Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Temperature sensitive water-in-water emulsions†
Marko
Pavlovic
a,
Alexander
Plucinski
b,
Lukas
Zeininger
 *a and
Bernhard V. K. J.
Schmidt
*a and
Bernhard V. K. J.
Schmidt
 *ab
*ab
aMax Planck Institute of Colloids and Interfaces, Department of Colloid Chemistry, Am Mühlenberg 1, 14476 Potsdam, Germany. E-mail: Lukas.zeiningner@mpikg.mpg.de
bSchool of Chemistry, University of Glasgow, Joseph Black Building, G128QQ Glasgow, UK. E-mail: Bernhard.schmidt@glasgow.ac.uk
First published on 20th May 2020
Abstract
A novel approach for a temperature-sensitive stabilization of water-in-water (W/W) emulsions is described. Specifically, we leveraged the thermal induced conformation change of tailored thermoresponsive block copolymers to reversibly stabilize and destabilize water–water interfaces. In addition, we investigated our approach to reversibly tune the reaction kinetics of enzymes compartmentalized within aqueous two-phase systems.
Aqueous emulsions have wide-spread applications in a wide variety of biomedical, cosmetic, food, and performance products.1,2 Emulsions are kinetically stabilized multiphase systems. Traditionally, to prevent breaking of the droplet network by droplet coalescence and Ostwald ripening, emulsions are stabilized by surfactants or particles.3 Recently, aqueous two-phase systems (ATPS) and water-in-water (W/W) emulsions have received increased attention,4–9 which is based on an observation by Beijerinck in 1896.10 Phase separation in water systems is enabled for mixtures of two polymers but also for polymer–salt mixtures.11 Due to high diversity and, more importantly biocompatibility, ATPS has gained broad attention in various application fields, including biomacromolecule extraction, purification and separation or to produce microreactors for biomimetic reactions.12,13 Although macroscopically similar to O/W and W/O emulsions, W/W emulsions exhibit significantly different properties as a result of their ultralow interfacial tensions at water–water interfaces (order of μN m−1), in comparison to water–oil interfaces (order of mN m−1).14–16
Consequently, due to the related low interfacial tension small surfactant molecules and copolymers cannot accumulate at the water–water interface to sufficiently kinetically stabilize these emulsions.17 Larger particles, i.e. Pickering emulsifiers however, can stabilize such interfaces due to a different adsorption mechanism. Although aqueous multiphase systems have ultralow interfacial tensions, particles can reduce free energy at the interface by adsorption as particle kinetic energy is significantly lower than binding energy.18 In such systems ions, surfactants, water molecules or large biomolecules can freely pass through the interface, which possesses a considerable advantage over traditional oil-based emulsions.19–21 As such, enzymatic reactions in W/W droplets do not require expensive transmembrane proteins or any other sort of channels to enable substrate and/or product diffusion between the phases. This membrane-less separation and enrichment of functionalities in aqueous phases with different properties featuring permeable boundaries is employed also by biological cells to increase local concentration of substrates or enzymes while maintaining communication with the environment.
Recently, various types of particles were introduced in W/W emulsion systems, including latex particles,22 nanocrystals,7 montmorillonite clays,18 polydopamine particles,23,24 amphiphilic block copolymer micelles25 or liposomes20 with radius >10 nm. In order to reversibly tune the kinetics of chemical reactions or for using W/W emulsions as microcontainers for the delivery of active agents, recent interest has been drawn towards a controlled formation and disruption of W/W emulsions. In a recent example, de Freitas and coworkers reported on the stabilization of aqueous emulsions using pH responsive polysaccharide-coated protein particles leading to a switchable formation and destabilization of the droplet network.26
Herein, we report on a temperature-triggered reversible and switchable stabilization of W/W emulsions (Scheme 1). We make use of water-soluble double hydrophilic block copolymer (DHBC) that can be switched to an amphiphilic block copolymer above the cloud point (TCP). We synthesized poly(N,N-dimethylacrylamide)-b-poly(N,N-diethylacrylamide) (PDMA-b-PDEA) DHBC with different ratios of the polymer blocks (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2
1 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), where the PDEA block can be switched from the coiled to the globule state above TCP leading to the formation of micelles and, subsequently, supramolecular aggregates. In the micellar state, the DHBCs successfully stabilized poly(ethylene glycol) (PEG) 35k–dextran (Dex) 40k W/W emulsions, which enabled tuning of the emulsion state by small temperature variations. The resulting stimuli-sensitive emulsions will help to pave the way towards dynamically triggerable purely aqueous based systems for drug delivery purposes or dynamic reaction control. To showcase this potential, we studied the kinetics of horseradish peroxidase (HRP) mediated oxidation of guaiacol within stable W/W emulsions in comparison to bulk ATPS.
