Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Raman reporters derived from aryl diazonium salts for SERS encoded-nanoparticles†
Yun
Luo
*a,
Yu
Xiao
a,
Delphine
Onidas
a,
Laura
Iannazzo
a,
Mélanie
Ethève-Quelquejeu
 a,
Aazdine
Lamouri
b,
Nordin
Félidj
a,
Aazdine
Lamouri
b,
Nordin
Félidj
 b,
Samia
Mahouche-Chergui
c,
Thibault
Brulé
d,
Nathalie
Gagey-Eilstein
e,
Florence
Gazeau
b,
Samia
Mahouche-Chergui
c,
Thibault
Brulé
d,
Nathalie
Gagey-Eilstein
e,
Florence
Gazeau
 f and
Claire
Mangeney
f and
Claire
Mangeney
 *a
*a
aUniversité de Paris, LCBPT, UMR 8601, F-75006 Paris, France. E-mail: claire.mangeney@parisdescartes.fr; yun.sun@parisdescartes.fr
bUniversité de Paris, ITODYS, UMR 7086, 75013 Paris, France
cUniversité Paris-Est, ICMPE, UMR 7182, 94320 Thiais, France
dHORIBA France SAS, 14 Boulevard Thomas Gobert, Passage Jobin Yvon, 91120 Palaiseau, France
eUniversité de Paris, COMETE, CNRS UMR 8638, F-75006 Paris, France
fUniversité de Paris, MSC, CNRS UMR 7057, 75013 Paris, France
First published on 14th May 2020
Abstract
Surface-enhanced Raman scattering (SERS) tags are usually prepared by immobilizing Raman reporters on plasmonic nanoparticles (NPs) via thiol-based self-assembled monolayers. We describe here the first example of SERS tags obtained by combining gold NPs and aryl diazonium salts. This strategy results in robust Au–C covalent bonds between the Raman reporter and the NPs, thus ensuring a high stability of the nanohybrid interface.
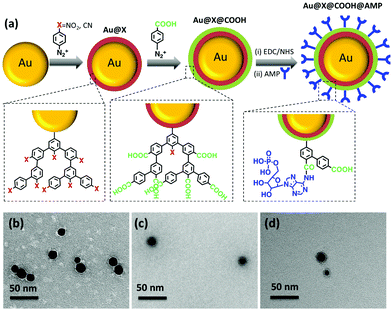
The popularity of surface-enhanced Raman scattering (SERS) tags has increased in recent decades, as an alternative to fluorescent probes, due to their attractive properties.1 Indeed, unlike the broad spectral feature of fluorescence probes, SERS tags provide multiple sets of narrow peaks, resulting in low spectral overlap and high multiplexing ability. Besides, a single light source can be used to excite multiple Raman reporters, with negligible photobleaching, low background and a high sensitivity that competes with that of fluorescence. Moreover, their low toxicity, compared to quantum dots, has boosted their attractiveness for in vitro and in vivo biosensing.2 SERS tags are based on several key elements:3 a metal core providing the source of SERS enhancement,4,5 an adsorbed Raman reporter molecule giving the spectral fingerprints, a protective shell to enhance the stability of the adsorbed reporter molecule and a targeting entity to improve specificity. The stacking of all these elements into a single SERS tag is a multi-step process which is usually challenging.6 In particular, reduction of the colloidal stability of plasmonic nanoparticles during the adsorption of Raman codes may lead to uncontrolled particle agglomeration. To solve this problem, the use of polymers or the co-adsorption of small Raman reporters and long-chain thiols was proposed, preserving colloidal stability all along the codification process.7,8 However, the chemical stability of thiolate self-assembled monolayers (SAMs) is a critical issue for applications in ambient and aqueous environments. Indeed, several studies have shown that SAMs desorb spontaneously from the surface in aqueous solutions after only few days9–11 and degrade upon laser excitation.12 Significant progress in stability have been obtained by using longer-chain thiols,13 small amount of amphiphilic surfactant molecules10 or multidentate sulfur-based adsorbates.14 Nevertheless, the development of alternative surface chemical approaches enabling robust covalent attachment of Raman reporters to plasmonic NPs can be expected to result in more significant improvements of interfacial stability. Aryl diazonium salts have emerged in recent years as a new generation of surface modifiers for plasmonic nanoparticles,15–19 in alternative to thiol-based SAMs. These molecules present several advantages such as their ease of preparation, rapid grafting, large choice of reactive functional groups and robust metal–C interfacial bonds with the surface. Their grafting on the surface of plasmonic NPs is a two-stage process:20,21 (i) first, the spontaneous reduction of the diazonium salts generates an aryl radical that binds to the surface, and (ii) in a second step, due to their high reactivity, these radicals react with the first grafted layer to give nanometer to micrometer thick polyaryl layers. Aryl diazonium salts were used to modify plasmonic NPs in order to design NPs-coated electrodes,22 colorimetric nanosensors,23 antimicrobial agents24 and functional lithographic arrays.25–27 In the latter case, they were shown to result in regioselective grafting of organic patches located specifically in the regions of maximum near field enhancement around nanoparticles, via site-selective