Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
An efficient, stereocontrolled and versatile synthetic route to bicyclic partially saturated privileged scaffolds†
Hannah L.
Stewart
 a,
Abigail R.
Hanby
a,
Thomas A.
King
a,
Abigail R.
Hanby
a,
Thomas A.
King
 a,
Andrew D.
Bond
a,
Andrew D.
Bond
 a,
Thomas A.
Moss
b,
Hannah F.
Sore
a,
Thomas A.
Moss
b,
Hannah F.
Sore
 a and
David R.
Spring
a and
David R.
Spring
 *a
*a
aDepartment of Chemistry, University of Cambridge, Lensfield Road, Cambridge, CB2 1EW, UK. E-mail: spring@ch.cam.ac.uk
bAstraZeneca UK Ltd, 310 Cambridge Science Park, Milton Road, Cambridge, CB4 0FZ, UK
First published on 11th May 2020
Abstract
Herein, we describe the development of a simple, high yielding and stereocontrolled strategy for the synthesis of a series of triazolopiperazines and other biologically relevant fused scaffolds from optically active amino acids. This route was applied to the synthesis of 22 scaffolds containing new, previously inaccessible vectors and used to access a novel analogue of ganaplacide.
“Privileged” scaffolds, have an inherent ability to bind and modulate the activity of protein targets, making them highly favourable scaffolds for drug discovery and as such have been utilised in a variety of discovery programs.1 Many synthetic drugs also share common scaffolds, which led to Black's suggestion that “The most fruitful basis for the discovery of a new drug is to start with an old drug.”2 The key to the construction of privileged scaffold libraries for drug discovery is the development of reactions of broad scope with stereocontrol and that allow access to multiple vectors in a scaffold.3
Heterocycles with increased levels of three-dimensionality present an underexplored and underrepresented class of privileged scaffolds. The incorporated heteroatoms enable potential binding interactions, and the limited structural flexibility of the cyclic scaffold reduces the entropic binding penalty resulting in an increased possibility to form productive binding interactions.4 As a result there has been a recent surge in the development of methodologies to access three-dimensional heterocyclic scaffolds.5
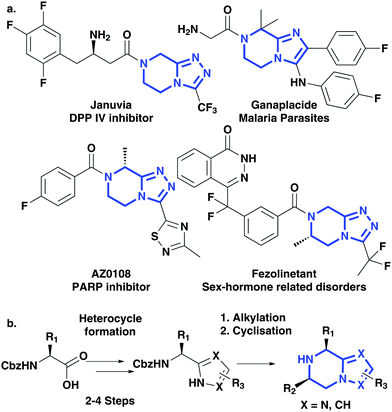
Partially saturated bicyclic piperazine-based privileged scaffolds are present in a number of drugs for a variety of clinical indications including treatments of malaria, hyperglycaemia, sex-hormone related disorders and cancer (Fig. 1a).6 Despite the promising and varied applications for these privileged scaffolds, there exists no consistent synthetic strategy for accessing them. Published routes include annulating the 5-membered heterocycle onto the 6-membered piperazine ring; selectively reducing the pyrazine ring of the fully aromatic triazolopyrazine and forming a 6-membered lactam on the 5-membered heterocycle.6,7 These varied routes lack the ability to generate scaffolds with enantioselective substitutions around the piperazine ring.
 | ||
| Fig. 1 (a) Fused piperazine scaffolds (highlighted in blue) utilised in FDA approved drugs and clinical candidates;6 (b) synthetic strategy developed in this work to access these fused piperazine scaffolds. | ||
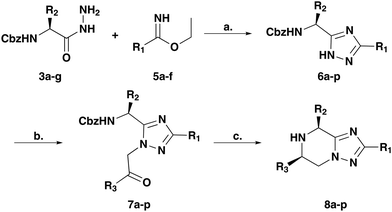
We describe a highly modular, efficient and versatile approach to synthesising fused bicyclic heteroaromatic privileged scaffolds starting from optically pure amino acids (Fig. 1b). We anticipated that assembling the 5-membered heterocycle first would provide the scaffold around which the partially saturated piperazine ring could be constructed. Alkylation of the bridging nitrogen would set up the scaffold for a one-pot deprotection and reductive amination with cis-diastereoselective control driven by the pre-set amino acid chiral centre (see ESI,† 1.2.6. for model).
