DOI:
10.1039/C9FO02157D
(Paper)
Food Funct., 2020,
11, 534-543
Green tea polyphenol epigallocatechin-3-gallate improves the antioxidant capacity of eggs
Received
16th September 2019
, Accepted 5th December 2019
First published on 6th December 2019
Abstract
It has been shown that supplementation of layers’ diets with epigallocatechin-3-gallate (EGCG) can improve egg albumen quality, but the underlying mechanisms behind this response are unclear. In this study, we investigate the effect of EGCG on egg antioxidative activity, free amino acid and fatty acid profiles, and the underlying relationship between the EGCG and oxidant-sensitive mitogen-activated protein kinase (MAPK) signaling pathway in laying hens. 288 hens (35-weeks-old) were fed 0 and 165 mg kg−1 of EGCG diets over 8 weeks. EGCG led to an increase in the albumen height, Haugh unit, and activity of glutathione S-transferase (GST) and a reduction in MDA content in plasma (P < 0.05). Egg white tryptophan and yolk carotenoid content was also increased by EGCG (P < 0.05). Eggs from EGCG fed layers had higher total antioxidant capacity (T-AOC), reducing power (RP), and oxygen radical absorbance capacity (ORAC), and lower albumen and yolk MDA content (P < 0.05). Also, liver gene and protein expression of P-38MAPK, nuclear factor erythroid 2-related 2 (Nrf2) and hemeoxygenase 1 (HO-1) was up-regulated by EGCG. Our findings suggest that dietary EGCG increased the antioxidant activity of eggs and regulated the MAPK/Nrf2 signaling pathway.
Introduction
As an important source of nutrients, eggs have several biological activities such as anti-bacterial, anti-fungal, anti-viral, anti-carcinogenic, anti-mutagenic, anti-inflammatory, anti-hypertensive, and antioxidant properties.1,2 Eggs have various natural occurring antioxidant compounds including ovalbumin, ovotransferrin, lysozyme, peptides and amino acids in egg white, as well as phosvitin, vitamin E (α-tocopherol), carotenoids, and free aromatic amino acids in egg yolk.3,4
Epigallocatechin-3-gallate (EGCG), as the most abundant biologically active substance (about 50–70% of the catechin) in green tea, is an effective scavenger of reactive oxygen series (ROS) in vitro.5–7 It has been observed that supplementing laying hen diets with a green tea extract diet (400 to 600 mg kg−1 tea extract or 200 to 400 mg kg−1 EGCG) improved egg production, feed efficiency,8–10 egg white quality and the antioxidant capacity of eggs during the late laying period or heat stress.9,11 However, the majority of the tea polyphenols or EGCG studies have investigated changes in lipid metabolism and anti-oxidant and pro-oxidant functions in humans or mice models, whereas the effect of EGCG on the antioxidant capacity of eggs and its mechanism is rather scarce. Nuclear factor erythroid 2-related 2 (Nrf2) is a key transcriptional factor that upregulates antioxidant response element-mediated expression of antioxidant enzymes and cytoprotective proteins;12 once activated, it translocates from the cytoplasm to the nucleus, inducing the transcription of target genes involved in the regulation of the antioxidant defense system, such as hemeoxygenase 1 (HO-1), glutathione S-transferase (GST), and NAD(P)H:quinone oxidoreductase 1 (NQO1). Moreover, compelling evidence concluded that EGCG may modulate antioxidant defense by activating the Nrf2 signaling pathway.6,13–15 So, we hypothesized that dietary EGCG supplementation of layers’ diets would increase the antioxidant capacity of their eggs as a consequence of up-regulation of the Nrf2 signaling pathway.
Therefore, the aim of this study is to investigate the response of hens to dietary EGCG supplementation by evaluation of the egg antioxidant capacity and Nrf2 signaling pathway expression.
Materials and methods
Chemicals
EGCG (>98% purity), anhydrous monobasic sodium phosphate, trichloroacetic acid, 6-hydraoxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (trolox), amino acid standards, and ethanolamine (EA) were obtained from Sigma (Sigma-Aldrich, St Louis, MO, USA). All antibodies were purchased from Cell signaling Technology (Danvers, MA, USA).
Animals and study design
All animal procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of Sichuan Agricultural University (SYXK2014-187) and approved by the Animal Ethics Committee of the State Council of the People's Republic of China. 288 35-week-old Lohmann laying hens were chosen from 2200 hens and divided into 2 groups according to the similar average egg production rate. One group was given a basal diet (control group: CON) and the other group was given a 165 mg kg−1 EGCG (EGCG group) supplemented diet for 8 weeks [this dose is according to those used in previous in vivo studies].9,10 There were 12 replicates for each treatment with 4 adjacent cages (3 hens per cage, 38.1 cm width × 50 length × 40 height) representing a replicate. As shown in Table 1, all diets were formulated complying with the recommendations of published NRC (1994) and manual of the Lohmann hens and given in mashed form. Both groups were kept in a windowless room at a temperature of 22 °C, humidity of 60%, and light of 16 h. Feed and water were supplied to allow ad libitum consumption throughout the whole experiment. The EGCG content in the experimental group was analyzed using reversed phase HPLC with UV-absorbance detection and it was 163, 168, 161, 166, 165, 171, 164 and 167 mg kg−1 through week 1 to week 8, respectively.
Table 1 Composition and nutrient levels of the basal diet (as-fed basis)
| Ingredients |
Content, % |
|
Provided per kilogram of diet: vitamin A, 8000 IU; vitamin D, 1600 IU; vitamin E, 5 IU; vitamin B1, 0.8 mg; vitamin B2, 2.5 mg; vitamin B6, 0.1 mg; vitamin B12, 0.009 mg; D-pantothenic acid, 2.2 mg, folic acid 0.25 mg; nicotinic acid 20 mg; biotin 0.1 mg.
Provided per kilogram of diet: 6 mg (MnO2); 80 mg (ZnSO4·7H2O); 8 mg (CuSO4·5H2O); 60 mg (FeSO4·7H2O); 0.35 mg (KI) and 0.3 mg (Na2SeO3·5H2O).
