Therapeutic effects of noni fruit water extract and polysaccharide on oxidative stress and inflammation in mice under high-fat diet
Xiaobing
Yang
 a,
Chunrui
Lin
a,
Chunrui
Lin
 a,
Shuang
Cai
a,
Shuang
Cai
 a,
Wenzhi
Li
a,
Wenzhi
Li
 b,
Jian
Tang
b,
Jian
Tang
 b and
Xiaoyong
Wu
b and
Xiaoyong
Wu
 *c
*c
aSchool of Public Health, Guangdong Pharmaceutical University, Guangzhou 510310, China
bInfinitus (China) Co. Ltd, Xinhui 529156, China
cSchool of Food Science, Guangdong Pharmaceutical University, Zhongshan 528453, China. E-mail: wuxiaoyong@gdpu.edu.cn
First published on 27th November 2019
Abstract
This study aims to compare the therapeutic effects of noni fruit water extract (NFW) and noni fruit polysaccharide (NFP) on oxidative stress and inflammation in mice under high-fat diet. In this study, mice were induced to develop oxidative stress and inflammation through high-fat diet. Treatment was performed via the administration of NFW (10 mL per kg bw) and NFP (50, 100, and 200 mg per kg bw) for 4 weeks. The results indicated that the NFW and NFP reduced the body weight gain, liver relative weight, and abdominal fat relative weight of mice under high-fat diet. Moreover, the NFW and NFP reduced the liver malondialdehyde level and increased the liver trolox equivalent antioxidant capacity level. The NFP effectively increased the liver superoxide dismutase and glutathione peroxidase activities, and the administration of NFP at 100 and 200 mg per kg bw effectively increased the hepatic nuclear factor erythroid-2 related factor level, thus presenting improved antioxidant activity. The NFW and NFP restrained the elevation of tumor necrosis factor alpha, interleutin-6, and nitric oxide levels in the liver and serum. All NFP doses prominently decreased the hepatic nuclear factor kappa B level, and the NFP at 100 and 200 mg per kg bw presented high anti-inflammatory activity. These results suggested that the NFW and NFP alleviated oxidative stress and inflammation in mice under high-fat diet, and the NFP at 100 mg per kg bw had a better effect than NFW with a similar polysaccharide dosage, illustrating that NFP may be an important component in the NFW.
1. Introduction
The negative effects of high-fat diet on human health are self-evident.1 Epidemiological studies have shown that obesity and obesity-related complications are highly correlated with high-fat diet.2–4 Framingham Heart Research states that the risk for cardiovascular and cerebrovascular diseases, such as hypertension, coronary heart disease, and stroke, increases when abdominal obesity is present.5 Obesity is closely related to diseases that threaten human health, such as hyperlipidemia, type 2 diabetes and fatty liver disease.6Long-term high-fat diet, especially excessive intake of foods rich in saturated fatty acids (SFAs), can lead to adipose tissue accumulation and lipid accumulation in the liver.7 The accumulation of lipids in the liver can cause structural and functional changes in the hepatocytes and stimulate the release of reactive oxygen species (ROS) and inflammatory cytokines, ultimately triggering oxidative stress and inflammation.8–10 Oxidative stress and inflammation play an important role in the occurrence and development of obesity, type 2 diabetes, cardiovascular disease, and fatty liver disease.9 Therefore, oxidative stress and inflammation induced by high-fat diet should be prevented and alleviated to promote human health and reduce the risk of related diseases.
Dietary supplementation with antioxidant ingredients can ameliorate oxidative stress and inflammation induced by high-fat diet.11 Studies have shown that cinnamon polyphenols can inhibit the expression of transcription factors (e.g., SREBP-1c and LXR-α) and activate the antioxidant defense signaling pathways in high-fat diet rats, thereby alleviating oxidative stress, inflammation, and hyperlipidemia.12 Apple pomace polysaccharides can successfully ameliorate oxidative stress induced by high-fat diet, which may be attributed to their capability of scavenging mitochondrial ROS and improving mitochondrial function.13 Compound extracts of black tea polyphenols and polysaccharides can effect weight loss, in which polysaccharides mainly inhibit body weight gain, whereas polyphenols mainly improve the levels of blood and liver lipids.14 Total green tea extract, polysaccharides, and polyphenols exhibit anti-inflammatory effects, and synergistic effects on anti-inflammation and body weight loss are found between polysaccharides and polyphenols.15
Noni (Morinda citrifolia L.) is a perennial tree of the Rubiaceae family. Its roots, branches, leaves, and fruits have various pharmacological activities.16,17 Noni fruit juice is one of the first natural foods approved by the new EU food regulations in 1997.18 Studies have shown that noni fruit juice is rich in bioactive ingredients, such as polysaccharides, flavonoids, iridoids, anthraquinones, and carotenoids, and has antioxidant, anti-inflammatory, antitumor, and immunomodulatory effects.17,19 Polysaccharides, which have antitumor, antioxidant, anti-inflammatory, and other physiological activities, are considered to be one of the main bioactive ingredients in noni fruit juice.20–23 Our previous research showed that the noni fruit polysaccharide (NFP) at 10 mg per kg bw can promote the expression levels of intestinal tight junction proteins (ZO-1 and occludin proteins) and the secretion of mucus, thus repairing the damage induced by DSS to the colonic mucosal barrier and presenting a potential effect in treating inflammatory bowel disease.24 In this study, mice were induced to develop oxidative stress through high-fat diet, and the effects of the noni fruit water extract (NFW) and NFP on the oxidative stress and inflammation in mice under high-fat diet were investigated to provide evidence for the antioxidant and anti-inflammatory activities of the NFP and confirm that the NFP is an important bioactive component of the NFW.
2. Materials and methods
2.1 Materials and reagents
Dehydrated sliced noni fruit was produced by Hainan Xisha Noni Biotechnology Co., Ltd (Hainan, China). NKA-9 macroporous resin was purchased from Hao Ju Resin Technology Co., Ltd (Tianjin, China). Total triglyceride (TG) and total cholesterol (TC) assay kits were purchased from Guangzhou Huaxin Technology Co., Ltd (Guangdong, China). High-density lipoprotein cholesterol (HDL-c) and low-density lipoprotein cholesterol (LDL-c) assay kits were purchased from Shanghai Kehua Bio-engineering Co., Ltd (Shanghai, China). The assay kits of total protein (TP), trolox equivalent antioxidant capacity (TEAC), malondialdehyde (MDA), superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and nitric oxide (NO) were obtained from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Enzyme linked immunosorbent assay (ELISA) kits for mouse interleutin-6 (IL-6), tumor necrosis factor alpha (TNF-α), nuclear factor erythroid-2 related factor (Nrf2), and nuclear factor kappa B (NF-κB) were all obtained from the Nanjing Jiancheng Bioengineering Institute (Nanjing, China). High-fat diet (17% kcal protein, 37% kcal fat, 46% kcal carbohydrate, and 4.4 kcal g−1 energy) and regular chow (23% kcal protein, 11% kcal fat, 66% kcal carbohydrate, and 3.5 kcal g−1 energy) were produced by the Guangdong Medical Laboratory Animal Center (Guangdong, China).2.2 Preparation of the NFW and NFP