Initial studies were carried out towards the synthesis of 5,6,7,8-tetrahydro-[1,2,4]triazolo[1,5-a]pyrazine scaffolds. To this end, a number of exemplar amino acids 1a–g were converted into the corresponding amino hydrazides 3a–gvia the corresponding amino ester intermediates 2a–g. In parallel, the required imidate building blocks 5a–f were formed from the corresponding nitriles 4a–f using the Markovnikov transformation8 (Scheme 1).
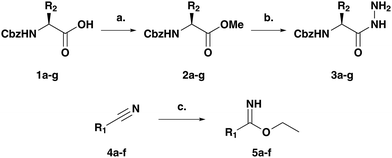 | ||
| Scheme 1 Building block synthesis (see Table 1 for R1 and R2): (a) SOCl2, MeOH, 3 h, rt, 96–100%; (b) NH2NH2·H2O, MeOH, 12 h, rt, 82–100%; (c) AcCl, EtOH, 12 h, 0 °C to rt, 66–100%. | ||
The combination of the amino hydrazide (3a–g) and imidate (5a–f) building blocks in the presence of acetic acid gave triazoles 6a–p (Table 1).9 Subsequent alkylation of the triazoles with α-bromoketones gave the cyclisation precursors 7a–p. Treatment of intermediates 7a–p with catalytic palladium dihydroxide and ammonium formate enabled the deprotection of the pendant nitrogen, unveiling it for the reductive amination. A 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of methanol and water was used, the addition of water was thought to increase the rate of imine reduction and thus suppress the undesired ketone reduction.10 The final scaffolds (8a–p) were generated in 51–91% and >20
1 ratio of methanol and water was used, the addition of water was thought to increase the rate of imine reduction and thus suppress the undesired ketone reduction.10 The final scaffolds (8a–p) were generated in 51–91% and >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 d.r. Significantly, the methodology enabled the incorporation of functionalities that provide vectors for compound elaboration, including amino, alcohol and sulphur groups in the final triazole-based 5,6-bicyclic compounds (8a–p). In addition, common functionalities used in drug discovery were included, such as trifluoromethyl and cyclopropyl groups. Due to the reductive conditions of the final step, alkene moieties were reduced to simple alkyl chains. To access a primary amine substituent, it was necessary to use a nitro group to mask this reactive moiety. Finally, for access to sulphur containing substituents it was necessary to increase the catalytic amount of palladium dihydroxide to excess such that the poisoning effect of the sulphur could be overcome. The desired cis-geometry between the two piperazine substituents of the 5,6-bicyclic scaffolds 8a–p was conclusively demonstrated by X-ray crystallography of compound 8n (see ESI†). The X-ray structure also confirmed the conservation of the stereochemistry of the chiral centre derived from the starting amino acid.11
1 d.r. Significantly, the methodology enabled the incorporation of functionalities that provide vectors for compound elaboration, including amino, alcohol and sulphur groups in the final triazole-based 5,6-bicyclic compounds (8a–p). In addition, common functionalities used in drug discovery were included, such as trifluoromethyl and cyclopropyl groups. Due to the reductive conditions of the final step, alkene moieties were reduced to simple alkyl chains. To access a primary amine substituent, it was necessary to use a nitro group to mask this reactive moiety. Finally, for access to sulphur containing substituents it was necessary to increase the catalytic amount of palladium dihydroxide to excess such that the poisoning effect of the sulphur could be overcome. The desired cis-geometry between the two piperazine substituents of the 5,6-bicyclic scaffolds 8a–p was conclusively demonstrated by X-ray crystallography of compound 8n (see ESI†). The X-ray structure also confirmed the conservation of the stereochemistry of the chiral centre derived from the starting amino acid.11
| Compound | Step (%) | ||||||
|---|---|---|---|---|---|---|---|
| R1 | R2 | R3 | 6 | 7 | 8 (d.r.) | ||
a i. EtOH, 6 h, reflux; ii. AcOH, 2 h, reflux.
b α-Bromoketone, K2CO3, acetone or DMF, 12 h, rt.
