Solvation properties of protic ionic liquids and molecular solvents†
Dilek
Yalcin
 ,
Calum J.
Drummond
,
Calum J.
Drummond
 and
Tamar L.
Greaves
and
Tamar L.
Greaves
 *
*
School of Science, College of Science, Engineering and Health, RMIT University, GPO Box 2476, Melbourne, Victoria 3001, Australia. E-mail: tamar.greaves@rmit.edu.au
First published on 2nd December 2019
Abstract
Ionic liquids (ILs) are highly tailorable solvents with many potential applications. Knowledge about their solvation properties is highly beneficial in the utilization of ILs for specific tasks, though for many ILs this is currently unknown. In this study, we have investigated the solvation properties of 12 protic ionic liquids (PILs) and 9 molecular solvents based on the Kamlet–Abboud–Taft′ (KAT) multi-parameter solvation scales. The KAT parameters, which are dipolarity/polarizability (π*), HBD acidity (α), HBA basicity (β), and the electronic transition energy (ET) were first obtained for the molecular solvents with an extensive set of 11 solvatochromic probe dye molecules. Based on these results the dyes which exhibited the highest sensitivities to polarity changes, and had the greatest chemical stability, were used to determine the KAT parameters of 12 PILs which contained alkyl-, dialkyl-, alkanol-, or dialkanolammonium cations paired with nitrate, formate or acetate anions. Solvation parameters were also obtained for the PILs using the three fluorescent probes pyrene, Coumarin 153 and Nile red for comparison. The PILs containing nitrate anions showed the greatest polarity, polarizability and HBD acidity followed by those containing formates and acetates. Almost all the PILs were found to have solvation properties comparable to water and single short chain alcohols like methanol and ethanol. The relative order of the IL polarities was similar for the solvatochromic and fluorescent probes. Through this study, in addition to the well-known distinct solvent properties of alkylammonium cation PILs, the high solvation capability of these PILs has been explicitly shown, which makes this class of ILs desirable for solvent-sensitive applications which require high polarity and H bonding ability.
Introduction
Ionic liquids (ILs) are important solvents due to the ability to design them to have low viscosity, low vapor pressure, low melting point and/or high thermal stability.1,2 Beside several other tailorable properties, many also have the ability to dissolve both polar and non-polar compounds, despite most of them being classified as ‘polar’ solvents according to general measures of overall polarity.3–7 Based on polarity and hydrogen bonding ability, it is possible to understand and predict the solubility, miscibility and dissolution equilibria6,8 and hence to design optimum solvent environments for targeted applications. However, these properties are not known for a broad range of ionic liquids, particularly for the protic ionic liquids (PILs), which are the focus of this study.There are several ways to characterize solvents, including ILs, in terms of their solvation properties such as absorption,9,10 fluorescence,11–13 electron spin resonance,141H & 13C NMR15 and vibrational spectroscopies,16 octanol–water partition,17 chromatography17–19 and via solvent effects upon chemical reactions20–25 or measuring physical properties like dielectric constants, refractive index, dipole moment, and relative permittivity.1,6,26,27 The overall polarity of conventional molecular solvents is commonly determined via measuring dielectric constant. However, this requires a zero conducting medium and hence is not applicable for ILs due to their ionic nature.6,28 In general, refractive index and molar refractivity29,30 are used to determine the overall polarity of ILs at a macroscopic level. However, these macroscopic properties cannot sufficiently describe the solvation strength governed through intermolecular, electrostatic and polarization forces between solute and solvent environment.31,32
The empirical scales based on the shifts in maximum absorbance or fluorescence of several solvatochromic/solvatofluorochromic probe molecules upon solvent change have been widely accepted by researchers for both molecular solvents and also ionic liquids.29,30,33,34 In addition, these single probe scales have been used in the characterization and investigations of micro-heterogenous systems such as micelles, vesicles, higher order self-assembled structures, sol–gels, porous materials, and polymer films, where specific hydrophilic and hydrophobic domains are present.35–39
Kamlet, Abboud and Taft (KAT) developed a solvatochromic comparison method where more than 45 different solvatochromic probe molecules were tested across more than 200 molecular solvents including amphiprotic, hydrogen bond acceptor and non-hydrogen bonding solvents.40–44 From this, a “Multi Parameter Approach” was established for the identification of specific intermolecular interactions, such as Coulombic, dipole/dipole, H-bonding and electron pair acceptor–donor interactions. In addition, the Linear Solvation Energy Relationship (LSER) between single parameter scales has been developed, as given in eqn (1);
| XYZ = (XYZ)0 + Sπ* + Aα + Bβ | (1) |
The ET(30) scale, based on a single dye known as Reichardt's betaine dye, gives a measure of not only solvent dipolarity/polarizability, but also hydrogen bond donating ability.26 Reichardt et al. have reported ET(30) values for more than 360 solvents, including ionic liquids, binary and ternary solvent systems,29 however the other 3 KAT parameters have not been reported for many solvents. According to the ET(30) values reported by Reichardt29 and others,17,18,33,45–52 the polarity of many ILs are within the ET(30) range of 42–63 kcal mol−1 with corresponding normalized ENT values of 0.35–1.00, where normalisation was done between 0 for tetramethylsilane and 1.0 for water.29 These values are comparable to those of dipolar non-H bond donating and H bond donating solvents. The results are well correlated with those from other empirical parameters of solvation such as fluorescent dyes13,33,53 as well as chromatographic techniques.18,19 Based on the ENT values, the primary, secondary and tertiary alkyl ammonium class of ILs are among the highest in polarity. This is followed by the imidazolium, pyrrolidinium, pyridinium, tetraalkylammonium and phosphonium classes of ILs with ENT values ranging from 0.35 (like acetone and DMSO) to 0.9 (comparable to methanol, ethanol and trifluoroethanol) depending on their structure.29
To date, more than 200 different ILs, which are mostly imidazolium and pyridinium class of ILs, have been characterized in terms of their solvation properties through employing a multiparameter approach. For these, the resulting ET(30) values along with the other 3 KAT parameters (π*, α and β values) have been reported.30,52,54–56 However, very few of these studies have included alkyl ammonium ILs, and of those that did, most focused on the aprotic tetraalkylammonium ILs and salts.17,18,47,48 The reported solvation properties of protic ionic liquids (PILs) containing primary, secondary or tertiary alkylammonium cations are limited to those of ethyl-, propyl-, butyl-, tributyl-, dimethyl-, dipropyl- and diethanolammonium cations in combination with nitrate, formate, dimethylcarbamate, and thiocyanate anions.52,55 However, none of these studies has explored the relationships between the PIL cation and anion structure and their solvation properties.
In this study, we have investigated the solvation behavior and properties of 12 PILs containing an alkylammonium cation in combination with formate, acetate or nitrate anions, of which the chemical structures are given in Fig. 1. Many have been previously well-characterized in terms of their thermal, structural and macroscopic physicochemical properties.57–62 To our knowledge, this is the first time in the literature that the solvation properties of alkylammonium PILs have been investigated in a systematic way to identify the specific anion and cation effect on the resulting PIL solvation capability. Since there are well known discrepancies between the results obtained in multiparameter polarity determination studies through the use of probe molecules, we first investigated 9 molecular solvents with anticipated solvation properties similar to those of PILs by using a collection of 11 solvatochromic dyes to initially assess the dye sensitivity to solvent changes. The chemical structures of 4 of these solvatochromic dyes, along with 3 fluorescent dyes including Nile red, which exhibits both absorbance and fluorescence properties, are given in Fig. 2 while the chemical structures of the other 7 solvatochromic dyes are provided in Fig. S1 in the ESI.† As the solvation parameters of these molecular solvents have been very well characterized, based on their results, we have selected the best set of dyes to be further used for the investigation of solvation properties of neat PILs. The resulting ET(30) values and KAT parameters have been compared with those obtained by using fluorescent dyes (pyrene and Coumarin 153) as well as Nile red. These were compared with reported literature values where available. All findings on the solvation properties of the alkylammonium class of PILs have been discussed in terms of the effects of PIL structure, dye molecule and the technique used.