1), where the PDEA block can be switched from the coiled to the globule state above TCP leading to the formation of micelles and, subsequently, supramolecular aggregates. In the micellar state, the DHBCs successfully stabilized poly(ethylene glycol) (PEG) 35k–dextran (Dex) 40k W/W emulsions, which enabled tuning of the emulsion state by small temperature variations. The resulting stimuli-sensitive emulsions will help to pave the way towards dynamically triggerable purely aqueous based systems for drug delivery purposes or dynamic reaction control. To showcase this potential, we studied the kinetics of horseradish peroxidase (HRP) mediated oxidation of guaiacol within stable W/W emulsions in comparison to bulk ATPS.
We started by synthesizing switchable DHBC via RAFT polymerization.27 PDMA-b-PDEA block copolymers were synthesized with two ratios of PDMA/PDEA, i.e. 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2
1 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
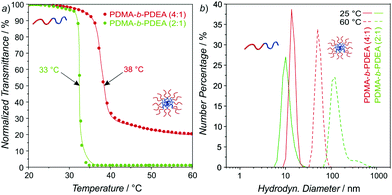
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and characterized with size exclusion chromatography (SEC) and 1H-NMR (Fig. S1–S4 and Table S1, ESI†). The PDEA block exhibits a TCP value ranging from 32 °C to 41 °C, depending on polymer molar mass, heating rate and polymer concentration.28 Therefore, initially TCP of both block copolymers was measured via turbidimetry (Fig. 1a).
1 and characterized with size exclusion chromatography (SEC) and 1H-NMR (Fig. S1–S4 and Table S1, ESI†). The PDEA block exhibits a TCP value ranging from 32 °C to 41 °C, depending on polymer molar mass, heating rate and polymer concentration.28 Therefore, initially TCP of both block copolymers was measured via turbidimetry (Fig. 1a).
As it was expected, PDMA-b-PDEA (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in comparison to PDMA-b-PDEA (4
1) in comparison to PDMA-b-PDEA (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), exhibited 5 °C lower TCP value. Interestingly, PDMA-b-PDEA (2
1), exhibited 5 °C lower TCP value. Interestingly, PDMA-b-PDEA (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) gave lower transmittance above TCP in comparison to the other block copolymer, which was attributed to a certain degree of aggregation of the 4
1) gave lower transmittance above TCP in comparison to the other block copolymer, which was attributed to a certain degree of aggregation of the 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 copolymer at ambient temperature. Oppositely, supramolecular aggregates formed by 2
1 copolymer at ambient temperature. Oppositely, supramolecular aggregates formed by 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 block copolymers are larger in diameter above TCP leading to increased scattering intensity.
1 block copolymers are larger in diameter above TCP leading to increased scattering intensity.
To further evaluate the block copolymer behavior at different temperatures, the hydrodynamic diameter was monitored via dynamic light scattering (DLS) at 25 °C and 60 °C (Fig. 1b). DLS experiments correlated well with turbidimetry measurements (Fig. 1a). PDMA-b-PDEA (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) showed higher differences in radii below and above Tcp in comparison to the second copolymer, i.e. the 4
1) showed higher differences in radii below and above Tcp in comparison to the second copolymer, i.e. the 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 block copolymer increased in diameter from 14 nm to 52 nm, whereas 2
1 block copolymer increased in diameter from 14 nm to 52 nm, whereas 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 block copolymer increases from 10 nm to 119 nm. Approximately doubling in diameter of aggregated 2
1 block copolymer increases from 10 nm to 119 nm. Approximately doubling in diameter of aggregated 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 block copolymer can be understood from the perspective of longer PEDA chains that form a larger hydrophobic micelle core compared to 4
1 block copolymer can be understood from the perspective of longer PEDA chains that form a larger hydrophobic micelle core compared to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 block copolymer. In addition, it should be noted that 2
1 block copolymer. In addition, it should be noted that 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 block copolymer also showed higher order aggregates illustrated by the shoulder towards higher diameters in the particle size distribution. For the purpose of emulsion stabilization such aggregates are advantageous due to the fact that larger spheres exhibit improved performances as a Pickering stabilizer.
1 block copolymer also showed higher order aggregates illustrated by the shoulder towards higher diameters in the particle size distribution. For the purpose of emulsion stabilization such aggregates are advantageous due to the fact that larger spheres exhibit improved performances as a Pickering stabilizer.
