Open Access Article
Open Access ArticleSmart lanthanide antennas for sensing water†
Francisco
Fueyo-González‡
a,
Emilio
Garcia-Fernandez
 b,
David
Martínez
b,
Lourdes
Infantes
b,
David
Martínez
b,
Lourdes
Infantes
 c,
Angel
Orte
c,
Angel
Orte
 b,
Juan A.
González-Vera
b,
Juan A.
González-Vera
 *ab and
Rosario
Herranz
*ab and
Rosario
Herranz
 *a
*a
aInstituto de Química Médica (CSIC), Juan de la Cierva 3, Madrid 28006, Spain. E-mail: rosario@iqm.csic.es
bDepartmento de Fisicoquímica, Unidad de Excelencia de Química aplicada a Biomedicina y Medioambiente, Facultad de Farmacia, Universidad de Granada, Campus Cartuja, Granada 18071, Spain. E-mail: gonzalezvera@ugr.es
cInstituto de Química Física Rocasolano, IQFR-CSIC, Serrano 119, Madrid 28006, Spain
First published on 21st April 2020
Abstract
Two new families of lanthanide antennas are described. 8-Methoxy-4,5-dihydrocyclopenta[de]quinolin-2(1H)-one phosphonates or carboxylates behave as selective antennas exhibiting Eu3+ luminescence in organic solvents, while quinolin-2(1H)-one analogues selectively sensitize the Tb3+ emission. These emissions are quenched by H2O addition. Based on this behaviour, the new lanthanide antennas can be used as highly sensitive water sensors.
Water is the most common impurity in organic solvents and its detection is important in diverse industrial processes, such as pharmaceutical and petrochemical manufacturing and food processing, and in the fine chemical industry.1–4 Classic and current industrial methods for the determination of H2O content are based on electrochemical and electrophysical sensing mechanisms, but optical sensors are emerging as sensitive, faster, and robust humidity sensors, in particular luminescent ones.5 The emission characteristics of lanthanide cations (Ln3+) are particularly interesting for sensor development, because they emit narrow and element specific bands, not perturbed by the environment, due to f–f electronic transitions, which are generally Laporte forbidden.6 This forbidden character is responsible for the low lanthanide absorbance and their long luminescence lifetimes that result in interesting and useful applications in time-resolved luminescence techniques. The low absorbance of Ln3+ can be overcome by indirect excitation through organic chromophores, which transfer energy to these cations by an “antenna effect”.7,8 Interestingly, the luminescence of lanthanide cations is very sensitive to quenching by water molecules.9,10 This effect can be exploited to develop water sensors, paving the way for a new application of lanthanide probes, since reports on the use of this strategy to design water sensors have been very scarce.2,11–14
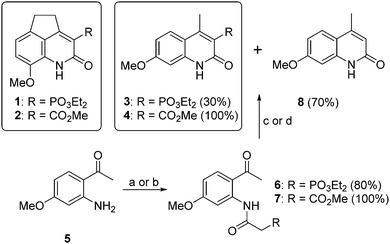
Even though β-dicarbonyl compounds are among some of the most studied ligands for lanthanides and quinolin-2-ones are among the most widely used sensitizing antennas,15–20 up to date, no quinolin-2(1H)-one-derived antennas capable of assembling directly with lanthanide ions in solution have been described. In general, antennas that directly assemble lanthanide cations are bulky multidentate ligands able to coordinate the cations with a high coordination number.7,8,17,21,22 However, herein we explore the potential of small-size quinolin-2(1H)-one derivatives with β-dicarbonyl character, concretely, 8-methoxy-4,5-dihydrocyclopenta[de]quinolin-2(1H)-ones 1 and 223 and their carbostyril analogues 3 and 4 (Chart 1) as self-assembled lanthanide sensitizers. Importantly, such complexes exhibit an extraordinary ability to sense H2O impurities in organic solvents. Analogues 3 and 4 were specifically designed taking into account the fact that substituent positions affect their absorption spectra, and hence the lanthanide sensitizing efficiency. Specifically, previous studies on lanthanide antennas derived from quinolin-2(1H)-ones (carbostyrils) have shown that the MeO substituent at position 7 gave the highest molar absorption coefficient (ε).18,24 These quinolin-2(1H)-ones were synthesized from the commercial 2-amino-4-methoxy-acetophenone (5), by acylation with diethyl phosphonoacetic acid for 3 or methyl 3-chloro-3-oxopropionate for 4, followed by base promoted cyclization in the presence of NaH or K2CO3 (Chart 1). It is interesting to note that in the cyclization of the intermediate carboxamide 6 in the presence of NaH the phosphonate 3 was obtained as a minor compound (30%), along with 8 (70%), which resulted from the removal of diethyl hydrogen phosphite. However, the carboxylate analogue 4 was obtained quantitatively.
