Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Engineering macrocyclic high performance pentagonal bipyramidal Dy(III) single-ion magnets†
Angelos B.
Canaj
 a,
Sourav
Dey
a,
Sourav
Dey
 b,
Claire
Wilson
b,
Claire
Wilson
 a,
Oscar
Céspedes
c,
Gopalan
Rajaraman
a,
Oscar
Céspedes
c,
Gopalan
Rajaraman
 *b and
Mark
Murrie
*b and
Mark
Murrie
 *a
*a
aSchool of Chemistry, University of Glasgow, University Avenue, Glasgow, G12 8QQ, UK. E-mail: mark.murrie@glasgow.ac.uk
bDepartment of Chemistry, Indian Institute of Technology Bombay, Powai, Mumbai, Maharashtra 400076, India. E-mail: rajaraman@chem.iitb.ac.in
cSchool of Physics and Astronomy, University of Leeds, Leeds LS2 9JT, UK
First published on 3rd September 2020
Abstract
We generate a new air-stable pseudo-D5h Dy(III) Single-Molecule Magnet (Ueff = 1108 K, TB = 14 K) by combining a weak equatorial ligand field from a macrocyclic LN5 ligand with a strong axial ligand field. Based on our synthetic blueprint, we use ab initio calculations to show the vast scope for macrocyclic engineering of magnetic anisotropy.
Molecular systems which display the ability to retain magnetisation, in the absence of an external magnetic field, resulting in the appearance of magnetic memory of molecular origin, are known as Single-Molecule Magnets (SMMs).1 Lanthanide-based SMMs are often associated with large magnetic moments and large magnetic anisotropy. In 4f-SMMs, the energy barrier to reorientation of the magnetisation (Ueff) is strongly determined by the control of the coordination environment at the level of a single metal ion.2 Specifically, the use of the Dy(III) ion in targeted coordination environments that promote strong uniaxial symmetry stabilizes the largest mJ = ±15/2 ground state and gives a large separation from the excited mJ states.2 Monometallic complexes with axial symmetry such as square antiprismatic,3 trigonal bipyramidal,4 pentagonal bipyramidal,5,6 and hexagonal bipyramidal,7,8 are an effective way to favour slower relaxation of the magnetisation. Furthermore, sandwich type ligands (e.g. cyclopentadienyl (Cp) anionic ligands), have generated organometallic compounds with impressive blocking temperatures showing coercivity up to 30 K,9 48 K,10 55 K,11 60 K,12 66 K13 and 80 K.14 Additionally, a unique family of Ln-SMMS are the endohedral metallofullerenes (EMFS).15
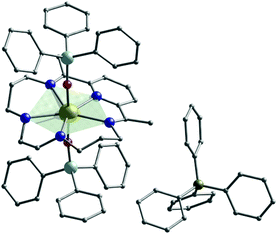
We have explored how the ligand electronics can tune SMM properties5,7,16,17 and recently first introduced a blueprint for engineering strong uniaxial magnetic anisotropy for Dy(III) ions in a hexagonal bipyramidal geometry,7 boosting the magnetisation reversal barrier from ∼50 K18 to ∼1100 K, by using the macrocyclic ligand LN6 (Scheme 1 left). Implementing further the flexibility of our synthetic approach towards the engineering of new quasi-Dnh systems, we demonstrate the isolation of a new Single-Ion Magnet (SIM) with pentagonal bipyramidal geometry, [DyIII(LN5)(Ph3SiO)2](BPh4)·CH2Cl2 (1). Compound 1 shows out-of-phase peaks in the ac susceptibility up to 80 K under zero dc field, a high magnetization reversal barrier of 1108 K and hysteresis, M(H), loops open up to 14 K, measured at an average sweep rate of 0.01 T s−1 (see Table S1, ESI†). 1 belongs to a small group of pentagonal bipyramidal complexes that are both air-stable and have a Ueff above 1000 K (Table S1, ESI†). In our carefully designed step-by-step synthetic approach we first targeted the formation of a weak N5-pentagonal plane by employing the macrocyclic LN5 ligand (Scheme 1, right), unused in 4f chemistry, formed from 2,6-diacetylpyridine and N,N′-bis-(3-aminopropyl)-ethylendiamine.19 We then used the anion of triphenylsilanol, Ph3SiO−, as stronger anionic donors at both axial positions to generate the pentagonal bipyramidal architecture (Fig. 1).
