Assembly strategies of organic-based imaging agents for fluorescence and photoacoustic bioimaging applications
Hong-Bo
Cheng†
a,
Yuanyuan
Li†
b,
Ben Zhong
Tang
 *b and
Juyoung
Yoon
*b and
Juyoung
Yoon
 *a
*a
aDepartment of Chemistry and Nanoscience, Ewha Womans University, Seoul 120-750, Korea. E-mail: jyoon@ewha.ac.kr; Fax: +82-2-3277-3419; Tel: +82-2-3277-2400
bDepartment of Chemistry, Hong Kong Branch of Chinese National Engineering Research Center for Tissue Restoration & Reconstruction, and Institute for Advanced Study, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, China. E-mail: tangbenz@ust.hk
First published on 4th December 2019
Abstract
The results of numerous studies have led to the development of supramolecular (assembled) organic substances for use in biomedical imaging as part of comprehensive approaches to the diagnosis of diseases. This review summarizes recent advances that have been made in the design and fabrication of assembled organic dyes for fluorescence and photoacoustic bioimaging.
Key learning points1. The general concept and importance of supramolecular organic imaging agents.2. Types and characteristics of supramolecular organic imaging agents. 3. The main approaches used to construct supramolecular organic imaging agents. 4. The recent advances made in the development of supramolecular organic imaging agents. 5. The opportunities and challenges that remain in the field of supramolecular organic imaging agents. |
1. Introduction
Precise imaging techniques are needed to monitor and analyze biological species and processes in vivo with high spatial resolution.1 Unfortunately, conventional fluorescence contrast agents/probes developed for these purposes have several significant drawbacks that limit their use in high-precision and high-resolution imaging in biological systems. The major sources of these shortcomings are off-target effects, biological auto-fluorescence and low signal-to-background responses that interfere with the signals associated with the targets.2 Moreover, the preparation of the current contrast agents/probes normally requires laborious synthetic protocols, which limits applications in real-time settings.3 Therefore, the development of simple contrast agents/probes that have properties that make them suitable for high-precision and high-resolution bioimaging remains a challenging task.Recent advances made in materials sciences demonstrate that supramolecular chemistry can be employed as a general “bottom-up” approach for designing smart materials that possess advanced performances and/or functions.4 In addition, it has been shown that assembly driven molecular aggregation plays an important role in regulating physical, chemical and/or biological properties of dyes, and that the photophysical properties of assembled dyes can be regulated by controlling their packing arrangements. Most organic dyes used as nanoprobes and/or contrast agents have reduced fluorescence efficiencies in aqueous solution because of the operation of aggregation-caused quenching (ACQ).5 It is difficult to apply the ACQ phenomenon to the design of fluorescence-based probes. Fortunately, other types of fluorescence phenomena, such as aggregation-induced emission (AIE)5 and J-type aggregate formation, do serve as foundations of strategies used for constructing functional dye-based contrast agents/probes.2
The concept of AIE was developed by Tang et al. in 2001.6 In this effort, Tang coined the term AIEgens to describe substances that are non- or weakly fluorescent when dissolved in compatible solvents but emit light strongly when in an aggregated state (Fig. 1). The prototypical AIEgen, tetraphenylethene (TPE), serves as an example to describe the underlying principles of the AIE phenomenon. In the solution state, dynamic free-rotation of the four phenyl groups linked to the doubly bonded carbons in TPE causes non-radiative dissipation of the excited energy (radiationless decay) and quenching of fluorescence. However, in the aggregated state TPE fluoresces strongly as a consequence of the synergistic effects of restriction of intramolecular motion (RIM) and prevention of intramolecular π–π interactions owing to its twisted conformation. Based on this proposal, it can be deduced that in addition to aggregation, structural rigidification, such as that caused by high viscosity or supramolecular interactions, would also result in strong emission from AIEgens.
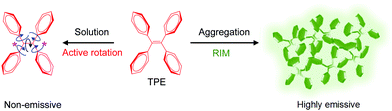 | ||
| Fig. 1 The propeller-shaped luminogen, tetraphenylethene (TPE), is non-emissive when in the solution state but becomes fluorescent when in the aggregated state. | ||
In addition to approaches that rely on fluorescence responses, photoacoustic (PA) techniques have also been incorporated into the design of precise biomedical imaging systems. One of the main reasons for this is that ultrasound signals arising from PA-based probes are less attenuated by biological media than the light responses associated with fluorescence based systems.7 In general, ideal contrast agents for both fluorescence and PA-based imaging absorb light in the near-infrared (NIR) region with high molar-extinction coefficients. However, unlike those based on fluorescence, PA probes have low fluorescence quantum yields and correspondingly high radiationless decay efficiencies.8 In addition, NIR-absorbing organic substances developed for use in both fluorescence and PA imaging contain highly conjugated π-systems and typically undergo aggregation in aqueous media. Many recent studies have demonstrated that PA imaging techniques utilizing assembled organic fluorophores can be used to detect and monitor biologically important species in vitro and in vivo.
In response to the rapid pace with which assembled organic fluorescent probes are being developed, we have created this tutorial review to summarize very-recent progress that has been made in the design and preparation of representative assembled organic fluorophores and their applications to fluorescence and PA imaging (Fig. 2).