The UV absorption and emission parameters of compounds 1–4 were determined in solvents of increasing polarity from toluene to H2O and are summarized in Table S1 (ESI†). The four compounds showed two absorption maxima with just slight changes in energy and molar absorptivity values. The dihydrocyclopenta[de]quinolin-2(1H)-one derivatives 1 and 2 emitted at longer wavelengths (30–100 nm) than 3 and 4 and their emission maxima were bathochromically shifted with increasing solvent polarity (≈40 nm from toluene to H2O). Interestingly, the fluorescence quantum yields (ΦF) of the four compounds significantly increased with solvent polarity.
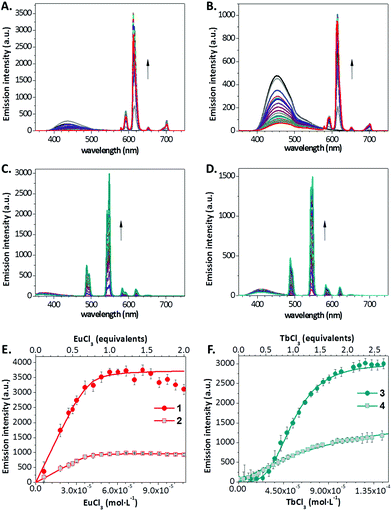
Then, we tested the ability of compounds 1–4 to directly bind lanthanide ions in solution, sensitizing their emission. First we carried out a qualitative evaluation by observing the emission of 1–4 (0.1 mM) in CH3CN upon addition of TbCl3, EuCl3, DyCl3 or SmCl3 (0.1 mM) on a UV transilluminator (λex = 365 nm, Fig. 1), and then we carried out a quantitative analysis in a spectrofluorometer. Regarding compounds 1 and 2, we collected emission spectra of these dyes (54 μM) dissolved in CH3CN after the addition of 1 and 2 equivalents of TbCl3, EuCl3, DyCl3 and SmCl3 (ESI,† Fig. S1 and S2). The addition of EuCl3 led to the appearance of significant bands of sensitized luminescence of the lanthanide cation, mainly the 5D0 → 7F2 Eu3+ band at 612 nm (Fig. 2A and B). We also performed a more systematic titration of solutions of 1 and 2 in CH3CN with increasing concentrations of EuCl3, showing, in both cases, that luminescence saturation is achieved for amounts of europium close to 1 equivalent (Fig. 2E). Besides, experimental data fit satisfactorily to a binding model considering the possibility of single or multiple binding events (see the ESI†). The fittings provided values for the apparent binding constants of 1.1 × 106 and 3.1 × 106 for the complexes formed with compounds 1 and 2, respectively. Interestingly, stoichiometries between 1 and 2 molecules of antenna per lanthanide ion are obtained for both complexes, indicating that a mixture of complexes with different stoichiometries may coexist in solution. In this regard, a double emission band (612 and 615 nm) was detected, whose relative intensity varied with the antenna:EuCl3 ratio, pointing out probably to the diverse contribution of the different complexes (Fig. S3, ESI†). The absolute luminescence quantum yields of the respective Eu3+ complexes of 1 and 2, measured with an integrating sphere, were 0.24 and 0.08, respectively. With the addition of EuCl3, the emission of the antenna decreased, due to energy transfer toward the cation, with the concomitant increase in the Eu3+ emission. The luminescence lifetimes (τ) of the Eu3+ emission for the complexes with 1 and 2 were 0.55 ± 0.04 and 0.24 ± 0.01 ms, respectively.
