Open Access Article
Open Access ArticleRed-light-sensitive BODIPY photoprotecting groups for amines and their biological application in controlling heart rhythm†
Kaja
Sitkowska
ab,
Martijn F.
Hoes
 c,
Michael M.
Lerch
c,
Michael M.
Lerch
 a,
Lucien N.
Lameijer
a,
Lucien N.
Lameijer
 ad,
Peter
van der Meer
ad,
Peter
van der Meer
 c,
Wiktor
Szymański
c,
Wiktor
Szymański
 *ad and
Ben L.
Feringa
*ad and
Ben L.
Feringa
 *a
*a
aCentre for Systems Chemistry, Stratingh Institute for Chemistry, University of Groningen, Nijenborgh 4, 9747 AG, Groningen, The Netherlands. E-mail: b.l.feringa@rug.nl
bUniversity of Warsaw, Faculty of Chemistry, Pasteura 1, 02-093 Warsaw, Poland
cDepartment of Cardiology, University of Groningen, University Medical Centre Groningen, Hanzeplein 1, 9713 GZ Groningen, The Netherlands
dDepartment of Radiology, University of Groningen, University Medical Center Groningen, Hanzeplein 1, 9713 GZ, Groningen, The Netherlands. E-mail: w.szymanski@umcg.nl
First published on 20th April 2020
Abstract
Control of biological function by the use of photoremovable protecting groups (PPGs) is a gateway towards many new medical developments. Herein, we report the synthesis and application of efficient and biocompatible BODIPY-based photoprotecting groups for amines, which are cleavable with red light in the phototherapeutic window region (λ > 650 nm). We use the most promising PPG for the protection of dopamine and apply it to control the beating frequency of human cardiomyocytes.
The amine functionality plays a key role in many bioactive compounds and frequently determines, among others, their solubility and target binding. By dynamically controlling the activity of amine groups, one can therefore achieve control over the activity of a drug within a biological system in selected time and space. Among the diverse approaches towards targeted activation, the use of photoprotecting groups (PPGs) has emerged as a highly versatile, modular and efficient technique. It allows for the selective release and/or activation of compounds with light in a discrete manner to reduce side effects on the biological system. It has been successfully applied to e.g. gene activation and silencing,1 or protein interactions.2 Because of many potential non-invasive applications with enhanced selectivity, light controlled activation of biologically active compounds, such as antibiotics,3,4 anticancer agents,5 neurotransmitters,6 amino acids or proteins7 and kinase inhibitors8 is currently being explored.9
Since their introduction by Engels and Schlaeger10 for the protection of adenosine, PPGs have been extensively studied, and several key aspects have been determined as being crucial for their applicability in biological systems. In their recent seminal review, Klán et al. highlight the most important parameters:9 first, PPGs should exhibit a narrow and intense absorption band, preferably in the so called “phototherapeutic window” (650–900 nm),11 in which the light used for photodeprotection is less toxic and shows better penetration of biological tissues.12 Second, they should show sufficient solubility and stability in aqueous media. Finally, the photorelease reaction should proceed within a short time frame (minutes) and yield the cargo compound in its active form. Furthermore, the residual by-products from the removed PPG should not absorb at the irradiation wavelengths and should be biologically inert.