Next, we investigated the preparation of switchable W/W emulsions. For this purpose, we chose W/W emulsion based on 7 w/v% of PEG 35k and 10 w/v% of Dex 40k that are characterized by a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 volume ratio of the two aqueous phases, a comparatively fast phase separation in the absence of stabilizers and most importantly, the fact that this composition is far from the critical point of the system resulting in a high interfacial potential between the phases. At first, the macroscopic phase separation of PEG 35k–Dex 40k ATPS emulsion was followed over seven days (Fig. 2).
1 volume ratio of the two aqueous phases, a comparatively fast phase separation in the absence of stabilizers and most importantly, the fact that this composition is far from the critical point of the system resulting in a high interfacial potential between the phases. At first, the macroscopic phase separation of PEG 35k–Dex 40k ATPS emulsion was followed over seven days (Fig. 2).
In order to prove the stability of the obtained W/W emulsion at elevated temperatures, we compared it to ambient temperature, but also to a system without any stabilizer (Fig. 2). Both block copolymers dissolve in water at ambient temperature (ia, iia) or form a turbid dispersion above Tcp due to formation of polymeric supramolecular aggregates (ib, iib). There is no change over time in the first two samples (ia, iia, ib, iib) regardless of the temperature. Mixed ATPS without any block copolymers (iiia, iiib) and with each of the block copolymers (iva, va, ivb, vb) were prepared. The control sample of pure ATPS (iiia, iiib) exhibited complete phase separation in less than 60 min and 5 min, at ambient and elevated temperatures, respectively. Increased rate of coalescence can be mainly attributed to higher diffusion rates of the polymers, but there is also a moderate effect of the increased interfacial tension caused by a temperature shift of binodal.16 In case of the last two samples (iva, va, ivb, vb), the stability of mixed ATPS containing each of PDMA-b-PDEA block copolymers is critically dependent on temperature. At ambient temperature polymeric chains are in a coiled-state, therefore they cannot cover W/W interface and prevent coalescence, and as a result they behaved as poor stabilizers (iva, va). On the other hand, at temperatures above TCP, polymers tended to form micelles and supramolecular aggregates that are much higher in size and act as Pickering-stabilizer for aqueous emulsions (ivb, vb). Complete phase separation on macroscopic scale did not occur in these samples even after 7 days, although there were some initial stages of destabilization present in the sample stabilized by 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 block copolymer (ivb) where the formation of a clear phase on top could be observed. This effect demonstrated that polymeric micelles showed preference to one of the phases.
1 block copolymer (ivb) where the formation of a clear phase on top could be observed. This effect demonstrated that polymeric micelles showed preference to one of the phases.
The temperature-dependent emulsion stability was further investigated by optical microscopy (Fig. 3 and Fig. S5, ESI†). According to images taken at two different magnifications, droplet coalescence was prevented at temperatures above Tcp. As soon as samples reached temperatures below 38 °C (Tcp of PDMA-b-PDEA 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) droplet coalescence occurred, which is directly macroscopically reflected as phase separation (unstable emulsion) over time. In these experiments we observed only the beginning of the rapid coalescence at 30 °C, while at 20 °C large droplets were formed. The same effect was observed for PDMA-b-PDEA 2
1) droplet coalescence occurred, which is directly macroscopically reflected as phase separation (unstable emulsion) over time. In these experiments we observed only the beginning of the rapid coalescence at 30 °C, while at 20 °C large droplets were formed. The same effect was observed for PDMA-b-PDEA 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. S5, ESI†).
1 (Fig. S5, ESI†).
We anticipated that the formed micelles prefer the PEG phase over the Dex phase and therefore would preferably stabilize a Dex in PEG emulsion. In order to prove this statement confocal laser scanning microscopy (CLSM) was employed. Accordingly, emulsions were prepared in presence of the block copolymers and stained with either FITC-labeled PEG or rhodamine-labeled Dex (Fig. 4a). As evident from confocal micrographs a Dex-in-PEG emulsion was formed regardless of the type of stabilizer used. Droplets exhibit moderate uniformity, except for the highly polydisperse sample of rhodamine-labeled Dex emulsion stabilized with 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 block copolymer. Because experiment temperature was very close to the TCP of this stabilizer, we attributed this observation to a local cooling effect that promoted droplet coalescence. Nevertheless, as soon as temperature was equilibrated, droplets remained constant in radius of 54 μm ± 23 μm and 24 μm ± 13 μm for 2
1 block copolymer. Because experiment temperature was very close to the TCP of this stabilizer, we attributed this observation to a local cooling effect that promoted droplet coalescence. Nevertheless, as soon as temperature was equilibrated, droplets remained constant in radius of 54 μm ± 23 μm and 24 μm ± 13 μm for 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 4
1 and 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 block copolymers, respectively (measured from FITC-PEG labeled samples).