c NH4·CO2H, Pd(OH)2/C (20 mol%), MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (3 H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v), 12 h, rt.
d Reduction of the double bond observed, yield in parenthesis refers to R1 as a n-pentyl chain.
e 2 eq. of Pd(OH)2.
f Reduction of the nitro group observed, yield in parenthesis refers to R3 as a p-aminophenyl. 1 v/v), 12 h, rt.
d Reduction of the double bond observed, yield in parenthesis refers to R1 as a n-pentyl chain.
e 2 eq. of Pd(OH)2.
f Reduction of the nitro group observed, yield in parenthesis refers to R3 as a p-aminophenyl.
|
|||||||
| a | Ph | Bn | Ph | 95 | 84 | 91 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| b | (CH2)3CHCH2 | Bn | Ph | 86 | 65 | (60)d | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| c | Cyclopropyl | Bn | Ph | 81 | 53 | 74 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| d | p-PhCF3 | Bn | Ph | 85 | 73 | 59 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| e | p-PhOMe | Bn | Ph | 87 | 77 | 77 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| f | Ph | H | Ph | 63 | 72 | 82 | n/a |
| g | Ph | CH2OH | Ph | 66 | 55 | 82 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| h | Ph | CH(CH3)2 | Ph | 82 | 79 | 84 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| i | Ph | CH2-indole | Ph | 74 | 81 | 93 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| j | Ph | CH2-p-PhOH | Ph | 83 | 33 | 83 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| k | Ph | (CH2)3-N | Ph | 70 | 80 | 60 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| l | Ph | (CH2)2SCH3 | Ph | 98 | 93 | 51e | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| m | Ph | Bn | Adamantyl | — | 87 | 91 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| n | Ph | Bn | p-PhCF3 | — | 66 | 71 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| o | Ph | Bn | m-PhOMe | — | 50 | 77 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| p | Ph | Bn | p-PhNO2 | — | 50 | (71)f | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Thus we have developed an efficient, high yielding synthetic strategy towards the synthesis of triazolopiperazines with stereocontrol and substitution at 3 of a possible 4 positions, leaving the amine unsubstituted for derivatisation. To expand the scope of this work and explore the potential to generate closely related privileged scaffolds with novel vectors in a stereocontrolled manner, other 5-membered heterocycles were used to form the fused ring system. To this end a synthetic strategy was sought which continued to utilise optically pure amino acids as an effective chiral source and our methodology to drive the stereo-control (Scheme 2).
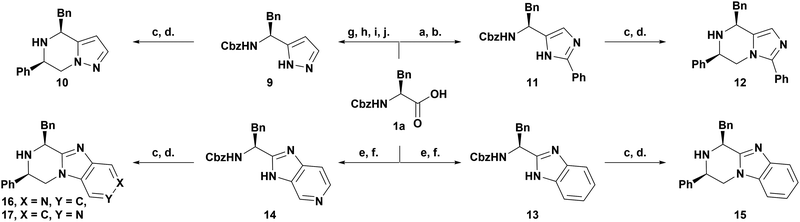 | ||
Scheme 2 A synthesis of other bicyclic scaffolds: (a) 2-aminoacetophenone hydrochloride, EDC, HOBt, NMM, DCM, 3 h, 0 °C to rt; (b) NH4OAc, xylene, 8 h, reflux; (c) 2-bromoacetophenone, K2CO3, acetone, 12 h, rt; (d) NH4·CO2H, Pd(OH)2/C (20 mol%), MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (3 H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v), 12 h, rt; (e) o-phenylenediamine or 3,4-diaminopyridine, HATU, DIPEA, DCM, 12 h, rt; (f) AcOH 2 h, 40 °C; (g) HNMeOMe·HCl, HOBt, HBTU, DIPEA, DCM, 12 h, rt; (h) phenylethylene magnesium bromide, THF, 12 h, −78 °C to rt; (i) HNEt2, DCM, 12 h, rt; (j) N2H4·H2O, HCl, 3 h, reflux (see ESI† for yields). 1 v/v), 12 h, rt; (e) o-phenylenediamine or 3,4-diaminopyridine, HATU, DIPEA, DCM, 12 h, rt; (f) AcOH 2 h, 40 °C; (g) HNMeOMe·HCl, HOBt, HBTU, DIPEA, DCM, 12 h, rt; (h) phenylethylene magnesium bromide, THF, 12 h, −78 °C to rt; (i) HNEt2, DCM, 12 h, rt; (j) N2H4·H2O, HCl, 3 h, reflux (see ESI† for yields). | ||
Pleasingly we successfully synthesised pyrazolopiperazine12 (10), imidazolopiperazine13 (12), benzimidazopiperzine14 (15) and pyridoimidazopiperazine (16 & 17) scaffolds in 4–6 steps including alkylation and reductive deprotection and amination of heterocyclic intermediates 9, 11, 13 and 14.
