Is soy protein effective in reducing cholesterol and improving bone health?
Kelli S.
George
ab,
Joseph
Muñoz
ab,
Neda S.
Akhavan
ab,
Elizabeth M.
Foley
ab,
Shalom C.
Siebert
ab,
Gershon
Tenenbaum
c,
Dania A.
Khalil
a,
Sheau C.
Chai
 d and
Bahram H.
Arjmandi
d and
Bahram H.
Arjmandi
 *ab
*ab
aDepartment of Nutrition, Food and Exercise Sciences, Florida State University, Tallahassee, FL, USA. E-mail: barjmandi@fsu.edu
bCenter for Advancing Exercise and Nutrition Research on Aging (CAENRA), College of Human Sciences, Florida State University, Tallahassee, FL, USA
cDepartment of Educational Psychology and Learning Systems, Florida State University, Tallahassee, FL, USA
dDepartment of Behavioral Health and Nutrition, University of Delaware, Newark, DE, USA
First published on 18th December 2019
Abstract
Hyperlipidemia associated with cardiovascular health, and bone loss with regard to osteoporosis contribute to increased morbidity and mortality and are influenced by diet. Soy protein has been shown to reduce cholesterol levels, and its isoflavones may improve bone health. The objective of this study was to determine the effects of soy protein on lipid profiles and biomarkers of bone metabolism and inflammation. Ninety men and women (aged 27–87) were randomly assigned to consume 40 g of soy or casein protein daily for three months. Both soy and casein consumption significantly reduced bone alkaline phosphatase (P = 0.011) and body fat % (P < 0.001), tended to decrease tartrate-resistant acid phosphatase (P = 0.066), and significantly increased serum insulin-like growth factor-I (IGF-1) (P < 0.001), yet soy increased IGF-1 to a greater extent (P = 0.01) than casein. Neither treatment affected total cholesterol, HDL cholesterol, LDL cholesterol, or C-reactive protein. These results demonstrate that daily supplementation of soy and casein protein may have positive effects on indices of bone metabolism and body composition, with soy protein being more effective at increasing IGF-1, an anabolic factor, which may be due to soy isoflavones’ role in upregulating Runx2 gene expression, while having little effect on lipid profiles and markers of inflammation.
Introduction
Cardiovascular disease (CVD) and osteoporosis are two common age-related diseases associated with increased morbidity and mortality.1 Hyperlipidemia is a major risk factor for the development of CVD, which remains the leading cause of death in the United States (U.S.).2 In osteoporotic individuals, the rate of bone resorption exceeds the rate of bone formation, leading to significant, albeit gradual bone loss, which increases the risk of fracture. Furthermore, women with osteoporosis have been reported to have an increased risk of CVD when compared to non-osteoporotic women.3 Though CVD and osteoporosis display different symptoms, the two diseases are thought to be related to one another4 and likely share similar etiologies, specifically in regard to inflammation.5Inflammatory cytokines and markers of systemic inflammation, such as C-reactive protein (CRP), are known to play a role in the bone remodeling process and have been associated with increased fracture risk6 as well as incidence of CVD.7 Although a positive association between CRP and an increased risk of fractures has been observed, these findings are inconsistent and not as well established in the general population without skeletal diseases;8,9 thus, there is a continued need for further investigation.
Both dyslipidaemia and bone loss are influenced by diet, including the source of protein. Among plant-based proteins, soy protein is a complete protein, and is still backed by the U.S. Food and Drug Administration health claim that 25 g daily soy protein consumption lowers total cholesterol, thereby decreasing the risk of cardiovascular disease.10 Additionally, soy protein, with its naturally occurring isoflavones, may contribute to a decreased risk of bone loss. The authors wish to express their view that isoflavones may be effective in terms of bone health, only within the context of soy protein. Other investigators, such as Alekel et al.,11 have used isoflavones alone in long-term studies, where no long-term beneficial effects were seen on bone. Therefore, we believe that there is a synergistic effect of soy isoflavones and soy protein in terms of beneficial effects on bone.
To date, soy has been reported to have positive effects on both bone metabolism and cardiovascular health in postmenopausal women,12–14 though these results are not conclusive.13,15–17 Chronic soy consumption has led to improvements in bone biomarkers such as insulin like growth factor 1 (IGF-1), which stimulates osteoblast activity, bone alkaline phosphatase (BALP), a marker of bone turnover, and tartrate-resistant acid phosphatase (TRAP), a marker of bone resorption.13,18 Furthermore, isoflavone-rich soy products have also been shown to increase serum estradiol in postmenopausal women,19 which can positively influence BMD and bone microarchitecture.20 In part, these beneficial effects of soy may be attributed to its isoflavone content, in which genistein, daidzein, and glycetin are found in high concentrations.21,22 Previously, our laboratory has shown that soy isoflavones in conjunction with soy protein at low (9.5 mg kg−1), medium (19 mg kg−1), and high (38 mg kg−1) doses were able to prevent increases in total plasma cholesterol in an ovariectomized golden Syrian hamster model.23 We have also reported that soy protein was able to prevent loss of bone after orchidectomy in a male rat model of bone loss.24 With previous data suggesting that soy has positive effects on cardiovascular and bone health in older populations, the ability of soy to prevent bone loss and CVD in the general population has not been extensively researched. Hence, the purpose of this study was two-fold; with anecdotal evidence both supporting and opposing the viewpoint on the beneficial properties of soy in regard to both bone loss and CVD, we aimed to observe the effects of three months of soy protein consumption on the biomarkers of bone metabolism, inflammation and lipid profile in normocholesterolemic and mildly hypercholesterolemic adult men and women. The mechanism of action by which soy protein and its isoflavones may exert their effects on bone and cardiovascular health will then be discussed.