1 block copolymers, respectively (measured from FITC-PEG labeled samples).
To showcase the potential of having control over the stability of aqueous emulsions we investigated enzyme kinetics in various W/W systems. Specifically, we introduced HRP to ATPSs with the different block copolymers (Fig. 4b). All experiments were performed at a temperature of 37 °C, i.e. in between the TCP of the two different copolymers, and guaiacol was utilized as substrate for measuring the enzymatic activity (Scheme S3, ESI†). In our system, HRP preferably partitions to the Dex phase with a partitioning coefficient (K) of 0.63.8 In turn, the reaction substrate and product are more hydrophobic and consequently partition in the PEG phase (Fig. S6, ESI†). Therefore, we anticipated that the emulsion state and the associated different contact areas between the two phases would result in changed reaction kinetics. As displayed in Fig. 4b, when compared to the enzyme turnover rate in the pure ATPS system, the introduction of the hydrophilic block copolymer in the coiled form (PDMA-b-PDEA 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with TCP;4
1 with TCP;4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 = 38 °C) promoted the enzymatic reaction. This increase in the maximum rate of the reaction was attributed to a higher contact area of two phases as a result of decelerated phase separation of the two phases. In contrast, when introducing the second block copolymer in the aggregated state (PDMA-b-PDEA 2
1 = 38 °C) promoted the enzymatic reaction. This increase in the maximum rate of the reaction was attributed to a higher contact area of two phases as a result of decelerated phase separation of the two phases. In contrast, when introducing the second block copolymer in the aggregated state (PDMA-b-PDEA 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with TCP;2
1 with TCP;2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 = 33 °C), we observed a decrease of the reaction kinetics, despite a largely increased contact area between the two phases as a result of the formation of emulsion droplets. In this scenario, the presence of micelles caused encapsulation of the hydrophobic substrates, i.e. guaiacol,29 and this confinement slowed down the mass exchange between the phases. The latter observation is supported by experiments where we investigated the enzymatic reactivity in the pure constituent phases of the ATPS which revealed a slowed down reaction rate even in the pure Dex phase. These experiments reveal that the enzymatic activity is mutually controlled by the interfacial area between the phases of an ATPS as well as by the substrate encapsulation within micelles. Thus, small temperature variations in a fine range of 5 °C around TCP of the copolymers served to gain control over the reactivity of enzymatic transformations in solution.
1 = 33 °C), we observed a decrease of the reaction kinetics, despite a largely increased contact area between the two phases as a result of the formation of emulsion droplets. In this scenario, the presence of micelles caused encapsulation of the hydrophobic substrates, i.e. guaiacol,29 and this confinement slowed down the mass exchange between the phases. The latter observation is supported by experiments where we investigated the enzymatic reactivity in the pure constituent phases of the ATPS which revealed a slowed down reaction rate even in the pure Dex phase. These experiments reveal that the enzymatic activity is mutually controlled by the interfacial area between the phases of an ATPS as well as by the substrate encapsulation within micelles. Thus, small temperature variations in a fine range of 5 °C around TCP of the copolymers served to gain control over the reactivity of enzymatic transformations in solution.
In summary, we presented a novel approach towards stimuli-sensitive W/W emulsions. The emulsions were stabilized using synthesized PDMA-b-PDEA block copolymers with a thermoresponsive PDEA block. The block copolymers demonstrated utility as a competent long-term stabilizer for W/W emulsion based on PEG 35k and Dex 40k at temperatures above Tcp. However, decreasing the temperature below TCP resulted in rapid droplet coalescence and macroscopic phase separation. In addition, enzymatic reaction kinetics of HRP were tested in the stable and unstable emulsion samples, which potentially serves as a promising path to tune enzymatic reactions in ATPS.
The authors are grateful for funding from the Max-Planck society. MP acknowledges financial support from the Swiss National Science Foundation (project no: P2GEP2_181528). LZ is grateful for financial support through the Emmy-Noether program of the German Research Foundation (DFG) (grant no: ZE1121-3). BS and AP acknowledge funding from the DFG (grant no: SCHM 3282/3-1). Open Access funding provided by the University of Glasgow.
Conflicts of interest
There are no conflicts to declare.Notes and references
- C. C. Berton-Carabin and K. Schroën, Annu. Rev. Food Sci. Technol., 2015, 6, 263–297 CrossRef CAS PubMed.
- F. Goodarzi and S. Zendehboudi, Can. J. Chem. Eng., 2019, 97, 281–309 CrossRef CAS.