In this context, in particular primary amines attracted our attention as PPG attachment points, as they are not only prevalent in bioactive compounds but also highly versatile functional groups in synthesis. However, previous attempts at building release systems for amine groups have shown that they are poor leaving groups and therefore an additional linker between PPG and amine, such as a carbamate, is often needed to enable their function in the desired manner.13 This approach was exemplified by the photodeprotection of histamine,14 dopamine14 and Vemurafenib.3
A common issue with most widely used PPGs (o-nitrobenzene, coumarin and benzoin derivatives)9 is that their removal typically requires high-energy light (650–900 nm), which can potentially lead to tissue damage. A direct solution to this problem, i.e. red-shifting the absorption band of the PPGs by extending the π-systems,15 has an inherent drawback of making the PPG molecules larger and less polar, thus decreasing their solubility in aqueous media.16 Recently, Bao et al. used styryl-conjugated coumarins for the protection of alcohols,17 while Etchenique and co-workers18 approached the problem of protection of amines by using ruthenium bipyridine complexes. Both PPGs show bathochromically shifted absorption maxima (λmax) in comparison with commonly used photoprotecting groups, but not enough to absorb in the phototherapeutic window. To the best of our knowledge, no efficient, red-light-sensitive systems employing these methods have been developed for amines. An alternative method for using red light in photodeprotection is two-photon excitation.19,20 However, compared to one photon excitation, irradiation with higher intensity of light is usually needed as the probability of such a transition is smaller.20,21
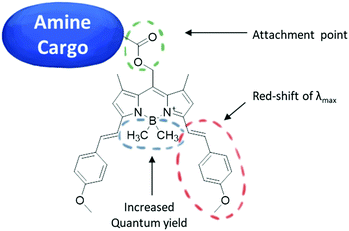
Facing these important challenges and limitations in designing a red-light responsive cage for amines, and inspired by the work of the group of Winter and Weinstain and our previous reports,14,17,22–25 describing a red-sensitive BODIPY PPG for esters, we set out to design and obtain analogous PPGs for amines applicable in biological setting. For this purpose, we choose the BODIPY (boron-dipyrromethene) core because of its: (i) easy synthesis, (ii) near-ideal photochemical properties; (iii) a narrow absorption band; and (iv) high ε values (∼105 M−1 cm−1).24 Next, we envisioned extending the π-system of the compound by adding styryl groups23 which leads to a significant red shift of the λmax. In our design, we also exchanged the fluorine atoms on the boron in the BODIPY core, taking advantage of the finding by Klán and co-workers26 that this increases the uncaging quantum yield. As PPG attachment point, we swapped the ester moiety at the meso position22 for an activated carbamate, which should allow to readily attach the amines and enable efficient release (Fig. 1). After initial studies with BODIPY-photoprotected 4-fluorobenzyl amine as the model compound, we prepared an analogous carbamate derivative for dopamine, a commonly used cardiac drug.27 We then tested the activity of the obtained compound before and after irradiation with red light (λ = 652 nm) on in vitro beating human embryonic stem cell (hESC) derived cardiomyocytes using multi-electrode array (MEA) methodology and compared the obtained values (beating frequency) with those obtained for dopamine. The results presented here suggest a potential application of PPGs in medical therapies.
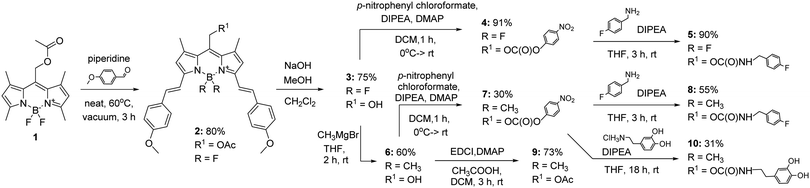
To obtain derivatives of biologically relevant amines whose activity could be controlled by irradiation with red light, we designed a synthetic route for the preparation of BODIPY-PPG based on reactive carbamates. The synthesis of the desired compounds started with the preparation of BODIPY ester 1 as reported previously25 (Fig. 2), followed by a Knoevenagel condensation leading to compound 2.
The latter reaction was initially run under standard Dean–Stark conditions, allowing us to obtain compound 2 in 43% yield.28 However, the yield was greatly improved by placing the neat reaction mixture under vacuum, presumably by efficiently removing water23 (80% yield). Ester 2 was then hydrolysed using NaOH(aq) in methanol to provide alcohol 3 in 75% yield, followed by reaction with p-nitrophenyl chloroformate to provide the activated carbonate 4 in 91% yield. Finally, this substrate was converted with 4-fluorobenzylamine to the desired carbamate in 90% yield. A similar strategy was used to obtain compound 8. To install CH3 groups on the boron at the BODIPY moiety, alcohol 3 was reacted with CH3MgBr at room temperature following a modified literature protocol,26 providing compound 6 in 60% yield. The formation of carbamate 8 from carbonate 7 afforded the desired compound in 55% yield. For comparison, compound 6 was reacted with acetic acid in the presence of EDCI and DMAP to give the corresponding acetate 9 (73% yield).