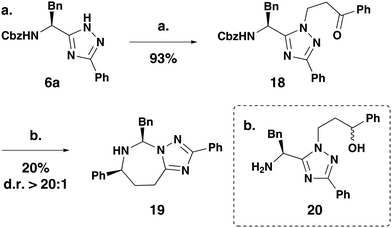
The methodology was further applied to the synthesis of 5,7-bicycles (Scheme 3). The 5-membered heteroarene (6a) was alkylated with β-haloketones to furnish the cyclisation precursor 18 in an excellent 93% yield. The 5,7-heterocycle 19 was achieved in a 20% yield and >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 diastereoselectivity, which was a pleasing result given the slower rate of cyclisation for larger rings. The by-product of the reaction was identified as 20 resulting from reduction of the uncyclised ketone.
1 diastereoselectivity, which was a pleasing result given the slower rate of cyclisation for larger rings. The by-product of the reaction was identified as 20 resulting from reduction of the uncyclised ketone.
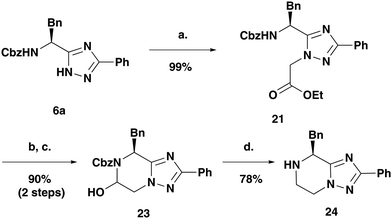
Synthesis of final scaffolds without an R3 substituent was explored (see Table 1). Diverging from the previous synthesis after formation of heterocycle 6a, the alkylation was carried out with ethyl bromoacetate to furnish ester 21 (Scheme 4). Reduction, followed by oxidation with IBX furnished the hemi-aminal 23. Application of the reductive palladium dihydroxide conditions yielded the desired amine 24 in a good yield.
The importance of this work is highlighted by the lead optimisation work carried out in the development of ganaplacide by Novartis, a drug currently in phase II clinical trials for the treatment of malaria. The SAR studies previously conducted were limited to double substitutions of the same group at each position around the piperazine ring due to the methodology available at the time, preventing exploration of various substitution patterns, functional groups and stereoselective control of the substitution (see ESI†).6a,b These limitations could be overcome by using our approach. To demonstrate its applicability, the synthesis of a novel analogue with a single substituent in the R2- and R3-positions was completed (Scheme 5). The key intermediate (32) of a ganaplacide analogue was achieved with substitution patterns and functional groups not accessible with the previous synthetic routes utilised. Intermediate 32 could be easily elaborated into the ganaplacide analogue 50 in 3 steps (see ESI†). Our modular synthetic route has the potential to access a wider range of novel analogues of ganaplacide than were previously inaccessible, such as those with single substitution patterns.
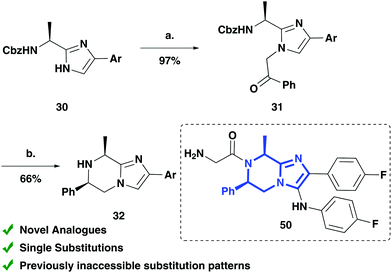 | ||
Scheme 5 Synthesis of ganaplacide analogue intermediate (full synthetic route for ganaplacide and analogue in the ESI†): (a) 2-bromoacetophenone, K2CO3, acetone, 97%; (b) NH4·CO2H, Pd(OH)2/C (20 mol%), MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (3 H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v), 66%. Ar = p-PhF. 1 v/v), 66%. Ar = p-PhF. | ||
In conclusion, we have developed a simple, highly scalable route to a series of fused piperazine- and diazepane-based scaffolds, which would enable the efficient construction of fragment-like, lead-like or drug-like screening libraries. We illustrated the utility of this work in the synthesis of a previously accessible analogue of ganaplacide. The reliable and highly scalable strategy developed therefore provides a means of expanding the diversity of these privileged but thus far relatively unexplored partially saturated bicyclic heteroaromatic scaffolds. These scaffolds have the potential to provide hits with excellent physicochemical properties for novel and challenging targets within drug discovery; furthermore, they provide access to a variety of vectors, enabling rapid hit development.