 | ||
| Fig. 2 Assembled organic fluorophore probes have advantageous properties for fluorescence and photoacoustic imaging. | ||
2. Strategies for the design of assembled organic fluorescent and PA probes based on diverse supramolecular models
2.1. Basic features of supramolecular assembled fluorescent and PA probes
Supramolecular chemistry is an area of science that focuses on chemical phenomena that take place beyond the molecular level.9 In 1987, Pedersen, Cram, and Lehn received the Nobel Prize for their contributions to supramolecular chemistry. Moreover, in 2016, the Nobel Prize in Chemistry was awarded to the supramolecular chemists, Stoddart, Feringa and Sauvage. The considerable effort devoted to this scientific area since its first formulation is a consequence of its close relationship to modern nanoscience and technology. The formation of supramolecular systems takes place by molecular assembly driven by a variety of noncovalent dynamic intermolecular attractive forces associated with π–π stacking, electrostatic, hydrogen-bonding, hydrophobic/hydrophilic and van der Waals interactions.10 Particularly interesting in this regard is the observation that supramolecular assembly can be utilized for facile “bottom-up” construction of biomaterials for nanomedicine11 that have unique properties such as high stability, long circulation times, and targetable biological distributions.10,122.2. Organic fluorophores formed by assembly of hydrophilic organic dyes
Supramolecular chemistry provides new strategies for the design of water-soluble nanosystems that can be employed as probes.13,14 Typically, cationic or anionic moieties are incorporated into organic fluorophores so that they will possess hydrophilicity or water-solubility. Moreover, the probes function by undergoing in situ assemblies in the presence of targeted analytes in conjunction with AIE caused by changes in electrostatic, hydrophobic and coordinative interactions.15 For improved target specificity, bioconjugates are created between target cleavable biomolecules, such as DNA, peptides, proteins and carbohydrate derivatives, with AIEgens.16 Owing to the hydrophilic properties of biomolecules, the conjugates are non-emissive in water but they produce AIEgens when they are cleaved by targeted analytes. An example of this type of probe is the light-up apoptosis sensor developed by Liu and Tang for evaluation of the therapeutic response of cells to a chemotherapeutic Pt(IV) prodrug. The conjugates designed for this purpose contain the cRGD tripeptide for targeting cancer cells and the apoptosis sensor TPS-DEVD composed of tetraphenylsilole (TPS) as the AIEgen and the caspase-3 specific DEVE peptide.17 Following cRGD guided selective uptake in cancer cells, the Pt(IV) moiety in the conjugate is reduced to form Pt(II) along with a simultaneous release of TPS-DEVD. The Pt(II) containing drug then induces cell apoptosis leading to the activation of caspase-3, which selectively cleaves the DEVD peptide. These events cause a release of the hydrophobic AIEgen TPS, whose aggregation triggers AIE via RIM.Amphiphilic polymers containing organic fluorophores assemble to form micelles that can be used for bioimaging and monitoring drug release. For example, a pH-responsive polymeric probe consisting of the AIEgen TPS and pheophorbide A (PheA) as a photosensitizer (PS), and possessing the ACQ property was developed for tracing the process involved in cancer therapy.18 In a pH 7.4 aqueous solution, the probe assembles to form nanoparticles (NPs), which display green AIE. In contrast, the probe disassembles in a solution at pH = 5.0 that mimics the acidic environment in cancer cells. This phenomenon gives rise to red emission resulting from ACQ after entrapment of the probe in lysosome. Under light irradiation and apoptosis, lysosome disruption takes place causing the probe to enter the cytoplasm (pH = 7.2) and display AIE for visualization of the therapeutic response.
Host–guest complexation has served as the basis for the effective design of supramolecular organic imaging agents.19,20 In systems developed using this strategy, traditional organic dyes that do not emit light in aqueous solution become fluorescent upon formation of host–guest complexes. In one approach, host–guest interactions effectively restrict the intramolecular motion of the AIEgen resulting in the turn-on of fluorescence.21 In a recent study of a system created using this design principle, Liu and his co-workers showed that host–guest interactions between cucurbit[8]uril and the anthracyl-pyridinium derivative ENDT promotes formation of supramolecular NPs that fluoresce in cellular environments.22
2.3. Organic fluorophores formed by assembly of hydrophobic organic dyes
Nanoprecipitation has become a widely used, straightforward and reproducible method for assembling hydrophobic organic dyes to form nanoparticles.23 In this process, injection of a solution of an AIEgen and an amphiphilic polymer in a water-miscible organic solvent (THF or acetone) along with sonication leads to the spontaneous formation of NPs. When used in combination with two- and three-dimensional microfluidic devices, it is possible to gain precise size control of the NPs and to obtain narrow size distributions on a large scale.24 In addition, substances that are only soluble in water-immiscible solvents can also be encapsulated into nanoparticles by utilizing an advanced microfluidic nanoprecipitation process which enables tuning the miscibility of the solvents by incorporating menthol in the water-immiscible solvents. Overall, nanoprecipitation has become a powerful and simple method for assembling hydrophobic AIEgens.Coordination-driven assembly is yet another approach for the construction of assembled organic dyes. For example, Stang et al. demonstrated that a TPE-based rhomboidal Pt(II) metallacycle, modified by incorporating a four-armed amphiphilic copolymer, assembles into different morphologies under various experimental conditions.25 Furthermore, assemblies generated in this manner can be used for loading and stimulated release of anticancer drugs. Recently, Lee developed a green mass production method to form NPs that is based on an ice-template-assisted strategy.26 Specifically, this effort showed that NPs can be controllably formed by dropping a solution of an organic substance into an ice template, followed by subsequent melting or freeze-drying. This method has a higher production yield than the reprecipitation method. Although this approach has not yet been applied to hydrophobic AIE dyes, it should be useful for this purpose.
3. Recent examples of assembled organic fluorophores for bioimaging
3.1. Nanosystems for fluorescence imaging
Fluorescence sensing techniques have excellent sensitivity27 and, as a result, imaging methods based on fluorescence are uniquely applicable to monitoring and analyzing disease-related metabolites in vitro and in vivo with high spatiotemporal resolution. AIE based assembled fluorescent probes have been developed for fluorescence bioimaging. “Off–On” fluorescence probes of this type that absorb and emit light in the NIR region are highly suitable for in vivo applications.Recently, Zhu designed an ultra-sensitive off–on NIR AIE-activated probe for mapping Aβ plaques in situ (Fig. 3). The probe, QM-FN-SO3, contains a lipophilic π-conjugated thiophene-bridge and a hydrophilic sulfonate group28 and it possesses advantageous features including long-wavelength emission with an enhancement of blood–brain-barrier (BBB) penetrability, and a high hydrophilicity to maintain it in a fluorescence-off state before binding to Aβ plaques (Fig. 3a and b). Owing to these properties, QM-FN-SO3 displays a minimal initial background signal and an ultra-high signal-to-noise ratio (SNR) for detection of the plaques. The enhanced fluorescence efficiency is attributed to the insertion of the lipophilic part of QM-FN-SO3 into hydrophobic pockets and the resulting interaction with aggregated amyloid fibrils, which leads to a decrease in conformational freedom and restriction of rotational motion. A study of in vivo mapping of Aβ plaques suggests that the fluorescence intensity of QM-FN-SO3 in Alzheimer's disease (AD)-model mice is higher than that in the control of wild-type mice (Fig. 3c). By overcoming the dilemma between the lipophilic and aggregation behavior from water to protein fibrillogenesis, the use of QM-FN-SO3 was a breakthrough in high-fidelity detection of Aβ plaques. It should be possible to utilize this strategy in designing AIE-activated probes for in vivo monitoring.