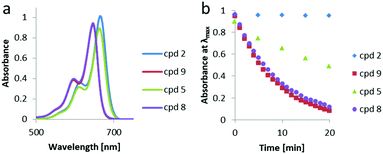
The photochemical properties of photocaged compounds 2, 5, 8 and 9 were then tested under standardized conditions (see ESI,† Fig. S1–S9). First, to determine if the compounds were indeed photoactive, we proceeded to measure their UV-vis spectra before and under irradiation with red LED (λmax = 652 nm). For most of studied compounds, a decrease in absorption was observed (Fig. 3b). As expected, ester 2 reacted slowly. Unfortunately, the same was observed for compound 5, which, even after installing the carbamate linker, needed around 1 h of irradiation to fully react under the standardized irradiation conditions. Additionally, both compounds 2 and 5 had fairly low solubility (limited to low μM range) in the used media (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of acetonitrile/aq. phosphate buffer) and had the most red-shifted λmax values of all the obtained compounds (Table 1).
1 mixture of acetonitrile/aq. phosphate buffer) and had the most red-shifted λmax values of all the obtained compounds (Table 1).
| Entry | Cpd no. | X | ε max × 10−3 [M−1 cm−1] | ε 650 × 10−3 [M−1 cm−1] | Half-lifea [min] | λ max [nm] |
|---|---|---|---|---|---|---|
| a Under irradiation with Sahlmann Photochemical Solutions LED system (3 × 400 mW, λmax = 652 nm, FWHM 26.4 nm from 0.2 cm distance), in 50% acetonitrile/5 mM phosphate buffer pH = 7.5. | ||||||
| 1 | 2 | F | 98 | 67 | — | 665 |
| 2 | 5 | F | 73 | 54 | 9.5 | 663 |
| 3 | 8 | CH3 | 47 | 45 | 2.7 | 646 |
| 4 | 9 | CH3 | 46 | 44 | 2.5 | 646 |
| 5 | 10 | CH3 | 63 | 61 | 1.6 | 643 |
Much better results were obtained for BODIPYs 8 and 9 with a B(CH3)2 group instead of BF2. Both compounds readily responded to irradiation with red light in about 20 min. The measured quantum yield for compound 8 under the irradiation with UV light (standard K3[Fe(C2O4)3] actinometry, λ = 365 nm, for details see ESI†) was 0.01%, at 20 °C. For a model carboxylic acid, caged with the same group, a roughly 10-times higher quantum yield was measured.22 Although the λmax of compounds 8 and 9 was slightly blue-shifted (646 vs. 663 nm) when compared to compounds 2 and 5 (Table 1), the CH3-substituted derivatives were reacting much faster and their solubility in the used media was relatively higher, making them far more promising PPGs for biological applications. While, compared to other PPGs for amines, these molecules show lower quantum yields, they do absorb light at longer wavelength. The solvent that was used in our system was, however, still not fully representative for a biological setting.
To assess the feasibility of the designed photoprotecting group in selective activation of drugs with light in biological systems, we synthesized compound 10 (Fig. 2), a BODIPY-protected dopamine derivative, and tested its light-dependent activity on spontaneously beating, hESC-derived cardiomyocytes as an in vitro model of a beating heart. Dopamine, aside from being a known neurotransmitter,29 plays a significant role in regulating cardiac function by increasing heart rate and contractile force.30 As patients using dopamine can suffer from severe adverse effects of the drug, ranging from mild nausea to anxiety and life-threatening arrhythmias,31 we envisioned that using a derivative of dopamine which could be selectively activated therapeutically at a chosen site, could not only help to control the patients’ cardiac function, but also limit the occurrence of the adverse effects of the original drug.
For this cause, the methodology of using PPGs to hinder the activity of the drug until it is needed seems to be an attractive approach. Even though synthetic routes of protecting dopamine with PPGs for neurological applications do exist,14,32 none of the reported compounds has been used to control cardiac rhythm with light within the phototherapeutic window (650–900 nm), which would enable safe biological use.