Materials and methods
The chemicals were all used as received. The amines used were ethylamine (70%, Sigma-Aldrich), ethanolamine (99.5%, Sigma-Aldrich), propylamine (99.5%, Sigma-Aldrich), butylamine (99.5%, Sigma-Aldrich), and pentylamine (99%, Sigma-Aldrich). Acids were formic acid (98%, Merck), acetic acid (99%, Sigma-Aldrich), and nitric acid (70%, AjaxFineChem). The 12 PILs shown in Fig. 1 were synthesized and dried according to our previously reported method.57,61 The water content of the neat PILs was determined using a Mettler Toledo C20 Karl–Fischer titrator and provided in the ESI.†As shown in Fig. 2 and Fig. S1 in the ESI,† the dye molecules were N,N-diethyl-4-nitroaniline (DE4A) (99%, Santa Cruz Biotechnology), 4-nitroaniline (4NA) (99%, Fluka), Reichardt's Dye 30 (RD30) (90%, Sigma-Aldrich), Reichardt's Dye 33 (RD33) (99%, Aurora Fine Chemicals), Phenol blue (PB) (97%, Sigma-Aldrich), N,N-dimethyl-4-nitroaniline (DM4A) (98%, Alfa-Aesar), 4-nitrophenol (4NP) (99.5%, Fluka), 4-nitroanisole (4NAni) (97%, Aldrich), N-methyl-2-nitroaniline (M2A) (98%, Aldrich), N,N-dimethyl-2-Nitroaniline (DM2A) (99%, Santa Cruz Biotechnology), pyrene (Pyr) (99%, Sigma), Coumarin 153 (C153) (99%, Aldrich) and Nile red (NR) (technical grade, Sigma-Aldrich). The 9 traditional molecular solvents used in this study were water, methanol (99.9%, Alfa-Aesar), ethanol (99.8%, Sigma-Aldrich), 2-propanol (99.5%, Sigma-Aldrich), cyclohexanol (99%, Sigma-Aldrich), dimethylsulfoxide (DMSO) (99.9%, Sigma-Aldrich), acetonitrile (99.8%, Sigma-Aldrich), cyclohexane (99.9%, Sigma-Aldrich), and hexamethyl phosphoramide (HMPA) (99%, Sigma-Aldrich).
Prior to absorbance and fluorescence measurements, stock solutions of all dyes were prepared in anhydrous methanol, which was used as a dye transferring solvent. The dye concentrations in the stock solutions are provided in Table S1 in the ESI† along with their molecular weights, types of hydrogen bonding and solvatochromic (or solvatofluorochromic) shift. An appropriate amount of dye solution in methanol (0.3 ml) was first transferred into Eppendorf tubes and then the methanol was immediately removed under vacuum at 40 °C. Next, the same amount of solvent under study was added to these dye containing tubes under vigorous shaking. The dye concentrations in each of the solvents was sufficient to allow an absorbance greater than 0.1.
All spectroscopic measurements were performed using a PerkinElmer EnSight Multimode plate reader. The spectral range for absorbance measurements was 250–950 nm. The maximum excitation wavelengths (λmaxex) for the fluorescent dyes were set at 530, 335 and 415 nm for NR, Pyr and C153, respectively, whereas ranges of the emission wavelength were 545–700 nm, 350–450 nm and 435–600 nm for the same dyes. The bandwidth was 1 nm for all absorbance and fluorescence measurements.
Results
In this study, we initially employed the single probe polarity approach to 9 molecular solvents using 11 solvatochromic dyes including Nile red, which is the only dye molecule exhibiting absorbance and fluorescence upon solvent change, to observe the dye sensitivities to different solvents and hence, to narrow down the number of dyes. The molecular solvents have been carefully selected as solvents with anticipated similar properties to the alkylammonium PILs. The polarity range of the PILs was expected to resemble that of simple alcohols such as methanol, ethanol and of non-protic H bond acceptor solvents like DMSO and acetonitrile. Cyclohexane and HMPA were included as common reference solvents. The absorbance spectra of each of the 11 absorbent dyes in each of the 9 molecular solvents were acquired, and the wavelengths of absorbance maxima are given in Table S2 in the ESI.† By means of the single probe approach, certain types of dyes have been compared to each other to evaluate the optimal sensitivity of dyes according to the two primarily important scales, which are the electronic transition energy or electrophilicity (ET(30) values) and dipolarity/polarizability (π*). In this manner, we selected the best set of dyes with optimal sensitivity and used them to characterize the solvation properties of 12 PILs containing alkylammonium cations.Dye selection (via absorbance)
| ET (kcal mol−1) = hcvmaxNA = (2.8591 × 10−3)vmax = 28591.5/λmax | (2) |
First, the transition energies calculated based on the two common Reichardt's dyes, RD30 and RD33 were compared to each other. The experimental ET(30) values for the given solvents exhibited good agreement with those reported by Reichardt.26 However, RD30 has limitations in water and acidic conditions where it has poor solubility, or gets protonated, leading to the band disappearing.64 The closely related dye, RD33, does not have these same limitations. Through a simple linear regression, the ET values from RD33 and RD30 correlated to each other, as shown in Fig. S2 in the ESI.† The obtained correlation equation for ET(30) and ET(33) values was given in eqn (3).
| ET(30) = 0.9442ET(33) − 5.7329. | (3) |
| vmax = v0 + sπ* | (4) |
All 4 non-HBD dyes demonstrate a similar trend upon the change of dipolarity/polarizability of molecular solvents, however, the values varied significantly. When compared with literature values, the two homomorphic dyes DE4A and DM4A appeared to have the greatest consistency. Since it is incredibly hard to find a solvatochromic dye which has sufficient non-specific interactions with the solvent but zero hydrogen bonding, the π* values have been averaged across 45 dye molecules in the development of the original π* scale.44 Of those 45 dyes, 2 solvatochromic dyes, 4NAni and DE4A have been extensively used by the IL community, although debate remains on which are the most suitable. For instance, Jessop et al. have stated that some groups prefer using 4NAni, due to DE4A suffering from poor band-shape in low polarity solvents.55 Ab Rani et al. have tested a broad collection of solvatochromic dyes including 4NAni and DE4A and concluded that even though the results obtained with these two dyes were quite close and similar to each other, the use of DE4A could provide a better consistency with the literature when comparing and applying the LSER approach.54 For these reasons, DE4A has been selected in this study as the π* indicator to be further used in PILs.
The other 2 KAT parameters, α (HBD acidity) and β (HBA basicity), were calculated based on the selected set of dyes according to the ET and π* values. In KAT LSERs, HBD acidity, α was introduced to complete the terms that involve the specific interactions between solvent and solute. The α value describes the ability of solvent to donate a proton in a solvent-to-solute H bond. Here, the α values were calculated based on ET(30) and π* values by the simplified equation given below,30 where ET(30)calc and πDE4A* values were taken from Tables S3 and S5 (ESI†), respectively.