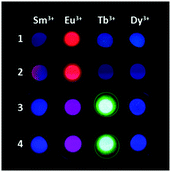 | ||
| Fig. 1 Luminescence of antennas 1–4 (0.54 μM) in CH3CN after addition of TbCl3, EuCl3, DyCl3 or SmCl3 (0.1 mM), upon UV irradiation on a transilluminator (λex = 365 nm). | ||
In the titration of the carbostyril derivatives 3 and 4 (54 μM) in CH3CN with TbCl3, EuCl3, DyCl3 and SmCl3, significant energy transfer was only observed for the Tb3+ cation, which was observed mainly in its 5D4 → 7F6 (490 nm) and 5D4 → 7F5 (540–550 nm) bands (Fig. 2C and D and Fig. S4 and S5, ESI†). The absolute luminescence quantum yields of the Tb3+ complexes of 3 and 4 were 0.21 and 0.08, respectively. The titration study for compounds 3 and 4 with Tb3+ showed that luminescence saturation is achieved progressively just after the addition of, at least, 2 equivalents of TbCl3 (Fig. 2F). Titrations with compounds 3 and 4 led to much lower binding constants, down to two orders of magnitude decreased when compared to those of the 1–2 antennas with Eu3+, indicating weak stability for these complexes, and poorer fit to the proposed binding model. Besides, the presence of a lag phase upon the increase of luminescence, particularly noticeable for compound 4, points out to the existence of supramolecular cooperative associations, and indeed, these curves better fitted to a positive cooperativity binding model (see the ESI†). Interestingly, in the case of these Tb3+ antennas, no significant influence of the binding equilibrium was observed on the relative intensity of the two bands at 540–550 nm (Fig. S6, ESI†) or on the lifetime of the Tb3+ luminescence. The luminescence lifetimes (τ) of the Tb3+ emission for the complexes with 3 and 4 were 1.58 ± 0.07 and 1.28 ± 0.06 ms, respectively.
To obtain additional information on the stoichiometry of the coordination of the antennas 1–4 with Eu3+ and Tb3+, we tried the crystallization of their respective complexes. We obtained good crystals for X-ray analysis of the dihydrocyclopenta[de]quinolin-2(1H)-ones 1 and 2 and their complexes with the Eu(OTf)3 salt.25 However, we could not obtain good crystals from the complexes of carbostyril derivatives 3 and 4 with Tb3+. The crystal and molecular structures of complexes Eu(1)2(H2O)4 and Eu(2)2(H2O)4OTf showed a (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) antenna:Eu3+ stoichiometry (Fig. 3, Fig. S11 and S12, ESI†). In the complex of phosphonate Eu(1)2(H2O)4, the cation presented a coordination number of 8, where Eu3+ binds two molecules of 1, through their 2-oxo group and the phosphonate oxygen, and four molecules of H2O. The two molecules of 1 are situated with their molecular planes forming an angle of 50°, on an opposite side to that of the H2O molecules. In the complex of carboxylate Eu(2)2(H2O)4OTf, the Eu3+ cation has a coordination number of 9, with the participation of two molecules of ligand 2, four molecules of H2O and one molecule of triflate. The molecules of 2 are orthogonal with their molecular planes forming an angle of 80° and situated with an opposite orientation. The stoichiometry of these complexes is in good agreement with that obtained in the fittings of the titration curves. Given that the dissociation equilibrium constants lie within the same order of magnitude of the concentration of the antenna and cation used in the titrations, the presence of sub-saturated species (1
1) antenna:Eu3+ stoichiometry (Fig. 3, Fig. S11 and S12, ESI†). In the complex of phosphonate Eu(1)2(H2O)4, the cation presented a coordination number of 8, where Eu3+ binds two molecules of 1, through their 2-oxo group and the phosphonate oxygen, and four molecules of H2O. The two molecules of 1 are situated with their molecular planes forming an angle of 50°, on an opposite side to that of the H2O molecules. In the complex of carboxylate Eu(2)2(H2O)4OTf, the Eu3+ cation has a coordination number of 9, with the participation of two molecules of ligand 2, four molecules of H2O and one molecule of triflate. The molecules of 2 are orthogonal with their molecular planes forming an angle of 80° and situated with an opposite orientation. The stoichiometry of these complexes is in good agreement with that obtained in the fittings of the titration curves. Given that the dissociation equilibrium constants lie within the same order of magnitude of the concentration of the antenna and cation used in the titrations, the presence of sub-saturated species (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry) is a reasonable result.
1 stoichiometry) is a reasonable result.