Calculated by NRC (1994).
|
| Corn |
59.064 |
| Wheat bran |
3.867 |
| Soybean oil |
1.500 |
| Soybean meal, 43% CP |
15.236 |
| Corn gluten meal |
5.000 |
| Corn DDGS |
5.000 |
| Limestone |
8.603 |
| CaHPO4 |
0.941 |
| NaCl |
0.250 |
| NaHCO3 |
0.100 |
|
L-Lysine |
0.162 |
|
DL-Met |
0.012 |
| Choline chloride |
0.100 |
| Vitamin premixa |
0.015 |
| Mineral premixb |
0.150 |
| Analyzed nutrient levels, % |
| Metabolizable energyc, Mcal kg−1 |
2.69 |
| Crude protein |
16.00 |
| Ether exact |
4.39 |
| Crude fiber |
2.69 |
| Calcium |
3.70 |
| Available phosphorus |
0.36 |
| Lysine |
0.65 |
| Methionine |
0.33 |
| Methionine + cysteine |
0.65 |
Productive performance and blood characteristics
Zootechnical performance of laying hens.
Feed consumption, egg production rate, and egg weight were recorded daily. The feed conversion ratio (FCR) was calculated by the ratio of feed consumed to per kilogram of eggs produced.
Plasma antioxidant enzyme activity assay.
Samples of blood were obtained via the wing vein of 1 hen from each replicate (12 replicates per treatment, 1 layer per replicate). After centrifugation (3000g, 10 min, 4 °C), plasma was separated and then stored at −20 °C pending further parameter analysis. Plasma malondialdehyde (MDA) concentration and antioxidant enzyme activities [total antioxidant capacity (T-AOC), GST, glutathione peroxidase (GPx), superoxide dismutase (SOD)] were determined by means of commercial kits (Nanjing Jiancheng Biotechnology, Nanjing, China).
Egg quality parameters
Egg samples (12 replicates per treatment, 3 eggs per replicate) were collected to analyze the egg quality at the end of the supplementation period. The shell quality was determined by observing the shell thickness, shell strength and shell color. Shell strength was measured by using egg shell force gauge model II, whereas shell strength was determined by using an eggshell thickness gauge (Robotmation Co., Ltd, Tokyo, Japan). A Minolta colorimeter (Konica Minolta Sensing Inc., Osaka, Japan) was used to measure the egg shell color value [lightness (L*), redness (a*), and yellowness (b*)]. Egg internal quality [including Haugh unit (HU), albumen height, and yolk color] was analyzed via an Egg Multi-tester (EMT-7300, Robotmation Co., Ltd, Tokyo, Japan). The yolk index value was calculated as 100 × [yolk height (cm)/yolk diameter (cm)]. The yolk ratio was calculated as yolk weight (g)/egg weight (g) × 100. The albumin ratio was computed as 100 × [albumin weight (g)/egg weight (g)].
Evaluation of the antioxidant substances and antioxidant capacities of eggs
Egg samples (12 replicates per treatment, 1 egg per replicate) were also collected at the end of the trial, and egg yolk and egg white were separated carefully and freeze-dried to measure the chemical composition and antioxidant capacity of eggs.
Analysis of amino acids in egg yolk and egg white.
Freeze-dried egg white and yolk powders were mixed with 88% formic acid and incubated overnight. The amino acid profiles were then determined by using an L-8900 automatic Amino Acid Analyzer. Each egg yolk and egg white were analyzed in triplicate.
Fatty acid profiles of egg yolk.
Egg yolk total lipids were extracted using chloroform/methanol (2/1, v/v) containing 0.01% butylated hydroxytoluene. Egg yolk lipids were esterified in hydrochloric acid (0.35 mmol L−1) and dimethoxypropane (0.40 mol L−1) containing methanol by heating for 35 min at 70 °C. The resulting fatty acid methyl esters were analyzed by using a Varian 450 gas chromatograph (GC) (GC-2010 Plus), using a capillary column (60 m × 0.25 mm diameters × 0.25 μm film thickness) and a flame ionization detector (FID). The temperature parameters and other settings used for the analysis were according the method of ref. 16.
Extraction and analysis of carotenoids and α-tocopherol.
Egg yolk total carotenoids were determined by spectrophotometry as described previously in ref. 17. The total carotenoid content was determined by summing up with the identified carotenoids, including β-apocarotenoic ester (E1% 2640), canthaxanthin (E1% 2200), lutein (E1% 2550), zeaxanthin (E1% 2540), and β-carotene (E1% 2540) on a spectrophotometer at 457, 466, and 450 nm wavelength, respectively. Moreover, the content of α-tocopherol was extracted with hot ethanol and determined using a standard Reverse Phase High Performance Liquid Chromatograph following the previous method.18
Oxygen radical absorbance capacity and reducing power assay.
The oxygen radical absorbance capacity (ORAC) assay was performed following the description of previous reports.19,20 The reducing power (RP) of egg yolk and white samples was measured according to the method as presented by the previous study.21 Briefly, the sample (1 mg mL−1) was added to 2.5 mL phosphate buffer saline (0.2 M) and K3[Fe(CN)6] solution (1%, w/v). After the mixture was incubated in a water bath (50 °C) for 20 min, the TCA solution (10%, w/v) was added and 2.5 ml of the supernatant was collected after centrifugation (10 min, 3000g). The mixture was then measured for absorbance at 700 nm wavelength after 10 min at room temperature.
Determination of T-AOC and MDA in eggs.
The T-AOC content in egg white and yolk was determined by colorimetric enzymatic assays and MDA concentration was measured by using malondialdehyde (MDA) by the 2-thiobarbituric acid (TBA) method using assay kits (T-AOC, A015-2-1; MDA, A003-1), which were purchased from Nanjing Jiancheng Bioengineering Institute of China. All assays were conducted and interpreted according to the manufacturer's manual without any modification.