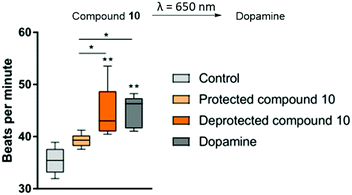
In our approach, the multi-electrode array (MEA) methodology was used for the determination of activity of compound 10 on spontaneously beating hESC derived cardiomyocytes before and after irradiation with red light (λ = 652 nm) by detecting the differences in the electrical signals that cause contractions of the cells. Therefore, we first prepared the hESC-derived cardiomyocytes in accordance with a method previously described.33 After 10 d of additional maintenance to assure syncytium formation, a baseline measurement for beating frequency was performed. Subsequently, pre-dilutions of PBS containing 10 μM compound 10 were irradiated with red light (λ = 652 nm) for 4 min periods. The cells were incubated for 20 min with 1 μM of compound 10, dopamine or 1 μM of deprotected compound 10 and their beating frequency was determined with the MEA (for the experimental details see ESI†). For each of the measurements, the data was collected for at least 30 s and compiled statistical data was analysed using the CiPA analysis tool (Axion Biosystems) and subjected to a one-way analysis of variance (ANOVA) followed by Tukey's multiple comparisons test (for exact values, please refer to the ESI†). We were able to observe significant differences in activity of irradiated and non-irradiated forms of compound 10 based on the beating frequency of the cardiomyocytes (Fig. 4). The activity of deprotected compound 10 was comparable to the activity of dopamine. Although the protected compound 10 still exhibited some residual activity, it was significantly lower when compared to both dopamine and deprotected compound 10.
In summary, we have presented the development of red-light-sensitive (λ = 652 nm, in the therapeutic window for light) photoprotecting groups for the amine functionality, which could be used in a biological setting for the control of bioactivity by light.
Fluorinated BODIPY derivatives 2 and 5 could be uncaged in aqueous media under red light irradiation in 10 to 20 min on average. The photodeprotection of methylated compounds 8, 9 and 10 occurred rapidly in aqueous media and the cargo molecules were released efficiently within a couple of minutes. These compounds proved to be superior to their fluorinated derivatives not only in terms of the deprotection efficiency, but also in terms of solubility in the used media.
To provide the proof of principle for the usefulness of our system in clinical applications, we employed compound 10, a BODIPY-protected dopamine derivative, on spontaneously beating hESC derived cardiomyocytes and observed a significant increase in their beating frequency after irradiating them for 1 min with red light (λ = 652 nm). These results prove that the developed photoprotecting groups can be successfully employed in a biological setting and offer future prospects in medical treatments.
We gratefully acknowledge the generous support from The Netherlands Organization for Scientific Research (NWO, VIDI grant no. 723.014.001 for W. S.), the Royal Netherlands Academy of Arts and Sciences Science (KNAW), the Ministry of Education, Culture and Science (Gravitation program 024.001.035), and the European Research Council (Advanced Investigator Grant, no. 694435 to B. L. F.).
Conflicts of interest
There are no conflicts to declare.Notes and references
- J. Hemphill, J. Govan, R. Uprety, M. Tsang and A. Deiters, J. Am. Chem. Soc., 2014, 136, 7152–7158 CrossRef CAS PubMed; J. Hemphill, E. K. Borchardt, K. Brown, A. Asokan and A. Deiters, J. Am. Chem. Soc., 2015, 137, 5642–5645 CrossRef PubMed; S. Pattanayak, L. A. Vázquez-Maldonado, A. Deiters and J. K. Chen, in Methods in Enzymology, ed. A. Deiters, Academic Press, 2019, vol. 624, pp. 69–88 CrossRef PubMed; W. A. Velema, A. M. Kietrys and E. T. Kool, J. Am. Chem. Soc., 2018, 140, 3491–3495 CrossRef PubMed.