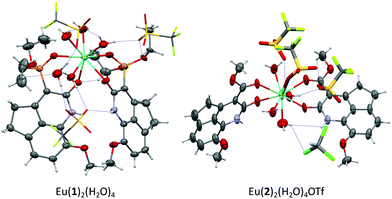 | ||
| Fig. 3 X-ray structures of the Eu3+ complexes with the 8-methoxy-4,5-dihydrocyclopenta[de]quinolin-2(1H)-ones 1 and 2. | ||
In general, the luminescence emission of lanthanide complexes is extremely sensitive to the presence of water or alcohols.9,10,26 While in aprotic organic solvents, the dihydrocyclopenta[de]quinolin-2(1H)-ones 1 and 2 are capable of sensitizing Eu3+ luminescence and the carbostyril analogues 3 and 4 that of Tb3+, in polar, acidic solvents, such as H2O, MeOH or EtOH, these compounds did not sensitize the lanthanide emission, but maintained their own intrinsic fluorescence emission. This quenching of the luminescence emission is due to the OH vibrations of solvent molecules bound to the central metal. This was confirmed by the large isotope dependency,26 as evidenced comparing the emission spectra of the Eu3+ complex of 1 in MeOH and MeOD (Fig. S8, ESI†). According to Horrocks’ equation (see the ESI†), the solvation parameter calculated for the europium complex of 1 (q = 2.8) revealed the presence of three coordinated methanol molecules (Fig. S9, ESI†). This result is in close agreement with the crystallographic results for water molecules, taking into account the larger volume of methanol molecules.
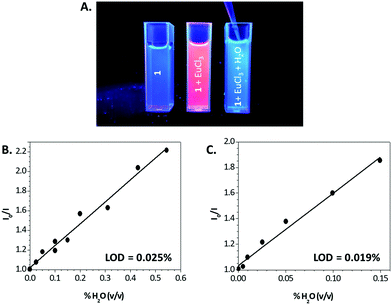
Interestingly, the addition of H2O or MeOH traces (<1%) to 0.1 mM solutions of the Eu3+ complexes of 1 or 2 dissolved in CH3CN turned off the characteristic red luminescence of the cation, as exemplarily shown in Fig. 4A. This suggested the potential of these new lanthanide antennas and complexes as H2O sensors. To evaluate this potential, we carried out the titrations of 54 μM solutions of the Eu3+ complex of 1 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 stoichiometry) and the Tb3+ complex of 3 (1
2 stoichiometry) and the Tb3+ complex of 3 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry) in CH3CN with increasing amounts of H2O (0–2% v/v). In both cases, the lanthanide luminescence started to decrease with the addition of 0.005% of H2O and completely disappeared with 2% of water. These results fit quite well with the Stern–Volmer equation for quenching (Fig. 4B and C). Simultaneously with the quenching in the lanthanide luminescence emission intensity, the lifetime decreased from 0.49 to 0.14 ms for the Eu3+ complex of 1 and from 1.6 to 0.51 ms for the Tb3+ complex of 3 (Fig. S10, ESI†). The linear regressions shown in Fig. 4 may be used as calibrations for the determination of water content. The limit of detection values [defined as LOD = (3.3 × SD)/KSV,27 where KSV is the Stern–Volmer constant] were 0.025% for the Eu3+ complex of 1 and 0.019% for the Tb3+ complex of 3. These values are comparable to those of the most sensitive water sensors described up to date (in the range of 0.002–0.2% for CH3CN),2,28 including some lanthanide complexes and metal–organic frameworks (MOFs, in the range of 0.003–0.05%).12,29,30
1 stoichiometry) in CH3CN with increasing amounts of H2O (0–2% v/v). In both cases, the lanthanide luminescence started to decrease with the addition of 0.005% of H2O and completely disappeared with 2% of water. These results fit quite well with the Stern–Volmer equation for quenching (Fig. 4B and C). Simultaneously with the quenching in the lanthanide luminescence emission intensity, the lifetime decreased from 0.49 to 0.14 ms for the Eu3+ complex of 1 and from 1.6 to 0.51 ms for the Tb3+ complex of 3 (Fig. S10, ESI†). The linear regressions shown in Fig. 4 may be used as calibrations for the determination of water content. The limit of detection values [defined as LOD = (3.3 × SD)/KSV,27 where KSV is the Stern–Volmer constant] were 0.025% for the Eu3+ complex of 1 and 0.019% for the Tb3+ complex of 3. These values are comparable to those of the most sensitive water sensors described up to date (in the range of 0.002–0.2% for CH3CN),2,28 including some lanthanide complexes and metal–organic frameworks (MOFs, in the range of 0.003–0.05%).12,29,30
In conclusion, we have presented herein two new families of lanthanide antennas that, interestingly, are among the smallest lanthanide antennas able to coordinate these cations, sensitizing their luminescence. The first type of antenna is based on 4,5-dihydrocyclopenta[de]quinolin-2(1H)-one phosphonates or carboxylates and selectively sensitizes the Eu3+ red luminescence, while the second type, based on carbostyril phosphonate or carboxylate derivatives, sensitizes the Tb3+ green emission. Moreover, complexes of these antennas with their respective lanthanides have resulted in highly sensitive sensors for sensing traces of H2O in organic solvents. Studies on the application of these sensors to determine water content in diverse food industry products are in progress and will be shortly published elsewhere. The ability of our antennas to efficiently sensitize lanthanide luminescence together with their small size will serve as a source of inspiration for the development of a variety of sensors for the study of complex biological systems.