We thank the EPSRC, AstraZeneca, The Cambridge Trust, BBSRC for funding, and acknowledge support from the Royal Society (Wolfson Research Merit Award), EPSRC (EP/P020291/1), EPSRC, BBSRC, MRC, Wellcome Trust, Royal Society and ERC (FP7/2007–2013; 279337/DOS).
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) L. Yet, Privileged Structures in Drug Discovery: Medicinal Chemistry and Synthesis, Wiley, 2018 CrossRef; (b) B. E. Evans, K. E. Rittle, M. G. Bock, R. M. DiPardo, R. M. Freidinger, W. L. Whitter, G. F. Lundell, D. F. Veber, P. S. Anderson, R. S. L. Chang, V. J. Lotti, D. J. Cerino, T. B. Chen, P. J. Kling, K. A. Kunkel, J. P. Springer and J. Hirshfieldt, J. Med. Chem., 1988, 31, 2235–2246 CrossRef CAS PubMed; (c) M. A. Koch and H. Waldmann, Drug Discovery Today, 2005, 10, 471–483 CrossRef CAS PubMed; (d) M. A. Koch, L. Wittenberg, S. Basu, D. A. Jeyaraj, E. Gourzoulidou, K. Reinecke, A. Odermatt and H. Waldmann, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 16721–16726 CrossRef CAS PubMed; (e) M. A. Koch, A. Schuffenhauer, M. Scheck, S. Wetzel, M. Casaulta, A. Odermatt, P. Ertl and H. Waldmann, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 17272–17277 CrossRef CAS PubMed.
- (a) T. N. K. Raju, Lancet, 2000, 355, 1022 CrossRef CAS PubMed; (b) C. Lipinski and A. Hopkins, Nature, 2004, 432, 855–861 CrossRef CAS PubMed.
- M. E. Welsch, S. A. Snyder and B. R. Stockwell, Curr. Opin. Chem. Biol., 2010, 14, 347–361 CrossRef CAS PubMed.
- (a) P. Ertl, S. Jelfs, J. Mühlbacher, A. Schuffenhauer and P. Selzer, J. Med. Chem., 2006, 49, 4568–4573 CrossRef CAS PubMed; (b) W. R. Pitt, D. M. Parry, B. G. Perry and C. R. Groom, J. Med. Chem., 2009, 52, 2952–2963 CrossRef CAS PubMed; (c) M. Aldeghi, S. Malhotra, D. L. Selwood and A. W. E. Chan, Chem. Biol. Drug Des., 2014, 83, 450–461 CrossRef CAS PubMed; (d) F. Lovering, J. Bikker and C. Humblet, J. Med. Chem., 2009, 52, 6752–6756 CrossRef CAS PubMed; (e) F. Lovering, Med. Chem. Commun., 2013, 4, 515–519 RSC; (f) T. E. Nielsen and S. L. Schreiber, Angew. Chem., Int. Ed., 2007, 47, 48–56 CrossRef PubMed.
- (a) D. G. Twigg, N. Kondo, S. L. Mitchell, W. R. J. D. Galloway, H. F. Sore, A. Madin and D. R. Spring, Angew. Chem., Int. Ed., 2016, 55, 12479–12483 CrossRef CAS PubMed; (b) A. Sveiczer, A. J. P. North, N. Mateu, S. L. Kidd, H. F. Sore and D. R. Spring, Org. Lett., 2019, 3–7 Search PubMed; (c) A. W. Hung, A. Ramek, Y. Wang, T. Kaya, J. A. Wilson, P. A. Clemons and D. W. Young, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 6799–6804 CrossRef CAS; (d) T. A. King, H. L. Stewart, K. T. Mortensen, A. J. P. North, H. F. Sore and D. R. Spring, Eur. J. Org. Chem., 2019, 5219–5229 CrossRef CAS PubMed; (e) N. Mateu, S. L. Kidd, L. Kalash, H. F. Sore, A. Madin and D. R. Spring, Chem. – Eur. J., 2018, 24, 13681–13687 CrossRef CAS PubMed.