Materials and methods
Participants
Men and women with diverse ethnic backgrounds were recruited for this three month intervention study. Pre-screening was conducted via a phone interview to identify anyone meeting exclusion criteria. Exclusion criteria included current presence of rheumatoid arthritis, joint pain due to injury, cancer or a history of cancer, insulin dependent diabetes mellitus, kidney disease, gastrointestinal or chronic digestive disorders, and allergy to milk, eggs, or soy. There were no special selection criteria regarding cholesterol levels, bone disorders or medications.Study design
In this double blind study, a total of 135 mobile individuals (65 men and 70 women), ranging in age from 27 to 87 years, were randomly assigned to consume 40 g of protein per day in the form of a powdered drink mix supplement containing either soy protein (donated by DuPont Nutrition and Health, formerly known as Solae, and Protein Technologies International, St Louis, MO) or casein (control). Of the 135 initial study participants, 64 consumed soy protein and 71 consumed casein. The protein supplements were consumed daily for three months. Both regimens supplied 40 g protein, 18 g carbohydrate, 0 g fat, and 240 calories daily. The soy protein supplement contained 2.4 milligrams isoflavones per gram of protein (96 mg isoflavones per 40 gram serving of protein). The rationale for choosing the soy protein dose (40 g day−1) was based on an effective amount previously used in clinical studies.13,25,26 It was made clear to the participants that they had an equal chance of being placed in either the soy protein or casein group, and both supplements were similar in appearance and taste to ensure that the participants were unaware of which supplement they were consuming. Compliance with the study protocol was monitored via two means: a monthly calendar for recording consumption of the provided supplements on a daily basis, as well as returning any unconsumed packets to the investigators during their monthly visits, where unused supplies were counted and recorded. Subjects were given access to a registered dietitian for advice on how to incorporate the supplement into their diets. The study protocol was approved by the Oklahoma State University IRB (HS-01-71). Participants signed a consent form after being provided with oral and written descriptions of the study. A complete medical history was obtained from all participants before initiating the treatments. A total of eighty-eight subjects (46 men and 42 women) completed the study with 44 subjects in each of the two treatment groups.Anthropometrics
Height, weight, and percent body fat were collected at the baseline and during the final visit. Percent body fat was measured using a Body Composition Analyzer (Biodynamics, Model 310e). Height and weight were used to calculate body mass index (BMI). Weight was monitored during each monthly follow-up visit. If weight gain was apparent, counselling was available to adjust the diet to prevent further gain.Blood collection and analyses
A fasting venous blood sample of 20 mL was collected from each subject at the baseline and at the end of the study. Serum and plasma were separated from the blood (centrifuged at 1500g for 20 minutes) within 2 hours of collection and stored at −80 °C until analysis.Serum triglycerides (TG) and total cholesterol (TC) were determined enzymatically using kits from Roche Diagnostic Systems (Somerville, NJ). Serum high density lipoprotein cholesterol (HDL-C) was determined by a direct method (Unimate HDL Direct, Roche Diagnostic Systems, Somerville, NJ). Low density lipoprotein cholesterol (LDL-C) was calculated using the Friedewald equation. Lipoprotein(a) was determined via immunoprecipitation (DiaSorin, Stillwater, MN). Each of these tests was performed on a Cobas-Fara II Clinical Analyzer (Montclair, NJ) following the manufacturer's instructions and using commercially available calibrators and quality control samples.
Serum BALP activity was measured using an Enzyme-Linked Immunosorbent Assay (ELISA) kit (Alkphase, Metra Biosystems, Mountain View, CA). Serum TRAP was measured using commercially available kits from Roche Diagnostics (Branchburg, NJ) and analyzed on a Cobas Fara II clinical analyzer (Montclair, NJ). Serum IGF-I was extracted and measured using a radioimmunoassay kit from Nichols Institute Diagnostics (San Juan Capistrano, CA). Serum levels of estradiol were assessed using double antibody assay for estradiol from Diagnostic Products Corporation (Los Angeles, CA).