RNA extraction and real-time PCR
Hens were randomly chosen (12 replicates per treatment, 1 hen per replicate) and sacrificed by cervical dislocation at the end of the experiment. Liver segments were immediately removed for real-time PCR. Total RNA was extracted by using a TRIzol reagent kit (Invitrogen, CA, USA). Forward and reverse primers directed towards Gallus Nrf2, HO-1, NQO1, P38-MAPK, ERK1/2, JNK, sMaf, and β-actin are described in Table 2, and β-actin gene was used as the housekeeping gene. Real-time PCR assay was performed in a 96-well plate using a SYBR Premix Ex TaqII and a 7500 fluorescence detection system (Applied Biosystems, Foster City, CA, USA). The relative quantification of mRNA expression was determined by the 2−ΔΔCT model, with the quantity of the control diet scale to 1.
Table 2 Gene-specific primers for real-time quantitative reverse transcription PCR
| Genes |
Primers (5′ to 3′) |
Genes numbera |
Product size, bp |
| Abbreviation indicated that: P38 MAPK = P38 mitogen activated protein kinases, ERK1/2 = extracellular regulated protein kinase 1 and 2, JNK = c-Jun N-terminal kinase, Nrf2 = nuclear factor erythroid-2 related factor 2, sMaf = small Maf, NQO1 = NAD(P)H: quinone dehydrogenase 1, HO-1 = heme oxygenase-1, β-actin = beta-actin (reference gene). GenBank accession number for sequence from which primers were designed. |
| Nrf2 |
Forward: TGTGTGTGATTCAACCCGACT |
NM_205117.1 |
143 |
| Reverse: TTAATGGAAGCCGCACCACT |
| HO-1 |
Forward: TTGGCAAGAAGCATCCAGA |
NM_205344.1 |
129 |
| Reverse: TCCATCTCAAGGGCATTCA |
| NQO1 |
Forward: GTTCAATGCCGTGCTCTCAC |
NM_001277619.1 |
146 |
| Reverse: CCGCTTCAATCTTCTTCTGC |
| P38-MAPK |
Forward: TGTGTTCACCCCTGCCAAGT |
AJ719744.1 |
149 |
| Reverse: GCCCCCGAAGAATCTGGTAT |
| ERK1/2 |
Forward: GGATCCCCTTCCCAAGAA |
NM_204150.1 |
108 |
| Reverse: CTTTGGGGTCAGCATTGG |
| JNK |
Forward: GCAGAAGCAAGCGTGACA |
XM_015288439.1 |
110 |
| Reverse: CGTTTCGCTCAAGGATGG |
| sMaf |
Forward: GCAGAAGCAAGCGTGACA |
NM_001044671 |
247 |
| Reverse: CGTTTCGCTCAAGGATGG |
| β-Actin |
Forward: TCAGGGTGTGATGGTTGGTATG |
NM_205518.1 |
152 |
| Reverse: TGTTCAATGGGGTACTTCAGGG |
Western blot analysis
The total protein content was extracted from liver and the protein content was determined and isolated by SDS-PAGE gel. After blocking for 1 h, membranes were incubated with rabbit polyclonal anti-phosphor-P38MAPK, anti-Nrf2, anti-HO-1, anti-phosphor-ERK1/2, anti-phosphor-JNK, and anti-GAPDH. After incubation with a goat anti-rabbit IgG-HRP conjugated to horseradish peroxidase for 1 h, the membranes were visualized with an ECL chemiluminescent substrate and images were obtained using an Odyssey Infrared Imaging System (Bio-Rad, CA, USA).
Statistical analysis
The data were subjected to one-way variance analysis (ANOVA), at a significant level of 5% using the GLM procedure of SAS 9.2 (SAS Institute). The results are presented as mean with their standard errors (SE). Comparison between two groups was performed using Student's t test.
Results
Production performance and egg quality
As shown in Tables 3 and 4, dietary EGCG supplementation improved the albumen height and Haugh unit (P < 0.05). No differences were observed in production performance (egg production rate, ADFI, egg weight, and FCR) and other egg quality parameters (strength, thickness and color of eggshell, yolk index, yolk color, albumen weight, yolk weight, and eggshell weight) among treatments (P > 0.05).
Table 3 Effect of dietary epigallocatechin-3-gallate supplementation on production performance of laying hensa
| Items |
CON |
EGCG |
SE |
P-Value |
|
Each mean represents 12 replicates, with 4 cages per replicate and 3 layers per cage.
|
| Egg production, % |
96.11 |
94.09 |
1.43 |
0.15 |
| Average daily feed intake, g |
103 |
105 |
2.42 |
0.45 |
| Egg weight, g |
59.8 |
59.2 |
0.52 |
0.35 |
| FCR |
1.86 |
1.88 |
0.02 |
0.51 |
Table 4 Effect of epigallocatechin-3-gallate supplementation on egg quality of laying hensa
| Items |
CON |
EGCG |
SE |
P-Value |
|
Each mean represents 12 replicates, with 3 eggs per replicate.
|
| Eggshell strength, kg cm−2 |
4.43 |
4.15 |
0.19 |
0.22 |
| Eggshell thickness, mm−2 |
37.71 |
37.42 |
0.69 |
0.42 |
| Haugh unit |
83.82 |
87.13 |
0.91 |
0.02 |
| Albumen height |
7.09 |
7.93 |
0.17 |
0.03 |
| Yolk color |
12.98 |
13.14 |
0.15 |
0.58 |
| Albumen weight, % |
60.58 |
61.16 |
0.27 |
0.19 |
| Yolk weight, % |
29.34 |
28.59 |
0.12 |
0.40 |
| Yolk index |
0.52 |
0.53 |
0.06 |
0.54 |
| Eggshell weight, % |
10.06 |
10.24 |
0.09 |
0.61 |
| Eggshell color |
L* |
75.42 |
75.36 |
0.40 |
0.45 |
|
a* |
6.19 |
6.03 |
0.23 |
0.18 |
|
b* |
14.85 |
14.44 |
0.24 |
0.11 |
Plasma antioxidative status
Supplementation of layers’ diets with EGCG resulted in a higher GST-ST activity and lower MDA concentration in plasma (Table 5; P < 0.05). No differences in T-SOD, CAT, GSH-Px and T-AOC activities were observed between the treatments and control groups (P > 0.05).