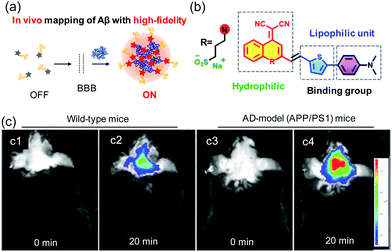 | ||
| Fig. 3 (a) In vivo mapping of Aβ plaques. (b) The structure of QM-FN-SO3. (c) In vivo mapping of Aβ deposition in AD-model mice. Reproduced from ref. 28 with permission from the American Chemical Society, copyright 2019. | ||
Specific chemical reactions that change the solubility of an AIEgen can also be employed to design probes. Tan and co-workers developed an enzyme responsive TPE derivative HTPQ (Fig. 4), which operates through an excited-state intramolecular proton transfer (ESIPT) mechanism.29 HTPQ is water-insoluble and highly emissive in the solid state. To enable it to be alkaline phosphatase (ALP) responsive, the phenolic hydroxyl group of the probe is incorporated into a phosphate mono-ester to prohibit the ESIPT process and block fluorescence (Fig. 4a). ALP cleavage of the resulting probe HTPQA releases free HTPQ, which precipitates from the solution during a short time period. Thus, signal reduction associated with the diffusion of the probe away from the target site is diminished. A comparison with the commercial probe 4-MUP, which releases water-soluble 4-MU upon ALP activation, shows that HTPQA undergoes in situ localization while 4-MUP diffuses over the entire cytoplasm (Fig. 4b and c).
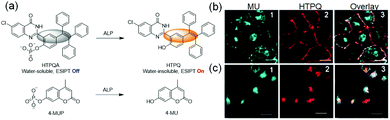 | ||
| Fig. 4 (a) Off–On response of ALP probes HTPQA and 4-MUP (control). (b and c) Co-incubation of HeLa cells with HTPQA (5 mm) and 4-MUP (10 mm) for 40 min in tris-buffered saline buffer without (b) and with (c) NaH2PO4.29 Copyright 2017, Wiley-VCH. | ||
Although imaging dynamic biological processes with light in the visible and first-NIR regions (400–900 nm) has high spatiotemporal resolution and sensitivity, light–tissue interactions still interfere with the process. New fluorophores have been uncovered that emit light in the second-NIR region (1000–1700 nm) and, thus, have deeper tissue penetration and higher spatial resolution for in vivo imaging. An example of a system of this type is the assembled NIR dots composed of F127 encapsulated TQ-BPN, which emits light beyond 900 nm with a tail out to 1200 nm (Fig. 5).30 Imaging with the NIR dots displays 3 μm spatial resolution and an 800 μm penetration depth. In an exploratory study assessing their use to diagnose diseases, the NIR-dots are employed to image brains undergoing photothrombotic ischemia (PTI). The results show that blocked blood flow can be monitored in the injured areas of the brain through an abnormal accumulation of NIR dots and an associated increase in the fluorescence intensity (green arrows in Fig. 5d).
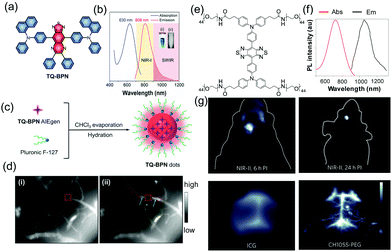 | ||
| Fig. 5 (a) Molecular structure of TQ-BPN. (b) Absorption and PL spectra of TQ-BPN dots in water. (c) Schematic illustration of the fabrication of AIE dots. (d) Fluorescence microscopic images of brain blood vessels.30 Copyright 2018, Wiley-VCH. (e) Chemical structure of CH1055 and the one-step synthesis of CH1055-PEG. (f) Absorbance and fluorescence emission of CH1055-PEG. (g) Through-skull non-invasive imaging of brain tumors at 4 mm depth with CH1055-PEG.31 Copyright 2016, Nature Publishing Group. | ||
To extend the wavelength into longer regions and accelerate excretion from the body, Dai and co-workers designed a water-soluble NIR-II imaging probe (emission peak at 1055 nm), which can not only be employed to delineate brain tumors in mice at a depth of 4 mm, but is also rapidly excreted through the kidneys within 24 h (Fig. 5e–g).31 Thus, the assembled NIR-II probes display significant advantages for monitoring and analysing diseases in vivo.