- V. Y. Grinberg and V. B. Tolstoguzov, Food Hydrocolloids, 1997, 11, 145–158 CrossRef CAS.
- J. Esquena, Curr. Opin. Colloid Interface Sci., 2016, 25, 109–119 CrossRef CAS.
- L. Tea, T. Nicolai and F. Renou, Langmuir, 2019, 35, 9029–9036 CrossRef CAS PubMed.
- Y. Beldengrün, J. Aragon, S. F. Prazeres, G. Montalvo, J. Miras and J. Esquena, Langmuir, 2018, 34, 9731–9743 CrossRef PubMed.
- K. R. Peddireddy, T. Nicolai, L. Benyahia and I. Capron, ACS Macro Lett., 2016, 5, 283–286 CrossRef CAS.
- M. Pavlovic, A. Plucinski, J. Zhang, M. Antonietti, L. Zeininger and B. V. K. J. Schmidt, Langmuir, 2020, 36, 1401–1408 CrossRef CAS PubMed.
- M. Pavlovic, M. Antonietti, B. V. K. J. Schmidt and L. Zeininger, J. Colloid Interface Sci., 2020, 575, 88–95 CrossRef CAS PubMed.
- M. W. Beijerinck, Z. Chem. Ind. Kolloide, 1910, 7, 16–20 CrossRef.
- R. Hatti-Kaul, Mol. Biotechnol., 2001, 19, 269–277 CrossRef CAS PubMed.
- Y. Chao and H. C. Shum, Chem. Soc. Rev., 2020, 49, 114–142 RSC.
- B. V. K. J. Schmidt, Macromol. Chem. Phys., 2018, 219, 1700494 CrossRef.
- E. Scholten, J. E. Visser, L. M. C. Sagis and E. van der Linden, Langmuir, 2004, 20, 2292–2297 CrossRef CAS PubMed.
- Y. A. Antonov, P. Van Puyvelde and P. Moldenaers, Int. J. Biol. Macromol., 2004, 34, 29–35 CrossRef CAS PubMed.
- D. Forciniti, C. K. Hall and M. R. Kula, J. Biotechnol., 1990, 16, 279–296 CrossRef CAS.
- B. T. Nguyen, W. Wang, B. R. Saunders, L. Benyahia and T. Nicolai, Langmuir, 2015, 31, 3605–3611 CrossRef CAS PubMed.
- W. J. Ganley, P. T. Ryan and J. S. van Duijneveldt, J. Colloid Interface Sci., 2017, 505, 139–147 CrossRef CAS PubMed.
- B. Davis, W. Aumiller, N. Hashemian, S. An, A. Armaou and C. Keating, Biophys. J., 2015, 109, 2182–2194 CrossRef CAS PubMed.
- D. C. Dewey, C. A. Strulson, D. N. Cacace, P. C. Bevilacqua and C. D. Keating, Nat. Commun., 2014, 5, 4670 CrossRef CAS PubMed.
- S. D. Hann, K. J. Stebe and D. Lee, ACS Appl. Mater. Interfaces, 2017, 9, 25023–25028 CrossRef CAS PubMed.
- H. Firoozmand, B. S. Murray and E. Dickinson, Langmuir, 2009, 25, 1300–1305 CrossRef CAS PubMed.
- J. Zhang, B. Kumru and B. V. K. J. Schmidt, Langmuir, 2019, 35, 11141–11149 CrossRef CAS PubMed.
- J. Zhang, J. Hwang, M. Antonietti and B. V. K. J. Schmidt, Biomacromolecules, 2019, 20, 204–211 CrossRef CAS PubMed.
- D. M. A. Buzza, P. D. I. Fletcher, T. K. Georgiou and N. Ghasdian, Langmuir, 2013, 29, 14804–14814 CrossRef CAS PubMed.
- R. A. de Freitas, T. Nicolai, C. Chassenieux and L. Benyahia, Langmuir, 2016, 32, 1227–1232 CrossRef CAS PubMed.
- B. V. K. J. Schmidt, M. Hetzer, H. Ritter and C. Barner-Kowollik, Macromolecules, 2011, 44, 7220–7232 CrossRef CAS.
- I. Idziak, D. Avoce, D. Lessard, D. Gravel and X. X. Zhu, Macromolecules, 1999, 32, 1260–1263 CrossRef CAS.
- A. Faridi Esfanjani and S. M. Jafari, Colloids Surf., B, 2016, 146, 532–543 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental Section, polymer characterization and additional microscopy images. See DOI: 10.1039/d0cc02171g |
| This journal is © The Royal Society of Chemistry 2020 |