Table 5 Effect of epigallocatechin-3-gallate supplementation on plasma characteristics of laying hensa
| Items |
CON |
EGCG |
SE |
P-Value |
|
Each mean represents 12 replicates, with 1 hen per replicate. Abbreviation represents: T-SOD = total superoxide dismutase, CAT = catalase, GST = glutathione S-transferase, GPx = glutathione peroxidase, T-AOC = total antioxidant capacity, MDA = malondialdehyde.
|
| T-SOD (U mg−1 prot) |
161.25 |
167.95 |
11.23 |
0.79 |
| CAT (U mg−1 prot) |
230.5 |
250.32 |
15.36 |
0.68 |
| GST (U mg−1 prot) |
190.18 |
231.48 |
19.49 |
0.02 |
| GPx (U mg−1 prot) |
3122.96 |
3207.63 |
110.20 |
0.64 |
| T-AOC (U mg−1 prot) |
5.70 |
6.23 |
0.89 |
0.69 |
| MDA (nmol mg−1 prot) |
7.93a |
5.37b |
0.56 |
0.01 |
Egg antioxidant substances and activities of eggs
Albumen tryptophan and yolk carotenoid concentrations were higher in the EGCG compared to the CON group (Tables 6, 7 and 8; P < 0.05). Eggs from EGCG fed layers had a higher content in T-AOC, RP, and ORAC activity, while a lower MDA content in egg albumen and yolk was lower in the eggs from the EGCG group (Fig. 1; P < 0.05). No differences in total fat, total phospholipids, fatty acid profiles, and α-tocopherol contents in egg yolk were observed between the CON and EGCG groups (Table 8; P > 0.05).
 |
| | Fig. 1 Effect of epigallocatechin-3-gallate supplementation on the antioxidant activities in egg albumen and yolk of laying hens. T-AOC = total antioxidant capacity; MDA = malondialdehyde; ORAC = oxygen radical absorbance capacity. All data are mean ± SE values. Different letters significant differences between samples of different treatments. | |
Table 6 Effect of epigallocatechin-3-gallate supplementation on free amino acid content of the albumen samples (mg g−1 egg albumen)a
| Items |
CON |
EGCG |
SE |
P-Value |
|
Each mean represents 12 replicates, with 1 egg per replicate. Abbreviation indicated: EAA = percentage of essential amino acid for humans (including lysine, leucine, valine, isoleucine, phenylalanine, threonine, methionine, and histidine), TAA = percentage of total amino acids.
|
| Lysine |
7.9 |
7.8 |
0.11 |
0.24 |
| Leucine |
12.2 |
11.8 |
0.24 |
0.19 |
| Valine |
8.1 |
8.4 |
0.67 |
0.33 |
| Isoleucine |
6.0 |
5.8 |
0.09 |
0.88 |
| Phenylalanine |
8.4 |
8.9 |
0.22 |
0.67 |
| Threonine |
7.1 |
7.3 |
0.20 |
0.32 |
| Methionine |
5.7 |
5.4 |
0.17 |
0.44 |
| Histidine |
2.9 |
2.7 |
0.03 |
0.49 |
| Glutamic acid |
14.4 |
14.5 |
0.12 |
0.18 |
| Tryptophan |
2.3 |
4.9 |
0.02 |
0.01 |
| Serine |
9.8 |
9.7 |
0.10 |
0.54 |
| Aspartic |
13.7 |
13.5 |
0.21 |
0.67 |
| Arginine |
8.1 |
7.9 |
0.17 |
0.25 |
| Tyrosine |
4.0 |
3.8 |
0.25 |
0.67 |
| Alanine |
5.6 |
5.4 |
0.11 |
0.55 |
| Glycine |
4.9 |
5.2 |
0.11 |
0.28 |
| Proline |
4.0 |
4.2 |
0.18 |
0.37 |
| Cysteine |
2.7 |
2.6 |
0.06 |
0.21 |
| EAA |
60.1 |
62.9 |
1.94 |
0.77 |
| TAA |
126.8 |
129.8 |
1.87 |
0.41 |
Table 7 Effect of epigallocatechin-3-gallate supplementation on free amino acid content of the yolk samples (mg g−1 egg yolk)a
| Items |
CON |
EGCG |
SE |
P-Value |
|
Each mean represents 12 replicates, with 1 egg per replicate. Abbreviation indicated: EAA = percentage of essential amino acid for humans (including lysine, leucine, valine, isoleucine, phenylalanine, threonine, methionine, and histidine), TAA = percentage of total amino acids.
|
| Lysine |
10.6 |
11.0 |
0.34 |
0.41 |
| Leucine |
12.4 |
12.5 |
0.24 |
0.64 |
| Valine |
8.7 |
8.8 |
0.11 |
0.29 |
| Isoleucine |
6.7 |
6.6 |
0.09 |
0.57 |
| Phenylalanine |
6.5 |
6.4 |
0.08 |
0.66 |
| Threonine |
7.5 |
7.7 |
0.11 |
0.19 |
| Methionine |
3.7 |
3.8 |
0.07 |
0.28 |
| Histidine |
3.7 |
3.6 |
0.10 |
0.65 |
| Tryptophan |
3.2 |
3.3 |
0.19 |
0.32 |
| Glutamic acid |
16.8 |
18.7 |
0.44 |
0.04 |
| Serine |
11.2 |
11.1 |
0.15 |
0.33 |
| Aspartic |
13.8 |
13.6 |
0.19 |
0.45 |
| Arginine |
9.2 |
8.9 |
0.14 |
0.37 |
| Tyrosine |
5.4 |
5.2 |
0.24 |
0.66 |
| Alanine |
6.8 |
6.9 |
0.18 |
0.87 |
| Glycine |
4.5 |
4.4 |
0.08 |
0.37 |
| Proline |
5.1 |
5.2 |
0.09 |
0.44 |
| Cysteine |
2.8 |
2.6 |
0.12 |
0.58 |
| ΣEAA |
63.0 |
62.9 |
0.45 |
0.21 |
| ΣTAA |
138.6 |
139.4 |
0.99 |
0.43 |
Table 8 Effect of epigallocatechin-3-gallate supplementation on egg yolk cholesterol and fatty acid profiles in laying hensa
| Items |
CON |
EGCG |
SE |
P-Value |
|
Each mean represents 12 replicates, with 1 egg per replicate.