Another strategy for overcoming the limitation of penetration depth relies on the nonlinear optical effects of the probes, including multi-photon fluorescence (MPF) and second/third-harmonic generation (S/THG), which are frequency upconversion processes utilizing long-wavelength excitation. MPF probes, which are two (or three or more) photon excitable light emitters, absorb two (or more) NIR photons (700–1500 nm) to generate excited states. The assembled AIE-dots used for MPF imaging have great advantages because they have large absorption cross-sections as well as high emission quantum yields, which lower the needed excitation power, minimize photodamage and reduce photobleaching. An example of this type of probe was developed by Tang and co-workers, who showed that ultradeep and high-resolution two-photon fluorescence (2PF) imaging can be achieved by crab-shaped AIE-dots possessing 1300 nm NIR-II excitation and NIR-I emission (810 nm) (Fig. 6a–c).23 By utilizing 2PF microscopic imaging with a 1300 nm excitation, small 5 μm vessels can be clearly visualized in a mouse brain at a depth of 1065 μm (Fig. 6d). Therefore, deep tissue monitoring can be accomplished using the MPF technique. In addition, THG imaging holds a considerable promise for deep-tissue and high-resolution bioimaging. Compared with three-photon fluorescence (3PF), THG is a third-order nonlinear optical phenomenon but 3PF belongs to a fifth-order nonlinear optical process. This suggests a much lower excitation threshold of THG than that of 3PF. Recently, Tang and co-workers reported assembled crystalline dots with enhanced THG properties, which enable visualization of small vessels of 2.7 μm at a depth of 800 μm in a mouse brain.32
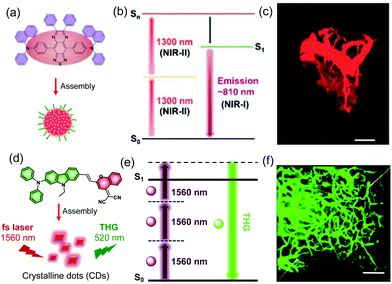 | ||
| Fig. 6 (a) Chemical structure of encapsulated AIE dots. (b) Schematic illustration of the AIE dots for NIR-II excited in vivo two-photon fluorescence imaging. (c) 3D reconstruction of brain vasculature using 2PF microscopy.23 Copyright 2018, American Chemical Society. (d) Chemical structure of AIE crystalline dots. (e) Schematic illustration of the THG process. (f) 3D reconstruction of the brain vasculature using THG microscopy.32 Copyright 2018, Wiley-VCH. | ||
Compared with normal fluorescence that arises from short lived singlet excited states and thus requires continuous light excitation for imaging to be performed, afterglow luminescence occurs for a long time after cessation of irradiation. Thus, methods based on these types of afterglow luminescence have lower background signals and higher SNRs. Generally, room-temperature phosphorescence (RTP), thermally activated delayed fluorescence (TADF) and chemo-afterglow luminescence are the major modes of emission used for time-resolved imaging. Numerous long lifetime RTP organic substances that emit light through triplet states with lifetimes on the order of milliseconds have been developed. These systems typically incorporate heavy atoms and host–guest designs, and emit light by way of H-aggregation and crystallization-induced routes. For instance, Li and colleagues developed the metal-free 10-phenyl-10H-phenothiazine 5,5-dioxide derivatives CS-F, whose RTP lifetimes in the crystalline state are as large as 410 ms (Fig. 7a and b).33 The water soluble organic nanoparticles, prepared by using a top-down approach, are activated by UV light irradiation and display phosphorescence 10 s after removal of irradiation. The RTP signal of a lymph node, 1 h post injection of CS-F and 10 s after activation by using a UV lamp, was clearly detected with a negligible background signal. In contrast, background during typical fluorescence imaging of the lymph node using CS-F was too strong to enable it to be distinguished from the signal.
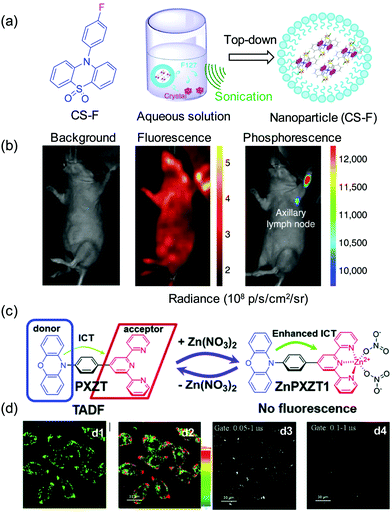 | ||
| Fig. 7 In vivo real-time excitation-free phosphorescent imaging of lymph nodes. (a) The structure of CS-F and the top-down approach to synthesize the water-soluble nanoparticles of CS-F. (b) Ultralong phosphorescence and fluorescence imaging of lymph node in living mice.33 Copyright 2018, Nature Publishing Group. (c) Proposed mechanism for zinc ion-assisted regulation of thermally activated delayed fluorescence. (d) Steady-state (d1), luminescence lifetime (d2) and time-gated (d3 and d4) imaging of HeLa cells incubated with ZnPXZT1.34 Copyright 2018, the Royal Society of Chemistry. | ||
TADF originates from reverse intersystem crossing (RICS) in charge transfer systems that have a thermally accessible energy gap between the lowest singlet (S1) and triplet (T1) excited states. Interestingly, the long nature of TADF lifetimes makes agents incorporating this phenomenon useful for fluorescence lifetime imaging. Recently, Yang reported the novel fluorophore PXZT, whose fluorescence is quenched by coordination with a zinc ion (ZnPXZT1) owing to enhanced intramolecular charge transfer (Fig. 7c).34 The TADF emission of PXZT is recovered upon dissociation of ZnPXZT1 and subsequent hydrophobic aggregation. Normal fluorescence and TADF imaging utilize different signals in different wavelength regions and, as a result, it is possible to separately detect the long lifetime delayed fluorescence of PXZT. Simple TADF emitters have become promising agents for time-resolved luminescence imaging. Using this technique, Zhao developed a nanosystem, composed of an amphiphilic cell-penetrating peptide (CPP) and TADF molecules.35 Aided by the CPP, the nanosystem assembles into well-dispersed NPs, whose delayed fluorescence can be observed in cells using an integral gate up to 0.5 μs.
Transformations of aggregated into disaggregated states are generally accompanied by an enhancement or recovery of emission. The reversal of aggregation-caused quenching (ACQ) can be utilized advantageously to design new emission ‘‘turn-on’’ probes. The number of new analyte-responsive ACQ fluorescent probes has grown substantially in recent years. Recently, Jiang et al. developed a small molecule, DPP-thiophene-4, based fluorescent nanoprobe that responds sharply to pH changes in a narrow range that causes switchable conversion of nonfluorescent nanoassemblies into fluorescent monomers (Fig. 8).36DPP-thiophene-4 is composed of a π-conjugated core (diketopyrrolo-pyrrole) and two alkyl chains possessing terminal quaternary ammonium groups. Interestingly, this substance forms a nonfluorescent nanosystem when in solutions at pH values greater than 7 and it reversibly disassembles to form fluorescent monomers at pH values less than 6.8. Owing to the slightly high acidity of tumor microenvironments, this fluorogenic nano-assembly can be employed to precisely differentiate between a number of malignant tumor types and normal tissues in vivo. This study has led to the development of a supramolecular strategy for selective diagnosis of malignant tumors.