- (a) A. Nagle, T. Wu, K. Kuhen, K. Gagaring, R. Borboa, C. Francek, Z. Chen, D. Plouffe, X. Lin, C. Caldwell, J. Ek, S. Skolnik, F. Liu, J. Wang, J. Chang, C. Li, B. Liu, T. Hollenbeck, T. Tuntland, J. Isbell, T. Chuan, P. B. Alper, C. Fischli, R. Brun, S. B. Lakshminarayana, M. Rottmann, T. T. Diagana, E. A. Winzeler, R. Glynne, D. C. Tully and A. K. Chatterjee, J. Med. Chem., 2012, 55, 4244–4273 CrossRef CAS PubMed; (b) T. Wu, A. Nagle, K. Kuhen, K. Gagaring, R. Borboa, C. Francek, Z. Chen, D. Plouffe, A. Goh, S. B. Lakshminarayana, J. Wu, H. Q. Ang, P. Zeng, M. L. Kang, W. Tan, M. Tan, N. Ye, X. Lin, C. Caldwell, J. Ek, S. Skolnik, F. Liu, J. Wang, J. Chang, C. Li, T. Hollenbeck, T. Tuntland, J. Isbell, C. Fischli, R. Brun, M. Rottmann, V. Dartois, T. Keller, T. Diagana, E. Winzeler, R. Glynne, D. C. Tully and A. K. Chatterjee, J. Med. Chem., 2011, 54, 5116–5130 CrossRef CAS PubMed; (c) D. Kim, L. Wang, M. Beconi, G. J. Eiermann, M. H. Fisher, H. He, G. J. Hickey, J. E. Kowalchick, B. Leiting, K. Lyons, F. Marsilio, M. E. McCann, R. A. Patel, A. Petrov, G. Scapin, S. B. Patel, R. S. Roy, J. K. Wu, M. J. Wyvratt, B. B. Zhang, L. Zhu, N. A. Thornberry and A. E. Weber, J. Med. Chem., 2005, 48, 141–151 CrossRef CAS PubMed; (d) D. Kim, J. E. Kowalchick, L. L. Brockunier, E. R. Parmee, G. J. Eiermann, M. H. Fisher, H. He, B. Leiting, K. Lyons, G. Scapin, S. B. Patel, A. Petrov, K. D. Pryor, R. S. Roy, J. K. Wu, X. Zhang, M. J. Wyvratt, B. B. Zhang, L. Zhu, N. A. Thornberry and A. E. Weber, J. Med. Chem., 2008, 51, 589–602 CrossRef CAS; (e) J. E. Kowalchick, B. Leiting, K. A. D. Pryor, F. Marsilio, J. K. Wu, H. He, K. A. Lyons, G. J. Eiermann, A. Petrov, G. Scapin, R. A. Patel, N. A. Thornberry, A. E. Weber and D. Kim, Bioorg. Med. Chem. Lett., 2007, 17, 5934–5939 CrossRef CAS PubMed; (f) H. R. Hoveyda, G. L. Fraser, G. Dutheuil, M. El Bousmaqui, J. Korac, F. Lenoir, A. Lapin and S. Noel, ACS Med. Chem. Lett., 2015, 6, 736–740 CrossRef CAS PubMed; (g) H. R. Hoveyda, G. L. Fraser, M. Roy, G. Dutheuil, F. Batt, M. El Bousmaqui, J. Korac, F. Lenoir, A. Lapin, S. Noel and S. Blanc, J. Med. Chem., 2015, 58, 3060–3082 CrossRef CAS PubMed; (h) J. W. Johannes, L. Almeida, K. Daly, A. D. Ferguson, S. E. Grosskurth, H. Guan, T. Howard, S. Ioannidis, S. Kazmirski, M. L. Lamb, N. A. Larsen, P. D. Lyne, K. Mikule, C. Ogoe, B. Peng, P. Petteruti, J. A. Read, N. Su, M. Sylvester, S. Throner, W. Wang, X. Wang, J. Wu, Q. Ye, Y. Yu, X. Zheng and D. A. Scott, Bioorg. Med. Chem. Lett., 2015, 25, 5743–5747 CrossRef CAS PubMed.