The dehydrated sliced noni fruit (160 g) was extracted twice with distilled water (3200 mL) at 90 °C for 55 min, and the filtrates were mixed and divided into two equal parts on the basis of our previous method.24 The first part was concentrated in a rotary evaporator (80 °C) under reduced pressure until the content of crude polysaccharide was approximately 10 mg mL−1, and the NFW (approximately 500 g) was obtained. The other half was concentrated to approximately 600 g under reduced pressure in a rotary evaporator (80 °C). The crude NFP was precipitated with absolute ethyl alcohol (final ethanol concentration, 30%) overnight at 4 °C, and the precipitate was collected via centrifugation (4000 rpm, 10 min). The precipitate was dissolved in distilled water and precipitated again under the same conditions. After the second alcohol precipitation, the NFP precipitate was dissolved in distilled water. Subsequently, the NFP solution (10 mg mL−1, 500 g, natural pH, 40 °C) was purified using a column (2.6 × 100 cm) packed with the pretreated NKA-9 macroporous resin (170 g) at a flow rate of 1.0 mL min−1. Here, the effluent was the purified NFP solution. The purified NFP solution was freeze-dried, and the refined NFP powder (approximately 5 g) was obtained.2.3 Chemical composition of the NFW
The crude polysaccharide was precipitated with absolute ethyl alcohol (final ethanol concentration, 80%) at 4 °C for 12 h, and the crude polysaccharide content of the NFW was determined at 490 nm using the phenol–sulfuric acid method with glucose as the reference standard.25 The water-soluble protein content of the NFW was determined by using the Coomassie Brilliant Blue method at 595 nm, and bovine serum albumin was set as the reference.26 The total flavonoid content of the NFW was evaluated at 510 nm through sodium nitrite–aluminum nitrate–sodium hydroxide colorimetry and expressed as milligrams of rutin equivalent (RE) per 100 mL of sample.27 The total phenol content of the NFW was measured using the Folin–Ciocalteu method at 765 nm and expressed as milligrams of gallic acid equivalent (GAE) per 100 mL of sample.28 The mineral content in the NFW was determined in accordance with GB 5009.268-2016 (a national food safety standard for the determination of multiple elements in food, which was promulgated by the State Food and Drug Administration of the National Health and Family Planning Commission of the People's Republic of China on December 26, 2016.).2.4 Chemical composition of the NFP
The moisture in the NFP was determined in accordance with GB 5009.3-2016 (a national food safety standard for the determination of moisture in food, which was promulgated by the State Food and Drug Administration of the National Health and Family Planning Commission of the People's Republic of China on August 31, 2016). The total carbohydrate content of the NFP was determined at 490 nm via the phenol–sulfuric acid method with glucose as the reference standard.29 The contents of soluble protein, total flavonoid, and total phenol in the NFP were measured using the methods described in the Chemical composition of the NFW section.2.5 Fourier transform infrared (FT-IR) analysis of the NFP
For the FT-IR analysis of the NFP, 2 mg of NFP dry powder was mixed with KBr powder at the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 and pressed into thin slices by using a tablet press. The FT-IR spectrum was recorded using an FT-IR spectrometer (Nicolet iS10, Thermo Fisher Scientific, USA) at a frequency ranging from 4000 cm−1 to 400 cm−1.26
9 and pressed into thin slices by using a tablet press. The FT-IR spectrum was recorded using an FT-IR spectrometer (Nicolet iS10, Thermo Fisher Scientific, USA) at a frequency ranging from 4000 cm−1 to 400 cm−1.26
2.6 Animals and diets
Forty-five healthy 8-week-old male Kunming mice (SCXK 2017-0125) were purchased from the Guangdong Medical Laboratory Animal Center (Guangdong, China). The mice were housed in an experimental animal room under a 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 h light–dark cycle at 23 ± 1 °C and fed normal laboratory chow and water ad libitum for 1-week acclimation. The mice were randomly divided into control (n = 9, normal diet containing 11% energy from fat) and high-fat diet (n = 36, high-fat diet containing 37% energy from fat) groups. At the end of the fifth week, three and six mice were randomly selected from the control and high-fat diet groups, respectively, and were used to evaluate whether oxidative stress and inflammation presented in the liver of the mice fed with high-fat diet for 5 weeks. The high-fat diet mice were randomly divided into five groups (n = 6 per group) for 4 weeks of treatment. Table 1 shows the grouping and administration dosage during treatment. During the experiment, the mice were provided access to chow and water, and their body weights were monitored daily. After 4 weeks of treatment, the mice underwent 12 h fasting, and their blood samples were taken to separate serum. The organs (liver and abdominal fat) were dissected and weighed on ice, and the organ weights were standardized on the basis of the weight of the corresponding animal (organ weight per body weight) to obtain a relative percentage. The liver tissue was prepared into 10% liver homogenate (w/v) with normal saline. Serum and liver homogenate samples were stored at −80 °C until analysis. All animal experiments were conducted in accordance with the management regulations for the laboratory animal (State Scientific and Technological Commission Publication Revised on March 1, 2017), and were approved by the Experimental Animal Ethics Committee Inspection of Guangdong Pharmaceutical University (Guangdong, China).
12 h light–dark cycle at 23 ± 1 °C and fed normal laboratory chow and water ad libitum for 1-week acclimation. The mice were randomly divided into control (n = 9, normal diet containing 11% energy from fat) and high-fat diet (n = 36, high-fat diet containing 37% energy from fat) groups. At the end of the fifth week, three and six mice were randomly selected from the control and high-fat diet groups, respectively, and were used to evaluate whether oxidative stress and inflammation presented in the liver of the mice fed with high-fat diet for 5 weeks. The high-fat diet mice were randomly divided into five groups (n = 6 per group) for 4 weeks of treatment. Table 1 shows the grouping and administration dosage during treatment. During the experiment, the mice were provided access to chow and water, and their body weights were monitored daily. After 4 weeks of treatment, the mice underwent 12 h fasting, and their blood samples were taken to separate serum. The organs (liver and abdominal fat) were dissected and weighed on ice, and the organ weights were standardized on the basis of the weight of the corresponding animal (organ weight per body weight) to obtain a relative percentage. The liver tissue was prepared into 10% liver homogenate (w/v) with normal saline. Serum and liver homogenate samples were stored at −80 °C until analysis. All animal experiments were conducted in accordance with the management regulations for the laboratory animal (State Scientific and Technological Commission Publication Revised on March 1, 2017), and were approved by the Experimental Animal Ethics Committee Inspection of Guangdong Pharmaceutical University (Guangdong, China).