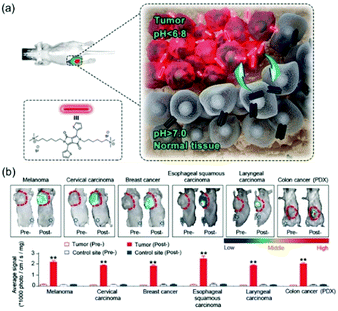 | ||
| Fig. 8 (a) The schematic diagram of DPP-thiophene-4 on imaging tumors in vivo. (b) Six malignant tumor models were used (breast cancer, colorectal cancer, cervical cancer, esophageal cancer, melanoma, and laryngeal carcinoma).36 Copyright 2017, American Chemical Society. | ||
3.2. Nanosystems for photoacoustic (PA) imaging
Photoacoustic (PA) imaging is an emerging technique that has several advantages over typical fluorescence-based approaches. For example, in addition to having higher in vivo spatial resolution PA approaches have deeper tissue penetration levels for in vivo imaging because they are not restricted by the optical diffusion limit (Fig. 9).37 Effective contrast agents for PA imaging need to absorb light in the NIR region with high molar-extinction coefficients, and have low fluorescence quantum yields and high photostability, high target affinity and low toxicity. In addition, the effectiveness of PA imaging techniques depends on the light-absorption coefficients of the contrast agents where an increase in the coefficient for near-infrared absorption leads to an increase in the photoacoustic imaging signal. Naturally occurring biological substances that absorb light in the NIR region such as hemoglobin and melanin can be used as endogenous contrast agents in PA based imaging methods to monitor physiological and anatomic changes occurring in diseases.38 Also, organic NIR absorbing dyes have been widely used for PA imaging because of their excellent biodegradability and biocompatibility.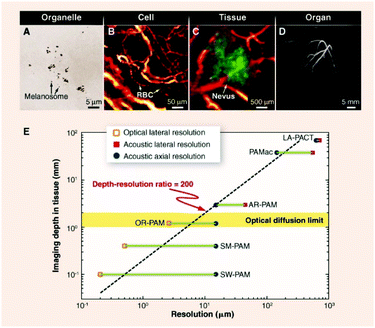 | ||
| Fig. 9 Multiscale photoacoustic tomography of organelles, cells, tissues, and organs in vivo.37 Copyright © 2012, American Association for the Advancement of Science. | ||
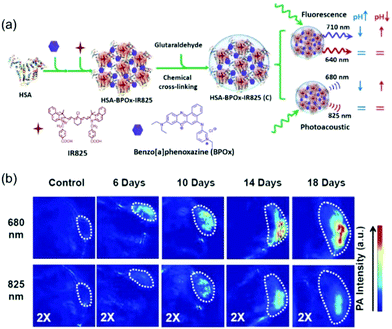 | ||
| Fig. 10 (a) A schematic illustration showing the formation of C-HAS-BPOx-IR825 and its application in pH sensing under both ratiometric fluorescence and photoacoustic imaging. (b) PA imaging of tumors with different sizes after mice were i.v. injected with C-HSA-BPOx-IR825. Images were recorded at excitation wavelengths of 680 and 825 nm.39 Copyright 2015, Wiley-VCH. | ||
In addition to stability, a PA imaging agent needs to have high biocompatibility. Recently, Yin et al. developed the water-soluble quaterrylenediimide (QDI) based photothermal agent P(QDI) that forms NPs (QDI-NPs) in aqueous solution owing to the presence of polyethylene glycol (PEG) groups tethered to the QDI core (Fig. 11).40 The QDI-NPs have small sizes (10.8 ± 1.4 nm) and good physiological and chemical stability in aqueous solution at 48 °C over a two-week period. Moreover, the core-expanded form of QDI in the NPs displays intense absorption at the NIR wavelength of 808 nm and they do not promote efficient reactive oxygen species (ROS) generation and they are weakly fluorescent because of heat producing vibrational relaxation of their excited states. It was shown that the irradiation of the QDI-NPs with different laser power densities leads to a remarkable increase in the temperature associated with a photothermal conversion efficiency (PTCE) of 64.7 ± 4%. Finally, also, the QDI-NPs effectively and passively target tumors and can be employed to visualize the tumor outline with a high degree of spatial resolution in vivo.
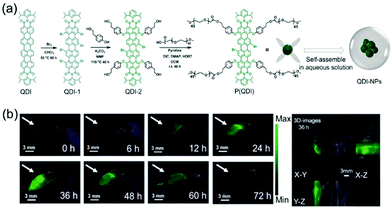 | ||
| Fig. 11 (a) Synthesis of the targeted product P(QDI) and the assembly of P(QDI) to form QDI-NPs in aqueous solution. (b) In vivo photoacoustic images of tumor tissue (arrows) at different times (left) and the 3D-images (right) at 36 h after the intravenous injection of QDI-NPs. Scale bars: 3 mm.40 Copyright 2019, Wiley-VCH. | ||
Zhao et al. designed the activatable photoacoustic probes BODPA1 and BODPA2 for real-time imaging of H2S in tumors of living mice (Fig. 12).41 These nanoprobes contain BODIPY hybrid dyes encapsulated in hydrophobic interiors of core–shells of silica nanocomposites. The strategy for the design of these probes is based on H2S promoted substitution reactions which lead to the formation of thiol derivatives that, unlike their chloro-substituted precursors, serve as NIR absorbing PA imaging agents. The nanoprobes display high levels of selectivity toward H2S, strong NIR absorption at 780 nm and excellent biocompatibility. The results of animal studies show that the H2S activated photoacoustic probes can be utilized to monitor endogenous H2S generation in a tumor-bearing mouse model.
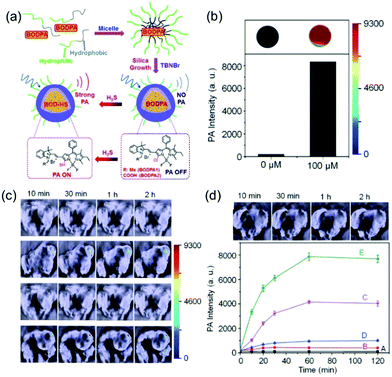 | ||
| Fig. 12 (a) Schematic illustration of the construction of activatable photoacoustic probes for H2S. (b) Representative photoacoustic images and amplitudes of Si@BODPA (80 mM BODPA1) in the absence and presence of NaHS (100 mM). (c) and (d) In vivo photoacoustic imaging of tumor-bearing mice using Si@BODPA (10 nmol BODPA1).41 Copyright 2017, the Royal Society of Chemistry. | ||
Pu et al. developed a similar aza-BODIPY dye based ratiometric nanoprobe, OSNs, for in vivo detection of peroxynitrite (ONOO−) (Fig. 13).42 The nanoprobe is composed of an aza-BODIPY dye as the reaction center responsible for ONOO− activated production of NIR absorption and PA. Importantly, this is the first activated PA nanoprobe developed for selective ROS based in vivo PA imaging.