Statistical analyses
Descriptive statistics were calculated for all variables and included means and standard deviations. The data were analyzed using IBM SPSS Statistics version 25. The primary outcome variables were serum lipid parameters, anthropometric parameters, and biochemical markers of bone formation and bone resorption. Treatment (soy protein vs. casein) effects were assessed using repeated measures analysis of variance (RMANOVA). Independent sample T-tests were used to assess treatment (soy protein vs. casein) effects and compare baseline values within groups. Statistical significance level was set at P < 0.05 for all analyses.Results
Baseline characteristics
Participants did not differ in age, weight, or percent body fat at the baseline between the two treatment groups (Table 1). The rate of attrition at the end of the study was 33%.| Soy | Casein | ||||
|---|---|---|---|---|---|
| Variable | Baseline | Final | Baseline | Final | P value |
| Values for sex are presented as frequencies; all other variables are mean ± SD. * indicates statistically significant values (P < 0.05). | |||||
| Sex (female) | 20 | 22 | — | ||
| Sex (male) | 24 | 22 | — | ||
| Age | 60.3 ± 12.0 | 60.6 ± 12.0 | — | ||
| Weight (lbs) | 210 ± 60.4 | 206 ± 70.3 | 198 ± 41.0 | 200 ± 41.9 | 0.642 |
| Body fat % | 36.6 ± 7.9 | 35.1 ± 7.1 | 36.2 ± 8.33 | 35.4 ± 8.14 | <0.001* |
| BMI (kg m−2) | 31.6 ± 8.4 | 31.2 ± 8.8 | 30.6 ± 6.74 | 30.9 ± 6.97 | 0.743 |
Anthropometrics
Anthropometric measurements are reported in Table 1. Daily consumption of 40 g soy protein or casein for a three-month period did not significantly affect body weight or BMI; however, both protein regimens had positive effects on percent body fat as indicated by a significant net change from the baseline (P < 0.001 for both soy and casein protein regimens; decreases of 4.1% and 2.2%, respectively).Serum biomarkers of bone and inflammation
Data regarding IGF-1, BALP, estradiol, TRAP, and CRP are presented in Table 2. Daily consumption of 40 g soy protein or casein for a three-month period did not affect serum estradiol concentrations (P = 0.66) or CRP (P = 0.45). Both treatments tended to increase serum TRAP (P = 0.066), significantly increased serum IGF-1 (P < 0.001), and decreased BALP (P = 0.009). However, soy protein increased IGF-I to a significantly (P = 0.011) greater extent than casein protein (85.2% and 26.1%, respectively).| Measures | Treatment group | Baseline values | Final values | P value |
|---|---|---|---|---|
| Values are mean ± SD. Baseline values are not different among the two groups. * indicates statistically significant values (P < 0.05); ⊥ indicates values tending toward significance (P < 0.1). | ||||
| TRAP (U L−1) | Soy | 2.97 ± 0.92 | 3.12 ± 0.83 | 0.066⊥ |
| Casein | 3.12 ± 0.65 | 3.35 ± 0.74 | ||
| BALP (ukat L−1) | Soy | 0.43 ± 0.15 | 0.40 ± 0.14 | 0.009* |
| Casein | 0.41 ± 0.14 | 0.40 ± 0.15 | ||
| IGF (ng ml−1) | Soy | 115 ± 54.8 | 213 ± 150 | <0.001* |
| Casein | 138 ± 69.1 | 174 ± 76.4 | ||
| Estradiol (pg ml−1) | Soy | 37.8 ± 44.3 | 43.6 ± 60.3 | 0.659 |
| Casein | 50.6 ± 71.5 | 49.5 ± 81.5 | ||
Serum lipids
Data regarding total cholesterol, HDL-C, LDL-C, and triglycerides are presented in Table 3. Both soy protein and casein had no effect on total, HDL-, or LDL-cholesterol. However, both dietary protein regimens significantly increased serum triglycerides (P = 0.018).| Measures | Treatment group | Baseline values | Final values | P value |
|---|---|---|---|---|
| Values are mean ± SD. Baseline values are not different among the two groups. * indicates statistically significant values (P < 0.05). | ||||
| TC (mg dl−1) | Soy | 221 ± 41.1 | 222 ± 38.5 | 0.946 |
| Casein | 226 ± 43.9 | 226 ± 41.4 | ||
| HDL (mg dl−1) | Soy | 53.8 ± 18.4 | 55.7 ± 13.6 | 0.179 |
| Casein | 57.6 ± 17.2 | 57.9 ± 17.5 | ||
| LDL (mg dl−1) | Soy | 126 ± 33.1 | 126 ± 33.7 | 0.303 |
| Casein | 137 ± 32.1 | 132 ± 28.0 | ||
| TAG (mg dl−1) | Soy | 191 ± 82.6 | 195 ± 85.2 | 0.018* |
| Casein | 166 ± 83.0 | 166 ± 83.0 | ||
Discussion
The participants randomized into the soy protein group consumed 2.4 mg of isoflavones per gram of soy protein (approximately 96 mg of soy isoflavones per day). Preliminary studies from our laboratory and others suggest that this amount of isoflavones, in the context of soy protein, is efficacious in improving the biomarkers of bone metabolism and lipid profiles in both human and rodent models of disease.27–29 It is important to note that potentially, the benefits of soy isoflavones may only be seen in the context of soy protein or whole soy. As previously mentioned, other investigators have examined the long term effects of isolated soy isoflavones with little to no evidence for their efficacy.11 For this reason, the authors of this manuscript would like to emphasize that they believe that soy isoflavones have a synergistic effect with soy protein in terms of benefiting bone health.Findings from this double-blind comparative control study suggest that daily supplementation of soy protein may have positive effects on bone metabolism as indicated by increased IGF-1 and small decreases in TRAP. Individuals who consumed soy showed increases in IGF-1 (P < 0.001) levels to a greater extent than casein as well as a tendency to decrease TRAP (P = 0.06). As a whole, functional food, soy and its isoflavones may work similarly to tamoxifen, a well-established breast cancer chemopreventive agent, in that it has weak estrogenic and anti-estrogenic effects.30 It is known that tamoxifen acts as an estrogenic agent in bone, reducing postmenopausal bone loss. Soy isoflavones are structurally similar to tamoxifen; thus, it can be one postulated mechanism of action.