hot-electron mediated reduction of aryl diazonium salts. However, the application of these surface agents as Raman reporters for SERS encoded-nanoparticles has never been reported so far, although aryl layers derived from diazonium salts were shown to exhibit intense Raman signals.28,29 In this work, we explore the potential of aryl diazonium salts to provide a new class of Raman reporters with several advantages, such as: (i) the formation of strong interfacial bonds with the supporting NPs, (ii) the presence of intense SERS fingerprints, (iii) the possibility to create multilayers with various functions. The formation of multilayers will be exploited here to introduce straightforwardly (i) several SERS labels along the grafted polyaryl chains and (ii) post-functionalization moieties at their end. As a proof of concept, we selected three different aryl diazonium salts. Two of them, bearing either nitro groups (4-nitrobenzenediazonium tetrafluoroborate, noted D-NO2) or cyano groups (4-cyanobenzenediazonium tetrafluoroborate, noted D-CN) were chosen to act as Raman reporters. The third one possesses carboxyl-terminated groups (4-carboxybenzene diazonium tetrafluoroborate, noted D-COOH) for post-functionalization with the nucleotide adenosine 5′-monophosphate (AMP) (see Fig. 1). Adenosine derivatives are important intermediary metabolites in cells, which have fundamental impacts on a wide range of cellular processes.30 Particularly, AMP was identified recently to be a novel tumour-targeting ligand, guiding nanoparticles to cancer cells via adenosine A1 receptor, offering promising prospects for clinical diagnostic and therapeutic applications.31 The functionalization of spherical gold NPs by the three aryl diazonium salts (–NO2, –CN, –COOH) and AMP was performed in water at room temperature, providing a simple method to create multifunctional SERS tags. The modified NPs were characterized by transmission electron microscopy (TEM), UV-vis absorption, X-ray photoelectron spectroscopy (XPS) and SERS.
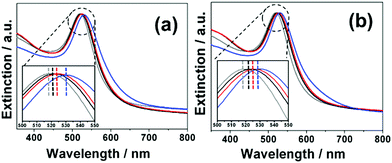
Spherical AuNPs with an average diameter of 15 ± 3 nm (see typical TEM image Fig. 1b), were prepared through the well-known Turkevich method,32 by citrate reduction of HAuCl4. The procedure for grafting the Raman reporters on the AuNPs consisted to add the corresponding diazonium salts (5 × 10−5 M) to the AuNPs aqueous dispersions ([AuNPs] = 8 × 10−9 M) and to let them react spontaneously during 60 min at room temperature. Multilayers were obtained by adding the diazonium salts successively, one after the other in a controlled sequence. The functionalized AuNPs were then purified through 3 cycles of centrifugation/redispersion in water. Post-functionalization with AMP was performed by mixing an aqueous AMP solution (10−4 M) with the resulting NPs, and adding 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) and N-hydroxysuccinimide (NHS), in the presence of 0.001 wt% polyvinylpyrrolidone (PVP), as a stabilizer. After reaction at 4 °C overnight, the particles were washed through 3 cycles of centrifugation/redispersion in water. Details on the functionalization experiments are given in ESI.† Transmission electron microscopy (TEM) images (see Fig. 1b–d and Fig. S1, ESI†) show individually dispersed particles indicating that the reaction of the AuNPs with the diazonium salts does not disturb their colloidal stability. Interestingly, it can be seen from TEM images that the functionalization process leads to the appearance of a thin layer covering the NPs, likely due to the presence of the polyaryl layers and AMP. An average thickness of the organic coating of ca. 3–4 nm was estimated using 100 NPs from different TEM images (see Table S1, ESI†). The observation of polyaryl layers around the gold cores can be explained by the grafting reaction mechanism. Indeed, it is based on the formation of aryl radicals by spontaneous cleavage of diazohydroxides Ar–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–OH that form from aryl diazonium salts in neutral aqueous media, as described previously.33 The formed aryl radicals bind to the AuNPs surface and to the first (and further) grafted aromatic groups via homolytic aromatic substitution, leading to polyaryl layers. The extinction profile of the AuNPs (Fig. 2a and b) retained its original shape upon functionalization confirming that the colloidal stability was preserved. Nevertheless, a progressive red shift of the plasmon band was observed after each functionalization step (see zoom in Fig. 2a and b and λmax values in Table S2, ESI†). This red shift increases with the number of grafted layers and reaches ca. 10 nm for AMP-modified NPs, likely due to the modification of the local dielectric environment around the Au cores.