| Group | Diet | Administration |
|---|---|---|
| Control | Normal diet: 11% energy from fat | Normal saline |
| HFD | High-fat diet: 37% energy from fat | Normal saline |
| NFW | NFW, 10 mL per kg bw | |
| NFP-50 | NFP, 50 mg per kg bw | |
| NFP-100 | NFP, 100 mg per kg bw | |
| NFP-200 | NFP, 200 mg per kg bw |
2.7 Measurement of serum lipid
Blood lipid levels, such as TG, TC, HDL-c, and LDL-c, were determined using a biochemical analyzer (Hitachi 7020, Japan) in accordance with the user instructions, and the results are expressed in mmol L−1.2.8 Determination of the liver lipid peroxidation level and antioxidant capacity
Liver MDA was used to determine the liver lipid peroxidation level, whereas the TEAC level and the activities of SOD and GSH-Px were used as the indices to measure the liver antioxidant capacity. The TP liver concentration was determined using the Coomassie Brilliant Blue method, and results are expressed in gprot L−1. The MDA content in the liver was determined using the thiobarbituric acid reactant method, and the results are expressed in nmol MDA per mg protein (nmol mg−1 protein). The capability of the TEAC level was determined under the absorbance of 405 nm and a standard curve for trolox on scavenging ABTS + radical formation capability was drawn to calculate the TEAC level in the liver; the results are expressed in mmol g−1 protein. SOD activity was detected using a water-soluble tetrazole-1 colorimetric method at 450 nm absorbance. One unit of SOD activity in liver tissue was defined as the corresponding enzyme amount when the SOD inhibition rate reached 50% in the reaction system, and the results are expressed in units per mg protein (U mg−1 protein). The GSH content was calculated by measuring the absorbance of 2-nitro-5-thiobenzoic acid at 412 nm. GSH-Px activity, that is, the reaction rate of catalytic GSH, was calculated by measuring the consumption of GSH, and the results are expressed in units per mg protein (U mg−1 protein). All steps were performed in accordance with the user instructions.2.9 Determination of serum and hepatic TNF-α, IL-6, and NO levels
The serum and hepatic TNF-α and IL-6 levels were measured through ELISA. The optical density values of each hole in the plate were read at 450 nm absorbance using a full wavelength microplate reader (DG3022A, USA) and converted to the TNF-α and IL-6 levels in the serum and liver using a standard curve, which are expressed as pg mL−1 and pg mg−1 protein, respectively. The NO content in the serum and liver was assayed using a nitrate reductase method. The serum NO level is expressed in μmol L−1, whereas the liver NO level is expressed in μmol g−1 protein. All steps were performed in accordance with the user instructions.2.10 Assay of hepatic Nrf2 and NF-κB levels
The hepatic Nrf2 and NF-κB levels were measured using the corresponding ELISA development kits in accordance with the user instructions, and the results are expressed in pg mg−1 protein.2.11 Statistical analysis
All values are expressed as mean ± standard error of the mean (SEM). Student's t-test was used to evaluate the differences between the high-fat diet and the corresponding control groups at the end of the fifth week. Significant differences were distinguished in more than three groups via one-way ANOVA and Tukey's post hoc test. P < 0.05 was considered as statistically significant, whereas P < 0.01 was extremely significant (* versus the control group, # versus the HFD group, and Δ versus the NFP-100 group). All statistical analyses of data were performed on SPSS software (version 23, SPSS, Inc., USA), and all graphs were drawn on GraphPad Prism software (version 5.0, GraphPad Software, Inc., USA).3. Results
3.1 Main chemical composition of the NFW
After extraction with hot water twice and concentration, the NFW with a solid content of 6.78% was obtained. As shown in Table 2, crude polysaccharide, water-soluble protein, total flavonoids, and total polyphenols were 1106.63 mg per 100 mL, 134.45 mg per 100 mL, 18.11 mg RE per 100 mL, and 151.24 mg GAE per 100 mL, respectively. With regard to the mineral profile in the NFW, the major component was potassium (K), followed by magnesium (Mg), sodium (Na), and calcium (Ca). Iron (Fe), zinc (Zn), manganese (Mn), and selenium (Se) were also analyzed in the NFW. Table 2 presents the corresponding dosages of the chemical components in the NFW. The results indicated that the NFW group has essentially the same noni fruit polysaccharide dosage as the NFP-100 group.| Items | Content | Dosage (mg per kg bw) |
|---|---|---|
| a RE, rutin equivalent. b GAE, gallic acid equivalent. | ||
| Crude polysaccharide (mg per 100 mL) | 1106.63 ± 17.87 | 110.66 |
| Water-soluble protein (mg per 100 mL) | 134.45 ± 4.14 | 13.45 |
| Total flavonoids (mg REaper 100 mL) | 18.11 ± 0.30 | 1.81 |
| Total phenols (mg GAEb per 100 mL) | 151.24 ± 0.45 | 15.12 |
| Potassium (K) (mg per 100 mL) | 235.75 | 23.58 |
| Magnesium (Mg) (mg per 100 mL) | 13.85 | 1.39 |
| Sodium (Na) (mg per 100 mL) | 17.20 | 1.72 |
| Calcium (Ca) (mg per 100 mL) | 12.20 | 1.22 |
| Iron (Fe) (mg per 100 mL) | 2.65 | 0.27 |
| Zinc (Zn) (mg per 100 mL) | 0.13 | 0.01 |
| Manganese (Mn) (mg per 100 mL) | 0.29 | 0.03 |
| Selenium (Se) (μg per 100 mL) | 7.50 | 0.75 μg per kg bw |
3.2 Chemical composition of the NFP
Crude polysaccharide (approximately 5.6 g) was obtained from the water extract of dehydrated sliced noni fruit via secondary ethanol precipitation. The NFP purified by NKA-9 macroporous resin was a relatively uniform polysaccharide with a number average molecular weight of 456 kDa.24 As shown in Table 3, the total carbohydrate content of NFP was 93.87% (w/w). In addition, the contents of water-soluble protein, total flavonoids, and total phenols were low, indicating that the purity of NFP is relatively high.3.3 Structural characterization of the NFP
On the basis of our preliminary research,24 the monosaccharide components of NFP were GalA (58.44%), Gal (4.44%), Glu (21.13%), Rha (4.84%), and Ara (2.16%). The NFP is an HG-type pectin polymerized by linear (1→4)-linked-α-GalAp, accounting for approximately 70% of the total. The NFP is composed of 15%–20% neutral polysaccharide mainly with (1→4)-linked α-Glc.
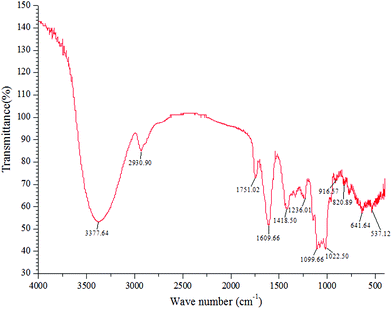
Fig. 1 shows the FT-IR analysis result of the NFP. The absorption peak at 3377.64 cm−1 was attributed to the O–H stretching vibrations of the hydroxyl groups, and the weak band at 2930.90 cm−1 was attributed to the stretching vibrations of the C–H bond. The two peaks were the characteristic absorption peaks of the saccharide compounds.26 The absorption peak at 1751.02 cm−1 was associated with the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration, indicating the presence of glucuronic acid.30 The strong absorption band at 1609.66 cm−1 was caused by the asymmetric stretching vibration of the C–O bond. The absorptions at 1022.50 and 1099.66 cm−1 were mainly attributed to the stretching vibrations of the C–O–C glycosidic bond and C–O–H side groups, indicating a pyranose unit in the NFP.30 The characteristic absorptions at 820.89 and 916.57 cm−1 demonstrated the presence of α- and β-configurations in the NFP.26