| α = 0.0649(ET(30)) − 0.72π* − 2.03 | (5) |
 | (6) |
Protic ionic liquids (via absorbance)
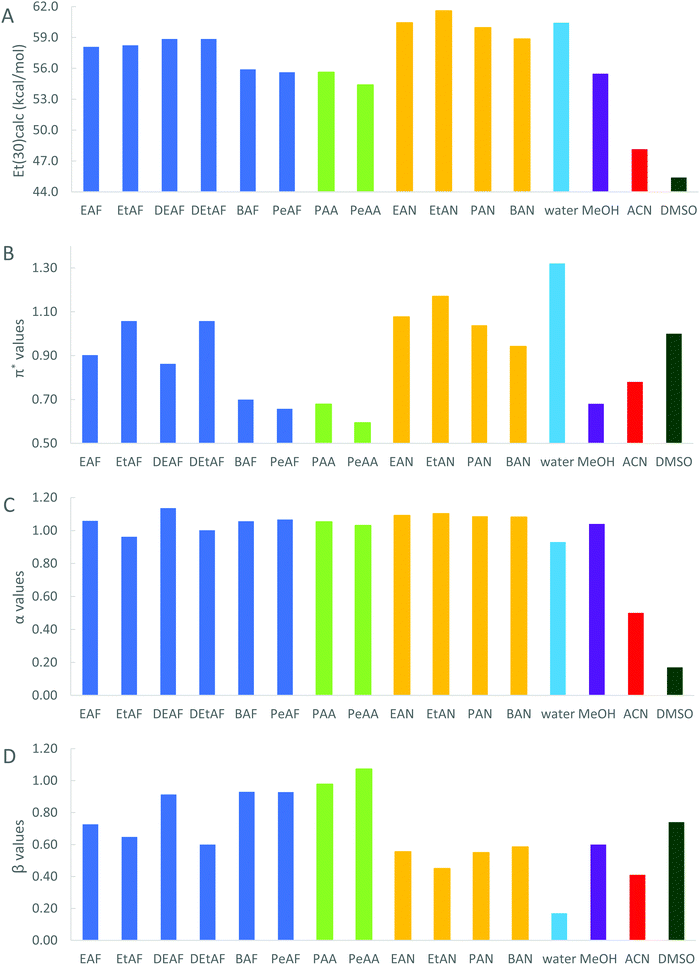
The multiparameter Kamlet, Abboud and Taft LSER approach was used to determine the solvation properties of the 12 alkylammonium cation containing PILs as shown in Fig. 1. The spectral data showing the wavelengths of absorption maxima of the selected dyes in PILs along with the water content of neat PILs are provided in Table S7 in the ESI.† Using eqn (2)–(6), the ET values and KAT parameters (π*, α and β values) for PILs were calculated based on the spectral data, and the results are given in Table 1, with a visual representation in Fig. 3.| E T(33) (kcal mol−1), (±0.02) | E T(30) (kcal mol−1), (±0.02) | E T(30)calca (kcal mol−1), (±0.02) | Dipolarity/polarizability, π*, (±0.02)b | HBD acidity, α, (±0.04) | HBA basicity, β, (±0.04) | |
|---|---|---|---|---|---|---|
| a E T(30)calc values were calculated through the linear correlation equation (ET(30) = 0.9442 × ET(33) − 5.7329) obtained for molecular solvents. b Data was normalized between 0 (for cyclohexane) and 1.0 (for DMSO). c Not determined due to the limited solubility of RD30 in these PILs. d Values for molecular solvents were taken from the data given in the ESI. e Transition energies were obtained based on the solvatochromic band shift of Nile red (NR).65 The name of other ILs listed here are [B3N][N]: tributylammonium nitrate, [BN][SCN]: butylammonium thiocyanate, EAC: Ethylammonium chloride, DMAC: dimethylammonium chloride, DEAN: diethylammonium nitrate, [N][tFA]: ammonium trifluoroacetate. | ||||||
| PILs | ||||||
| EAF | 67.59 | ndc | 58.09 | 0.90 | 1.06 | 0.73 |
| EtAF | 67.75 | 59.81 | 58.24 | 1.06 | 0.96 | 0.65 |
| DEAF | 68.40 | ndc | 58.85 | 0.86 | 1.14 | 0.91 |
| DEtAF | 68.40 | ndc | 58.85 | 1.06 | 1.00 | 0.60 |
| BAF | 65.28 | 57.99 | 55.90 | 0.70 | 1.06 | 0.93 |
| PeAF | 64.98 | 56.96 | 55.62 | 0.66 | 1.07 | 0.93 |
| PAA | 64.98 | 56.96 | 55.62 | 0.68 | 1.05 | 0.98 |
| PeAA | 63.68 | 55.73 | 54.39 | 0.59 | 1.03 | 1.07 |
| EAN | 70.08 | 61.75 | 60.43 | 1.08 | 1.09 | 0.55 |
| EtAN | 71.30 | 62.02 | 61.59 | 1.17 | 1.10 | 0.45 |
| PAN | 69.57 | 61.36 | 59.95 | 1.04 | 1.09 | 0.55 |
| BAN | 68.40 | 60.32 | 58.85 | 0.94 | 1.08 | 0.58 |
| Molecular solventsd | ||||||
| Water | 70.08 | ndc | 60.43 | 1.32 | 0.93 | 0.17 |
| Methanol | 64.83 | 55.52 | 55.48 | 0.68 | 1.04 | 0.60 |
| Acetonitrile | 57.07 | 47.34 | 48.15 | 0.78 | 0.50 | 0.41 |
| DMSO | 54.15 | 45.24 | 45.40 | 1.00 | 0.17 | 0.74 |
| ILs by others | ||||||
| EAN | 61.6;18 59.848 | 1.2418 | 1.1051; 0.8518 | 0.4618 | ||
| PAN | 60.618 | 1.1718 | 0.8818 | 0.5218 | ||
| [B3N][N] | 56.718 | 0.9718 | 0.8418 | |||
| [BN][SCN] | 61.418 | 1.2318 | 0.9218 | |||
| EAC | 62.347 | |||||
| DMAC | 60.347 | |||||
| DEAN | 65.547 | |||||
| [N][tFA] | 43.647 | |||||
| DEAAe | 50.265 | |||||
| DEAFe | 50.665 | |||||
| DEtAFe | 50.165 | |||||
From Table 1 and Fig. 3, it is clear that the solvation parameters of the PILs exhibited certain trends depending on the cation and anion. In the following sections, we discuss each parameter in detail and compare the findings with the reported literature values for the same and other class of ILs, where available.
The strong anion dependency of the ET(30) values of these PILs can be clearly seen from Fig. 3A and decreases in the order of nitrates > formates > acetates. Shukla et al. have studied alkyl imidazolium PILs and found that the formate anion led to higher polarity than the acetate anion for the same PIL cation. Increasing the alkyl chain length on the PIL cation also had an effect on polarity, but to a lesser extent. Polarity decreased from 1-methylimidazolium to butylimidazolium with the same anion.66 Beniwal et al. have also investigated the polarity of the same protic imidazolium ILs and found the same trend for the PILs with the formate and acetate anions.67 Harrod et al. have reported the ET(30) and π* values for a broad range of protic and aprotic ammonium and phosphonium class ILs including the eutectic salts and concluded that the polarity of salts/ILs increased with the increasing size of the anion.47 Although the anion dependency of transition energies is obvious, Hallett et al. have stated that the degree of difference in ET values for ILs with the same cation but different anions actually varies by the cations.6 This is due to the antagonistic behavior of the IL anions in determining the overall hydrogen-bond donating properties of the ionic liquids.6
The specific cation and anion effect on the overall polarity of PILs can also be seen from Fig. 3A. The stronger coulombic force between the PIL cation and anion reduces ET(30) through reducing the ability of the cation to interact with the phenoxy moiety,30,68 the HBA center, of RD30. This also affects the α values, since α is calculated by separating out the polarizability term from the overall polarity. However, the PILs with nitrate anions exhibited greater overall polarity, polarizability and HBD ability than those with formate and acetate anions. This is attributed in part to the nitrate anion being able to coordinate with the pyridinium cation of RD30, leading to an increase in the intramolecular charge transfer energy.
