foundation Grant. Study foods were prepared and supplied by Sanitarium Health and Wellbeing Company, Otaway Pasta Company and Il Granino Bakery. The authors thank the participants who took part in the study. Jonathan Hodgson and Trevor Mori are supported by National Health and Medical Research Council of Australia Senior Research Fellowships.References
- J. P. Despres, Body fat distribution and risk of cardiovascular disease: an update, Circulation, 2012, 126, 1301–1313 CrossRef PubMed.
- F. B. Hu, Globalization of diabetes: the role of diet, lifestyle, and genes, Diabetes Care, 2011, 34, 1249–1257 CrossRef PubMed.
- J. Y. Dong, Z. L. Zhang, P. Y. Wang and L. Q. Qin, Effects of high-protein diets on body weight, glycaemic control, blood lipids and blood pressure in type 2 diabetes: meta-analysis of randomised controlled trials, Br. J. Nutr., 2013, 110, 781–789 CrossRef CAS PubMed.
- C. B. Villarino, V. Jayasena, R. Coorey, S. Chakrabarti-Bell and S. K. Johnson, Nutritional, Health, and Technological Functionality of Lupin Flour Addition to Bread and Other Baked Products: Benefits and Challenges, Crit. Rev. Food Sci. Nutr., 2016, 56, 835–857 CrossRef CAS PubMed.
- S. K. Johnson, J. Clements, C. B. J. Villarino and R. Coorey, Lupins: Their unique nutritional and health-promoting attributes, in The Gluten-free Ancient Grains: Cereals, Pseudocereals and Legumes - Sustainable, Nutritious and Health-promoting Foods for the 21st Century, ed. J. R. N. Taylor, Elsevier, Amsterdamn, 2017 Search PubMed.
- R. S. Hall, S. J. Thomas and S. K. Johnson, Australian sweet lupin flour addition reduces the glycaemic index of a white bread breakfast without affecting palatability in healthy human volunteers, Asia Pac. J. Clin. Nutr., 2005, 14, 91–97 Search PubMed.
- E. R. Dove, T. A. Mori, G. T. Chew, A. E. Barden, R. J. Woodman, I. B. Puddey, S. Sipsas and J. M. Hodgson, Lupin and soya reduce glycaemia acutely in type 2 diabetes, Br. J. Nutr., 2011, 106, 1045–1051 CrossRef CAS PubMed.
- Y. P. Lee, T. A. Mori, S. Sipsas, A. Barden, I. B. Puddey, V. Burke, R. S. Hall and J. M. Hodgson, Lupin-enriched bread increases satiety and reduces energy intake acutely, Am. J. Clin. Nutr., 2006, 84, 975–980 CrossRef CAS PubMed.
- R. S. Hall and S. K. Johnson, Sensory acceptibility of foods containing Australian sweet lupin (Lupinus angustifolius) flour, J. Food Sci., 2004, 69, SNQ92–SNQ97 CAS.
- R. Belski, T. A. Mori, I. B. Puddey, S. Sipsas, R. J. Woodman, T. R. Ackland, L. J. Beilin, E. R. Dove, N. B. Carlyon, V. Jayaseena and J. M. Hodgson, Effects of lupin-enriched foods on body composition and cardiovascular disease risk factors: a 12-month randomized controlled weight loss trial, Int. J. Obes., 2011, 35, 810–819 CrossRef CAS PubMed.
- S. A. Bajorek and C. M. Morello, Effects of dietary fiber and low glycemic index diet on glucose control in subjects with type 2 diabetes mellitus, Ann. Pharmacother., 2010, 44, 1786–1792 CrossRef CAS PubMed.
- M. T. Streppel, L. R. Arends, P. van ‘t Veer, D. E. Grobbee and J. M. Geleijnse, Dietary fiber and blood pressure: a meta-analysis of randomized placebo-controlled trials, Arch. Intern. Med., 2005, 165, 150–156 CrossRef PubMed.
- C. M. Rebholz, E. E. Friedman, L. J. Powers, W. D. Arroyave, J. He and T. N. Kelly, Dietary protein intake and blood pressure: a meta-analysis of randomized controlled trials, Am. J. Epidemiol., 2012, 176(Suppl 7), S27–S43 CrossRef PubMed.
- Y. P. Lee, T. A. Mori, I. B. Puddey, S. Sipsas, T. R. Ackland, L. J. Beilin and J. M. Hodgson, Effects of lupin kernel flour-enriched bread on blood pressure: a controlled intervention study, Am. J. Clin. Nutr., 2009, 89, 766–772 CrossRef CAS.
- N. Ward, A. Sahebkar, M. Banach and G. Watts, Recent perspectives on the role of nutraceuticals as cholesterol-lowering agents, Curr. Opin. Lipidol., 2017, 28, 495–501 CrossRef CAS PubMed.
- J. M. Hodgson, Y. P. Lee, I. B. Puddey, S. Sipsas, T. R. Ackland, L. J. Beilin, R. Belski and T. A. Mori, Effects of increasing dietary protein and fibre intake with lupin on body weight and composition and blood lipids in overweight men and women, Int. J. Obes., 2010, 34, 1086–1094 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9fo01778j |
| ‡ Clinical Trial Registration: ACTRN12615000297527 |
| This journal is © The Royal Society of Chemistry 2020 |