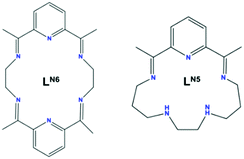 | ||
| Scheme 1 The macrocyclic ligand LN6 (left)7 and LN5 (right). | ||
Importantly, our synthetic strategy offers vast synthetic flexibility for carefully engineering the equatorial crystal field in order to (i) further improve the relaxation dynamics and (ii) to target the isolation of new quasi-Dnh systems with enhanced magnetic anisotropy. In a quest to identify new promising directions towards high temperature SMMs we also investigate the new in silico models 1-N3O2 and 1-O5 (vide infra), inspired by 1. From our systematic study, we find that the proposed in silico models are extremely promising as new target systems and have the potential to show improved SMM properties, with the magnetisation reversal barrier boosted up to ca. 1400 K and 1800 K, respectively (vide infra).
Compound 1 (Fig. 1) crystallises in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (Table S2, ESI†) with the asymmetric unit containing two crystallographically independent molecules with a Dy⋯Dy distance of 11.667 Å. Both Dy(III) centres are found in an axially compressed pentagonal bipyramidal geometry, as confirmed via SHAPE analysis (see Table S4 and Fig. S3, ESI†).20 Two Ph3SiO− ligands occupy the axial positions providing the shortest axial Dy–O bond lengths of 2.157(3) Å and 2.136(4) Å for Dy1A and 2.161(4) Å and 2.158(4) Å for Dy1B (see Table S3 and Fig. S2, ESI†). In addition, the axial O–Dy–O angle is 176.54(15)° and 173.13(15)° for Dy1A and Dy1B, respectively. In the equatorial plane of the LN5 ligand (Fig. S2, ESI†) the Dy–N bonds fall in the range of 2.400(5)–2.570(5) Å for Dy1A and 2.457(5)–2.564(5) Å for Dy1B (Table S3, ESI†).
(Table S2, ESI†) with the asymmetric unit containing two crystallographically independent molecules with a Dy⋯Dy distance of 11.667 Å. Both Dy(III) centres are found in an axially compressed pentagonal bipyramidal geometry, as confirmed via SHAPE analysis (see Table S4 and Fig. S3, ESI†).20 Two Ph3SiO− ligands occupy the axial positions providing the shortest axial Dy–O bond lengths of 2.157(3) Å and 2.136(4) Å for Dy1A and 2.161(4) Å and 2.158(4) Å for Dy1B (see Table S3 and Fig. S2, ESI†). In addition, the axial O–Dy–O angle is 176.54(15)° and 173.13(15)° for Dy1A and Dy1B, respectively. In the equatorial plane of the LN5 ligand (Fig. S2, ESI†) the Dy–N bonds fall in the range of 2.400(5)–2.570(5) Å for Dy1A and 2.457(5)–2.564(5) Å for Dy1B (Table S3, ESI†).