N–OH that form from aryl diazonium salts in neutral aqueous media, as described previously.33 The formed aryl radicals bind to the AuNPs surface and to the first (and further) grafted aromatic groups via homolytic aromatic substitution, leading to polyaryl layers. The extinction profile of the AuNPs (Fig. 2a and b) retained its original shape upon functionalization confirming that the colloidal stability was preserved. Nevertheless, a progressive red shift of the plasmon band was observed after each functionalization step (see zoom in Fig. 2a and b and λmax values in Table S2, ESI†). This red shift increases with the number of grafted layers and reaches ca. 10 nm for AMP-modified NPs, likely due to the modification of the local dielectric environment around the Au cores.
It is noteworthy that the addition of a small amount of PVP (0.001 wt%) in the colloidal dispersion at the post-functionalization step was necessary in order to maintain a perfect colloidal stability, as described in ESI† (see Fig. S2a). However, this parameter should be carefully controlled to ensure that the NP surface is still accessible for post-functionalization with AMP. Indeed, above a certain [PVP] value, the grafting of AMP is no more detected by SERS after the post-functionalization step (see Fig. S2b, ESI†). The stability of the nanoprobes was evaluated in various media: aqueous solutions with a high ionic force, acid or basic solutions. No sign of aggregation could be detected, as shown in Fig. S3 (ESI†), regardless the medium to which the particles were exposed, demonstrating an extended colloidal stability. The surface chemical composition of the modified AuNPs was investigated by XPS (see Table S3 and Fig. S4, ESI†). The XPS spectra revealed large modifications after functionalization and confirmed the successful covering of the gold cores by the functional polyaryl layers and AMP.
The SERS properties of the encoded particles were studied at 633 nm laser excitation and compared to the Raman spectra of the free diazonium salts (Fig. S5, ESI†). Several key features can be pointed out: first, the intense N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N stretching peak, observed in the Raman spectra of the diazonium salt powders at ca. 2280–2300 cm−1 disappears completely for all nanoprobes, confirming the removal of the diazonium group during the grafting reaction. At the same time, one notices the appearance of a small peak at ca. 410–416 cm−1, assigned to the Au–C stretching vibration, evidencing the covalent grafting of the aryl layers. The SERS spectra display also the characteristic signature of phenyl-derivatives stemming from diazonium salts, particularly the aryl ring stretching peak at ca. 1580–1595 cm−1. For the nanoprobes Au@NO2 and Au@CN, the characteristic NO2 and CN stretching modes appear at 1330 cm−1 and 2220 cm−1, respectively. Interestingly, thanks to the radical coupling mechanism in diazonium salt chemistry, different functional layers could be grafted one after another around the gold core, forming multifunctional layers with a wide variety of SERS signatures. For example, Fig. 3 compares the SERS spectra and the corresponding Raman images of Au@CN, Au@NO2, Au@NO2@CN and Au@CN@NO2, deposited as dried drops on a glass slide. Each type of NPs reveals distinct characteristic spectra, which can be associated to one particular color in Raman imaging. The spectra of Au@CN are associated here to the magenta color, Au@NO2 to the blue, Au@NO2@CN to the green and Au@CN@NO2 to the red. The blackline corresponds to the glass slide.