|
| Total fat |
4.92 |
4.94 |
0.32 |
0.63 |
| Total phospholipids, g |
1.68 |
1.71 |
0.15 |
0.84 |
| Carotenoids, mg |
0.22 |
0.29 |
0.02 |
0.04 |
| α-Tocopherol, mg |
1.2 |
1.8 |
0.10 |
0.54 |
| Total cholesterol, mg |
127.1 |
123.5 |
6.1 |
0.95 |
| % total fatty acid |
|
| Myristic acid (C14:0) |
0.27 |
0.21 |
0.01 |
0.45 |
| Palmitic acid (C16:0) |
30.27 |
29.48 |
1.55 |
0.38 |
| Palmitoleic acid (C16:1) |
3.12 |
2.45 |
0.15 |
0.57 |
| Stearic acid (C18:0) |
7.45 |
7.11 |
0.14 |
0.74 |
| Oleic acid (C18:1) |
36.56 |
37.11 |
1.23 |
0.44 |
| Linoleic acid (C18:2) |
18.26 |
19.85 |
2.28 |
0.80 |
| α-Linolenic acid (C18:3) |
0.58 |
0.64 |
0.16 |
0.76 |
| Eicosaenoic acid (C20:0) |
0.07 |
0.06 |
0.01 |
0.47 |
| Arachidonic acid (C20:4) |
0.2 |
0.18 |
0.05 |
0.62 |
| Docosahexaenoic acid (C22:6) |
— |
— |
— |
— |
| Others |
1.95 |
1.41 |
0.47 |
0.78 |
| ΣPolyunsaturated fatty acid |
19.11 |
20.73 |
0.28 |
0.41 |
| ΣMonounsaturated fatty acid |
39.68 |
39.56 |
1.54 |
0.71 |
| ΣSaturated fatty acid |
38.06 |
36.86 |
0.98 |
0.67 |
| ΣUnsaturated fatty acid |
58.79 |
60.29 |
1.98 |
0.77 |
Antioxidative status related gene expression and protein levels in liver
Liver mRNA expression of P38MAPK, Nrf2 and HO-1 was up-regulated in EGCG compared to the CON group (Fig. 2; P < 0.05); similarly, the corresponding protein levels of p-P38 MAPK, Nrf2 and HO-1 were also higher in the EGCG group compared to the CON group (Fig. 3; P < 0.05). No difference in ERK1/2, JNK and sMaf mRNA gene expression and their protein levels were noted between the CON and EGCG groups (P > 0.05).
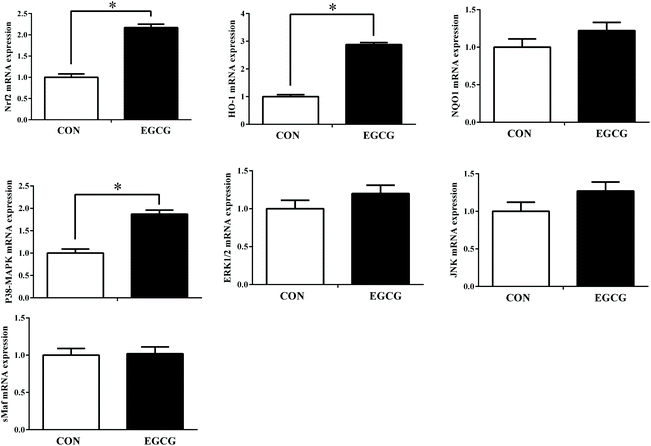 |
| | Fig. 2 Effect of epigallocatechin-3-gallate supplementation on the antioxidative stress-related gene expression in livers of laying hens. P38 MAPK (P38) = P38 mitogen activated protein kinases, ERK1/2 = extracellular regulated protein kinase 1 and 2, JNK = c-Jun N-terminal kinase, Nrf2 = nuclear factor erythroid-2 related factor 2, sMaf = small musculoaponeurotic fibrosarcoma protein, NQO1 = NAD(P)H quinone dehydrogenase 1, HO-1 = heme oxygenase-1. | |
 |
| | Fig. 3 Effect of epigallocatechin-3-gallate supplementation on the antioxidative stress-related protein levels in livers of laying hens. P38 MAPK (P38) = P38 mitogen activated protein kinases, p-P38 = phosphate-P38, ERK1/2 = extracellular regulated protein kinase 1 and 2, JNK = c-Jun N-terminal kinase, Nrf2 = nuclear factor erythroid-2 related factor 2, HO-1 = heme oxygenase-1. | |
Discussion
Green tea EGCG has antioxidant properties in light of its ability to scavenge reactive oxygen (ROS), reactive nitrogen species (NOS) and chelating redox active transition metal ions resulting in an anti-inflammatory effect.5,22–25 The results gathered in this study indicate that dietary EGCG was effective in improving egg albumen (egg white) quality after 8 weeks of feeding; however no influences were observed or made in production performance. Similarly, Wang et al.9 observed that feeding hens with 200 mg kg−1 tea polyphenols during the late laying phase increased egg production performance and albumen quality. Sahin et al.11 also noted that supplementing 200 to 400 mg kg−1 EGCG in the diet of heat-stressed quails increased the egg production rate. Moreover, we also reported that inclusion of 600 or 1000 mg kg−1 tea polyphenols during the late laying phase can increase egg production and egg albumen quality in layers during an oxidative stress challenge.10 The lack of significant changes in production performance observed in our study could not only be ascribed to the different EGCG or total polyphenol inclusion levels used in the above studies, but also to the different physiological status of layers, suggesting that dietary EGCG can be more effective when layers are physiologically challenged (aging or oxidative stress). On the other hand, our results suggest that dietary EGCG can improve egg albumen quality. It has been demonstrated that ovomucin polymers and the ovomucin-lysozyme complex and their structures were one of the main determinants for the albumen jelly-like property and its height.26 Polyphenols are reported to bind with proteins and metals, such as lysozymes, ovomucin, ovalbumin (OVA), gelatin and bovine serum albumin.27 Moreover, previous studies have suggested that the structure of these proteins can be changed by the binding of polyphenols.28,29 Wang et al.30 found that supplementation of tea polyphenols in layer's diets increased the ovomucin fraction. Similarly, in our previous study, we also found that EGCG led to a reduction in the ovalbumin-related X protein and higher level of the ovalbumin (OVA)-related Y protein in an oxidative stress model.