The static dc magnetic susceptibility and magnetization measurements for complex 1 are shown in the ESI† (Fig. S4 and S5). Upon cooling the χMT profile of 1 decreases steadily from the room temperature value of 14.1 cm3 mol−1 K to a value of 13.05 cm3 mol−1 K at 10 K followed by a sharp drop below 10 K (Fig. S4, ESI†). The field-cooled (FC) and zero-field cooled (ZFC) magnetic susceptibility (Fig. S6, ESI†) diverge at 7 K for 1 with the maximum observed at ∼5 K, indicative of a magnetic blocking temperature, TB.21 The magnetic hysteresis measurements, M(H) loops, performed on a microcrystalline powder sample of 1 remain open up to 14 K, measured at a sweep rate of 0.01 T s−1 (Fig. 2 and Fig. S7, ESI†). The characteristic waist-restricted shape of the loops is strongly affected by the faster relaxation around zero field, due to the presence of unsuppressed Quantum Tunnelling of the Magnetisation (QTM).3
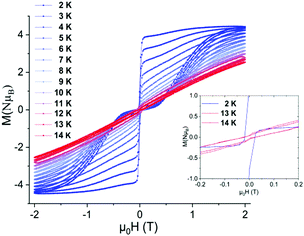 | ||
| Fig. 2 Powder magnetic hysteresis measurements for 1 with an average sweep rate of 0.01 T s−1. Inset: M(H) loops around zero field region open up to 14 K. | ||
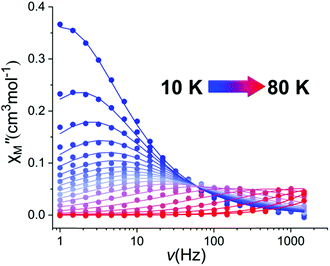
Alternating current (ac) susceptibility measurements were performed in order to investigate the magnetic relaxation in 1 (Fig. 3 and Fig. S8–S11, ESI†). Under zero dc field, the out-of-phase χM′′ susceptibility data exhibit well-defined maxima, clearly observable at temperatures up to 80 K (Fig. 3 and Fig. S8, ESI†), indicative of slow magnetic relaxation and a high magnetisation reversal barrier. The magnetisation relaxation times, τ, were extracted by fitting the Argand plots of χM′′ vs. χM′ using the generalized Debye model (Fig. S11, ESI†).22 The α-parameters found are in the range of 0.17–0.43 (2–80 K) showing a relatively wide distribution of relaxation times. The τ−1vs. T data were fitted using the equation τ−1 = τQTM−1 + CTn + τ0−1exp(−Ueff/T), in which C and n are the parameters of the Raman process and τQTM is the rate of QTM.3,21 The best fit gives a magnetisation reversal barrier Ueff = 1108 K, τ0 = 1.56 × 10−11 s, n = 2.05, C = 0.03 K−n s−1, τQTM = 0.5 s, under zero dc field (Fig. S12, ESI†). The values of τ0, C and n are within the commonly observed range for Dy(III) SMMs.3 The exponent n of the Raman process has a smaller value than expected for a Kramers ion (i.e., n = 9) suggesting the presence of Raman processes involving optical acoustic phonons.22
Ab initio calculations on 1, using the CASSCF/RASSI-SO/SINGLE_ANISO approach implemented in MOLCAS 8.2 (see ESI†),23 reveal that the eight Kramers Doublets (KDs) span an energy range of ∼1520 K. Inspection of the calculated g-tensors (Table S5, ESI†) show a highly anisotropic ground state (mJ = ±15/2) with strong axiality (gzz = 19.979, gxx, gyy = 0.001 for Dy1A and gzz = 19.984, gxx, gyy = 0.000 for Dy1B). The main anisotropy axis in 1 is nearly collinear with the shortest O–Dy–O bonds, stabilised by the stronger donor Ph3SiO− ligands located above and below the equatorial plane of the LN5 ligand (Fig. 1 and Fig. S13, ESI†).
The first excited state (mJ = ±13/2), located at ∼600 K (594 K for Dy1A and 602 K for Dy1B) and the second excited state (mJ = ± 11/2) located at ∼1040 K (1033 K for Dy1A and 1040 K for Dy1B) are also axial in nature (Fig. S14 and Table S5, ESI†). The maximum calculated relaxation barrier, Ucal, for compound 1 is estimated at ∼1040 K; in excellent agreement with the experimentally determined magnetisation reversal barrier (Ueff) of 1108 K found in zero dc field.
One can imagine changing the planarity and rigidity of the LN5 macrocycle by using different building blocks (or the application of pressure) to allow access to new pseudo-D5h environments. Our ongoing efforts are focused on fully exploring the vast synthetic flexibility in the design that our approach offers in order to improve the relaxation dynamics. In this regard, the LoProp26 charges on 1, computed using the CASSCF wavefunction, show that the negative charges at the secondary amine –NH– groups are of similar magnitude to those found for the axial oxygen atoms (Fig. S15, ESI†). Hence, replacing the secondary amine –NH– groups with tertiary amines has the potential to result in smaller negative LoProp charges in the equatorial plane, which may have a significant impact on further improving the magnetic properties of 1.