N stretching peak, observed in the Raman spectra of the diazonium salt powders at ca. 2280–2300 cm−1 disappears completely for all nanoprobes, confirming the removal of the diazonium group during the grafting reaction. At the same time, one notices the appearance of a small peak at ca. 410–416 cm−1, assigned to the Au–C stretching vibration, evidencing the covalent grafting of the aryl layers. The SERS spectra display also the characteristic signature of phenyl-derivatives stemming from diazonium salts, particularly the aryl ring stretching peak at ca. 1580–1595 cm−1. For the nanoprobes Au@NO2 and Au@CN, the characteristic NO2 and CN stretching modes appear at 1330 cm−1 and 2220 cm−1, respectively. Interestingly, thanks to the radical coupling mechanism in diazonium salt chemistry, different functional layers could be grafted one after another around the gold core, forming multifunctional layers with a wide variety of SERS signatures. For example, Fig. 3 compares the SERS spectra and the corresponding Raman images of Au@CN, Au@NO2, Au@NO2@CN and Au@CN@NO2, deposited as dried drops on a glass slide. Each type of NPs reveals distinct characteristic spectra, which can be associated to one particular color in Raman imaging. The spectra of Au@CN are associated here to the magenta color, Au@NO2 to the blue, Au@NO2@CN to the green and Au@CN@NO2 to the red. The blackline corresponds to the glass slide.
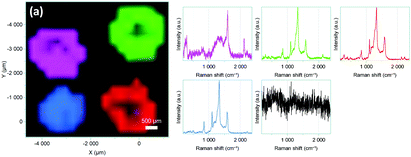 | ||
| Fig. 3 Raman images and corresponding SERS spectra of Au@CN (magenta), Au@NO2 (blue), Au@CN@NO2 (green) and Au@NO2@CN (red), deposited as dried drops on a glass slide. | ||
A layer of carboxyl-terminated functions was then added to the nanoconstructs to obtain a post-functionalizable platform. The successive grafting of the different layers for Au@CN@COOH and Au@NO2@COOH could be assessed by following the increase in intensity of the aryl ring stretching peak, Iaryl at 1580–1595 cm−1 (see Fig. S6 and S7, ESI†) and the evolution of ICN/IAu–C, INO2/IAu–C and Iaryl/IAu–C band intensity ratios (see Fig. S8, ESI†). An activation step using EDC/NHS was necessary to activate the carboxyl groups for direct conjugation to the nucleotide AMP, via amide bonds. The presence of the grafted AMP generates new SERS characteristic peaks34 (see Fig. 4) at ∼733 cm−1 (in-plane ring breathing of adenine ring), ∼1332 cm−1 (ring stretching mode) and ∼1460 cm−1 (C–N stretching).
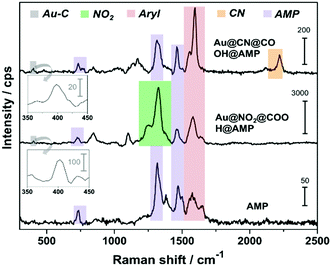 | ||
| Fig. 4 SERS spectra of Au@CN@COOH@AMP and Au@NO2@COOH@AMP. The Raman spectrum of AMP is used as a reference. Inset: Zoom in the spectral range 350–450 cm−1, revealing the presence of νAu–C peak. | ||
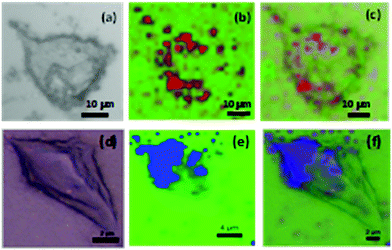
The other peaks remain unchanged demonstrating the robustness of the grafted layers derived from diazonium salts at the surface of the nanoconstruct. This new generation of SERS encoded NPs, based on AuNPs functionalized by aryl diazonium salts, was then evaluated as labels for Raman bioimaging inside cells (see Fig. 5).