In the current study, biomarkers of bone health improved over the three-month period under both experimental conditions. High levels of BALP in older individuals are associated with an increased level of bone turnover.31 Our results show a significant time effect (P = 0.009) on BALP, indicating that both treatments decrease BALP over time, which is in accordance with previous research demonstrating that high protein diets can attenuate blood biomarkers associated with bone turnover, including BALP.32 There was no significant treatment × time interaction, indicating that decreases in BALP were not different between the groups and most likely due to protein consumption and not isoflavone content.
A significant time effect (P < 0.001) indicates that both protein regimens significantly increased IGF-1. The significant treatment × time interaction (P = 0.011) indicates that the magnitude of change in IGF-1 was greater in the soy group than in the casein group, suggesting that soy protein is more effective at increasing IGF-1 compared to casein protein. In vitro studies suggest that soy may play a part in anabolic processes due to its isoflavone content and can significantly increase mRNA levels of both IGF-1 and IGF-1 receptors.33 Moreover, our laboratory has previously demonstrated that soy protein effectively increases IGF-1, more so than milk protein, in clinical trials in both men and women.13,34 Our animal studies have also shown that increases in IGF-1 are greater in rats consuming a diet supplemented with soy versus casein protein.28 These results suggest that while protein, regardless of its source, has anabolic properties such as increasing IGF-1, additional benefits from soy may be attributed to its isoflavone content. This is further supported by animal studies showing that diets supplemented with soy isoflavones are sufficient to improve indices of bone health and increase IGF-1.28,35 Rudman et al.36 demonstrated that increasing plasma IGF-1 levels of men over 65 years of age who previously had low levels of IGF-1 resulted in significantly increased lumbar vertebral bone density, in addition to increases in muscle mass and a reduction in adiposity.
Soy isoflavones are thought to inhibit protein tyrosine kinase, suppress angiogenesis, have antioxidant effects,37 and prevent cell growth by interfering with signal transduction.38 Genistein itself binds to ERβ, initiates gene transcription of ERα, inhibits DNA topoisomerases I and II, and inhibits 17β-hydroxysteroid dehydrogenase.39 There is some evidence that isoflavones in vitro may also inhibit aromatase and 5α-reductase,40 the rate-limiting enzyme in estrogen synthesis in humans. Thus, these data suggest that one underlying mechanism by which isoflavones act is hormonal, but this is not likely the sole mechanism.
The effects of growth hormone on bone are likely mediated locally through IGFs.41 Bone cells synthesize both IGF-I and IGF-II, while IGF-I is more potent in stimulating osteoblasts,42 increasing collagen synthesis, and matrix apposition.43 Serum IGF-I declines with age in both sexes44 but also has been shown to decline immediately after menopause.45 Administration of IGF-I stimulates bone turnover,46 similar to growth hormone,47 and is thought to play a role in regulating bone remodelling.48 Additionally, IGF-I concentrations are correlated positively with bone mass in pre-,45 peri-,49 and post-48 menopausal women, but are lower in osteoporotics.50 Whether this decline is due to estrogen deficiency51 or to aging per se has not been resolved. Additionally, though IGF-I levels have been reported to have a positive association with different types of cancer,52 it is important to note that this is true in the case of abnormally high levels of IGF-I, but not in the case of increased IGF-I levels within the normal limits.
This story is complicated by the finding that IGF-I, but not growth hormone,47 stimulates the production of 1,25(OH)2 vitamin D in vivo,46 likely through enhancing renal 1α-hydroxylase activity. Complicating this picture further is the influence of binding proteins on IGF action. The major serum binding protein for IGF-I, IGFBP-3,53 decreases with age, but these changes are not necessarily associated with decreases in estrogen. In perimenopausal women, IGF-I is inversely related to serum IGFBP-2 and is directly related to IGFBP-3, despite the lack of direct relation of IGFBP-2 to bone density.49 In contrast, serum IGFBP-3 concentrations were reduced in young osteoporotic men,54 suggesting a role of IGF binding proteins in addition to IGF-I in bone metabolism. Circulating IGFBPs not only may influence the effects of IGF-I on bone, but also other hormones may alter the concentration of these binding proteins or modulate IGF receptors.55 We have published a study28 examining the effects of feeding soy protein with isoflavones versus reduced isoflavones to ovariectomized rats with established bone loss. The results indicated that IGF-I mRNA (isolated from femurs) transcripts were increased (P < 0.001) by both diets, suggesting an effect of soy on protein synthesis, but by greater percentages (P < 0.05) with soy and its natural isoflavone content. Similarly, in the present study, though both groups received the same amount of protein, soy and its isoflavones increased IGF-I to a greater extent. These results confirm the additive effects of soy isoflavones on increasing IGF-I within normal limits.