Furthermore, the ability to discover promising new candidate systems that have the potential to show improved SMM properties and could be targeted by experimental chemists is of key importance. Following on from the insight gained from the LoProp charges, using 1 as a blueprint we have created the in silico model systems 1-N3O2 and 1-O5 (Fig. S16 and S17, ESI†). In the new model systems we examine how changes in the first coordination sphere of 1, at the equatorial positions, affect the magnetic anisotropy. In both model systems 1-N3O2 and 1-O5 we have maintained the same coordination environment at the axial positions as in 1 but the equatorial ligand LN5 has been modified in silico to LN3O2 and the 16-crown-5 ligand (i.e., the closest candidate of the crown ether family to the LN5) (Fig. S16 and S17, ESI†). Firstly, we modified in silicoLN5 (Schiff base ligand formed from 2,6-diacetylpyridine and N,N′-bis-(3-aminopropyl)-ethylendiamine, see Scheme 1) to LN3O2 (Schiff base ligand formed from 2,6-diacetylpyridine and 3,3′-(ethane-1,2-diylbis(oxy))bis(propan-1-amine)). This is an alternative strategy to replace the two –NH– groups that have the larger LoProp charges, with two oxygen groups (Fig. S16, ESI†). In the model system 1-N3O2 (Fig. S16, ESI†) the first excited state is calculated at 658 K (mJ = ±13/2, gzz = 17.046, gxx = 0.028, gyy = 0.032) with the second (mJ = ±11/2) located at 1190 K and the third (0.62|±1/2 > +0.22|±3/2>) excited state at 1438 K (Table S6, ESI†). The maximum calculated relaxation barrier, Ucal, for model system 1-N3O2 is estimated at ∼1400 K (Fig. 4, upper). In addition, the computed LoProp charges on the oxygen atoms are found to be 50% smaller than those on the –NH– groups in 1 (Fig. S18, ESI†). Next we employed a crown ether ligand in the equatorial plane of our model system because of its neutral nature, long metal–ligand distances, the popularity of crown ether ligands in 4f chemistry24 and to compare it with LN5 and LN3O2. In the model system 1-O5 the strongly axial excited states are higher in energy than that of 1 (Table S7, ESI†). In addition, the transverse components for model system 1-O5 are weaker giving lower gxx/gyy values (Table S7, ESI†). The QTM probabilities calculated for the first three KDs (0.45 × 10−4, 0.49 × 10−2, 0.68 × 10−1μB, respectively) are lower compared to 1 with the magnetization relaxing via the fourth KD giving a calculated magnetization reversal barrier of 1788 K (Fig. 4, lower). The ratio between the axial B20 parameter and the corresponding non-axial crystal field parameters also increases 1 < 1-N3O2 < 1-O5 (Table S8, ESI†) supporting the increase of Ucal from 1 (∼1040 K) to 1-N3O2 (∼1400 K) and 1-O5 (∼1800 K) (Fig. S20, ESI†).
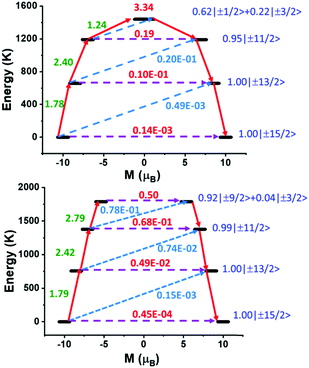 | ||
| Fig. 4 Ab initio calculated relaxation dynamics for 1-N3O2 (Upper) and 1-O5 (Lower). The black line indicates the KDs as a function of magnetic moments. The violet dashed arrow represents QTM (QTM = quantum tunnelling of the magnetisation) via the ground state and TA-QTM (TA-QTM = thermally assisted QTM) via excited states. The blue dashed arrow indicates possible Orbach processes. The red arrows indicate the mechanism of magnetic relaxation (Orbach/Raman). The numbers above each arrow represent corresponding transverse matrix elements for the transition magnetic moments.25 | ||
In conclusion, we report [DyIII(LN5)(Ph3SiO)2](BPh4)·CH2Cl2 (1) using the macrocyclic ligand LN5, which is a strongly axial Dy(III) single-ion magnet in a pentagonal bipyramidal geometry showing out-of-phase peaks up to 80 K under zero field, a high anisotropy barrier of 1108 K, and hysteresis loops open up to 14 K (@ 0.01 T s−1). This novel compound was engineered following our flexible synthetic approach where five long Dy–N bonds are formed by the macrocyclic ligand LN5, while two strong donor Ph3SiO− ligands are used for the axial positions, creating the short O–Dy–O bonds. Future work will involve modifying the planarity and rigidity of the LN5 macrocycle and investigating the new pentagonal bipyramidal families identified through our computational studies, showing the vast scope for macrocyclic engineering of magnetic anisotropy.