Proof of concept experiments consisted to expose different cell lines (EGI-1 and CT-26 cells) to Au@CN@COOH@AMP and Au@NO2@COOH@AMP (details of the experiments are given in ESI†). Fig. 5 shows that, when internalized inside cells, the SERS tags could be perfectly detected as bright spots, probably due to accumulation into endosomal compartments. These results demonstrate that this new generation of SERS labels offers promising prospects for multiplex SERS-based Raman imaging.
In summary, Raman reporters derived from aryl diazonium salts were grafted on gold NPs to design a new generation of SERS encoded-nanoparticles. AuNPs were modified by three different aryl diazonium salts, bearing either nitro or cyano groups and carboxyl-terminated function. These nanoprobes were shown to combine unique SERS spectra and post-functionalization capacity for the immobilization of the nucleotide AMP. Unlike thiol-derived Raman reporters forming monolayers around plasmonic NPs, this approach enables the formation of strongly attached multilayers around the gold cores, with various chemical functions, providing a breakthrough strategy to create multifunctional SERS tags. It thus offers several advantages over conventional methods of SERS tag preparation, such as: (i) the formation of strong covalent bonds between the AuNP surface and the Raman labels and (ii) the growing of multifunctional layers around the gold cores, using a simple and straightforward process (in air, at room temperature). Therefore, this aryl diazonium salt-based approach will not only pave a new way for the functionalization of AuNPs by multilayers but also provide a general strategy to design SERS-encoded NPs.
The authors thank G. Pehau-Arnaudet (Institut Pasteur) for TEM analysis, Horiba for Raman imaging, L. Fouassier (Sorbonne Université) and A. Nicolas Boluda (Univ. de Paris) for providing cells.
Conflicts of interest
There are no conflicts to declare.Notes and references
- L. Fabris, ChemNanoMat, 2016, 2, 249 CrossRef CAS.
- Y. Wang, B. Yan and L. Chen, Chem. Rev., 2013, 113, 1391 CrossRef CAS PubMed.
- R. A. Alvarez-Puebla and L. M. Liz-Marzán, Small, 2010, 6, 604 CrossRef CAS PubMed.
- M. Tabatabaei, D. McRae and F. Lagugné-Labarthet, Recent advances of plasmon-enhanced spectroscopy at bio-Interfaces. Frontiers of Plasmon Enhanced Spectroscopy, 2016, vol. 1246, pp. 183–207 Search PubMed.
- Y. B. Rus, L. Galmiche, P. Audebert, A. Courty, E. Maisonhaute and F. Miomandre, ChemistrySelect, 2019, 4, 1298 CrossRef.
- L. A. Lane, X. Qian and S. Nie, Chem. Rev., 2015, 115, 10489 CrossRef CAS PubMed.
- E. Lenzi, D. Jimenez de Aberasturi and L. M. Liz-Marzán, ACS Sens., 2019, 4, 1126 CrossRef CAS PubMed; J. Gao, X. Huang, H. Liu, F. Zan and J. Ren, Langmuir, 2012, 28, 4464 CrossRef PubMed.
- B. Mir-Simon, I. Reche-Perez, L. Guerrini, N. Pazos-Perez and R. A. Alvarez-Puebla, Chem. Mater., 2015, 27, 950 CrossRef CAS.
- N. Bhatt, P.-J. J. Huang, N. Dave and J. Liu, Langmuir, 2011, 27(10), 6132 CrossRef CAS PubMed.
- G. Yang, N. A. Amro, Z. B. Starkewolfe and G.-Y. Liu, Langmuir, 2004, 20, 3995 CrossRef CAS PubMed.
- C. Vericat, M. E. Vela, G. Benitez, P. Carro and R. C. Salvarezza, Chem. Soc. Rev., 2010, 39, 1805 RSC.
- J. Walia, J.-M. Guay, O. Krupin, F. Variola, P. Berini and A. Weck, Phys. Chem. Chem. Phys., 2018, 20, 238 RSC.
- S.-W. Tam-Chan, H. A. Biebuyck, G. M. Whitesides, N. Jeon and R. G. Nuzzo, Langmuir, 1995, 11, 4371 CrossRef.
- P. Chinwangso, A. C. Jamison and T. R. Lee, Acc. Chem. Res., 2011, 44, 511 CrossRef CAS PubMed.
- L. Laurentius, S. R. Stoyanov, S. Gusarov, A. Kovalenko, R. B. Du, G. P. Lopinski and M. T. McDermott, ACS Nano, 2011, 5, 4219 CrossRef CAS PubMed.