O stretching vibration, indicating the presence of glucuronic acid.30 The strong absorption band at 1609.66 cm−1 was caused by the asymmetric stretching vibration of the C–O bond. The absorptions at 1022.50 and 1099.66 cm−1 were mainly attributed to the stretching vibrations of the C–O–C glycosidic bond and C–O–H side groups, indicating a pyranose unit in the NFP.30 The characteristic absorptions at 820.89 and 916.57 cm−1 demonstrated the presence of α- and β-configurations in the NFP.26
3.4 Oxidative stress and inflammation in mice induced by high-fat diet
Studies have shown that high-fat diet initiates gut microbiota dysbiosis by increasing the levels of oxidative stress and changes the indigenous opportunistic bacteria in the interior of Peyer's patches, thereby inducing the production of intestinal inflammatory cytokines and causing systemic inflammatory response and metabolic syndrome.31–33 As shown in Table 4, feeding of high-fat diet for 5 weeks induced evident changes in the mice, such as high body weight and liver fat, and high levels of serum lipids. These adverse effects of high-fat diet resulted in decreased antioxidant system activity (TEAC level and SOD activity) in the liver and increased MDA and key inflammatory markers (TNF-α, IL-6, and NO). These results indicated that 5 weeks of high-fat diet induced oxidative stress and inflammatory response in the mice.| Group | ||
|---|---|---|
| Control | HFD | |
| a Weight of the mice from the first week to the fifth week. b Relative weight of the liver and abdominal fat. c Serum lipid level. d Oxidative stress in the liver. e Key inflammatory markers in the liver. Data are expressed as the mean ± SEM, *P < 0.05, **P < 0.01, ***P < 0.001 versus the control group. | ||
| Growth performancea | ||
| Initial body weight (g) | 22.72 ± 0.42 | 23.28 ± 0.25 |
| Final body weight (g) | 44.04 ± 0.74 | 47.68 ± 0.78 |
| Body weight gain (g) | 21.32 ± 1.07 | 24.40 ± 0.74* |
| Relative weight of organsb | ||
| Liver (%) | 4.77 ± 0.21 | 5.85 ± 0.25* |
| Abdominal fat (%) | 1.65 ± 0.25 | 3.75 ± 0.30** |
| Serum lipid levelc | ||
| TC (mmol L−1) | 3.07 ± 0.39 | 5.70 ± 0.31* |
| TG (mmol L−1) | 1.56 ± 0.06 | 0.97 ± 0.14 |
| HDL-c (mmol L−1) | 2.91 ± 0.92 | 2.57 ± 0.07 |
| LDL-c (mmol L−1) | 0.10 ± 0.02 | 0.47 ± 0.02*** |
| Oxidative stress in the liverd | ||
| MDA (nmol mg−1 protein) | 0.17 ± 0.01 | 0.53 ± 0.08** |
| TEAC (mmol g−1 protein) | 0.73 ± 0.17 | 0.51 ± 0.07 |
| SOD (U mg−1 protein) | 389.8 ± 7.80 | 347.6 ± 9.41* |
| Key inflammatory markers in the livere | ||
| TNF-α (pg mg−1 protein) | 10.50 ± 0.60 | 24.21 ± 1.72** |
| IL-6 (pg mg−1 protein) | 3.40 ± 0.74 | 6.35 ± 0.51* |
| NO (μmol g−1 protein) | 0.09 ± 0.01 | 0.19 ± 0.03* |
3.5 NFW and NFP restrained the body weight gain of mice under high-fat diet
Table 5 shows the body weight of the mice during the treatment (from the sixth to the ninth week) and the relative weight of the organs (liver and abdominal fat). The results indicated that high-fat diet could significantly increase (P < 0.01) the body weight gain of the mice by increasing (P < 0.05) the weight of their livers and abdominal fat compared with the normal control mice. The result was consistent with the results of other related studies.34,35 The body weight gain and the relative weight of the liver and abdominal fat of the mice under high-fat diet significantly decreased (P < 0.05) after 4-week administration of NFW and NFP. These results indicated that NFW and NFP restrained the weight gain of the mice under high-fat diet, which was mainly related to the decrease in abdominal fat accumulation and lipid accumulation in the liver.| Group | ||||||
|---|---|---|---|---|---|---|
| Control | HFD | NFP-50 | NFP-100 | NFP-200 | NPW | |
| a Weight of the mice at the end of the sixth week. b Weight of the mice at the end of the ninth week. Data are expressed as mean ± SEM (n = 6). *P < 0.05, **P < 0.01 versus the control group, #P < 0.05, ##P < 0.01 versus the HFD group. | ||||||
| Initial body weighta (g) | 45.10 ± 1.63 | 49.95 ± 2.25 | 48.18 ± 0.53 | 49.28 ± 1.00 | 48.58 ± 0.36 | 51.30 ± 1.79* |
| Final body weightb (g) | 47.70 ± 1.65 | 56.53 ± 2.64* | 51.90 ± 1.00 | 51.24 ± 1.15 | 52.18 ± 0.60 | 54.90 ± 1.44* |
| Body weight gain (g) | 2.60 ± 0.40 | 6.58 ± 0.59** | 3.73 ± 0.74# | 1.96 ± 0.24## | 3.60 ± 0.65## | 3.60 ± 0.42## |
| Liver (%) | 4.31 ± 0.11 | 6.16 ± 0.47** | 5.22 ± 0.26 | 4.81 ± 0.29# | 4.96 ± 0.20# | 4.64 ± 0.05# |
| Abdominal fat (%) | 2.43 ± 0.40 | 5.02 ± 0.62* | 3.96 ± 0.30 | 2.61 ± 0.41# | 2.77 ± 0.46# | 2.05 ± 0.23## |
3.6 Effects of the NFW and NFP on the serum lipid levels of mice under high-fat diet
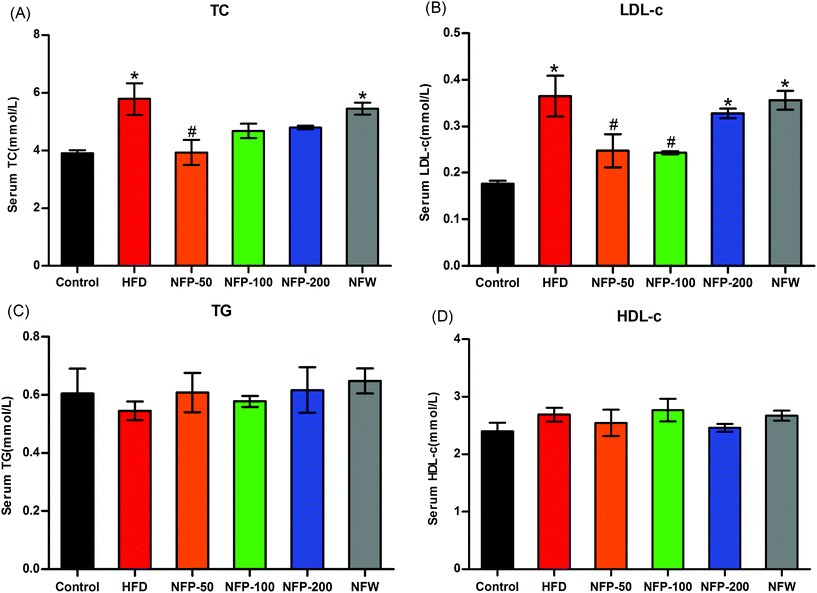
Lipid research clinic primary prevention trials have shown that elevated serum TC and TG levels, especially high LDL-c levels induced by high-fat diet, are closely related to cardiovascular disease, nonalcoholic fatty liver disease, and type 2 diabetes.36 As shown in Fig. 2, the serum TC and LDL-c levels of the HFD mice were significantly higher (P < 0.05) than that of the normal control mice, whereas the serum TG and HDL-c levels of the HFD mice and the normal control mice showed no significant differences (P > 0.05). Compared with the HFD group, the serum TC and LDL-c levels of the NFP-50 group effectively reduced (P < 0.05) (32% decrease). The serum TC and LDL-c levels of the NFP-100 group decreased by 19% (P > 0.05) and 35% (P < 0.05), respectively. The numerical results showed that the serum TC and LDL-c levels of the NFP-100 group were slightly lower than those of the NFW group, but showed no significant differences (P > 0.05). The same result was obtained between the NFP-200 and HFD groups. The serum TG and HDL-c levels of the mice in the HFD, NFW, and all doses of the NFP groups showed no significant differences (P > 0.05). This condition might be because the high-fat diet used in this experiment did not have significant effects on the TG and HDL-c levels of the mice used in this study.3.7 NFW and NFP reduced the hepatic oxidative stress of mice under high-fat diet
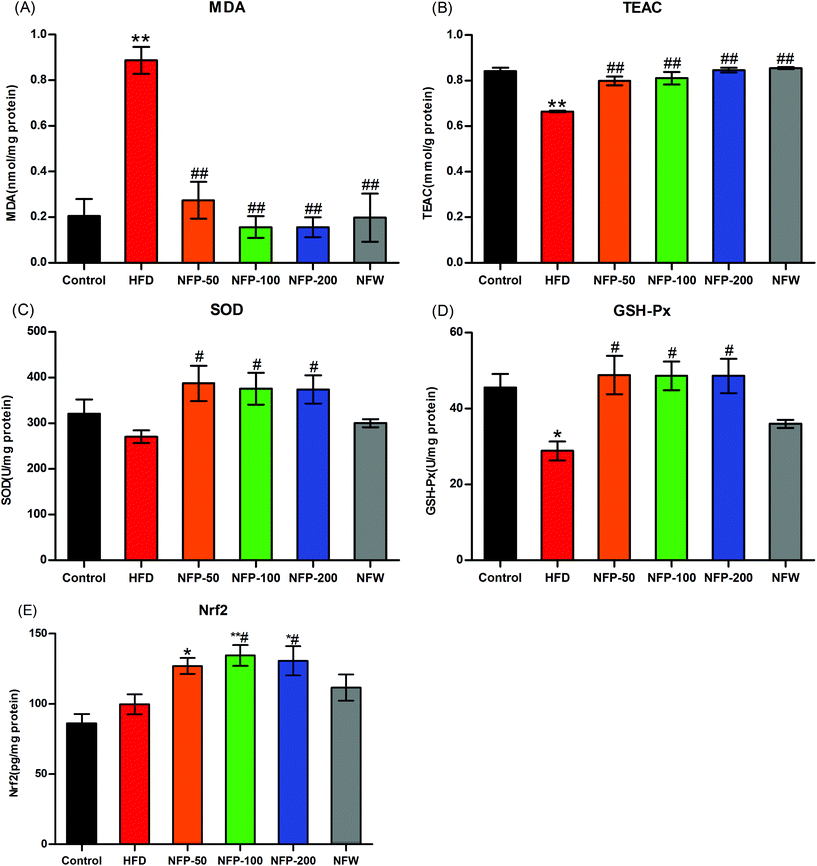
High SFA consumption can affect the β-oxidation of mitochondria and increase the production of ROS, thereby causing lipid peroxidation and decreasing the activity of the antioxidant system.13,37 As shown in Fig. 3(A), the hepatic MDA level of the HFD mice was significantly higher (P < 0.01) than that of the normal control mice, the hepatic MDA levels of the NFW and NFP groups were significantly lower (P < 0.01) compared with those of the HFD group, and no significant differences were found between them (P > 0.05). As shown in Fig. 3(B), the hepatic TEAC level of the HFD mice was significantly lower (P < 0.01) than that of the normal control mice. However, the administration of NFW and NFP effectively increased (P < 0.01) the TEAC level, and no significant differences were found between them (P > 0.05).The results in Fig. 3(C) and (D) showed that the hepatic SOD activity of the HFD mice was slightly lower (P > 0.05) than that of the normal control group, and the hepatic GSH-Px activity of the HFD mice was significantly lower (P < 0.05) than that of the normal control group. NFP administration significantly increased (P < 0.05) the hepatic SOD and GSH-Px activities of the mice under high-fat diet, and no statistical difference (P > 0.05) was found among different dosages. By contrast, NFW administration had no significant (P > 0.05) effect on the hepatic SOD and GSH-Px activities of the mice under high-fat diet.