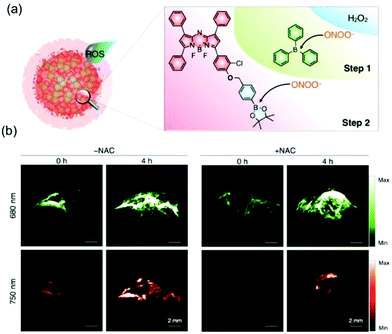 | ||
| Fig. 13 (a) Schematic illustration of reaction mechanisms of the bulky borane reinforced nanoprobe (OSN-B1) with ROS (ONOO− and H2O2). (b) In vivo ratiometric PA imaging of ONOO−.42 Copyright 2017, Wiley-VCH. | ||
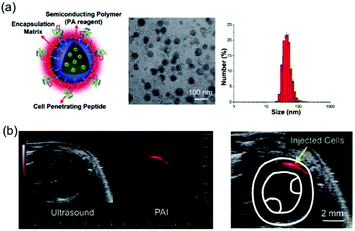 | ||
| Fig. 14 (a) Characterization of semiconducting polymer (SP)-based photoacoustic nanoparticles (PANPs). (b) PAI of the injection and engraftment of the PANP-labeled hESC-CMs in mouse hearts in vivo.44 Copyright 2018, Wiley-VCH. | ||
Bian and Pu et al. also developed positively charged organic semiconducting polymer nanoparticles (OSPNs+) for stem cell tracking and PA imaging in the NIR-II range (Fig. 15).45 The OSPNs+ are prepared by depositing an anionic inner layer on a SP core followed by deposition of a cationic shell composed of a co-assembly of amphiphilic poly-(styrene maleic anhydride) (PSMA) and poly(L-lysine) (PLL). The OSPNs+, which produce a PA signal from their SP core under NIR-II laser excitation, have optimized surface properties, outstanding biocompatibility, and appropriate sizes (113.2 ± 4.8 nm). Moreover, OSPN+ undergoes cellular uptake for PA imaging of stem cells. In vivo investigations show that this probe creates PA contrast enhancements of 21.7 and 40.6-fold compared with unlabeled cells when present in the brain and subcutaneous human mesenchymal stem cells (hMSCs), respectively.
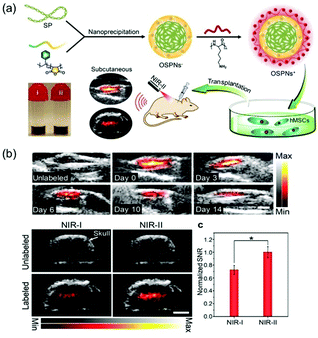 | ||
| Fig. 15 (a) Illustration of the procedure for preparation of OSPNs+ and the photoacoustic labelling of hMSCs after transplantation. (b) PA imaging in vivo.45 Copyright 2018, American Chemical Society. | ||
A simple cobalt protoporphyrin IX (CoPP) based “mesopore-induced aggregation” method was devised by Wu and Chen et al. to produce an enhanced PA imaging probe (Fig. 16).46 Porphyrin derivatives (Pds) play key roles in a variety of biochemical processes. However, Pds have limited application in PA imaging because they only weakly absorb light in the NIR range and they have poor solubilities in aqueous solution. It is expected that Pds tightly packed in assemblies would display improved performance in PA imaging.47 Wu and Chen et al. observed that CoPP@aMSNs, formed by loading CoPP into functionalized silica nanoparticles (MSNs), strongly absorb light in the NIR region as a consequence of intermolecular aggregation. Moreover, observation of a signal associated with Co in an element mapping image showed that CoPP in CoPP@aMSNs is encapsulated into the aMSN. CoPP@aMSNs has a high surface area (631.3 m2 g−1) and an average size of 578 nm. Confirmation of the feasibility of using this system to label BMSCs came from the observation that BMSCs incubated with this probe internalize about 80% of CoPP@aMSNs and they strongly resist lysosomal degradation. Interestingly, CoPP@aMSNs can be used to effectively monitor BMSCs in the body.
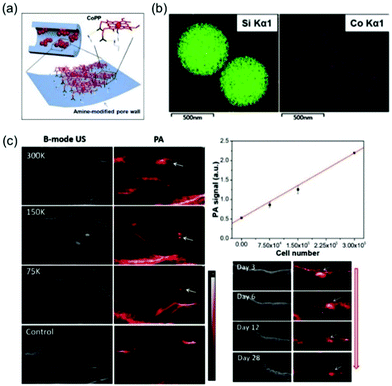 | ||
| Fig. 16 (a) The schematic shows the interactions between the adsorbed porphine cores and amino groups on the pore wall surface. (b) Element mapping images of CoPP@aMSNs. (c) US/PA images of the subcutaneous regions injected with CoPP@aMSNs-labeled BMSCs.46 Copyright 2018, Wiley-VCH. | ||
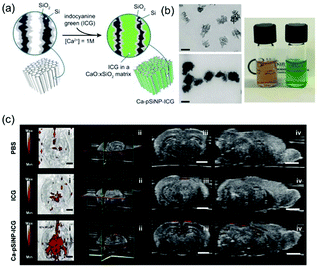 | ||
| Fig. 17 (a) Preparation and (b) properties of ICG-containing porous Si nanoparticles. (c) Photoacoustic images of fixed mouse brain compared with free ICG versus a comparable quantity of ICG encapsulated in a nanoparticle.48 Copyright 2018, Wiley-VCH. | ||
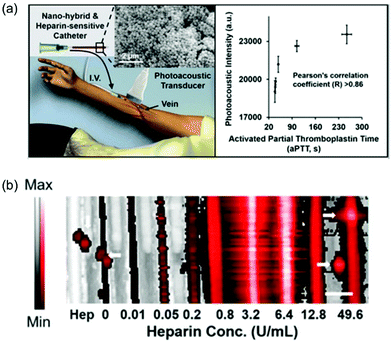 | ||
| Fig. 18 (a) Schematic of use of photoacoustics to image anticoagulation therapy. (b) The imaging data of a phantom using 0.6 mM methylene blue and increasing concentrations of heparin.49 Copyright 2016, American Chemical Society. | ||
4. Conclusion and outlook