Endogenous antioxidants include enzymatic and non-enzymatic antioxidants. Members of the enzymatic antioxidant system, such as SOD, CAT, GSH-ST and GPx, play important roles in protecting the organism from oxidative damage. In this study, we observed that dietary EGCG supplementation increased the activity of GST, and concurrently reduced plasma concentration of MDA, a well-known product of lipid peroxidation. In agreement with our results are the observations that green tea intake resulted in an increased hepatic GPx and oxidized glutathione enzyme activities as well as glutathione (GSH) content and higher T-AOC.31 Similarly, in a previous study we also reported that tea polyphenols enhanced GSH-ST and GSH-Px activities and reduced the MDA content in livers of layers.10 It could be plausible that this overall improved redox balance could be responsible for the better egg quality observed in our study.
Eggs are considered as a good source of dietary antioxidants.3 The DPPH radical-scavenging capacity assay, RP and the ORAC assay are the main methods used to evaluate antioxidant effects.32 In this study, we observed that both the reducing power and ORAC were improved by dietary EGCG supplementation. We also noted that dietary EGCG resulted in lower MDA and higher T-AOC in both egg white and egg yolk. These observations suggest that the antioxidant capacity of eggs was improved by EGCG supplementation. In agreement with our observations are findings reported by Wang et al.,31 who found that tea polyphenols decreased the protein carbonyl content of albumen. Moreover, it was observed that tea polyphenols enhanced the ovomucin content in egg white.30 Recently, it has been demonstrated that egg proteins, peptides, aromatic amino acids (tryptophan and tyrosine), phospholipids, vitamin E (α-tocopherol), carotenoids and phosvitin are the main compounds responsible for the egg antioxidant activities.20,33–35 In the current study we found that dietary EGCG increased the tryptophan in egg white and carotenoid content in egg yolk, which may have contributed to the higher antioxidative capacity of eggs. It could be argued that the abundance of hydrophobic amino acids, such as tryptophan, observed in the EGCG group might have contributed to the enhanced antioxidant capacity of the eggs by increasing the solubility of peptides in lipids which facilitates accessibility to radical species. Also, the reason that EGCG can improve the content of tryptophan and carotene in eggs is not clear and it may be because EGCG prevents tryptophan and carotene from oxidation,36,37 which may increase its deposition in eggs. Further studies, however, are required to clarify the previse mechanism of action for this effect.
Nrf2 is a redox-sensitive transcription factor, which plays a pivotal role in the modulation of the defensive response to redox stress.7 Previous studies have reported that EGCG modulated redox balance through the Nrf2 pathway. For example, Wang et al.,30 observed an increase that caused EGCG to increase Nrf2 and Nrf2-targeting gene expression, including HO-1, NQO1 and GST in the liver of EGCG supplementation mice. As the upstream effectors in antioxidant responses, mitogen-activated protein kinases (MAPKs), including P38MAPK, ERK, and JNK, manifest in the activation of many transcription factors, including Nrf2.14,31,38 In the current study, dietary EGCG enhanced the hepatic P38MAPK, Nrf2, together with HO-1 mRNA and protein expression, indicating that EGCG was able to activate the MAPK/Nrf2 signaling pathway. Also, it was also observed that EGCG increased the phosphorylation of P38, ERK1/2, JNK and Nrf2 in cells in previous studies.39,40 This suggested that EGCG activated the phosphorylation of P38, and sequentially regulated the activation of Nrf2 and this down-stream pathway.
Conclusion
Taken together, it is indicated in our study that dietary supplementation with EGCG increases the antioxidant capacity of eggs as reflected by the up-regulation of the antioxidant system and by the increase in the concentration of tryptophan and carotenoids. This observation could be ascribed to a positive modulation of the MAPK-Nrf2 pathway as reflected by the up-regulation of the P38MAPK, Nrf2 and HO-1 mRNA expression (Fig. 4).
 |
| | Fig. 4 The overview of the effect of epigallocatechin-3-gallate supplementation in layers. Dietary supplementation with EGCG led to an increase in the antioxidant activity and antioxidant chemical substance including tryptophan and carotenoid contents of eggs. This may be associated with its increasing effect on the oxidative stress related gene and protein levels of P38MAPK, Nrf2 and HO-1 expression. | |
Author contribution
J.P., R.J., and K.Y. conceived and designed the experiments; J.P., R.J., X. M., and S. P. performed the experiments; J.P., R.J., and Q.F. analyzed the data; J.P. wrote the paper; P. C. and X.M. helped revise this manuscript. All authors read and approved the final manuscript.
Conflicts of interest
We confirm that there are no known conflicts of interest that have been associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Acknowledgements
The authors deeply acknowledge the National Natural Science Foundation of China (Grant No. 31872792 and 31402031), and Sichuan Provincial Science and Technology Projects (Grant No. 2018NZ20009, 2019YFH0062, 2014BAD13B04, 2014NZ0043, 2014NZ0002, and 2013NZ0054) for financial support.
References
- J. Kovacs-Nolan, M. Phillips and Y. Mine, Advances in the value of eggs and egg components for human health, J. Agric. Food Chem., 2005, 53(22), 8421–8431 CrossRef CAS.
- K. Erdmann, B. W. Y. Cheung and H. Schröder, The possible roles of food derived bioactive peptides in reducing the risk of cardiovascular disease, J. Nutr. Biochem., 2008, 19, 643–654 CrossRef CAS PubMed.