Under the stimulation of ROS, Nrf2 entered the nucleus and bound with the antioxidant response elements (AREs) of the target gene promoter to reduce the oxidative stress of the mice.38 In this study, the hepatic Nrf2 level was measured to evaluate the oxidative stress of the mice. As shown in Fig. 3(E), the hepatic Nrf2 level of the HFD group was 15% higher than that of the normal control group, and no statistical difference was found between them (P > 0.05). The hepatic Nrf2 level of the NFW group was 12% higher than that of the HFD group, and no statistical difference was found between them (P > 0.05). However, the hepatic Nrf2 level of the NFP-50 group was 27% (P > 0.05) higher than that of the HFD group, and the hepatic Nrf2 levels of NFP-100 and NFP-200 groups were 35% (P < 0.05) and 31% (P < 0.05) higher than that of the HFD group, respectively.
3.8 NFW and NFP attenuated the inflammation in mice under high-fat diet
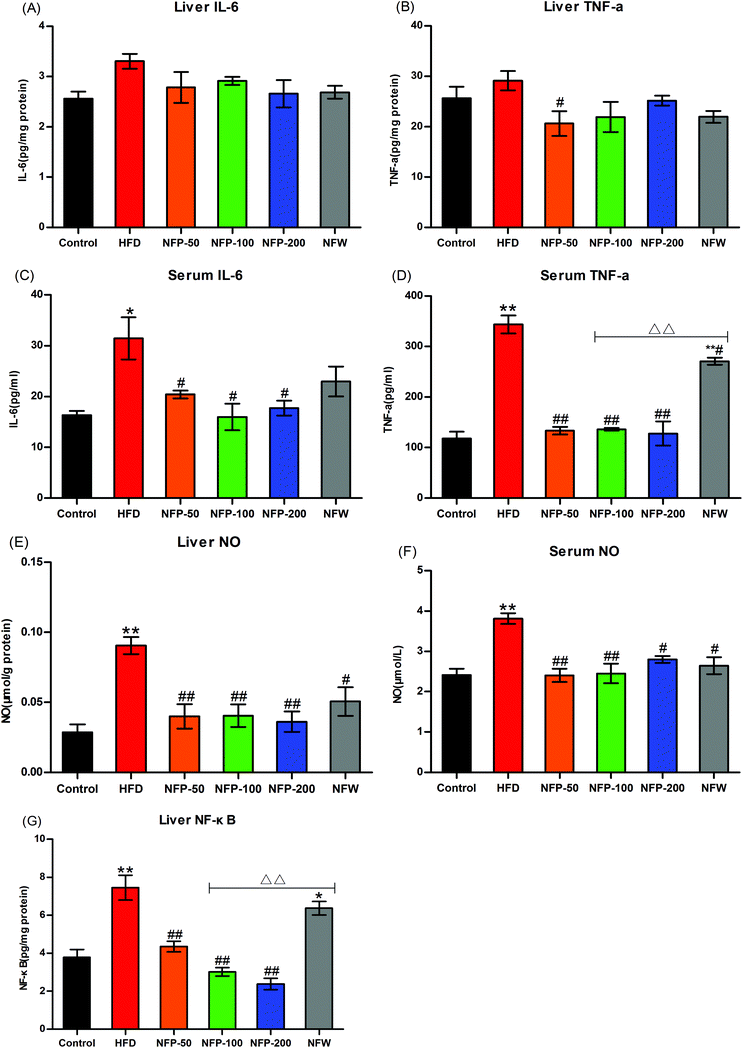
High-fat diet may induce lipid accumulation and oxidative stress in the liver, thereby triggering inflammation.10 As shown in Fig. 4(A) and (B), the hepatic IL-6 and TNF-α levels of the HFD group were slightly higher than those of the normal control group (P > 0.05), indicating that a certain degree of inflammation occurred in the mice of the HFD group. However, the hepatic IL-6 and TNF-α levels of the NFW group and all NFP groups were similar to those of the normal control group, indicating that the administration of NFW and NFP attenuated the inflammation in mice under high-fat diet to some extent.As shown in Fig. 4(C) and (D), the serum IL-6 and TNF-α levels of the HFD group were significantly higher (P < 0.05 and P < 0.01) than those of the normal control group, whereas the serum IL-6 and TNF-α levels of the NFP groups were significantly lower (P < 0.05) than those of the HFD group. The serum IL-6 level in the NFW group was lower than that in the HFD group, and no statistical difference (P > 0.05) was found between them. The serum TNF-α level in the NFW group was significantly lower than that in the HFD group (P < 0.05), but significantly higher than that in the NFP-100 group (P < 0.01). The above results indicated that the anti-inflammatory effect of the NFP was better than that of the NFW with a similar polysaccharide content.
NO, produced by the overexpression of NO synthase during lipid metabolism, was used to measure the inflammatory condition of the animal.39 As shown in Fig. 4(E) and (F), the hepatic and serum NO levels of the HFD group were significantly higher (P < 0.01) than those of the normal control group. However, the hepatic and serum NO levels of all NFP groups were significantly lower (P < 0.01) than those in the HFD group, and no statistical difference (P > 0.05) was observed among different dosages. The hepatic and serum NO levels in the NFW group were lower (P < 0.05) than those in the HFD group, indicating that the NPW had a modest anti-inflammatory effect, but the effect was lower than that in the NFP-100 group.
Transcription factor NF-κB plays an important role in the regulation of inflammatory response. The NF-κB level in the animals is positively correlated with the inflammation level.40 The results (Fig. 4(G)) indicated that the hepatic NF-κB level of the HFD group was significantly higher (P < 0.01) than that of the normal control group. However, the hepatic NF-κB levels of the NFP-50, NFP-100, and NFP-200 groups were 42%, 60%, and 68% lower than that of the HFD group, respectively, with a dose–effect relationship. The hepatic NF-κB level of the NFW group decreased by 15% compared with that of the HFD group, but no statistical difference was found between them (P > 0.05). The hepatic NF-κB level of the NFW group was significantly higher (P < 0.01) than that of the NFP-100 group, indicating that the anti-inflammatory effect of the NFW was inferior to that of the NFP.
4. Discussion
Long-term high-fat diet can increase weight gain and lipid accumulation, thereby inducing oxidative stress and inflammation in mice.41,42 Short-chain fatty acids derived from pectin polysaccharides of the intestinal flora can regulate the transcription of lipid metabolism-related enzymes (e.g., ChREBP, FAS, and ACC) in the liver, thereby inhibiting fat accumulation and preventing excessive weight gain in the mice.43,44 The results of this study indicated that the supplementation of NFW and NFP could significantly restrain the weight gain by reducing the lipid accumulation in the mice under high-fat diet. The NFP had a better effect than the NFW. As a type of pectin polysaccharide,24 NFP may be converted into short-chain fatty acids through the intestinal flora, regulate lipid metabolism, and reduce abnormal lipid levels in the mice under high-fat diet.Long-term high-fat diet can lead to excessive fat supply, and large amounts of fat, combined with circulating lipids and lipoproteins, enter the liver directly through the portal vein, making the liver to be the susceptible organ to lipotoxicity.12,45 Free fatty acids can upregulate the expression of bile acid synthase (CYP7A1) and activate the nuclear receptor PPAR-α to influence the β-oxidation of fatty acids, thereby stimulating ROS formation.10,46 Excess ROS will increase the lipid peroxidation levels of the hepatocytes. Excess ROS will cause the separation of the nuclear transcription factor Nrf2 from its inhibitor Keap1, activate the Nrf2 to interact with antioxidant elements (AREs) further, and mediate the transcription of various antioxidant enzymes (e.g., SOD, CAT, and GSH-Px), ultimately leading to oxidative stress.47 High-fat diet alone does not alter the expression of Nrf2 over Keap-1,48 whereas many bioactive components from herbal medicines can activate the Nrf2 signaling pathway, thereby improving the Nrf2 expression and reducing the Keap-1 expression.49 In this study, the hepatic Nrf2 levels of the HFD and normal control groups showed no statistical difference (P > 0.05), whereas the hepatic Nrf2 levels of the NFP-100 and NFP-200 groups were significantly higher than that of the HFD group, indicating that the NFP had a regulatory effect on the Nrf2 signaling pathway and exerted antioxidant effects in the mice. The results of this research also showed that high-fat diet consumption increased the liver lipid peroxidation level and reduced the TEAC level and the activities of SOD and GSH-Px in the liver, thereby causing severe oxidative stress in the liver. The NFW and NFP effectively reduced the hepatic MDA level and increased the hepatic TEAC level in the mice under high-fat diet. In addition, the NFP significantly increased the SOD and GSH-Px activities in the liver, indicating that NFP is an important antioxidant in the NFW.