In regard to changes in the PIL cation structure, alkyl and –OH substitution showed a slight increasing effect on polarity. The ET(30) value of EAF was found to be 58.09 kcal mol−1 and it increased to 58.85 kcal mol−1 when the cation was replaced with its diethyl-substituent (Fig. 3A). A similar incremental change has been observed from EtAF to DEtAF. Harrod et al. reported an ET(30) value of 65.5 kcal mol−1 for diethylammonium nitrate (DEAN),47 which is higher than that of EAN (60.43 kcal mol−1), suggesting a similar increasing effect on alkyl substitution. Zhang et al. have also found that introducing an –OH group to 1-ethyl-3-methylimidazolium ILs increased the polarity and its anion dependency remarkably.69
Increasing alkyl chain length on the cation and/or also anion led to a decrease in the PILs polarity. This is consistent with other classes of ILs, where Chiappe et al. have reviewed the transition energies of several class of ILs and concluded that the change in alkyl chain length has a moderate effect whereas substitution of –OH groups on the cation increases the polarity to a greater extent.30
Using the solvatochromic dye Nile red (NR), Chen et al. studied the relationship between the polarity and hygroscopicity of PILs to design a PIL with tunable water solubility. The library in their study included 9 different PILs, 8 of which are diethyl-, diethanol- and dimethoxymethyl ammonium PILs in combination with formate, acetate, sulfate, sulfamate and phosphate anions and pyrrolidonium acetate. Based on the solvatochromic shifts of NR, they only examined the overall polarity responses of the given PILs whereas the KAT parameters have not been studied. They found that diethanolammonium sulfate [DEtAS] and dimethoxymethyl acetate have the greatest and the lowest polarities, respectively. Similar to our findings, hydroxyl (–OH) substitution on the cation increased the polarity; however, they observed that diethylammonium acetate (DEAA) had a greater polarity than DEAF.65 As a general trend, Hallett et al. have stated that more basic anions (like acetate compared to formate) lead to a decrease in polarity determined by the solvatochromic shifts of Nile red.6
The π* values showed a similar trend to the transition energies observed for PILs with nitrate and acetate anions, and differed for the alkyl substituted PILs with the formate anion. Fig. 3B clearly shows that PILs with nitrate anions had the highest π* values, followed by formates and acetates. This is consistent with the literature where it is known that small and highly acidic anions lead to high π* values.54 Poole et al. have also indicated that alkylammonium nitrate and thiocyanate are more dipolar/polarizable than DMSO, water and other class of ILs.52 Regarding the anion effect, Shukla et al. have observed a similar trend and reported higher dipolarity/polarizability for 1-methylimidazolium and 1-butylimidazolium with formate anions compared to those with acetate anions. They have also stated that increasing the alkyl chain length on the cation leads to a decrease of π* for ILs with the same anions.66
In addition to its anion dependency, we have found that –OH substitution on the cation showed the greatest increasing effect on the polarizability of PILs, for formates in particular. The π* values for EtAF, DEtAF and EtAN were found to be far greater than their non-hydroxyl derivatives. This –OH functionalization, on either the cation or anion of a PIL, can lead to an increase in π* values due to the enhanced interaction between the PIL hydroxyl and the dye nitro (–NO2) functional group.54 High π* values indicate a better stabilization of charge for DE4A in its excited state, and hence favorable interaction between the PIL and the dye. Other modifications on the cation such as increasing chain length and alkyl substitution affected the dipolarity/polarizability of PILs with alkyl substitution from EAF to DEAF led to π* values decreasing from 0.90 to 0.86, whereas it did not change from EtAF to DEtAF (Fig. 3B). In other class of ILs such as morpholinium, imidazolium, pyridinium, pyrrolidinium and phosphonium, alkyl substitution led to a decrease in π* values.54 Moreover, Ab Rani et al. have also noted that the π* values of different classes of IL cations with the same anion generally show the following order of morpholinium > imidazolium > pyridinium > pyrrolidinium > phosphonium.54
Among all these PILs, PeAA (β = 1.07) was found to be the greatest H bond acceptor while EtAN (β = 0.45) was the lowest. Increasing the alkyl chain length on the PIL cation led to a slight increase in HBA basicities in PILs with nitrates, and a larger increase for PILs with organic anions. The basicities ranged between 0.45–0.58 for nitrates, 0.60–0.93 for formates and 0.98–1.07 for acetates (Fig. 3D). The introduction of an –OH group on the cation reduced the HBD and HBA ability of EtAF and DEtAF relative to EAF and EAF respectively, and the HBA ability of EtAN compared to EAN, though the HBD ability showed an increase for the PILs with a nitrate anion (Fig. 3C and D). This suggests that the HBD ability of alkylammonium PILs with organic anions has a stronger dependency on the cation. Surprisingly, –OH functionalization on the cation with formate anions decreased the basicities and the acidities, however an opposite trend was observed for the nitrates, where introducing a hydrogen bond donor group increased the acidity and decreased the basicity.
There is only one previous literature study that we are aware of where the acidity and basicity of alkylammonium PILs was reported, and this was by Poole et al. where they investigated the solvation properties of EAN and PAN along with nitrates of other ammonium and phosphonium cations.18 Their data is provided in Table 1. They found that the H bond donating (α) and accepting (β) abilities of PILs were similar to water and simple aliphatic alcohols. The alkylammonium nitrate and thiocyanate salts are all strong hydrogen-bond donors (α = 0.84–0.97) and moderate hydrogen-bond acceptors (β = 0.39–0.52), as reported by Poole C,52 which is in good agreement with our findings.
With respect to HBD and HBA abilities, different observations have been reported for other classes of ILs, with some discrepancies. In the protic alkylimidazolium ILs, Shukla et al. have reported that PILs with the inorganic sulfate anion exhibited the highest HBD acidity and lowest HBA basicity.66 They also found that the α values of 1-methylimidazolium PILs decreased on changing the anion from sulfate to formate or acetate. Unlike ours, increasing the alkyl chain length on the alkylimidazolium cation had an increasing effect on α values. However, Kurnia et al. have stated that increasing the alkyl chain length in [CnC1im] [NTf2] from n = 1 to 9 decreased HBD acidity and increased HBA basicity,70 which agreed well with our findings for PILs with nitrate and acetate anions. As a more general view, they have also compared the KAT parameters obtained for ILs with different type of cations, where imidazolium ILs showed the greatest acidity followed by tetraalkylammonium > sulfonium > pyridinium > pyrrolidinium > piperidinium > tetraalkylphosphonium based ILs with an opposite order for basicity.70 However, Hallett et al. have said that lengthening the alkyl chains caused acidities to decrease for –OH substituted imidazolium, pyridinium and pyrrolidinium cation ILs.6
Jessop et al. have reported KAT parameters for many classes of ILs and some exhibited unexpected trends upon change of either the cation or anion. For example, the reported HBD acidities and HBA basicities of butyl-, hexyl-, and octyl pyridinium with a [NTf2] anion did not follow a general trend. Similarly, there was no obvious relationship on increasing the cation chain length for methylpropyl-, methylpentyl- and methylhexylpyrrolidinium ILs with the [NTf2] anion.55
Consequently, the results on HBD acidity and HBA basicity seem to vary remarkably depending on the cationic and anionic class of ILs, the dye molecules used in LSERs, impurities present, and on how precisely the sample preparation was done. Therefore, a more sophisticated approach and techniques are required for quantifying the strengths of IL acidity and basicity, and hence also for developing structure–property relationships for these two parameters. This is discussed in more detail in the following sections. In addition, there is a need for larger data sets collected in a consistent manner to enable broader comparisons.