Recent advances made in designing assembled organic dyes have led to novel approaches to highly spatiotemporal and non-invasive fluorescence and photoacoustic imaging. It is interesting that fluorescence and PA methods take advantage of two competitive decay processes of the electronic excited states of dyes. Thus, it is usually difficult to maximize both processes simultaneously in one system by varying the structures and/or substituents of organic molecules. Strategies based on limiting or enhancing molecular motions hold potential promise in addressing this issue and for creating on–off sensing signals.50 Specifically, enhancing molecular motion of organic substances by using reversible nano-assembly can promote molecular motion induced radiationless decay, fluorescence quenching and consequent heat generation for PA imaging. On the other hand, restriction of molecular motion of organic substances by controlling nano-assembly states results in radiative decay for fluorescence imaging. Thus, it is clear why the assembly phenomena of organic dyes have served as the foundation of strategies for the design of fluorescence and PA imaging agents. Another problem addressed by using assemblies is associated with the fact that visible and NIR absorbing organic dyes used for fluorescence and PA imaging typically contain extended π-conjugated systems. As a result, they suffer from ready oxidative decomposition, which results in lowered imaging efficiencies. This problem is lessened by incorporation of dyes in nano-assemblies.Continuing studies in this area will no doubt lead to the development of new photostable NIR-absorbing imaging agents. Moreover, most assembled organic imaging agents developed to date absorb light in the NIR-I region. It is anticipated that future efforts will focus on designing assemblies that display NIR-II activated fluorescence and PA and consequently have deeper penetration and higher spatiotemporal resolution.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This investigation was supported by the National Research Foundation of Korea (NRF), which is funded by the Korean government (MSIP) (No. 2012R1A3A2048814 for J. Y.). B. Z. Tang thanks the financial support from the Innovation and Technology Commission of Hong Kong (ITC-CNERC14SC01) and the National Natural Science Foundation of China (21788102).Notes and references
- J. Chan, S. C. Dodani and C. J. Chang, Nat. Chem., 2012, 4, 973–984 CrossRef CAS PubMed.
- K. Zhang, Y. J. Gao, P. P. Yang, G. B. Qi, J. P. Zhang, L. Wang and H. Wang, Adv. Healthcare Mater., 2018, 7, 1800344 CrossRef CAS PubMed.
- Z. Guo, S. Park, J. Yoon and I. Shin, Chem. Soc. Rev., 2014, 43, 16–29 RSC.
- J.-M. Lehn, Chem. Soc. Rev., 2017, 46, 2378–2379 RSC.
- J. Mei, N. L. Leung, R. T. Kwok, J. W. Lam and B. Z. Tang, Chem. Rev., 2015, 115, 11718–11940 CrossRef CAS.
- J. Luo, Z. Xie, J. W. Lam, L. Cheng, H. Chen, C. Qiu, H. S. Kwok, X. Zhan, Y. Liu, D. Zhu and B. Z. Tang, Chem. Commun., 2001, 1740–1741 RSC.
- L. V. Wang and S. Hu, Science, 2012, 335, 1458–1462 CrossRef CAS.
- H. F. Zhang, K. Maslov, G. Stoica and L. V. Wang, Nat. Biotechnol., 2006, 24, 848–851 CrossRef CAS PubMed.
- E. Mattia and S. Otto, Nat. Nanotechnol., 2015, 10, 111–119 CrossRef CAS PubMed.
- H.-B. Cheng, Y.-M. Zhang, Y. Liu and J. Yoon, Chem, 2019, 5, 553–574 CAS.
- X. Ma and Y. Zhao, Chem. Rev., 2015, 115, 7794–7839 CrossRef CAS.
- I. V. Kolesnichenko and E. V. Anslyn, Chem. Soc. Rev., 2017, 46, 2385–2390 RSC.
- W. Fan, B. Yung, P. Huang and X. Chen, Chem. Rev., 2017, 117, 13566–13638 CrossRef CAS PubMed.
- H.-B. Cheng, Y. Cui, R. Wang, N. Kwon and J. Yoon, Coord. Chem. Rev., 2019, 392, 237–254 CrossRef CAS.
- T. Zhang, Y. Li, Z. Zheng, R. Ye, Y. Zhang, R. T. K. Kwok, J. W. Y. Lam and B. Z. Tang, J. Am. Chem. Soc., 2019, 141, 5612–5616 CrossRef CAS PubMed.
- G. Feng and B. Liu, Acc. Chem. Res., 2018, 51, 1404–1414 CrossRef CAS.
- Y. Yuan, R. T. Kwok, B. Z. Tang and B. Liu, J. Am. Chem. Soc., 2014, 136, 2546–2554 CrossRef CAS.
- Y. Yuan, R. T. Kwok, B. Z. Tang and B. Liu, Small, 2015, 11, 4682–4690 CrossRef CAS.
- R. N. Dsouza, U. Pischel and W. M. Nau, Chem. Rev., 2011, 111, 7941–7980 CrossRef CAS PubMed.
- B. Shi, K. Jie, Y. Zhou, J. Zhou, D. Xia and F. Huang, J. Am. Chem. Soc., 2016, 138, 80–83 CrossRef CAS PubMed.
- J. Wu, S. Sun, X. Feng, J. Shi, X.-Y. Hu and L. Wang, Chem. Commun., 2014, 50, 9122–9125 RSC.
- X. M. Chen, Y. Chen, Q. Yu, B. H. Gu and Y. Liu, Angew. Chem., Int. Ed., 2018, 57, 12519–12523 CrossRef CAS PubMed.
- J. Qi, C. Sun, D. Li, H. Zhang, W. Yu, A. Zebibula, J. W. Y. Lam, W. Xi, L. Zhu, F. Cai, P. Wei, C. Zhu, R. T. K. Kwok, L. L. Streich, R. Prevedel, J. Qian and B. Z. Tang, ACS Nano, 2018, 12, 7936–7945 CrossRef CAS.
- B. Guo, Z. Feng, D. Hu, S. Xu, E. Middha, Y. Pan, C. Liu, H. Zheng, J. Qian, Z. Sheng and B. Liu, Adv. Mater., 2019, 31, e1902504 CrossRef PubMed.
- G. Yu, M. Zhang, M. L. Saha, Z. Mao, J. Chen, Y. Yao, Z. Zhou, Y. Liu, C. Gao, F. Huang, X. Chen and P. J. Stang, J. Am. Chem. Soc., 2017, 139, 15940–15949 CrossRef CAS.