Lipid accumulation in the liver and abdominal fat suggests that inflammation may occur in obese individuals.50,51 High-fat diet can alter the gut microbiota, resulting in increased permeability and influx of microbial components.52 In the liver, lipopolysaccharide and bacterial DNA activate toll-like receptors 4 and 9 (TLR4 and TLR9), thereby further activating the NF-κB signal transduction and leading to abnormal secretion of proinflammatory factors and inflammation in the mice.52,53 Some studies have reported that acetic, propionic, and butyric acids produced by pectin polysaccharide degradation are negatively correlated with the NF-κB expression to some extent, which could protect the mice under high-fat diet from inflammation injury.44 Short-chain fatty acids can effectively inhibit the oxidative stress and inflammation, thus improving the organ function and viability.54 High-fat diet induced inflammation in mice, which was mainly reflected in the increased levels of IL-6, TNF-α, and NO. However, with the treatment of the NFW and NFP, the levels of IL-6, TNF-α, and NO in the serum and liver were reduced, and the effects of the NFP were better than those of the NFW. In addition, the NFW and NFP reduced the hepatic NF-κB level in mice under high-fat diet, and the effect of NFP was better than that of NFW. In general, as a pectin polysaccharide, the NFP may be degraded into short-chain fatty acids through the intestinal bacteria, thereby repairing the intestinal mucosal barrier,24 reducing the flow of LPS and pathogenic bacteria into the blood, inhibiting the activation of the NF-κB pathway, and presenting the potential anti-inflammatory effect.
Studies have been conducted on the hypolipidemic, antioxidative, and anti-inflammatory effects of fermented noni fruit juice.41,55,56 We observed that the lipid lowering effect of NFW in our study was worse than that of noni fruit juice reported by them with a similar dosage, but their antioxidant and anti-inflammatory effects were equivalent. This condition may be related to the differences among their phytochemical compositions. The contents of polysaccharides and minerals (K and Mg) in the fermented noni fruit juice were higher than those in the NFW, and tannin and ascorbic acid were detected. The contents of the flavonoids, polyphenols, and minerals (Ca, Fe, Mn, and Se) in the NFW were higher than those in fermented noni fruit juice. Assi et al. (2017) reported that the chemical composition of noni fruit juice depends on the method of juice extraction.57 Hence, we inferred that the monosaccharide compositions and structures of polysaccharides in the fermented noni fruit juice may be transformed by the microbials during fermentation, thus showing better hypolipidemic effect than NFW.
Macrominerals, such as K, Mg, Na, and Ca, and trace minerals, such as Zn, Mn, and Se, are necessary and beneficial for human health. Mn and Se are the cofactors of SOD and GSH-Px, respectively. Maintenance of Zn in the serum and liver is important to decrease liver fibrosis.58 Studies have shown that polyphenols and flavonoids can improve oxidative stress and inflammatory response.12,45 We observed that the noncarbohydrate compounds in the NFW were higher than those in the NFP by comparing Tables 2 and 3. However, the NFP had better antioxidant and anti-inflammatory effects in mice under high-fat diet compared with NFW in this study. Studies have reported that Ganoderma lucidum reduces the body weight, inflammatory response, and insulin resistance in mice under high-fat diet probably because of the regulation of the intestinal flora by its high molecular weight polysaccharide component (>300 kDa).59 Therefore, these noncarbohydrate compounds in NFW may disturb the biological activities of polysaccharides, which may be due to the negative effects of other ingredients on certain polysaccharide-targeted intestinal flora.
High-fat diet-induced obesity and related complications is a slow and gradual process. Therefore, ameliorating the related metabolic changes caused by high-fat diet (e.g., lipid accumulation, oxidative stress, and inflammation) combined with specific exercises is an appropriate therapy, theoretically reversing or retarding the progress of obesity and related complications to an approximately healthy condition.60,61 The results of this study showed that supplementation of the NFW and NFP could restrain excessive body weight gain and lipid accumulation, thereby reducing oxidative stress and inflammation in mice under high-fat diet. Therefore, the NFW and NFP are suitable for the prevention and treatment of related metabolic diseases caused by high-fat diet (e.g., obesity, fatty liver disease, and type 2 diabetes).
5. Conclusion
The NFW and NFP were found to have remarkable effects not only on enhancing antioxidative status but also on ameliorating inflammation in this study. This study confirmed that the overall effect of the NFP was better than that of the NFW, indicating that the NFP is an important ingredient in the NFW. The NFP at 100 and 200 mg per kg bw exhibited remarkable effects in restraining the body weight gain, alleviating the oxidative stress, and attenuating the inflammation in mice under high-fat diet. Therefore, the NFP can be used as an alternative dietary supplement or a functional food to prevent obesity and other related complications.Abbreviations
| SFAs | Saturated fatty acids |
| ROS | Reactive oxygen species |
| TG | Total triglyceride |
| TC | Total cholesterol |
| HDL-c | High-density lipoprotein cholesterol |
| LDL-c | Low-density lipoprotein cholesterol |
| TP | Total protein |
| TEAC | Trolox equivalent antioxidant capacity |
| MDA | Malondialdehyde |
| SOD | Superoxide dismutase |
| GSH-Px | Glutathione peroxidase |
| NO | Nitric oxide |
| IL-6 | Interleutin-6 |
| TNF-α | Tumor necrosis factor alpha |
| Nrf2 | Nuclear factor erythroid-2 related factor |
| NF-κB | Nuclear factor kappa B |
| FT-IR | Fourier transform infrared |
| ABTS+ | 2,2′-Azino-di-(3-ethylbenzothiazoline)-6-sulfonic acid |
| ELISA | Enzyme linked immunosorbent assay |
Conflicts of interest
The authors declare no conflict of interest. The authors alone are responsible for the content and the writing of this paper.References
- E. T. Heinrichsen, H. Zhang, J. E. Robinson, J. Ngo, S. Diop, R. Bodmer, W. J. Joiner, C. M. Metallo and G. G. Haddad, Metabolic and transcriptional response to a high-fat diet in Drosophila melanogaster, Mol. Metab., 2014, 3, 42–54 CrossRef CAS.
- M. Selassie and A. C. Sinha, The epidemiology and aetiology of obesity: A global challenge, Best Pract. Res., Clin. Anaesthesiol., 2011, 25, 1–9 CrossRef.
- L. Cordain, S. B. Eaton, A. Sebastian, N. Mann, S. Lindeberg, B. A. Watkins, J. H. O'Keefe and J. Brand-Miller, Origins and evolution of the Western diet: health implications for the 21st century, Am. J. Clin. Nutr., 2005, 81, 341–354 CrossRef CAS.
- S. Bleich, D. Cutler, C. Murray and A. Adams, Why is the developed world obese?, Annu. Rev. Public Health, 2008, 29, 273–295 CrossRef.
- S. S. Mahmood, D. Levy, R. S. Vasan and T. J. Wang, The Framingham Heart Study and the epidemiology of cardiovascular disease: a historical perspective, Lancet, 2014, 383, 999–1008 CrossRef.
- G. Medina-Gomez and A. Vidal-Puig, Gateway to the metabolic syndrome, Nat. Med., 2005, 11, 602–603 CrossRef CAS.
- A. K. Leamy, R. A. Egnatchik and J. D. Young, Molecular mechanisms and the role of saturated fatty acids in the progression of non-alcoholic fatty liver disease, Prog. Lipid Res., 2013, 52, 165–174 CrossRef CAS.
- L. I. Onofrio, A. R. Arocena, A. F. Paroli, M. E. Cabalen, M. C. Andrada, R. C. Cano and S. Gea, Trypanosoma cruzi infection is a potent risk factor for non-alcoholic steatohepatitis enhancing local and systemic inflammation associated with strong oxidative stress and metabolic disorders, PLoS Neglected Trop. Dis., 2015, 9, e0003464 CrossRef.
- G. S. Hotamisligil, Inflammation and metabolic disorders, Nature, 2006, 444, 860–867 CrossRef CAS.