| PILs | Pyr (II/IIII)a | λ em (C153)b | λ em (NR)c |
|---|---|---|---|
| a λ ex = 335 nm. b λ ex = 415 nm. c λ ex = 530 nm. Numbers in parenthesis were extracted from literature. | |||
| EAF | 1.025 | 536 | 635 |
| EtAF | 1.119 | 545 | 642 |
| DEAF | 1.090 | 538 | 634 |
| DEtAF | 1.122 | 535 | 641 |
| BAF | 0.979 | 529 | 629 |
| PeAF | 0.955 | 530 | 628 |
| PAA | 0.987 | 525 | 625 |
| PeAA | 0.951 | 528 | 621 |
| EAN | 1.148 | 544 (55071) | 647 |
| EtAN | 1.495 | 548 | 650 |
| PAN | 1.019 | 544 | 645 |
| BAN | 0.990 | 539 | 642 |
| Molecular solvents | |||
| Water | 0.92 (1.9633–1.4669) | (54872) | 666 (666.133) |
| Methanol | 0.86 (1.5033–1.3552–1.2469) | 533 (53673–53274) | 635 (641.333) |
| Acetonitrile | 1.02 (1.8833–1.7952–1.5069) | 520 (52172) | 619 (620.033) |
| DMSO | 1.22 (1.9033–1.9552) | 528 | 628 (640.033) |
According to the pyrene results, the PILs with nitrate anions exhibited the highest dipolarity, followed by formates and acetates, which agrees very well with the π* values obtained from DE4A. Of all the 12 PILs, EtAN was found to be the most dipolar and PeAA the least. Increasing the alkyl chain length on the cation led to a reduction in dipolarity whereas –OH group and alkyl substitution on the cation showed a polarity increasing effect. The polarities of almost all the PILs were found to be close to that of acetonitrile, whereas EAN and EtAN were more dipolar and reminiscent of DMSO. Poole et al. have studied 3 non-ionic fluorescent dyes in several ammonium and phosphonium class of ILs, however, they reported the dipolarity responses for EAN and PAN only based on the probe, benzoperylene.52 The dipolarity of EAN and PAN based on benzoperylene were both 1.20, which is similar to polar non-ionic solvents like acetonitrile (1.23).52 They have also stated that the dipolarities of primary alkylammonium nitrates were higher than those of alkylammonium thiocyanates but lower than those of tertiary and quaternary alkyl ammonium ILs.52
Further discussion
A linear free energy relationship can be utilized to describe the solvation process of a solute in three stages: (1) a suitable size of cavity should be formed in the solvents, in PILs in our case, (2) solute is replaced in solvents and polarizes them, and (3) due to the polarization, reorganization of solvent molecules around the cavity takes place through various solvent–solute interaction.19,31 The polarization takes place in different ways in molecular solvents and ILs, which are described as orientational and translational polarization.76 Orientational polarization occurs when the molecular solvents are attracted towards the solute by dispersion forces, and oriented around the cavity by the solute local charge. In ionic solvents such as ILs, anions and cations are placed towards the positive and negative sides of a solute leading to an inhomogeneity with polar and non-polar domains.76The solvation of various compounds, such as aromatics, non-charged and charged solutes3,77,78 or other solvents78–81 in PILs highly depends on the nano segmentation of PILs, which consist of polar and non-polar domains. The mesoscopic models of liquid nanostructure of PILs based on X-ray and neutron scattering data can be a good measure of interactions between the solute molecules and either polar or non-polar domains of PILs.3 These mesoscopic models are generally consistent with the findings through solvatochromic methods as well as the molecular dynamics simulations.3 Moreover, MD simulations are more likely to quantify the strength and elucidate the role of H-bonding and H-bonded network of PILs to understand their solvation capabilities.77 Many PILs of interest in this study were previously characterised in terms of their liquid nanostructure and the intermediate range correlation distances between polar and non-polar segments have been reported.7,60 When the correlation distances are linked to our present findings for solvation properties, there are clear trends between the liquid nanostructure and the solvation data. For the series of primary alkylammonium cation containing PILs of EAF, BAF, PeAF; EAN, BAN and PeAN, it is observed that the solvation parameters of ET, π* and α values decrease with increasing alkyl chain length, while the β values increase. A similar trend is also observed for the overall polarity data obtained based on the fluorescent dye molecules. The solvation parameters of PILs containing nitrate anions were less affected by increasing correlation distances than those obtained for PILs with formate anions. Overall, the less structured PILs appear to be better solvents to solvate compounds such as zwitterionic, non-H bonding, charged and polar compounds. This might also explain why the –OH functionalised and/or dialkyl-substituted PILs with smaller or negligible correlation distances but enhanced H-bonding are considered as more ‘water-like’ solvents and capable of dissolving a broad range of solutes.60,77,78
Although solvatochromic methods were originally developed for describing the solvation properties of molecular solvents, they have been widely employed by the IL community for comparing the IL properties and behaviours with well-characterized conventional solvents. However, while comparing the results, great care should be taken since these parameters are highly sensitive to the method, probe molecules, presence of impurities, and even sample preparation.29 Depending on many variables, the solvation parameters vary widely. For example, the reported relative polarity values for one of the commonly used ionic liquid [BMIM][NTf2] varied in the range of 0.645–0.840.20,30
Employing a multiparameter approach has remarkable advantages compared to single probe scales/parameters. However, selection of the dyes/probes is critical in either single or multiparameter scales. The zwitterionic Reichardt's dye have been the most widely used probe in the determination of overall polarity, and it senses the dipolarity/polarizability and also H bond donating ability of solvents. Due to its zwitterionic nature, it is believed that the betaine dye is affected by the coulombic interactions with ILs, which is not the case with molecular solvents. Some researchers have preferred using other probe molecules, such as N,N-dimethylbenzamide,54 Nile red,27,65 tetramethyl-piperidine-1-oxyl,14 4-hydroxy2,2,6,6-tetramethylpiperidine-1-oxyl14 or spiropyran derivatives.82,83 Others have tried fluorescent dyes such as pyrene,52,69 dansylamide,69 coumarin derivatives,74 4-aminophthalimide53 and 4-(N,N-dimethylamino)phthalimide53 claiming they have higher sensitivity to solvent change and are capable of overcoming the problems encountered for betaine dyes, such as their large size, relative high concentrations (causing a possible dye–dye interaction), and their zwitterionic nature.69 All these parameters of solvent polarity have been successfully applied in the analysis of solvent related relationships, processes, solvation dynamics and equilibria and in most cases, they have been well correlated with the findings obtained based on Reichardt's betaine dyes.29 For these reasons, the set of dye molecules in this study, and in many others, appear to be more suitable ones in establishing the polarity scales for moderately and/or highly polar protic solvents. It was found that using the N,N-diethyl-4-nitroaniline/4-nitroaniline pair, along with Reichardt's dye 30, provided the greatest internal consistency in the polarity analysis. They are also the most commonly used set of dyes in the literature, which gives the greatest availability of comparison with other solvents. However, it is still possible for two homomorphic dyes to give quite different parameter values.6 The main reason is that the correlation equations are obtained from average values of the dye molecules in hundreds of different solvents, which is not feasible for each new solvent being investigated.40–44,84
The solvation behavior and capability of ILs also depends upon the nature of the dissolved probe, mainly if it is non-polar or polar, and non-ionic or charged. The polarity of ILs obtained using a polar probe is significantly different from that obtained by a non-polar probe. Moreover, understanding the effect of coulombic contribution is very important. If the probe molecule is charged, then there would be a strong coulombic contribution to the IL–dye interactions.6,66 If it is neutral, the coulombic contribution could be negligible. Consequently, the empirical scales developed based upon charged or polar probes usually give higher polarity values for ILs than those based upon neutral or non-polar probes. In our results, it was found that the coulombic contribution in PIL–dye interactions was significantly higher, regardless of whether the probe molecule was non-polar or not, resulting in the indication of a higher degree of dye solvation capability relative to that of molecular solvents. While there is no absolute scale, or method, to compare different scales, some tentative generalizations are possible. In the IL community, it is generally accepted that the alkylammonium class of ILs has the highest dipolarity/polarizability values and greater than those of molecular solvents, due to the coulombic interactions along with dipole/polarizability effects.29 Moreover, HBD acidity and HBA basicity values cover quite a large range and vary significantly depending on the IL structure. HBD acidities are primarily controlled by the IL cation whereas HBA basicities are mainly determined by the IL anion.85
Despite the quantification of the strength of HBD acidity and HBA basicity, these remain the most challenging aspect of empirical KAT scales. Some researchers have found correlations between the reported α and β values of ILs and their behaviours as reaction solvents, such as the highest selectivity observed for ILs with the strongest hydrogen bond donor capacity, combined with the weakest hydrogen bond acceptor ability.20
Conclusion
The solvation behaviour of 12 PILs as well as 9 traditional molecular solvents were investigated through Kamlet, Abboud and Taft LSER methods. An initial screening was conducted using 11 solvatochromic dyes across all molecular solvents. From this, Reichardt's dye 30, Reichardt's dye 33, 4-nitroaniline and N,N-diethyl-4-nitroaniline were found to be the optimal set of dyes for determining the solvatochromic parameters of PILs, which are ET, π*, α and β. The 12 PILs consisted of an alkyl-, dialkyl, alkanol-, or dialkanolammonium cation paired with a nitrate, formate or acetate anion. These were all found to be strongly polar with high H bond donating and moderate H bond accepting abilities. Certain trends upon changing the ionic species were observed. All solvation parameters exhibited a stronger dependency on the PIL anion than the cation. Nitrate containing PILs led to the highest polarities and H bond donating abilities followed by formates and acetates. Hydroxyl substitution on the PIL cation increased the molar transition energy and dipolarity while decreasing the H bond donating and accepting abilities. The solvation properties obtained from using absorbent dyes were also compared with those obtained by using three fluorescent probes, which were pyrene, Coumarin 153 and Nile red. Although the relative polarity order of PILs found with respect to fluorescent dyes was quite similar to each other and those based on absorbent dyes, the neutral molecule pyrene was found to be the most sensitive to even small changes in the polarity of PILs.As an emerging solvent class, many ILs have been extensively identified and characterised in terms of their physicochemical, thermal and solvation properties. However, this is the first time that the solvation properties of the alkylammonium cation class of ILs has been experimentally investigated in a systematic way.