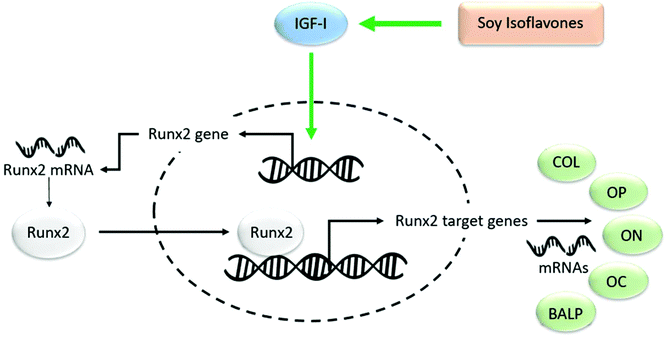
It is postulated that soy isoflavones exhibit positive effects on bone through increased transcription of the Runx2 gene.56 Increased transcription of Runx2 increases mRNA production of Runx2 target genes, namely type I collagen, osteopontin, osteocalcin, osteonectin, and alkaline phosphatase57,58 (Fig. 1). Increased production of these genes promotes differentiation of progenitor cells to osteoblasts. Furthermore, genistein specifically is capable of downregulating 18 bone turnover-related genes, including IL-6, IL-1β, and MMP13.59
Both casein and soy contributed to significant increases in triglycerides (P = 0.018) and significant decreases in BALP (P = 0.009), which gives different insights into the properties of soy and casein within this population of ethnically diverse men and women between the ages of 27 and 87. We have no way of explaining significant increases in TG. Other studies, including some of our own, have not supported this conclusion.16,60,61 Soy has been shown to decrease intestinal absorption of cholesterol, increase fecal sterol elimination, and increase the production of bile acids from cholesterol.62 Reasons for discrepancies in results may include the fluctuation in the levels of isoflavones between studies, the ratios of isoflavones used in each study, and the form of soy used. When these two elements (quantity and ratio) become consistent, the authors believe that isoflavones will only be beneficial in the context of whole soy or soy protein, not in an isolated form. However, this comes from years of experience working with soy and seeing the inconsistencies as a result; consequentially, this is speculative and needs to be confirmed. These results indicate that soy protein with its isoflavones should lower cholesterol levels, and as such it remains an FDA approved health claim.
Other investigators, such as Setchell et al.,63–66 believe that humans are either equol converters or non-equol converters, yet there are other investigators, such as Anderson et al.,67 who suggest that soy isoflavones behave differently in the presence and absence of estrogen; for example, they exert positive effects in the absence of estrogen and negative or no effects in the presence of estrogen on tissues such as bone. We have performed one 3-month study in which postmenopausal women not on HRT saw greater benefits in bone health with soy protein supplementation. An additional factor may be that when soy and its isoflavones are consumed on a regular basis, physical activity will make a difference. The rationale for this statement is that we noticed a significant difference in the right femoral BMD of women who consumed 40 g soy protein with normal isoflavone content for one year (unpublished data). We also noticed that all the women were right leg dominant; hence, this suggests that physical activity has some association with soy. Again, this is speculative and we have no other way of interpreting our earlier findings.
This wide age range of participants allowed us to effectively target the general population; however, this may be a limitation of the study as well. It should be noted that in this study a true placebo is missing, and casein is used as a comparative control, which is one of the limitations of this study. Hence, any inferences regarding time effects could be attributed to the treatments. Furthermore, a true placebo group should be implemented in similar future studies. Having said that, our data show a significant time effect on body fat percentage (P < 0.001), indicating that both treatments decrease body fat percentage over time. However, there was no significant interaction, indicating that the magnitude of change in body fat percentage was not different between the two groups. Thus, decreases in body fat percentage may be due to the protein content of both treatments and not soy isoflavones. This may be due, in part, to the satiating effects of protein.68–70 Additionally, clinical studies have demonstrated that increased protein consumption is associated with improvements in body composition, including a reduction in body fat percentage and increases in lean mass.71 In our study, changes in body fat percentage were accompanied by no significant differences in body weight or BMI, indicating potential retention or a possible increase in muscle mass, although these results cannot be conclusively made since muscle mass was not assessed.
Our findings indicate no relationship between either protein regimen and levels of inflammation, measured here through CRP. With our observed significant improvements in biomarkers of bone health, this does not indicate any relationship between levels of inflammation and bone health in this representation of the general population. However, since this study did not measure bone density, further studies are warranted to support this conclusion.
Conclusions
It is well established that both cardiovascular disease and osteoporosis majorly affect the quality of life in men and women; therefore, in this study we looked at how soy protein supplementation may affect the markers of bone metabolism and lipid profiles in the general population. Our study population was highly representative of the general population with a wide age range and an almost equal number of both men and women in each treatment group. Soy protein consumption led to significant reductions in percent body fat, and as expected, protein supplementation increased IGF-1, irrespective of the source. Nonetheless, soy protein was able to increase the levels of IGF-1 significantly more than casein. Our findings suggest that a daily supplement of soy protein has positive effects on some indices of bone metabolism, but has little effect on total-, LDL-, and HDL-cholesterol in normolipidemic and mildly hyperlipidemic men and women. The results from this analysis suggest that daily supplementation of soy protein has positive effects on bone metabolism as indicated by increased IGF-I and small decreases in TRAP, but little effect on blood cholesterol levels. However, a limitation of this study is that only bone biomarkers were assessed and not bone density. Therefore, it is difficult to say whether improvements in biomarkers are reflective of improvements in bone density. Based on the biomarkers of bone turnover, our data suggest that soy protein with its isoflavones can favorably alter bone metabolism.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors of this manuscript would like to acknowledge DuPont Nutrition and Health for providing both supplements used in this study. Additionally, they thank Oklahoma Center for the Advancement of Science and Technology for funding this study.References
- E. Jaul and J. Barron, Age-Related Diseases and Clinical and Public Health Implications for the 85 Years Old and Over Population, Front. Public Health, 2017, 5, 335 CrossRef PubMed.
- R. H. Nelson, Hyperlipidemia as a risk factor for cardiovascular disease, Prim. Care, 2013, 40, 195–211 CrossRef PubMed.
- G. N. Farhat and J. A. Cauley, The link between osteoporosis and cardiovascular disease, Clin. Cases Miner. Bone Metab., 2008, 5, 19–34 Search PubMed.