- Y. Yuan, H. Naito, X. Jia, K. Kitamori and T. Nakajima, Combination of Hypertension Along with a High Fat and Cholesterol Diet Induces Severe Hepatic Inflammation in Rats via a Signaling Network Comprising NF-κB, MAPK, and Nrf2 Pathways, Nutrients, 2017, 9, 1018 CrossRef.
- S. Hogan, C. Canning, S. Sun, X. Sun and K. Zhou, Effects of grape pomace antioxidant extract on oxidative stress and inflammation in diet induced obese mice, J. Agric. Food Chem., 2010, 58, 11250–11256 CrossRef CAS.
- Z. Tuzcu, C. Orhan, N. Sahin, V. Juturu and K. Sahin, Cinnamon Polyphenol Extract Inhibits Hyperlipidemia and Inflammation by Modulation of Transcription Factors in High-Fat Diet-Fed Rats, Oxid. Med. Cell. Longevity, 2017, 2017, 1583098 Search PubMed.
- L. Chen, L. Liu, C. Li, C. Hu, F. Su, R. Liu, M. Zeng, D. Zhao, J. Liu, Y. Guo and J. Long, A mix of apple pomace polysaccharide improves mitochondrial function and reduces oxidative stress in the liver of high-fat diet-induced obese mice, Mol. Nutr. Food Res., 2017, 61, 1600433 CrossRef.
- T. Wu, Y. Guo, R. Liu, K. Wang and M. Zhang, Black tea polyphenols and polysaccharides improve body composition, increase fecal fatty acid, and regulate fat metabolism in high-fat diet-induced obese rats, Food Funct., 2016, 7, 2469–2478 RSC.
- Y. Xu, M. Zhang, T. Wu, S. Dai, J. Xu and Z. Zhou, The anti-obesity effect of green tea polysaccharides, polyphenols and caffeine in rats fed with a high-fat diet, Food Funct., 2015, 6, 297–304 Search PubMed.
- R. K. Gupta and A. K. Patel, Do the health claims made for Morinda citrifolia (Noni) harmonize with current scientific knowledge and evaluation of its biological effects, Asian Pac. J. Cancer Prev., 2013, 14, 4495–4499 CrossRef.
- M. A. O. Torres, I. de Fatima Braga Magalhaes, R. Mondego-Oliveira, J. C. de Sa, A. L. Rocha and A. L. Abreu-Silva, One Plant, Many Uses: A Review of the Pharmacological Applications of Morinda citrifolia, Phytother. Res., 2017, 31, 971–979 CrossRef.
- European Commission, Commission decision of 5 June 2003 authorising the placing on the market of “noni juice” (juice of the fruit of Morinda citrifolia L.) as a novel food ingredient under regulation (EC) No 258/97 of the European parliament and of the council, Off. J. Eur. Union L 144, 2003, 46, 12 Search PubMed . Available online: http://data.europa.eu/eli/dec/2003/426/oj (accessed on 11 December 2017).
- B. J. West, S. Deng, F. Isami, A. Uwaya and C. J. Jensen, The Potential Health Benefits of Noni Juice: A Review of Human Intervention Studies, Foods, 2018, 7, 58 CrossRef.
- A. K. Bui, A. Bacic and F. Pettolino, Polysaccharide composition of the fruit juice of Morinda citrifolia (Noni), Phytochemistry, 2006, 67, 1271–1275 CrossRef CAS.
- E. Furusawa, A. Hirazumi, S. Story and J. Jensen, Antitumour potential of a polysaccharide-rich substance from the fruit juice of Morinda citrifolia (Noni) on sarcoma 180 ascites tumour in mice, Phytother. Res., 2003, 17, 1158–1164 CrossRef CAS.
- H. Zhang, J. Li, J. Xia and S. Lin, Antioxidant activity and physicochemical properties of an acidic polysaccharide from Morinda officinalis, Int. J. Biol. Macromol., 2013, 58, 7–12 CrossRef CAS.
- S. G. Sousa, L. A. Oliveira, D. de Aguiar Magalhaes, T. V. de Brito, J. A. Batista, C. M. C. Pereira, M. de Souza Costa, J. C. R. Mazulo, M. de Carvalho Filgueiras, D. F. P. Vasconselos, D. A. da Silva, F. C. N. Barros, V. G. Sombra, A. L. P. Freitas, R. C. M. de Paula, J. P. de Andrade Feitosa and A. L. Dos Reis Barbosa, Chemical structure and anti-inflammatory effect of polysaccharide extracted from Morinda citrifolia Linn (Noni), Carbohydr. Polym., 2018, 197, 515–523 CrossRef CAS.
- M. Jin, Y. Wang, X. Yang, H. Yin, S. Nie and X. Wu, Structure characterization of a polysaccharide extracted from noni (Morinda citrifolia L.) and its protective effect against DSS-induced bowel disease in mice, Food Hydrocolloids, 2019, 90, 189–197 CrossRef CAS.
- M. T. Wu, B. S. Tzang, Y. Y. Chang, C. H. Chiu, W. Y. Kang, C. H. Huang and Y. C. Chen, Effects of Antrodia camphorata on alcohol clearance and antifibrosis in livers of rats continuously fed alcohol, J. Agric. Food Chem., 2011, 59, 4248–4254 CrossRef CAS.
- S. Bi, Y. Jing, Q. Zhou, X. Hu, J. Zhu, Z. Guo, L. Song and R. Yu, Structural elucidation and immunostimulatory activity of a new polysaccharide from Cordyceps militaris, Food Funct., 2018, 9, 279–293 RSC.
- S. A. Baba and S. A. Malik, Determination of total phenolic and flavonoid content, antimicrobial and antioxidant activity of a root extract of Arisaema jacquemontii Blume, J. Taibah. Univ. Sci., 2015, 9, 449–454 CrossRef.
- A. Mahboubi, J. Asgarpanah, P. N. Sadaghiyani and M. Faizi, Total phenolic and flavonoid content and antibacterial activity of Punica granatum L. var. pleniflora flowers (Golnar) against bacterial strains causing foodborne diseases, BMC Complementary Altern. Med., 2015, 15, 366 CrossRef.
- M. Dubois, K. A. Gilles, J. K. Hamilton, P. A. Rebers and F. Smith, Colorimetric method for determination of sugars and related substances, Anal. Chem., 1956, 28, 350–356 CrossRef CAS.
- D. Ren, Y. Zhao, Q. Zheng, A. Alim and X. Yang, Immunomodulatory effects of an acidic polysaccharide fraction from herbal Gynostemma pentaphyllum tea in RAW264.7 cells, Food Funct., 2019, 10, 2186–2197 RSC.
- Y. Qiao, J. Sun, Y. Ding, G. Le and Y. Shi, Alterations of the gut microbiota in high-fat diet mice is strongly linked to oxidative stress, Appl. Microbiol. Biotechnol., 2013, 97, 1689–1697 CrossRef CAS.
- Y. Qiao, J. Sun, S. Xia, X. Tang, Y. Shi and G. Le, Effects of resveratrol on gut microbiota and fat storage in a mouse model with high-fat-induced obesity, Food Funct., 2014, 5, 1241–1249 RSC.
- Y. Qiao, J. Sun, Z. Xie, Y. Shi and G. Le, Propensity to high-fat diet-induced obesity in mice is associated with the indigenous opportunistic bacteria on the interior of Peyer's patches, J. Clin. Biochem. Nutr., 2014, 55, 120–128 CrossRef CAS.
- Z. Liang, Z. Yuan, G. Li, F. Fu and Y. Shan, Hypolipidemic, Antioxidant, and Antiapoptotic Effects of Polysaccharides Extracted from Reishi Mushroom, Ganoderma lucidum (Leysser: Fr) Karst, in Mice Fed a High-Fat Diet, J. Med. Food, 2018, 21, 1218–1227 CrossRef CAS.
- R. B. A. Kolsi, N. Jardak, F. Hajkacem, R. Chaaben, I. Jribi, A. E. Feki, T. Rebai, K. Jamoussi, L. Fki, H. Belghith and K. Belghith, Anti-obesity effect and protection of liver-kidney functions by Codium fragile sulphated polysaccharide on high fat diet induced obese rats, Int. J. Biol. Macromol., 2017, 102, 119–129 CrossRef.
- M. J. Chapman, H. N. Ginsberg, P. Amarenco, F. Andreotti, J. Boren, A. L. Catapano, O. S. Descamps, E. Fisher, P. T. Kovanen, J. A. Kuivenhoven, P. Lesnik, L. Masana, B. G. Nordestgaard, K. K. Ray, Z. Reiner, M. R. Taskinen, L. Tokgozoglu, A. Tybjaerg-Hansen and G. F. Watts, Triglyceride-rich lipoproteins and high-density lipoprotein cholesterol in patients at high risk of cardiovascular disease: evidence and guidance for management, Eur. Heart J., 2011, 32, 1345–1361 CrossRef CAS.