While there are limitations, the KAT and fluorescence methods enable us to investigate the solvation properties of ILs. Further investigations need to be done for pure ionic liquids, and also for ionic liquid containing systems such as their binary, or ternary mixtures with other solvents, to enable robust structure–property relationships to be developed for the solvation properties of IL containing solvents.
Conflicts of interest
There are no conflicts to declare.References
- T. Welton, Room-Temperature Ionic Liquids. Solvents for Synthesis and Catalysis, Chem. Rev., 1999, 99, 2071–2083 CrossRef CAS PubMed.
- N. D. Khupse and A. Kumar, Ionic liquids: new materials with wide applications, Indian J. Chem., 2010, 49A, 635–648 CAS.
- R. Hayes, G. G. Warr and R. Atkin, Structure and Nanostructure in Ionic Liquids, Chem. Rev., 2015, 115, 6357–6426 CrossRef CAS PubMed.
- T. L. Greaves and C. J. Drummond, Protic Ionic Liquids: Properties and Applications, Chem. Rev., 2008, 108, 206–237 CrossRef CAS PubMed.
- I. A. Sedov, T. I. Magsumov, T. M. Salikov and B. N. Solomonov, Solvation of apolar compounds in protic ionic liquids: the non-synergistic effect of electrostatic interactions and hydrogen bonds, Phys. Chem. Chem. Phys., 2017, 19(37), 25352–25359 RSC.
- J. P. Hallett and T. Welton, Room-temperature ionic liquids: solvents for synthesis and catalysis. 2, Chem. Rev., 2011, 111(5), 3508–3576 CrossRef CAS PubMed.
- T. L. Greaves and C. J. Drummond, Protic Ionic Liquids: Evolving Structure–Property Relationships and Expanding Applications, Chem. Rev., 2015, 115, 11379–11448 CrossRef CAS PubMed.
- V. G. Rao, C. Ghatak, R. Pramanik, S. Sarkar and N. Sarkar, Solvation and rotational dynamics of coumarin-153 in ethylammonium nitrate containing gamma-cyclodextrin, J. Phys. Chem. B, 2011, 115(35), 10500–10508 CrossRef CAS PubMed.
- J. F. Deye, T. A. Berger and A. G. Anderson, Nile Red as a Solvatochromic Dye for Measuring Solvent Strength in Normal Liquids and Mixtures of Normal Liquids with Supercritical and Near Critical Fluids, Anal. Chem., 1990, 62, 615–622 CrossRef CAS.
- M. A. Webb, B. C. Morris, W. D. Edwards, A. Blumenfeld, X. Zhao and J. L. McHale, Thermosolvatochromism of Phenol Blue in Polar and Nonpolar Solvents, J. Phys. Chem. A, 2004, 108, 1515–1523 CrossRef CAS.
- R. J. Cave and E. W. Castner, Time-Dependent Density Functional Theory Investigation of the Ground and Excited States of Coumarins 102, 152, 153, and 343, J. Phys. Chem. A, 2002, 106, 12117–12123 CrossRef CAS.
- Y. Ando, Y. Homma, Y. Hiruta, D. Citterio and K. Suzuki, Structural characteristics and optical properties of a series of solvatochromic fluorescent dyes displaying long-wavelength emission, Dyes Pigm., 2009, 83(2), 198–206 CrossRef CAS.
- H. Jin, G. A. Baker, S. Arzhantsev, J. Dong and M. Maroncelli, Solvation and Rotational Dynamics of Coumarin 153 in Ionic Liquids: Comparisons to Conventional Solvents, J. Phys. Chem. B, 2007, 111, 7291–7302 CrossRef CAS PubMed.
- V. Strehmel, A. Laschewsky, R. Stoesser, A. Zehl and W. Herrmann, Mobility of spin probes in ionic liquids, J. Phys. Org. Chem., 2006, 19(5), 318–325 CrossRef CAS.
- W. Guan, N. Chang, L. Yang, X. Bu, J. Wei and Q. Liu, Determination and Prediction for the Polarity of Ionic Liquids, J. Chem. Eng. Data, 2017, 62(9), 2610–2616 CrossRef CAS.
- K. Fumino, S. Reimann and R. Ludwig, Probing molecular interaction in ionic liquids by low frequency spectroscopy: Coulomb energy, hydrogen bonding and dispersion forces, Phys. Chem. Chem. Phys., 2014, 16(40), 21903–21929 RSC.
- P. H. Shetty, P. J. Youngberg, B. R. Kersten and C. F. Poole, Solvent Properties of Liquid Organic Salts Used as Mobile Phases in Micrpcolumn Reversed-Phase Liquid Chromatography, J. Chromatogr., 1987, 1, 61–79 CrossRef.
- S. K. Poole, P. H. Shatty and C. F. Poole, Chromatographic and Spectroscopic Studies of the Solvent Properties of a New Series of Room Temperature Liquid Tetraalkylammonium Sulfonates, Anal. Chim. Acta, 1989, 218, 241–264 CrossRef CAS.
- J. L. Anderson, J. Ding, T. Welton and D. W. Armstrong, Characterizing Ionic Liquids On the Basis of Multiple Solvation Interactions, J. Am. Chem. Soc., 2002, 124(47), 14247–14254 CrossRef CAS PubMed.
- R. Bini, C. Chiappe, V. L. Mestre, C. S. Pomelli and T. Welton, A rationalization of the solvent effect on the Diels–Alder reaction in ionic liquids using multiparameter linear solvation energy relationships, Org. Biomol. Chem., 2008, 6(14), 2522–2529 RSC.
- A. Jeličić, N. García, H.-G. Löhmannsröben and S. Beuermann, Prediction of the Ionic Liquid Influence on Propagation Rate Coefficients in Methyl Methacrylate Radical Polymerizations Based on Kamlet–Taft Solvatochromic Parameters, Macromolecules, 2009, 42(22), 8801–8808 CrossRef.
- L. Crowhurst, R. Falcone, N. Llewellyn Lancaster, V. Llopis-Mestre and T. Welton, Using Kamlet–Taft Solvent Descriptors To Explain the Reactivity of Anionic Nucleophiles in Ionic Liquids, J. Org. Chem., 2006, 71, 8847–8853 CrossRef CAS PubMed.
- G. Ranieri, J. P. Hallett and T. Welton, Nucleophilic Reactions at Cationic Centers in Ionic Liquids and Molecular Solvents, Ind. Eng. Chem. Res., 2008, 47, 638–644 CrossRef CAS.
- R. R. Hawker, R. S. Haines and J. B. Harper, The effect of varying the anion of an ionic liquid on the solvent effects on a nucleophilic aromatic substitution reaction, Org. Biomol. Chem., 2018, 16(18), 3453–3463 RSC.