- R. Boukhris and K. L. Becker, Calcification of the aorta and osteoporosis. A roentgenographic study, J. Am. Med. Assoc., 1972, 219, 1307–1311 CrossRef CAS.
- D. Sprini, G. B. Rini, L. Di Stefano, L. Cianferotti and N. Napoli, Correlation between osteoporosis and cardiovascular disease, Clin. Cases Miner. Bone Metab., 2014, 11, 117–119 Search PubMed.
- S. Ishii, et al., C-reactive protein, bone strength, and nine-year fracture risk: data from the Study of Women's Health Across the Nation (SWAN), J. Bone Miner. Res., 2013, 28, 1688–1698 CrossRef CAS PubMed.
- T. T. Idicula, J. Brogger, H. Naess, U. Waje-Andreassen and L. Thomassen, Admission C-reactive protein after acute ischemic stroke is associated with stroke severity and mortality: the ‘Bergen stroke study’, BMC Neurol, 2009, 9, 18 CrossRef PubMed.
- K. Nakamura, et al., C-reactive protein predicts incident fracture in community-dwelling elderly Japanese women: the Muramatsu study, Osteoporosis Int., 2011, 22, 2145–2150 CrossRef CAS PubMed.
- J. A. Pasco, et al., High-sensitivity C-reactive protein and fracture risk in elderly women, J. Am. Med. Assoc., 2006, 296, 1353–1355 CrossRef CAS PubMed.
- U.S. Food &, Drug Administration (2018).
- D. L. Alekel, et al., The soy isoflavones for reducing bone loss (SIRBL) study: a 3-y randomized controlled trial in postmenopausal women, Am. J. Clin. Nutr., 2010, 91, 218–230 CrossRef CAS PubMed.
- K. C. Maki, et al., Effects of soy protein on lipoprotein lipids and fecal bile acid excretion in men and women with moderate hypercholesterolemia, J. Clin. Lipidol., 2010, 4, 531–542 CrossRef PubMed.
- B. H. Arjmandi, et al., One year soy protein supplementation has positive effects on bone formation markers but not bone density in postmenopausal women, Nutr. J., 2005, 4, 8 CrossRef PubMed.
- H. Lee, R. Choue and H. Lim, Effect of soy isoflavones supplement on climacteric symptoms, bone biomarkers, and quality of life in Korean postmenopausal women: a randomized clinical trial, Nutr. Res. Pract., 2017, 11, 223–231 CrossRef CAS PubMed.
- R. M. Weggemans and E. A. Trautwein, Relation between soy-associated isoflavones and LDL and HDL cholesterol concentrations in humans: a meta-analysis, Eur. J. Clin. Nutr., 2003, 57, 940–946 CrossRef CAS PubMed.
- S. C. Campbell, D. A. Khalil, M. E. Payton and B. H. Arjmandi, One-year soy protein supplementation does not improve lipid profile in postmenopausal women, Menopause, 2010, 17, 587–593 Search PubMed.
- S. Levis, et al., Soy isoflavones in the prevention of menopausal bone loss and menopausal symptoms: a randomized, double-blind trial, Arch. Intern. Med., 2011, 171, 1363–1369 CrossRef CAS.
- A. García-Martín, et al., [Effect of milk product with soy isoflavones on quality of life and bone metabolism in postmenopausal Spanish women: randomized trial], Med. Clin., 2012, 138, 47–51 CrossRef PubMed.
- J. Huber, M. Imhof and M. Schmidt, Effects of soy protein and isoflavones on circulating hormone concentrations in pre- and post-menopausal women: a systematic review and meta-analysis, Hum. Reprod. Update, 2010, 16, 110–111 CrossRef CAS PubMed ; author reply 111–112.
- H. Ahn and Y. K. Park, Soy isoflavone supplementation improves longitudinal bone growth and bone quality in growing female rats, Nutrition, 2017, 37, 68–73 CrossRef CAS PubMed.
- E. Anupongsanugool, S. Teekachunhatean, N. Rojanasthien, S. Pongsatha and C. Sangdee, Pharmacokinetics of isoflavones, daidzein and genistein, after ingestion of soy beverage compared with soy extract capsules in postmenopausal Thai women, BMC Clin. Pharmacol., 2005, 5, 2 CrossRef PubMed.
- J. Yu, X. Bi, B. Yu and D. Chen, Isoflavones: Anti-Inflammatory Benefit and Possible Caveats, Nutrients, 2016, 8, E361 CrossRef PubMed.
- E. A. Lucas, D. A. Khalil, B. P. Daggy and B. H. Arjmandi, Ethanol-extracted soy protein isolate does not modulate serum cholesterol in golden Syrian hamsters: a model of postmenopausal hypercholesterolemia, J. Nutr., 2001, 131, 211–214 CrossRef CAS PubMed.
- D. A. Khalil, et al., Soy isoflavones may protect against orchidectomy-induced bone loss in aged male rats, Calcif. Tissue Int., 2005, 76, 56–62 CrossRef CAS PubMed.
- D. L. Alekel, et al., Isoflavone-rich soy protein isolate attenuates bone loss in the lumbar spine of perimenopausal women, Am. J. Clin. Nutr., 2000, 72, 844–852 CrossRef CAS PubMed.
- S. M. Potter, et al., Soy protein and isoflavones: their effects on blood lipids and bone density in postmenopausal women, Am. J. Clin. Nutr., 1998, 68, 1375S–1379S CrossRef CAS PubMed.