- K. Ben Haj Salah, B. Legrand, M. Bibian, E. Wenger, J.-A. Fehrentz and S. Denoyelle, Org. Lett., 2018, 20, 3250–3254 CrossRef CAS PubMed.
- V. K. Yadav and K. G. Babu, Eur. J. Org. Chem., 2005, 452–456 CrossRef CAS.
- D. F. Burdi, R. Hunt, L. Fan, T. Hu, J. Wang, Z. Guo, Z. Huang, C. Wu, L. Hardy, M. Detheux, M. A. Orsini, M. S. Quinton, R. Lew and K. Spear, J. Med. Chem., 2010, 53, 7107–7118 CrossRef CAS PubMed.
- (a) S. Gomez, J. A. Peters and T. Maschmeyer, Adv. Synth. Catal., 2002, 344, 1037–1057 CrossRef CAS; (b) Q. Lei, Y. Wei, D. Talwar, C. Wang, D. Xue and J. Xiao, Chem. – Eur. J., 2013, 19(12), 4021–4029 CrossRef CAS PubMed.
- CCDC 1914926 contains the supplementary crystallographic data for this paper†.
- (a) S. Pirc, D. Bevk, A. Golobič, B. Stanovnik and J. Svete, Helv. Chim. Acta, 2006, 89, 30–44 CrossRef CAS; (b) J. Pu, A. F. Kreft, S. H. Aschmies, K. P. Atchison, J. Berkowitz, T. J. Caggiano, M. Chlenov, G. Diamantidis, B. L. Harrison, Y. Hu, D. Huryn, J. Steven Jacobsen, M. Jin, K. Lipinski, P. Lu, R. L. Martone, K. Morris, J. Sonnenberg-Reines, D. R. Riddell, J. Sabalski, S. C. Sun, E. Wagner, Y. Wang, Z. Xu, H. Zhou and L. Resnick, Bioorg. Med. Chem. Lett., 2009, 17, 4708–4717 CrossRef CAS PubMed.
- (a) H. J. Breslin, T. A. Miskowski, B. M. Rafferty, S. V. Coutinho, J. M. Palmer, N. H. Wallace, C. R. Schneider, E. S. Kimball, S. P. Zhang, J. Li, R. W. Colburn, D. J. Stone, R. P. Martinez and W. He, J. Med. Chem., 2004, 47, 5009–5020 CrossRef CAS PubMed; (b) H. J. Breslin, C. J. Diamond, R. W. Kavash, C. Cai, A. B. Dyatkin, T. A. Miskowski, S. P. Zhang, P. R. Wade, P. J. Hornby and W. He, Bioorg. Med. Chem. Lett., 2012, 22, 4869–4872 CrossRef CAS PubMed.
- (a) A. P. Combs, W. Zhu, M. L. Crawley, B. Glass, P. Polam, R. B. Sparks, D. Modi, A. Takvorian, E. McLaughlin, E. W. Yue, Z. Wasserman, M. Bower, M. Wei, M. Rupar, P. J. Ala, B. M. Reid, D. Ellis, L. Gonneville, T. Emm, N. Taylor, S. Yeleswaram, Y. Li, R. Wynn, T. C. Burn, G. Hollis, P. C. C. Liu and B. Metcalf, J. Med. Chem., 2006, 49, 3774–3789 CrossRef CAS PubMed; (b) S. G. Gouin, J. F. Gestin, A. Reliquet, J. C. Meslin and D. Deniaud, Tetrahedron Lett., 2002, 43, 3003–3005 CrossRef CAS; (c) D. Zhang, Z. Wang, W. Xu, F. Sun, L. Tang and J. Wang, Eur. J. Med. Chem., 2009, 44, 2202–2210 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1914926. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0cc02728f |
| This journal is © The Royal Society of Chemistry 2020 |