- C. Nimalaratne and J. P. Wu, Hen egg as an antioxidant food commodity: a review, Nutrients, 2015, 7, 8274–8293 CrossRef CAS.
- C. Nimalaratne, D. Lopes-Lutz, A. Schieber and J. Wu, Free aromatic amino acids in egg yolk show antioxidant properties, Food Chem., 2011, 129(1), 155–161 CrossRef CAS.
- B. Frei and J. V. Higdon, Antioxidant activity of tea polyphenols in vivo: evidence from animal studies, J. Nutr., 2003, 133, 3275S–3284S CrossRef CAS PubMed.
- N. Sriram, S. Kalayarasan and G. Sudhandiran, Epigallocatechin-3-gallate augments antioxidant activities and inhibits inflammation during bleomycin-induced experimental pulmonary fibrosis through Nrf2-Keap1 signaling, Pulm. Pharmacol. Ther., 2009, 22(3), 221–236 CrossRef CAS.
- R. Dong, D. Wang, X. Wang, K. Zhang, P. Chen, C. S. Yang and J. Zhang, Epigallocatechin-3-gallate enhances key enzymatic activities of hepatic thioredoxin and glutathione systems in selenium-optimal mice but activates hepatic Nrf2 responses in selenium-deficient mice, Redox Biol., 2016, 10, 221–232 CrossRef CAS PubMed.
- M. Ariana, A. Samie, M. Edriss and R. Jahanian, Effects of powder and extract form of green tea and marigold, and α-tocopheryl acetate on performance, egg quality and egg yolk cholesterol levels of laying hens in the late phase of production, J. Med. Plants Res., 2011, 5, 2710–2716 CAS.
- J. Wang, Z. Yuan, K. Zhang, X. Ding, S. Bai, Q. Zeng, H. Peng and P. Celi, Epigallocatechin-3-gallate protected vanadium-induced eggshell depigmentation via the P38MAPK-Nrf2/HO-1 signaling pathway in laying hens, Poult. Sci., 2018, 97, 3109–3118 CrossRef CAS.
- J. P. Wang, X. Bai, X. M. Ding, S. P. Bai, Q. F. Zeng, X. B. Mao and K. Y. Zhang, Quantitative proteomic analysis reveals the role of tea polyphenol EGCG in egg whites in response to vanadium stress, Nutrition, 2017, 39–40, 20–29 CrossRef CAS PubMed.
- K. Sahin, C. Orhan, M. Tuzcu, S. Ali, N. Sahin and A. Hayirli, Epigallocathchin-3-gallate prevent lipid peroxidation and enhances antioxidant defense system via modulating hepatic nuclear transcription factors in heat-stressed quails, Poult. Sci., 2010, 89, 2251–2258 CrossRef CAS.
- T. Nguyen, P. Nioi and C. B. Pickett, The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress, J. Biol. Chem., 2009, 284, 13291–13295 CrossRef CAS PubMed.
- S. Gupta, K. Hastak, F. Afaq, N. Ahmad and H. Mukhtar, Essential role of caspases in epigallocatechin-3-gallate-mediated inhibition of nuclear factor kappa B and induction of apoptosis, Oncogene, 2004, 23(14), 2507–2522 CrossRef CAS.
- G. Shen, C. Xu, R. Hu, M. R. Jain, S. Nair, W. Lin, C. S. Yang, J. Y. Chan and A. N. Kong, Comparison of (−)-epigallocatechin-3-gallate elicited liver and small intestine gene expression profiles between C57BL/6J mice and C57BL/6J/Nrf2 (−/−) mice, Pharm. Res., 2005, 22, 1805–1820 CrossRef CAS PubMed.
- P. Y. Tsai, S. M. Ka, J. M. Chang, H. C. Chen, H. A. Shui, C. Y. Li, K. F. Hua, W. L. Chang, J. J. Huang, S. S. Yang and A. Chen, Epigallocatechin-3-gallate prevents lupus nephritis development in mice via enhancing the Nrf2 antioxidant pathway and inhibiting NLRP3 inflammasome activation, Free Radical Biol. Med., 2011, 51, 744–754 CrossRef CAS PubMed.
- M. Neijat, M. Suh, J. Neufeld and J. House, Hempseed products fed to hens
effectively increased n-3 polyunsaturated fatty acids in total lipids, triacylglycerol and phospholipid of egg yolk, Lipids, 2016, 51, 601–614 CrossRef CAS.
- K. M. S. Islam and F. J. Schweigert, Comparison of three spectrophotometric methods for analysis of egg yolk carotenoids, Food Chem., 2015, 172, 233–237 CrossRef CAS.
- E. Claeys, E. Vossen and D. De Smet, Determination of α-tocopherol by reversed-phase HPLC in feed and animals-derived foods without saponification, J. Sci. Food Agric., 2016, 96, 522–529 CrossRef.
- B. X. Ou, M. Hampsch-Woodill and R. L. Prior, Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe, J. Agric. Food Chem., 2001, 49, 4619–4626 CrossRef CAS.
- J. B. Liu, J. Yan, S. Y. Lin, G. S. Jones and F. Chen, Purification and identification of novel antioxidant peptides from egg white protein and their antioxidant activities, Food Chem., 2015, 175, 258–266 CrossRef CAS PubMed.
- D. Y. Ren, Y. Zhao, Y. Nie, X. S. Lu, Y. F. Sun and X. B. Yang, Chemical composition of Pleurotus eryngii polysaccharides and their inhibitory effects on high-fructose diet-induced insulin resistance and oxidative stress in mice, Food Funct., 2014, 10, 2609–2620 RSC.
- S. Uesato, Y. Kitagawa and M. Kamishimo, Inhibition of green tea catechins against the growth of cancerous human colon and hepatic epithelial cells, Cancer Lett., 2001, 170, 41–44 CrossRef CAS.
- Y. Wang, Y. Mei, D. Feng and L. Xu, (-)-Epigallocatechin-3-gallate protects mice from concanavalin A-induced hepatitis through suppressing immune-mediated liver injury, Clin. Exp. Immunol., 2006, 145, 485–492 CrossRef CAS PubMed.