- M. J. Hansen, F. M. Feringa, P. Kobauri, W. Szymanski, R. H. Medema and B. L. Feringa, J. Am. Chem. Soc., 2018, 140, 13136–13141 CrossRef CAS PubMed.
- R. Horbert, B. Pinchuk, P. Davies, D. Alessi and C. Peifer, ACS Chem. Biol., 2015, 10, 2099–2107 CrossRef CAS PubMed.
- W. A. Velema, J. P. van der Berg, W. Szymanski, A. J. M. Driessen and B. L. Feringa, Org. Biomol. Chem., 2015, 13, 1639–1642 RSC.
- M. Michael Dcona, Q. Yu, J. A. Capobianco and M. C. T. Hartman, Chem. Commun., 2015, 51, 8477–8479 RSC; L. F. Tietze, M. Müller, S.-C. Duefert, K. Schmuck and I. Schuberth, Chem. – Eur. J., 2013, 19, 1726–1731 CrossRef CAS PubMed.
- S. Passlick and G. C. R. Ellis-Davies, J. Neurosci. Methods, 2018, 293, 321–328 CrossRef CAS PubMed; S. Passlick, E. R. Thapaliya, Z. Chen, M. T. Richers and G. C. R. Ellis-Davies, J. Physiol., 2018, 596, 5307–5318 CrossRef PubMed; S. Passlick, P. F. Kramer, M. T. Richers, J. T. Williams and G. C. R. Ellis-Davies, PLoS One, 2017, 12, e0187732 CrossRef PubMed.
- T. Courtney and A. Deiters, Curr. Opin. Chem. Biol., 2018, 46, 99–107 CrossRef CAS PubMed; J. Liu, J. Hemphill, S. Samanta, M. Tsang and A. Deiters, J. Am. Chem. Soc., 2017, 139, 9100–9103 CrossRef PubMed.
- L. K. Shawver, D. Slamon and A. Ullrich, Cancer Cell, 2002, 1, 117–123 CrossRef CAS PubMed; S. J. Baker and E. P. Reddy, Mt. Sinai J. Med., 2010, 77, 573–586 CrossRef PubMed.
- P. Klán, T. Šolomek, C. G. Bochet, A. Blanc, R. Givens, M. Rubina, V. Popik, A. Kostikov and J. Wirz, Chem. Rev., 2013, 113, 119–191 CrossRef PubMed.
- J. Engels and E. J. Schlaeger, J. Med. Chem., 1977, 20, 907–911 CrossRef CAS PubMed.
- R. Weissleder and V. Ntziachristos, Nat. Med., 2003, 9, 123–128 CrossRef CAS PubMed.
- R. Richards-Kortum and E. Sevick-Muraca, Annu. Rev. Phys. Chem., 1996, 47, 555–606 CrossRef CAS PubMed.
- M. O. Axel Griesbeck and F. Ghetti, CRC Handbook of Organic Photochemistry and Photobiology, CRC Press, 3rd edn, 2012 Search PubMed.
- N. Rubinstein, P. Liu, E. W. Miller and R. Weinstain, Chem. Commun., 2015, 51, 6369–6372 RSC.
- I. Aujard, C. Benbrahim, M. Gouget, O. Ruel, J.-B. Baudin, P. Neveu and L. Jullien, Chem. – Eur. J., 2006, 12, 6865–6879 CrossRef CAS PubMed.
- M. Reinfelds, J. von Cosel, K. Falahati, C. Hamerla, T. Slanina, I. Burghardt and A. Heckel, Chem. – Eur. J., 2018, 24, 13026–13035 CrossRef CAS PubMed; Z. Guo, S. Park, J. Yoon and I. Shin, Chem. Soc. Rev., 2014, 43, 16–29 RSC.
- C. Bao, G. Fan, Q. Lin, B. Li, S. Cheng, Q. Huang and L. Zhu, Org. Lett., 2012, 14, 572–575 CrossRef CAS PubMed.
- L. Zayat, M. Salierno and R. Etchenique, Inorg. Chem., 2006, 45, 1728–1731 CrossRef CAS PubMed.