We thank EPSRC UK (EP/N01331X/1, EP/M000923/1) for funding. S. D. thanks UGC for an SRF fellowship. G. R. thanks DST/SERB for funding (CRG/2018/000430; DST/SJF/CSA03/2018-10; SB/SJF/2019-20/12).
Conflicts of interest
There are no conflicts to declare.Notes and references
- S. M. J. Aubin, M. W. Wemple, D. M. Adams, H.-L. Tsai, G. Christou and D. N. Hendrickson, J. Am. Chem. Soc., 1996, 118, 7746 CrossRef CAS; R. Sessoli, H. L. Tsai, A. R. Schake, S. Wang, J. B. Vincent, K. Folting, D. Gatteschi, G. Christou and D. N. Hendrickson, J. Am. Chem. Soc., 1993, 115, 1804 CrossRef; C. J. Milios, A. Vinslava, W. Wernsdorfer, S. Moggach, S. Parsons, S. P. Perlepes, G. Christou and E. K. Brechin, J. Am. Chem. Soc., 2007, 129, 2754 CrossRef.
- A. Castro-Alvarez, Y. Gil, L. Llanos and D. Aravena, Inorg. Chem. Front., 2020, 7, 2486 RSC; M. J. Heras Ojea, L. H. C. Maddock and R. A. Layfield, Lanthanide Organometallics as Single-Molecule Magnets, Springer, New York, NY, 2019, vol. 64 CrossRef CAS; F.-S. Guo and R. A. Layfield, Acc. Chem. Res., 2018, 51, 1880 CrossRef CAS; M. Feng and M.-L. Tong, Chem. – Eur. J., 2018, 24, 7574 CrossRef; A. K. Bar, P. Kalita, M. K. Singh, G. Rajaraman and V. Chandrasekhar, Coord. Chem. Rev., 2018, 367, 163 CrossRef.
- S. Bala, G.-Z. Huang, Z.-Y. Ruan, S.-G. Wu, Y. Liu, L.-F. Wang, J.-L. Liu and M.-L. Tong, Chem. Commun., 2019, 55, 9939 RSC; J. Wu, J. Jung, P. Zhang, H. Zhang, J. Tang and B. Le Guennic, Chem. Sci., 2016, 7, 3632 RSC; K. Katoh, S. Yamashita, N. Yasuda, Y. Kitagawa, B. K. Breedlove, Y. Nakazawa and M. Yamashita, Angew. Chem., Int. Ed., 2018, 57, 9262 CrossRef CAS.
- P. Zhang, L. Zhang, C. Wang, S. Xue, S.-Y. Lin and J. Tang, J. Am. Chem. Soc., 2014, 136, 4484 CrossRef CAS; K. L. M. Harriman, J. L. Brosmer, L. Ungur, P. L. Diaconescu and M. Murugesu, J. Am. Chem. Soc., 2017, 139, 1420 CrossRef; S.-M. Chen, Y.-Q. Zhang, J. Xiong, B.-W. Wang and S. Gao, Inorg. Chem., 2020, 59, 5835 CrossRef.
- A. B. Canaj, M. K. Singh, C. Wilson, G. Rajaraman and M. Murrie, Chem. Commun., 2018, 54, 8273 RSC.