- B. J. Butler and J. B. Harper, The effect of the structure of the anion of an ionic liquid on the rate of reaction at a phosphorus centre, J. Phys. Org. Chem., 2019, 32, 1 CrossRef.
- C. Reichardt, Solvatochromic Dyes as Solvent Polarity Indicators, Chem. Rev., 1994, 94, 2319–2358 CrossRef CAS.
- T. Y. Wu, S. G. Sua, S. T. Gunga, M. W. Lina, Y. C. Linc, W. C. Ou-Yangd, I. W. Suna and C. A. Laia, Synthesis and Characterization of Protic Ionic liquids Containing Cyclic Amine Cations and Tetrafluoroborate Anion, J. Iran. Chem. Soc., 2011, 8, 149–165 CrossRef CAS.
- M. Huang, Dielectric Properties of Ionic Liquids, Ruhr University Bochum, Germany, 2011 Search PubMed.
- C. Reichardt, Polarity of ionic liquids determined empirically by means of solvatochromic pyridinium N-phenolate betaine dyes, Green Chem., 2005, 7(5), 339–351 RSC.
- C. Chiappe, C. S. Pomelli and S. Rajamani, Influence of structural variations in cationic and anionic moieties on the polarity of ionic liquids, J. Phys. Chem. B, 2011, 115(31), 9653–9661 CrossRef CAS PubMed.
- R. W. Taft, I. L. M. Abboud, M. I. Kamlet and M. H. Abraham, Linear Solvation Energy Relations, J. Solution Chem., 1985, 11, 153–186 CrossRef.
- X. Wang, K. Chen, J. Yao and H. Li, Recent progress in studies on polarity of ionic liquids, Sci. China Chem., 2016, 59(5), 517–525 CrossRef CAS.
- K. A. Fletcher, I. A. Storey, A. E. Hendricks, S. Pandey and S. Pandey, Behavior of the solvatochromic probes Reichardts dye, pyrene, dansylamide, Nile red and 1-pyrenecarbaldehyde within the room-temperature ionic liquid bmimPF6, Green Chem., 2001, 3(5), 210–215 RSC.
- J. Figueras, Hydrogen Bonding, Solvent Polarity, and the Visible Spectrum of Phenol Blue and Its Derivatives, J. Am. Chem. Soc., 1971, 93, 3255–3263 CrossRef CAS.
- C. Reichardt, Pyridinium-N-phenolate betaine dyes as empirical indicators of solvent polarity: some new findings, Pure Appl. Chem., 2008, 80(7), 1415–1432 CAS.
- P. Hrdlovic, J. Donovalova, H. Stankovicova and A. Gaplovsky, Influence of polarity of solvents on the spectral properties of bichromophoric coumarins, Molecules, 2010, 15(12), 8915–8932 CrossRef CAS PubMed.
- R. Pardo, M. Zayat and D. Levy, E T(33) dye as a tool for polarity determinations: application to porous hybrid silica thin-films, J. Photochem. Photobiol., A, 2010, 210(1), 17–22 CrossRef CAS.
- M. S. Zakerhamidi and S. Shahrabi, Determination of solvatochromic solvent polarity parameters for the characterisation of some nematic liquid crystal in anisotropic and isotropic phases, Liq. Cryst., 2013, 40(1), 22–30 CrossRef CAS.
- C. J. Drummond and D. N. Furlong, Photochromism of a Surface-active Spirobenzopyran Moiety in Dioxane-Water Mixtures and Self-assembled Surfactant Aggregates, J. Chem. Soc., Faraday Trans., 1990, 86, 3613–3621 RSC.
- M. J. Kamlet and R. W. Taft, The Solvatochromic Comparison Method. I. The Beta Scale of Solvent Hydrogen-Bond Acceptor (HBA) Basicities, J. Am. Chem. Soc., 1976, 98, 377–383 CrossRef CAS.
- R. W. Taft and M. J. Kamlet, The Solvatochromic Comparison Method. 2. The Alpha-Scale of Solvent Hydrogen-Bond Donor (HBD) Acidities, J. Am. Chem. Soc., 1976, 98, 2886–2894 CrossRef CAS.
- T. Yokoyama, R. W. Taft and M. J. Kamlet, The Solvatochromic Comparison Method. 3. Hydrogen Bonding by Some 2-Nitroaniline Derivatives, J. Am. Chem. Soc., 1976, 98, 3233–3237 CrossRef CAS.
- R. R. Minesinger, M. E. Jones, R. W. Taft and M. J. Kamlet, The Solvatochromic Comparison Method. 5. Spectral Effects and Relative Strengths of the First and Second Hydrogen Bonds by 4-Nitroaniline to Hydrogen Bond Acceptor Solvents, J. Org. Chem., 1977, 42, 1929–1934 CrossRef CAS.
- M. J. Kamlet, J. L. Abboud and R. W. Taft, The Solvatochromic Comparison Method. 6. The Pi Scale of Solvent Polarities, J. Am. Chem. Soc., 1977, 99, 6027–6038 CrossRef CAS.
- S. N. Baker, G. A. Baker and F. V. Bright, Temperature-dependent microscopic solvent properties of ‘dry’ and ‘wet’ 1-butyl-3-methylimidazolium hexafluorophosphate: correlation with ET(30) and Kamlet–Taft polarity scales, Green Chem., 2002, 4(2), 165–169 RSC.
- J.-M. Lee, S. Ruckes and J. M. Prausnitz, Solvent Polarities and Kamlet–Taft Parameters for Ionic Liquids Containing a Pyridinium Cation, J. Phys. Chem. B, 2008, 112, 1473–1476 CrossRef CAS PubMed.
- W. B. Harrod and N. J. Pienta, Solvent Polarity Scales. 1. Determination of Et and pi* values for Phosphonium and Ammonium Melts, J. Phys. Org. Chem., 1990, 3, 534–544 CrossRef CAS.
- I. M. Herfort and H. Schneider, Spectroscopic studies of the solvent polarities of room temperature liquid ethylammonium nitrate and its mixtures with polar solvents, Liebigs Ann. Chem., 1991, 27–31 CrossRef CAS.
- M. J. Muldoon, C. M. Gordon and I. R. Dunkin, Investigations of solvent–solute interactions in room temperature ionic liquids using solvatochromic dyes, J. Chem. Soc., Perkin Trans. 2, 2001, 433–435 RSC.
- K. A. Fletcher and S. Pandey, Solvatochromic Probe Behavior within Neat and Cosolvent added Room-Temperature Ionic Liquid Solutions, ECS Proc., 2002, 2002–19, 244–256 CrossRef CAS.
- L. Crowhurst, P. R. Mawdsley, J. M. Perez-Arlandis, P. A. Salter and T. Welton, Solvent–solute interactions in ionic liquids, Phys. Chem. Chem. Phys., 2003, 5(13), 2790–2794 RSC.
- C. F. Poole, Chromatographic and spectroscopic methods for the determination of solvent properties of room temperature ionic liquids, J. Chromatogr. A, 2004, 1037(1–2), 49–82 CrossRef CAS PubMed.
- S. N. V. K. Aki, J. F. Brennecke and A. Samanta, How polar are room-temperature ionic liquids?, Chem. Commun., 2001, 413–414 RSC.
- M. A. Ab Rani, A. Brant, L. Crowhurst, A. Dolan, M. Lui, N. H. Hassan, J. P. Hallett, P. A. Hunt, H. Niedermeyer, J. M. Perez-Arlandis, M. Schrems, T. Welton and R. Wilding, Understanding the polarity of ionic liquids, Phys. Chem. Chem. Phys., 2011, 13(37), 16831–16840 RSC.
- P. G. Jessop, D. A. Jessop, D. Fu and L. Phan, Solvatochromic parameters for solvents of interest in green chemistry, Green Chem., 2012, 14(5), 1245–1259 RSC.
- S. Spange, R. Lungwitz and A. Schade, Correlation of molecular structure and polarity of ionic liquids, J. Mol. Liq., 2014, 192, 137–143 CrossRef CAS.