- J. A. Baum, et al., Long-term intake of soy protein improves blood lipid profiles and increases mononuclear cell low-density-lipoprotein receptor messenger RNA in hypercholesterolemic, postmenopausal women, Am. J. Clin. Nutr., 1998, 68, 545–551 CrossRef CAS PubMed.
- B. H. Arjmandi, et al., Bone-sparing effect of soy protein in ovarian hormone-deficient rats is related to its isoflavone content, Am. J. Clin. Nutr., 1998, 68, 1364S–1368S CrossRef CAS.
- B. H. Arjmandi, et al., Soy protein has a greater effect on bone in postmenopausal women not on hormone replacement therapy, as evidenced by reducing bone resorption and urinary calcium excretion, J. Clin. Endocrinol. Metab., 2003, 88, 1048–1054 CrossRef CAS.
- S. Ziaei and R. Halaby, Dietary Isoflavones and Breast Cancer Risk, Medicines, 2017, 4, E18 CrossRef PubMed.
- K. Mukaiyama, et al., Elevation of serum alkaline phosphatase (ALP) level in postmenopausal women is caused by high bone turnover, Aging: Clin. Exp. Res., 2015, 27, 413–418 Search PubMed.
- D. Jesudason, B. C. Nordin, J. Keogh and P. Clifton, Comparison of 2 weight-loss diets of different protein content on bone health: a randomized trial, Am. J. Clin. Nutr., 2013, 98, 1343–1352 CrossRef CAS.
- W. Zheng, M. L. Hemker, M. Xie, S. T. Soukup and P. Diel, Anabolic Activity of a Soy Extract and Three Major Isoflavones in C2C12 Myotubes, Planta Med., 2018, 84, 1022–1029 CrossRef CAS PubMed.
- B. H. Arjmandi, et al., Soy protein may alleviate osteoarthritis symptoms, Phytomedicine, 2004, 11, 567–575 CrossRef CAS PubMed.
- W. W. Wong, et al., Soy isoflavone supplementation and bone mineral density in menopausal women: a 2-y multicenter clinical trial, Am. J. Clin. Nutr., 2009, 90, 1433–1439 CrossRef CAS PubMed.
- D. Rudman, et al., Relations of endogenous anabolic hormones and physical activity to bone mineral density and lean body mass in elderly men, Clin. Endocrinol., 1994, 40, 653–661 CrossRef CAS PubMed.
- M. S. Kurzer and X. Xu, Dietary phytoestrogens, Annu. Rev. Nutr., 1997, 17, 353–381 CrossRef CAS.
- K. Higashi and H. Ogawara, Daidzein inhibits insulin- or insulin-like growth factor-1-mediated signaling in cell cycle progression of Swiss 3T3 cells, Biochim. Biophys. Acta, 1994, 1221, 29–35 CrossRef CAS.
- Y. Huang, et al., Decreased circulating levels of tumor necrosis factor-alpha in postmenopausal women during consumption of soy-containing isoflavones, J. Clin. Endocrinol. Metab., 2005, 90, 3956–3962 CrossRef CAS PubMed.
- H. Adlercreutz, et al., Inhibition of human aromatase by mammalian lignans and isoflavonoid phytoestrogens, J. Steroid Biochem. Mol. Biol., 1993, 44, 147–153 CrossRef CAS.
- J. I. Jones and D. R. Clemmons, Insulin-like growth factors and their binding proteins: biological actions, Endocr. Rev., 1995, 16, 3–34 CAS.
- R. W. Zhang, D. J. Simmons, R. S. Crowther, S. Mohan and D. J. Baylink, Contribution of marrow stromal cells to the regulation of osteoblast proliferation in rats: evidence for the involvement of insulin-like growth factors, Bone Miner., 1991, 13, 201–215 CrossRef CAS.
- J. M. Hock, M. Centrella and E. Canalis, Insulin-like growth factor I has independent effects on bone matrix formation and cell replication, Endocrinology, 1988, 122, 254–260 CrossRef CAS PubMed.
- H. Yamamoto, M. Sohmiya, N. Oka and Y. Kato, Effects of aging and sex on plasma insulin-like growth factor I (IGF-I) levels in normal adults, Acta Endocrinol., 1991, 124, 497–500 CAS.
- E. Romagnoli, et al., Effect of estrogen deficiency on IGF-I plasma levels: relationship with bone mineral density in perimenopausal women, Calcif. Tissue Int., 1993, 53, 1–6 CrossRef CAS PubMed.
- T. Bianda, et al., Effects of short-term insulin-like growth factor-I or growth hormone treatment on bone turnover, renal phosphate reabsorption and 1,25 dihydroxyvitamin D3 production in healthy man, J. Intern. Med., 1997, 241, 143–150 CrossRef CAS PubMed.
- K. Brixen, et al., Short-term treatment with growth hormone stimulates osteoblastic and osteoclastic activity in osteopenic postmenopausal women: a dose response study, J. Bone Miner. Res., 1995, 10, 1865–1874 CrossRef CAS PubMed.
- S. Boonen, et al., Relationship between baseline insulin-like growth factor-I (IGF-I) and femoral bone density in women aged over 70 years: potential implications for the prevention of age-related bone loss, J. Am. Geriatr. Soc., 1996, 44, 1301–1306 CrossRef CAS.