- T. Furuta, S. S.-H. Wang, J. L. Dantzker, T. M. Dore, W. J. Bybee, E. M. Callaway, W. Denk and R. Y. Tsien, Proc. Natl. Acad. Sci. U. S. A., 1999, 96, 1193–1200 CrossRef CAS PubMed.
- S. Piant, F. Bolze and A. Specht, Opt. Mater. Express, 2016, 6, 1679–1691 CrossRef CAS.
- A. P. Pelliccioli and J. Wirz, Photochem. Photobiol. Sci., 2002, 1, 441–458 RSC; K. Svoboda and R. Yasuda, Neuron, 2006, 50, 823–839 CrossRef CAS PubMed.
- J. A. Peterson, C. Wijesooriya, E. J. Gehrmann, K. M. Mahoney, P. P. Goswami, T. R. Albright, A. Syed, A. S. Dutton, E. A. Smith and A. H. Winter, J. Am. Chem. Soc., 2018, 140, 7343–7346 CrossRef CAS PubMed.
- A. H. Winter, C. L. Beck, K. M. Mahoney, T. R. Albright and P. P. Goswami, US Pat., US2016/228845, 2016 Search PubMed.
- P. P. Goswami, A. Syed, C. L. Beck, T. R. Albright, K. M. Mahoney, R. Unash, E. A. Smith and A. H. Winter, J. Am. Chem. Soc., 2015, 137, 3783–3786 CrossRef CAS PubMed.
- K. Sitkowska, B. L. Feringa and W. Szymański, J. Org. Chem., 2018, 83, 1819–1827 CrossRef CAS PubMed.
- T. Slanina, P. Shrestha, E. Palao, D. Kand, J. A. Peterson, A. S. Dutton, N. Rubinstein, R. Weinstain, A. H. Winter and P. Klán, J. Am. Chem. Soc., 2017, 139, 15168–15175 CrossRef CAS PubMed.
- D. De Backer, P. Biston, J. Devriendt, C. Madl, D. Chochrad, C. Aldecoa, A. Brasseur, P. Defrance, P. Gottignies and J.-L. Vincent, N. Engl. J. Med., 2010, 362, 779–789 CrossRef CAS PubMed.
- F. Ali, A. H. A, N. Taye, R. G. Gonnade, S. Chattopadhyay and A. Das, Chem. Commun., 2015, 51, 16932–16935 RSC.
- J. H. Ko and A. P. Strafella, Neuroscientist, 2011, 18, 149–168 CrossRef PubMed.
- V. Karajala, M. Raghavan, R. Venkataraman and J. A. Kellum, in Acute Heart Failure, ed. A. Mebazaa, M. Gheorghiade, F. M. Zannad and J. E. Parrillo, Springer London, London, 2008, pp. 577–582 DOI:10.1007/978-1-84628-782-4_53.
- J. E. Tisdale, R. Patel, C. R. Webb, S. Borzak and B. J. Zarowitz, Prog. Cardiovasc. Dis., 1995, 38, 167–180 CrossRef CAS PubMed.
- R. Araya, V. Andino-Pavlovsky, R. Yuste and R. Etchenique, ACS Chem. Neurosci., 2013, 4, 1163–1167 CrossRef CAS PubMed; T. H. Lee, K. R. Gee, E. H. Ellinwood and F. J. Seidler, J. Neurosci. Methods, 1996, 67, 221–231 Search PubMed.
- X. Lian, J. Zhang, S. M. Azarin, K. Zhu, L. B. Hazeltine, X. Bao, C. Hsiao, T. J. Kamp and S. P. Palecek, Nat. Protoc., 2012, 8, 162 CrossRef CAS PubMed; M. F. Hoes, N. Grote Beverborg, J. D. Kijlstra, J. Kuipers, D. W. Swinkels, B. N. G. Giepmans, R. J. Rodenburg, D. J. van Veldhuisen, R. A. de Boer and P. van der Meer, Eur. J. Heart Failure, 2018, 20, 910–919 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental, analytical and spectral data. See DOI: 10.1039/d0cc02178d |
| This journal is © The Royal Society of Chemistry 2020 |