- J. Zhang, W. Nie, R. Chen, J. Chelora, Y. Wan, X. Cui, X. Zhang, W. Zhang, X. Chen, H. Y. Xie and C. S. Lee, Nano Lett., 2019, 19, 658–665 CrossRef PubMed.
- X. P. He, Y. Zang, T. D. James, J. Li and G. R. Chen, Chem. Soc. Rev., 2015, 44, 4239–4248 RSC.
- W. Fu, C. Yan, Z. Guo, J. Zhang, H. Zhang, H. Tian and W. H. Zhu, J. Am. Chem. Soc., 2019, 141, 3171–3177 CrossRef CAS PubMed.
- H. W. Liu, K. Li, X. X. Hu, L. Zhu, Q. Rong, Y. Liu, X. B. Zhang, J. Hasserodt, F. L. Qu and W. Tan, Angew. Chem., Int. Ed., 2017, 56, 11788–11792 CrossRef CAS.
- J. Qi, C. Sun, A. Zebibula, H. Zhang, R. T. K. Kwok, X. Zhao, W. Xi, J. W. Y. Lam, J. Qian and B. Z. Tang, Adv. Mater., 2018, 30, e1706856 CrossRef PubMed.
- A. L. Antaris, H. Chen, K. Cheng, Y. Sun, G. Hong, C. Qu, S. Diao, Z. Deng, X. Hu, B. Zhang, X. Zhang, O. K. Yaghi, Z. R. Alamparambil, X. Hong, Z. Cheng and H. Dai, Nat. Mater., 2016, 15, 235–242 CrossRef CAS.
- Z. Zheng, D. Li, Z. Liu, H. Q. Peng, H. H. Y. Sung, R. T. K. Kwok, I. D. Williams, J. W. Y. Lam, J. Qian and B. Z. Tang, Adv. Mater., 2019, 31, 1904799 CrossRef CAS.
- J. Yang, X. Zhen, B. Wang, X. Gao, Z. Ren, J. Wang, Y. Xie, J. Li, Q. Peng, K. Pu and Z. Li, Nat. Commun., 2018, 9, 840 CrossRef.
- F. Ni, Z. Zhu, X. Tong, M. Xie, Q. Zhao, C. Zhong, Y. Zou and C. Yang, Chem. Sci., 2018, 9, 6150–6155 RSC.
- Z. Zhu, D. Tian, P. Gao, K. Wang, Y. Li, X. Shu, J. Zhu and Q. Zhao, J. Am. Chem. Soc., 2018, 140, 17484–17491 CrossRef CAS PubMed.
- Y. Liu, Z. Qu, H. Cao, H. Sun, Y. Gao and X. Jiang, ACS Nano, 2017, 11, 12446–12452 CrossRef CAS PubMed.
- L. V. Wang and S. Hu, Science, 2012, 335, 1458–1462 CrossRef CAS PubMed.
- J. Weber, P. C. Beard and S. E. Bohndiek, Nat. Methods, 2016, 13, 639–650 CrossRef CAS PubMed.
- Q. Chen, X. Liu, J. Chen, J. Zeng, Z. Cheng and Z. Liu, Adv. Mater., 2015, 27, 6820–6827 CrossRef CAS.
- C. Liu, S. Zhang, J. Li, J. Wei, K. Mullen and M. Yin, Angew. Chem., Int. Ed., 2019, 58, 1638–1642 CrossRef CAS PubMed.
- B. Shi, X. Gu, Q. Fei and C. Zhao, Chem. Sci., 2017, 8, 2150–2155 RSC.
- J. Zhang, X. Zhen, P. K. Upputuri, M. Pramanik, P. Chen and K. Pu, Adv. Mater., 2017, 29, 1604764 CrossRef PubMed.
- D. A. Robinton and G. Q. Daley, Nature, 2012, 481, 295–305 CrossRef CAS PubMed.
- X. Qin, H. Chen, H. Yang, H. Wu, X. Zhao, H. Wang, T. Chour, E. Neofytou, D. Ding, H. Daldrup-Link, S. C. Heilshorn, K. Li and J. C. Wu, Adv. Funct. Mater., 2018, 28, 1704939 CrossRef PubMed.
- C. Yin, G. Wen, C. Liu, B. Yang, S. Lin, J. Huang, P. Zhao, S. H. D. Wong, K. Zhang, X. Chen, G. Li, X. Jiang, J. Huang, K. Pu, L. Wang and L. Bian, ACS Nano, 2018, 12, 12201–12211 CrossRef CAS PubMed.
- M. Yao, M. Ma, H. Zhang, Y. Zhang, G. Wan, J. Shen, H. Chen and R. Wu, Adv. Funct. Mater., 2018, 28, 1804497 CrossRef.
- N. Muhanna, C. S. Jin, E. Huynh, H. Chan, Y. Qiu, W. Jiang, L. Cui, L. Burgess, M. K. Akens, J. Chen, J. C. Irish and G. Zheng, Theranostics, 2015, 5, 1428–1443 CrossRef CAS.
- J. Kang, D. Kim, J. Wang, Y. Han, J. M. Zuidema, A. Hariri, J. H. Park, J. V. Jokerst and M. J. Sailor, Adv. Mater., 2018, 30, e1800512 CrossRef PubMed.
- J. Wang, F. Chen, S. J. Arconada-Alvarez, J. Hartanto, L.-P. Yap, R. Park, F. Wang, I. Vorobyova, G. Dagliyan, P. S. Conti and J. V. Jokerst, Nano Lett., 2016, 16, 6265–6271 CrossRef CAS PubMed.
- S. Liu, X. Zhou, H. Zhang, H. Ou, J. W. Y. Lam, Y. Liu, L. Shi, D. Ding and B. Z. Tang, J. Am. Chem. Soc., 2019, 141, 5359–5368 CrossRef CAS PubMed.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2020 |




![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 times, with an h-index of 134. He received the State Natural Science Award (1st Class) from the Chinese Government in 2017. He is now serving as the Editor-in-Chief of Materials Chemistry Frontiers.
000 times, with an h-index of 134. He received the State Natural Science Award (1st Class) from the Chinese Government in 2017. He is now serving as the Editor-in-Chief of Materials Chemistry Frontiers.