- G. L. Tipoe, T. M. Leung, M. W. Hung and M. L. Fung, Green tea polyphenols as an antioxidant and anti-inflammatory agent for cardiovascular protection, Cardiovasc. Hematol. Disord.: Drug Targets, 2007, 7, 135–134 CrossRef CAS.
- M. C. Zhen, Q. Wang, X. H. Huang, L. Q. Cao, X. L. Chen, K. Sun, Y. J. Liu, L. Wen and L. J. Zhang, Green tea polyphenol epigallocatechin-3-gallate inhibits oxidative damage and preventive effects on carbon tetrachloride-induced hepatic fibrosis, J. Nutr. Biochem., 2007, 18, 795–805 CrossRef CAS.
- D. S. Robinson and J. B. Monsey, Changes in the composition of ovomucin during liquefaction of thick egg white, J. Sci. Food Agric., 1972, 23, 29–38 CrossRef CAS.
- M. C. Bohin, W. S. U. Roland, H. Gruppen, R. J. Gouka, H. T. can der Hijden, P. Dekker, G. Smit and J. P. Vincken, Evaluation of the bitter-masking potential of food proteins for EGCG by a cell-based human bitter taste receptor assay and binding studies, J. Agric. Food Chem., 2013, 61, 10010–10017 CrossRef CAS PubMed.
- Z. Yuksel, E. Avci and Y. K. Erdem, Characterization of binding interactions between green tea flavanoids and milk proteins, Food Chem., 2010, 121(2), 450–456 CrossRef CAS.
- C. D. Kanakis, I. Hasni, P. Bourassa, P. A. Tarantilis, M. G. Polissiou and H. A. Tajmir-Riahi, Milk beta-lactoglobulin complexes with tea polyphenols, Food Chem., 2011, 127(3), 1046–1055 CrossRef CAS.
- X. C. Wang, H. X. Wang, H. Wang, H. J. Zhang, S. G. Wu and G. H. Qi, Dietary tea polyphenols supplementation improved egg production performance, albumen quality, and magnum morphology of Hy-Line Brown hens during the late laying period, J. Anim. Sci., 2018, 96, 225–235 CrossRef.
- C. Wang, Y. Wang, X. Wan, C. S. Yang and J. Zhang, Green tea polyphenol (-)-epigallocatechin-3-gallate triggered hepatotoxicity in mice: responses of major antioxidant enzymes and the Nrf2 rescue pathway, Toxicol. Appl. Pharmacol., 2015, 283, 65–74 CrossRef.
- L. K. McDonald-Wicks, L. G. Wood and M. L. Garg, Methodology for the determination of biological antioxidant capacity in vitro: a review, J. Sci. Food Agric., 2006, 86, 2046–2056 CrossRef.
- A. Dávalos, M. Miguel, B. Bartolomé and R. López-Fandiño, Antioxidant activity of peptides derived from egg white proteins by enzymatic hydrolysis, J. Food Prot., 2004, 67(9), 1939–1944 CrossRef.
- R. Hargitai, Z. Matus, G. Hegyi, G. Michl, G. Tóth and J. Török, Antioxidants in the egg yolk of a wild passerine: differences between breeding seasons, Comp. Biochem. Physiol., Part B: Biochem. Mol. Biol., 2006, 143(2), 145–152 CrossRef PubMed.
- S. Katayama, X. Xu, M. Z. Fan and Y. Mine, Antioxidative stress activity of oligophosphopeptides derived from hen egg yolk phosvitin in Caco-2 cells, J. Agric. Food Chem., 2006, 54(3), 773–778 CrossRef CAS PubMed.
- J. Yang, L. Mao, W. Yang, C. X. Sun, L. Dai and Y. X. Gao, Evaluation of non-covalent ternary aggregates of lactoferrin, high methylated pectin, EGCG in stabilizing β–carotene emulsions, Food Chem., 2018, 240, 1063–1071 CrossRef CAS.
- S. Chaudhury, I. Ghosh, G. Saha and S. Dasgupta, GCG prevents tryptophan oxidation of cataractous ocular lens human γ-crystallin in presence of H2O2, Int. J. Biol. Macromol., 2015, 77, 287–292 CrossRef CAS PubMed.
- C. Wang, S. G. Wu, H. J. Zhang, H. Y. Yue, Y. G. Zhai and G. H. Qi, Study on oxidative stress-activated MAPK signaling pathway in broilers breast muscle satellite cells, Sci. Agric. Sin., 2010, 43, 4286–4294 CAS.
- M. I. Cerezo-Guisado, R. Zur, M. J. Lorenzo, A. Risco, M. A. Martin-Serrano, A. Alvarez-Barrientos, A. Cuenda and F. Centeno, Implication of Akt, ERK1/2 and alternative p38MAPK signalling pathways in human colon cancer cell apoptosis induced by green tea EGCG, Food Chem. Toxicol., 2015, 84, 125–132 CrossRef CAS.
- K. Sahin, M. Tuzcu, H. Gencoglu, A. Dogukan, M. Timurkan, N. Sahin, A. Aslan and O. Kucuk, Epigallocatechin- 3-gallate activates Nrf2/HO-1 signaling pathway in cisplatin induced nephrotoxicity in rats, Life Sci., 2010, 87, 240–245 CrossRef CAS.
Footnote |
| † These two author contributed to this work equally. |
|
| This journal is © The Royal Society of Chemistry 2020 |
Click here to see how this site uses Cookies. View our privacy policy here.  a,
Ru
Jia†
a,
Pietro
Celi
a,
Ru
Jia†
a,
Pietro
Celi
 bc,
Xuemei
Ding
bc,
Xuemei
Ding
 a,
Shiping
Bai
a,
Shiping
Bai
 a,
Qiufeng
Zeng
a,
Qiufeng
Zeng
 a,
Xiangbing
Mao
a,
Xiangbing
Mao
 a,
Shengyu
Xu
a,
Shengyu
Xu
 a and
Keying
Zhang
a and
Keying
Zhang
 *a
*a