- Y.-C. Chen, J.-L. Liu, L. Ungur, J. Liu, Q.-W. Li, L.-F. Wang, Z.-P. Ni, L. F. Chibotaru, X.-M. Chen and M.-L. Tong, J. Am. Chem. Soc., 2016, 138, 2829 CrossRef CAS; Y.-S. Ding, N. F. Chilton, R. E. P. Winpenny and Y.-Z. Zheng, Angew. Chem., Int. Ed., 2016, 55, 16071 CrossRef; J. Liu, Y.-C. Chen, J.-L. Liu, V. Vieru, L. Ungur, J.-H. Jia, L. F. Chibotaru, Y. Lan, W. Wernsdorfer, S. Gao, X.-M. Chen and M.-L. Tong, J. Am. Chem. Soc., 2016, 138, 5441 CrossRef; Y. Ma, Y.-Q. Zhai, Y.-S. Ding, T. Han and Y. Z. Zheng, Chem. Commun., 2020, 56, 3979 RSC; J. L. Liu, Y. C. Chen, Y. Z. Zheng, W. Q. Lin, L. Ungur, W. Wernsdorfer, L. F. Chibotaru and M. L. Tong, Chem. Sci., 2013, 4, 3310 RSC; Y.-S. Ding, T. Han, Y.-Q. Zhai, D. Reta, N. F. Chilton, R. E. P. Winpenny and Y.-Z. Zheng, Chem. – Eur. J., 2020, 26, 5893 CrossRef; S. K. Gupta, T. Rajeshkumar, G. Rajaraman and R. Murugavel, Chem. Sci., 2016, 7, 5181 RSC.
- A. B. Canaj, S. Dey, E. Regincós Martí, C. Wilson, G. Rajaraman and M. Murrie, Angew. Chem., Int. Ed., 2019, 58, 14146 CrossRef CAS; A. B. Canaj, S. Dey, E. Regincós Martí, C. Wilson, G. Rajaraman and M. Murrie, Angew. Chem., 2019, 131, 14284 CrossRef.
- Z.-H. Li, Y.-Q. Zhai, W.-P. Chen, Y.-S. Ding and Y.-Z. Zheng, Chem. – Eur. J., 2019, 25, 16219 CrossRef CAS.
- S. Demir, M. I. Gonzalez, L. E. Darago, W. J. Evans and J. R. Long, Nat. Commun., 2017, 8, 2144 CrossRef.
- P. Evans, D. Reta, G. F. Whitehead, N. F. Chilton and D. P. Mills, J. Am. Chem. Soc., 2019, 141, 19935 CrossRef CAS.
- C. Gould, K. R. McClain, J. Yu, T. J. Groshens, F. Furche, B. G. Harvey and J. R. Long, J. Am. Chem. Soc., 2019, 141, 12967 CrossRef CAS.
- A. P. Goodwin, F. Ortu, D. Reta, N. F. Chilton and D. P. Mills, Nature, 2017, 548, 439 CrossRef CAS; F.-S. Guo, B. M. Day, Y.-C. Chen, M.-L. Tong, A. Mansikkamaki and R. A. Layfield, Angew. Chem., Int. Ed., 2017, 56, 11445 CrossRef.
- K. R. McClain, C. A. Gould, K. Chakarawet, S. J. Teat, T. J. Groshens, J. R. Long and B. G. Harvey, Chem. Sci., 2018, 9, 8492 RSC.
- F.-S. Guo, B. M. Day, Y.-C. Chen, M.-L. Tong, A. Mansikkamäki and R. A. Layfield, Science, 2018, 362, 1400 CrossRef CAS.
- L. Spree and A. A. Popov, Dalton Trans., 2019, 48, 2861 RSC; W. Yang, G. Velkos, F. Liu, S. M. Sudarkova, Y. Wang, J. Zhuang, H. Zhang, X. Li, X. Zhang, B. Büchner, S. M. Avdoshenko, A. A. Popov and N. Chen, Adv. Sci., 2019, 1901352 CrossRef CAS; G. Velkos, W. Yang, Y.-R. Yao, S. M. Sudarkova, X. Liu, B. Büchner, S. M. Avdoshenko, N. Chen and A. A. Popov, Chem. Sci., 2020, 11, 4766 RSC; Y. Wang, J. Xiong, J. Su, Z. Hu, F. Ma, R. Sun, X. Tan, H.-L. Sun, B.-W. Wang, Z. Shi and S. Gao, Nanoscale, 2020, 12, 11130 RSC.