- F. Mirkhalaf, J. Paprotny and D. J. Schiffrin, J. Am. Chem. Soc., 2006, 128, 7400 CrossRef CAS PubMed.
- R. Ahmad, N. Felidj, L. Boubekeur-Lecaque, S. Lau-Truong, S. Gam-Derouich, P. Decorse, A. Lamouri and C. Mangeney, Chem. Commun., 2015, 51, 9678 RSC.
- L. Troian-Gautier, H. Valkenier, A. Mattiuzzi, I. Jabin, N. Van den Brande, B. Van Mele, J. Hubert, F. Reniers, G. Bruylants, C. Lagrost and Y. Leroux, Chem. Commun., 2016, 52, 10493 RSC.
- M. Bouriga, M. M. Chehimi, C. Combellas, P. Decorse, F. Kanoufi, A. Deronzier and J. Pinson, Chem. Mater., 2013, 25, 90 CrossRef CAS.
- J. Pinson and F. Podvorica, Chem. Soc. Rev., 2005, 34, 429 RSC.
- A. Berisha, M. M. Chehimi, J. Pinson and F. I. Podvorica, Electrode surface modification using diazonium salts, ed. A. J. Bard and C. G. Zoski, Electroanalytical Chemistry, CRC Press, Boca Raton, FL, 2016, vol. 26 Search PubMed.
- E. Gervais, Y. Aceta, P. Gros and D. Evrard, Electrochim. Acta, 2018, 261, 346 CrossRef CAS.
- J. Du, H. Du, X. Li, J. Fan and X. Peng, Sens. Actuators, B, 2017, 248, 318 CrossRef CAS.
- Y. Kalachyova, A. Olshtrem, O. A. Guselnikova, P. S. Postnikov, R. Elashnikov, P. Ulbrich, S. Rimpelova, V. Švorčík and O. Lyutakov, ChemistryOpen, 2017, 6, 254–260 CrossRef CAS PubMed.
- M. Nguyen, I. Kherbouche, S. Gam-Derouich, I. Ragheb, S. Lau-Truong, A. Lamouri, G. Levi, J. Aubard, P. Decorse, N. Felidj and C. Mangeney, Chem. Commun., 2017, 53, 11364 RSC.
- M. Nguyen, A. Lamouri, C. Salameh, G. Levi, J. Grand, L. Boubekeur-Lecaque, C. Mangeney and N. Felidj, Nanoscale, 2016, 8, 8633 RSC.
- V.-Q. Nguyen, Y. Ai, P. Martin and J.-C. Lacroix, ACS Omega, 2017, 2, 1947 CrossRef CAS PubMed.
- I. Tijunelyte, I. Kherbouche, S. Gam-Derouich, M. Nguyen, N. Lidgi-Guigui, M. Lamy de la Chapelle, A. Lamouri, G. Lévi, J. Aubard, A. Chevillot-Biraud, C. Mangeney and N. Felidj, Nanoscale Horiz., 2018, 3, 53 RSC.
- M. Supur, S. R. Smith and R. L. McCreery, Anal. Chem., 2017, 89, 6463 CrossRef CAS PubMed.
- L. Antonioli, C. Blandizzi, P. Pacher and G. Hasko, Nat. Rev. Cancer, 2013, 13, 842 CrossRef CAS PubMed.
- T. Dai, N. Li, F. Han, H. Zhang, Y. Zhang and Q. Liu, Biomaterials, 2016, 83, 37 CrossRef CAS PubMed.
- J. Turkevich, P. C. Stevenson and J. A. Hillier, Discuss. Faraday Soc., 1951, 11, 55 RSC.
- A. Berisha, C. Combellas, F. Kanoufi, P. Decorse, N. Oturan, J. Médard, M. Seydou, F. Maurel and J. Pinson, Langmuir, 2017, 33, 8730 CrossRef CAS PubMed.
- J. Kundu, O. Neumann, B. G. Janesko, D. Zhang, S. Lal, A. Barhoumi, G. E. Scuseria and N. J. Halas, J. Phys. Chem. C, 2009, 113, 14390 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0cc02842h |
| This journal is © The Royal Society of Chemistry 2020 |