- R. Mateos, E. Lecumberri, S. Ramos, L. Goya and L. Bravo, Determination of malondialdehyde (MDA) by high-performance liquid chromatography in serum and liver as a biomarker for oxidative stress. Application to a rat model for hypercholesterolemia and evaluation of the effect of diets rich in phenolic antioxidants from fruits, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2005, 827, 76–82 CrossRef CAS.
- Y. Fang, T. Su, X. Qiu, P. Mao, Y. Xu, Z. Hu, Y. Zhang, X. Zheng, P. Xie and Q. Liu, Protective effect of alpha-mangostin against oxidative stress induced-retinal cell death, Sci. Rep., 2016, 6, 21018 CrossRef CAS.
- C. S. Reiss and T. Komatsu, Does nitric oxide play a critical role in viral infections?, J. Virol., 1998, 72, 4547–4551 CrossRef CAS.
- H. Wang and C. H. Cho, Effect of NF-κB signaling on apoptosis in chronic inflammation-associated carcinogenesis, Curr. Cancer Drug Targets, 2010, 10, 593–599 CrossRef CAS.
- Y. L. Lin, Y. Y. Chang, D. J. Yang, B. S. Tzang and Y. C. Chen, Beneficial effects of noni (Morinda citrifolia L.) juice on livers of high-fat dietary hamsters, Food Chem., 2013, 140, 31–38 CrossRef CAS.
- Y. Y. Chang, C. H. Chou, C. H. Chiu, K. T. Yang, Y. L. Lin, W. L. Weng and Y. C. Chen, Preventive effects of taurine on development of hepatic steatosis induced by a high-fat/cholesterol dietary habit, J. Agric. Food Chem., 2011, 59, 450–457 CrossRef CAS.
- L. Tian, J. Scholte, K. Borewicz, B. van den Bogert, H. Smidt, A. J. Scheurink, H. Gruppen and H. A. Schols, Effects of pectin supplementation on the fermentation patterns of different structural carbohydrates in rats, Mol. Nutr. Food Res., 2016, 60, 2256–2266 CrossRef CAS PubMed.
- W. Li, K. Zhang and H. Yang, Pectin Alleviates High Fat (Lard) Diet-Induced Nonalcoholic Fatty Liver Disease in Mice: Possible Role of Short-Chain Fatty Acids and Gut Microbiota Regulated by Pectin, J. Agric. Food Chem., 2018, 66, 8015–8025 CrossRef CAS PubMed.
- P. S. Ferreira, L. C. Spolidorio, J. A. Manthey and T. B. Cesar, Citrus flavanones prevent systemic inflammation and ameliorate oxidative stress in C57BL/6J mice fed high-fat diet, Food Funct., 2016, 7, 2675–2681 RSC.
- E. Coccia, E. Varricchio, P. Vito, G. M. Turchini, D. S. Francis and M. Paolucci, Fatty acid-specific alterations in leptin, PPAR alpha, and CPT-1 gene expression in the rainbow trout, Lipids, 2014, 49, 1033–1046 CrossRef CAS.
- Y. Fang, T. Su, X. Qiu, P. Mao, Y. Xu, Z. Hu, Y. Zhang, X. Zheng, P. Xie and Q. Liu, Protective effect of alpha-mangostin against oxidative stress induced-retinal cell death, Sci. Rep., 2016, 6, 21018 CrossRef CAS.
- M. V. Barroso, A. Graca-Reis, I. Cattani-Cavalieri, L. B. Gitirana, S. S. Valenca and M. Lanzetti, Mate tea reduces high fat diet-induced liver and metabolic disorders in mice, Biomed. Pharmacother., 2019, 109, 1547–1555 CrossRef CAS.
- X. Yang, G. Hu, L. Lv, T. Liu, L. Qi, G. Huang, D. You and J. Zhao, Regulation of P-glycoprotein by Bajijiasu in vitro and in vivo by activating the Nrf2-mediated signalling pathway, Pharm. Biol., 2019, 57, 184–192 CrossRef CAS PubMed.
- M. Stepien, A. Stepien, R. N. Wlazel, M. Paradowski, M. Banach and J. Rysz, Obesity indices and inflammatory markers in obese non-diabetic normo- and hypertensive patients: a comparative pilot study, Lipids Health Dis., 2014, 13, 29 CrossRef.
- M. C. Amato, G. Pizzolanti, V. Torregrossa, G. Misiano, S. Milano and C. Giordano, Visceral adiposity index (VAI) is predictive of an altered adipokine profile in patients with type 2 diabetes, PLoS One, 2014, 9, e91969 CrossRef PubMed.
- V. Tremaroli and F. Backhed, Functional interactions between the gut microbiota and host metabolism, Nature, 2012, 489, 242–249 CrossRef CAS PubMed.
- J. Xiao, F. Xing, J. Huo, M. L. Fung, E. C. Liong, Y. P. Ching, A. Xu, R. C. Chang, K. F. So and G. L. Tipoe, Lycium barbarum polysaccharides therapeutically improve hepatic functions in non-alcoholic steatohepatitis rats and cellular steatosis model, Sci. Rep., 2014, 4, 5587 CrossRef CAS PubMed.
- V. Andrade-Oliveira, M. T. Amano, M. Correa-Costa, A. Castoldi, R. J. Felizardo, D. C. de Almeida, E. J. Bassi, P. M. Moraes-Vieira, M. I. Hiyane, A. C. Rodas, J. P. Peron, C. F. Aguiar, M. A. Reis, W. R. Ribeiro, C. J. Valduga, R. Curi, M. A. Vinolo, C. M. Ferreira and N. O. Camara, Gut Bacteria Products Prevent AKI Induced by Ischemia-Reperfusion, J. Am. Soc. Nephrol., 2015, 26, 1877–1888 CrossRef CAS PubMed.
- Y. L. Lin, C. H. Chou, D. J. Yang, J. W. Chen, B. S. Tzang and Y. C. Chen, Hypolipidemic and antioxidative effects of noni (Morinda citrifolia L.) juice on high-fat/cholesterol-dietary hamsters, Plant Foods Hum. Nutr., 2012, 67, 294–302 CrossRef CAS PubMed.
- A. Shoeb, M. C. Alwar, P. J. Shenoy and P. Gokul, Effect of Morinda citrifolia (Noni) Fruit Juice on High Fat Diet Induced Dyslipidemia in Rats, J. Clin. Diagn. Res., 2016, 10, FF06–FF10 CAS.
- R. A. Assi, Y. Darwis, I. M. Abdulbaqi, A. A. Khan, L. Vuanghao and M. H. Laghari, Morinda citrifolia (Noni): A comprehensive review on its industrial uses, pharmacological activities, and clinical trials, Arabian J. Chem., 2017, 10, 691–707 CrossRef.
- A. Szuster-Ciesielska, K. Plewka, J. Daniluk and M. Kandefer-Szerszen, Zinc supplementation attenuates ethanol- and acetaldehyde-induced liver stellate cell activation by inhibiting reactive oxygen species (ROS) production and by influencing intracellular signaling, Biochem. Pharmacol., 2009, 78, 301–314 CrossRef CAS.
- C.-J. Chang, C.-S. Lin, C.-C. Lu, J. Martel, Y.-F. Ko, D. M. Ojcius, S.-F. Tseng, T.-R. Wu, Y.-Y. M. Chen, J. D. Young and H.-C. Lai, Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota, Nat. Commun., 2015, 6, 7489 CrossRef CAS.
- J. Sun, Y. Qiao, C. Qi, W. Jiang, H. Xiao, Y. Shi and G. W. Le, High-fat-diet-induced obesity is associated with decreased antiinflammatory Lactobacillus reuteri sensitive to oxidative stress in mouse Peyer's patches, Nutrition, 2016, 32, 265–272 CrossRef CAS.
- J. Xiao, R. Guo, M. L. Fung, E. C. Liong and G. L. Tipoe, Therapeutic approaches to non-alcoholic fatty liver disease: past achievements and future challenges, Hepatobiliary Pancreatic Dis. Int., 2013, 12, 125–135 CrossRef.
| This journal is © The Royal Society of Chemistry 2020 |