- A. B. Canaj, M. K. Singh, E. Regincós Martí, M. Damjanović, C. Wilson, O. Céspedes, W. Wernsdorfer, G. Rajaraman and M. Murrie, Chem. Commun., 2019, 55, 5950 RSC.
- A. B. Canaj, S. Dey, C. Wilson, O. Céspedes, G. Rajaraman and M. Murrie, Chem. Commun., 2020, 56, 1533 RSC.
- J. Li, S. Gómez-Coca, B. S. Dolinar, L. Yang, F. Yu, M. Kong, Y.-Q. Zhang, Y. Song and K. R. Dunbar, Inorg. Chem., 2019, 58, 2610 CrossRef CAS; Q.-W. Li, R.-C. Wan, Y.-C. Chen, J.-L. Liu, L.-F. Wang, J.-H. Jia, N. F. Chilton and M.-L. Tong, Chem. Commun., 2016, 52, 13365 RSC; W. Zhao, H. Cui, X.-Y. Chen, G. Yi, L. Chen, A. Yuan and C.-L. Luo, Dalton Trans., 2019, 48, 5621 RSC.
- S. M. Nelson, S. G. McFall, M. G. B. Drew, A. H. Bin Othman and N. B. Mason, J. Chem. Soc., Chem. Commun., 1977, 167 RSC.
- M. Pinsky and D. Avnir, Inorg. Chem., 1998, 37, 5575 CrossRef CAS; D. Casanova, P. Alemany, J. M. Bofill and S. Alvarez, Chem. – Eur. J., 2003, 9, 1281 CrossRef.
- D. Gatteschi, R. Sessoli and J. Villain, Molecular Nanomagnets, Oxford University Press, Oxford, 2006 Search PubMed.
- K. N. Shrivastava, Phys. Status Solidi B, 1983, 117, 437 CrossRef CAS; S. Q. Wu, Y. Miyazaki, M. Nakano, S. Q. Su, Z. S. Yao, H. Z. Kou and O. Sato, Chem. – Eur. J., 2017, 23, 10028 CrossRef; A. Singh and K. N. Shrivastava, Phys. Status Solidi B, 1979, 95, 273 CrossRef; A. Chiesa, F. Cugini, R. Hussain, E. Macaluso, G. Allodi, E. Garlatti, M. Giansiracusa, C. A. P. Goodwin, F. Ortu, D. Reta, J. M. Skelton, T. Guidi, P. Santini, M. Solzi, R. De Renzi, D. P. Mills, N. F. Chilton and S. Carretta, Phys. Rev. B, 2020, 101, 174402 CrossRef.
- A. A. Granovsky, J. Chem. Phys., 2011, 134, 214113 CrossRef CAS; F. Aquilante, J. Autschbach, R. K. Carlson, L. F. Chibotaru, M. G. Delcey, L. De Vico, I. Fdez Galvan, N. Ferre, L. M. Frutos and L. Gagliardi, et al. , J. Comput. Chem., 2016, 37, 506 CrossRef; L. F. Chibotaru and L. Ungur, J. Chem. Phys., 2012, 137, 064112 CrossRef.
- Y. Gil, L. Llanos, P. Cancino, P. Fuentealba, A. Vega, E. Spodine and D. Aravena, J. Phys. Chem. C, 2020, 124, 5308 CrossRef CAS; Y.-S. Ding, T. Han, H. Yue-Qiao, X. Minwei, Y. Sen and Z. Yan-Zheng, Inorg. Chem. Front., 2016, 3, 798 RSC; M. Xemard, M. Cordier, F. Molton, C. Duboc, B. Le Guennic, O. Maury, O. Cador and G. Nocton, Inorg. Chem., 2019, 58, 2872 CrossRef.
- L. Ungur, M. Thewissen, J. P. Costes, W. Wernsdorfer and L. F. Chibotaru, Inorg. Chem., 2013, 52, 6328 CrossRef CAS.
- L. Gagliardi, R. Lindh and G. Karlstrom, J. Chem. Phys., 2004, 121, 4494 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section, magnetic studies, crystallographic details, ab initio studies. CCDC 2000246. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0cc04559d |
| This journal is © The Royal Society of Chemistry 2020 |