- M. Nasu, et al., Effect of natural menopause on serum levels of IGF-I and IGF-binding proteins: relationship with bone mineral density and lipid metabolism in perimenopausal women, Eur. J. Endocrinol., 1997, 136, 608–616 CAS.
- C. Wüster, W. F. Blum, S. Schlemilch, M. B. Ranke and R. Ziegler, Decreased serum levels of insulin-like growth factors and IGF binding protein 3 in osteoporosis, J. Intern. Med., 1993, 234, 249–255 CrossRef PubMed.
- A. J. Weissberger, K. K. Ho and L. Lazarus, Contrasting effects of oral and transdermal routes of estrogen replacement therapy on 24-hour growth hormone (GH) secretion, insulin-like growth factor I, and GH-binding protein in postmenopausal women, J. Clin. Endocrinol. Metab., 1991, 72, 374–381 CrossRef CAS PubMed.
- A. Grimberg, Mechanisms by which IGF-I may promote cancer, Cancer Biol. Ther., 2003, 2, 630–635 CAS.
- R. C. Baxter and C. T. Cowell, Diurnal rhythm of growth hormone-independent binding protein for insulin-like growth factors in human plasma, J. Clin. Endocrinol. Metab., 1987, 65, 432–440 CrossRef CAS PubMed.
- A. G. Johansson, et al., Reduced serum levels of the growth hormone-dependent insulin-like growth factor binding protein and a negative bone balance at the level of individual remodeling units in idiopathic osteoporosis in men, J. Clin. Endocrinol. Metab., 1997, 82, 2795–2798 CAS.
- R. Bouillon, Growth hormone and bone, Horm. Res., 1991, 36(Suppl 1), 49–55 CrossRef.
- J. Dai, et al., Genistein promotion of osteogenic differentiation through BMP2/SMAD5/RUNX2 signaling, Int. J. Biol. Sci., 2013, 9, 1089–1098 CrossRef PubMed.
- Z. Hou, et al., KLF2 regulates osteoblast differentiation by targeting of Runx2, Lab. Invest., 2019, 99, 271–280 CrossRef CAS PubMed.
- G. Z. Yang, et al., [Effect of overexpression of transcription factor Runx2 and Osterix on osteogenic differentiation of endothelial cells], Shanghai Kouqiang Yixue, 2017, 26, 353–357 Search PubMed.
- J. E. Pie, et al., Effect of genistein on the expression of bone metabolism genes in ovariectomized mice using a cDNA microarray, J. Nutr. Biochem., 2006, 17, 157–164 CrossRef CAS PubMed.
- Y. Wang, P. J. Jones, L. M. Ausman and A. H. Lichtenstein, Soy protein reduces triglyceride levels and triglyceride fatty acid fractional synthesis rate in hypercholesterolemic subjects, Atherosclerosis, 2004, 173, 269–275 CrossRef CAS.
- M. Nishimura, et al., Improvement of Triglyceride Levels through the Intake of Enriched-β-Conglycinin Soybean (Nanahomare) Revealed in a Randomized, Double-Blind, Placebo-Controlled Study, Nutrients, 2016, 8(8), 491 CrossRef PubMed.
- L. Normén, P. Dutta, A. Lia and H. Andersson, Soy sterol esters and beta-sitostanol ester as inhibitors of cholesterol absorption in human small bowel, Am. J. Clin. Nutr., 2000, 71, 908–913 CrossRef PubMed.
- K. D. Setchell, N. M. Brown and E. Lydeking-Olsen, The clinical importance of the metabolite equol-a clue to the effectiveness of soy and its isoflavones, J. Nutr., 2002, 132, 3577–3584 CrossRef CAS PubMed.
- K. D. Setchell and C. Clerici, Equol: history, chemistry, and formation, J. Nutr., 2010, 140, 1355S–1362S CrossRef CAS.
- K. D. Setchell, et al., Dietary factors influence production of the soy isoflavone metabolite s-(-)equol in healthy adults, J. Nutr., 2013, 143, 1950–1958 CrossRef CAS PubMed.
- N. M. Brown, et al., S-(-)equol production is developmentally regulated and related to early diet composition, Nutr. Res., 2014, 34, 401–409 CrossRef CAS.
- J. J. Anderson and S. C. Garner, Phytoestrogens and bone, Baillieres Clin. Endocrinol. Metab., 1998, 12, 543–557 CrossRef CAS.
- M. S. Westerterp-Plantenga, S. G. Lemmens and K. R. Westerterp, Dietary protein - its role in satiety, energetics, weight loss and health, Br. J. Nutr., 2012, 108(Suppl 2), S105–S112 CrossRef CAS.
- S. M. Nickols-Richardson, M. D. Coleman, J. J. Volpe and K. W. Hosig, Perceived hunger is lower and weight loss is greater in overweight premenopausal women consuming a low-carbohydrate/high-protein vs high-carbohydrate/low-fat diet, J. Am. Diet. Assoc., 2005, 105, 1433–1437 CrossRef PubMed.
- T. L. Halton and F. B. Hu, The effects of high protein diets on thermogenesis, satiety and weight loss: a critical review, J. Am. Coll. Nutr., 2004, 23, 373–385 CrossRef PubMed.
- K. M. Beavers, et al., Effect of protein source during weight loss on body composition, cardiometabolic risk and physical performance in abdominally obese, older adults: a pilot feeding study, J. Nutr., Health Aging, 2015, 19, 87–95 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2020 |