- T. L. Greaves, A. Weerawardena, C. Fong, I. Krodkiewska and C. J. Drummond, Protic Ionic Liquids: Solvents with Tunable Phase Behavior and Physicochemical Properties, J. Phys. Chem. B, 2006, 110, 22479–22487 CrossRef CAS PubMed.
- T. L. Greaves, A. Weerawardena, C. Fong and C. J. Drummond, Formation of Amphiphile Self-Assembly Phases in Protic Ionic Liquids, J. Phys. Chem. B, 2007, 111, 4082–4088 CrossRef CAS PubMed.
- T. L. Greaves, A. Weerawardena, I. Krodkiewska and C. J. Drummond, Protic Ionic Liquids: Physicochemical Properties and Behavior as Amphiphile Self-Assembly Solvents, J. Phys. Chem. B, 2008, 112, 896–905 CrossRef CAS PubMed.
- T. L. Greaves, D. F. Kennedy, S. T. Mudie and C. J. Drummond, Diversity Observed in the Nanostructure of Protic Ionic Liquids, J. Phys. Chem. B, 2010, 114, 10022–10031 CrossRef CAS PubMed.
- T. L. Greaves, K. Ha, B. W. Muir, S. C. Howard, A. Weerawardena, N. Kirby and C. J. Drummond, Protic Ionic Liquids (PILs) Nanostructure and Physicochemical Properties: Development of High-Throughput Methodology for PIL Creation and Property Screens, Phys. Chem. Chem. Phys., 2015, 17, 2357–2365 RSC.
- D. Yalcin, C. J. Drummond and T. L. Greaves, High-Throughput Approach to Investigating Ternary Solvents of Aqueous Non-Stoichiometric Protic Ionic Liquids, Phys. Chem. Chem. Phys., 2019, 21, 6810–6827 RSC.
- S. J. Cartwright, Solvatochromic dyes detect the presence of homeopathic potencies, Homeopathy, 2016, 105(1), 55–65 CrossRef PubMed.
- C. J. Drummond, F. Grieser, T. W. Healy and A. Single Spectroscopic, Probe for the Determination of Both the Interfacial Solvent Properties and Electrostatic Surface Potential of Model Lipid Membranes, Faraday Discuss. Chem. Soc., 1986, 81, 95–106 RSC.
- Y. Chen, Y. Cao, X. Lu, C. Zhao, C. Yan and T. Mu, Water sorption in protic ionic liquids: correlation between hygroscopicity and polarity, New J. Chem., 2013, 37, 7 Search PubMed.
- S. K. Shukla, N. D. Khupse and A. Kumar, Do anions influence the polarity of protic ionic liquids?, Phys. Chem. Chem. Phys., 2012, 14(8), 2754–2761 RSC.
- V. Beniwal and A. Kumar, Understanding positive and negative deviations in polarity of ionic liquid mixtures by pseudo-solvent approach, Phys. Chem. Chem. Phys., 2016, 18(34), 23853–23863 RSC.
- D. J. Eyckens, B. Demir, T. R. Walsh, T. Welton and L. C. Henderson, Determination of Kamlet–Taft parameters for selected solvate ionic liquids, Phys. Chem. Chem. Phys., 2016, 18(19), 13153–13157 RSC.
- S. Zhang, X. Qi, X. Ma, L. Lu and Y. Deng, Hydroxyl Ionic Liquids: The Differentiating Effect of Hydroxyl on Polarity due to Ionic Hydrogen Bonds between Hydroxyl and Anions, J. Phys. Chem. B, 2010, 114, 3912–3920 CrossRef CAS PubMed.
- K. A. Kurnia, F. Lima, A. F. Claudio, J. A. Coutinho and M. G. Freire, Hydrogen-bond acidity of ionic liquids: an extended scale, Phys. Chem. Chem. Phys., 2015, 17(29), 18980–18990 RSC.
- V. G. Rao, C. Ghatak, R. Pramanik, S. Sarkar and N. Sarkar, Solvent and rotational relaxation of Coumarin-153 in a micellar solution of a room-temperature ionic liquid, 1-butyl-3-methylimidazolium octyl sulfate, in ethylammonium nitrate, Chem. Phys. Lett., 2010, 499(1–3), 89–93 CrossRef CAS.
- T. J. V. Prazeres, M. Beija, F. V. Fernandes, P. G. A. Marcelino, J. P. S. Farinha and J. M. G. Martinho, Determination of the critical micelle concentration of surfactants and amphiphilic block copolymers using coumarin 153, Inorg. Chim. Acta, 2012, 381, 181–187 CrossRef CAS.
- M. Hof and P. Lianos, Structural Studies of Thin AOT Films by Using the Polarity Fluorescent Probe Coumarin-153, Langmuir, 1997, 13, 290–294 CrossRef CAS.
- D. Seth, S. Sarkar and N. Sarkar, Solvent and Rotational Relaxation of Coumarin 153 in a Protic Ionic Liquid Dimethylethanolammonium Formate, J. Phys. Chem. B, 2008, 112, 2629–2636 CrossRef CAS PubMed.
- L. R. Martins, A. Tamashiro, D. Laria and M. S. Skaf, Solvation dynamics of coumarin 153 in dimethylsulfoxide–water mixtures: Molecular dynamics simulations, J. Chem. Phys., 2003, 118(13), 5955–5963 CrossRef CAS.
- C. Chiappe, M. Malvaldi and C. S. Pomelli, Ionic liquids: Solvation ability and polarity, Pure Appl. Chem., 2009, 81(4), 767–776 CAS.
- H. J. Jiang, S. Imberti, B. A. Simmons, R. Atkin and G. G. Warr, Structural Design of Ionic Liquids for Optimizing Aromatic Dissolution, ChemSusChem, 2019, 12(1), 270–274 CrossRef CAS PubMed.
- I. A. Sedov, T. M. Salikov and B. N. Solomonov, Contrasting the solvation properties of protic ionic liquids with different nanoscale structure, J. Mol. Liq., 2019, 290 Search PubMed.
- R. Hayes, S. Imberti, G. G. Warr and R. Atkin, How water dissolves in protic ionic liquids, Angew. Chem., Int. Ed., 2012, 51(30), 7468–7471 CrossRef CAS PubMed.
- T. L. Greaves, D. F. Kennedy, N. Kirby and C. J. Drummond, Nanostructure Changes in Protic Ionic Liquids (PILs) Through Adding Solutes and Mixing PILs, Phys. Chem. Chem. Phys., 2011, 13, 13501–13509 RSC.
- T. L. Greaves, D. F. Kennedy, A. Weerawardena, N. M. Tse, N. Kirby and C. J. Drummond, Nanostructured Protic Ionic Liquids Retain Nanoscale Features in Aqueous Solution While Precursor Bronsted Acids and Bases Exhibit Different Behavior, J. Phys. Chem. B, 2011, 115, 2055–2066 CrossRef CAS PubMed.
- S. Coleman, R. Byrne, S. Minkovska and D. Diamond, Thermal reversion of spirooxazine in ionic liquids containing the [NTf2]− anion, Phys. Chem. Chem. Phys., 2009, 11(27), 5608–5614 RSC.
- R. Byrne, S. Coleman, S. Gallaghera and D. Diamond, Designer molecular probes for phosphonium ionic liquids, Phys. Chem. Chem. Phys., 2010, 12, 1895–1904 RSC.
- W. J. Cheong and P. W. Carr, Kamlet-Taft.pi.* polarizability/dipolarity of mixtures of water with various organic solvents, Anal. Chem., 1988, 60(8), 820–826 CrossRef CAS.
- A. F. Claudio, L. Swift, J. P. Hallett, T. Welton, J. A. Coutinho and M. G. Freire, Extended scale for the hydrogen-bond basicity of ionic liquids, Phys. Chem. Chem. Phys., 2014, 16(14), 6593–6601 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9cp05711k |
| This journal is © the Owner Societies 2020 |