This work was supported by the Spanish Ministerio de Economía y Competividad grants SAF2012-32209, FU2015-67284-R, the Ministerio de Ciencia e Innovación/Agencia Estatal de Investigación/European Regional Development Fund grants CTQ2017-85658-R, CTQ2015-63997-C2, and the CSIC grant 201580E073. X-ray experiments were performed at the BL13 beamline at ALBA Synchrotron with the collaboration of ALBA staff.
Conflicts of interest
There are no conflicts to declare.Notes and references
- H. Farahani, R. Wagiran and M. N. Hamidon, Sensors, 2014, 14, 7881–7939 CrossRef PubMed.
- H. S. Jung, P. Verwilst, W. Y. Kim and J. S. Kim, Chem. Soc. Rev., 2016, 45, 1242–1256 RSC.
- R. Nussbaum, D. Lischke, H. Paxmann and B. Wolf, Chromatographia, 2000, 51, 119–121 CrossRef CAS.
- E. D. Tsamis and J. N. Avaritsiotis, Sens. Actuators, A, 2005, 118, 202–211 CrossRef CAS.
- G. Korotcenkov, Handbook of Humidity Measurement, Volume 1: Spectroscopic Methods of Humidity Measurement, CRC Press, 2018 Search PubMed.
- M. H. V. Werts, Sci. Prog., 2005, 88, 101–131 CrossRef CAS PubMed.
- A. Thibon and V. C. Pierre, Anal. Bioanal. Chem., 2009, 394, 107–120 CrossRef CAS PubMed.
- S. V. Eliseeva and J.-C. G. Bunzli, Chem. Soc. Rev., 2010, 39, 189–227 RSC.
- Y. Haas and G. Stein, J. Phys. Chem., 1971, 75, 3668–3677 CrossRef CAS.
- Y. Haas and G. Stein, J. Phys. Chem., 1971, 75, 3677–3681 CrossRef CAS.
- F. Gao, F. Luo, X. Chen, W. Yao, J. Yin, Z. Yao and L. Wang, Microchim. Acta, 2009, 166, 163–167 CrossRef CAS.
- L. Song, Y.-W. Wu, W.-X. Chai, Y.-S. Tao, C. Jiang and Q.-H. Wang, Eur. J. Inorg. Chem., 2015, 2264–2271 CrossRef CAS.
- Y. Gao, P. Jing, N. Yan, M. Hilbers, H. Zhang, G. Rothenberg and S. Tanase, Chem. Commun., 2017, 53, 4465–4468 RSC.
- S. G. Dunning, A. J. Nuñez, M. D. Moore, A. Steiner, V. M. Lynch, J. L. Sessler, B. J. Holliday and S. M. Humphrey, Chem, 2017, 2, 579–589 CAS.
- D. Kovacs and K. E. Borbas, Coord. Chem. Rev., 2018, 364, 1–9 CrossRef CAS.
- K. Binnemans, Chem. Rev., 2009, 109, 4283–4374 CrossRef CAS PubMed.
- M. C. Heffern, L. M. Matosziuk and T. J. Meade, Chem. Rev., 2014, 114, 4496–4539 CrossRef CAS PubMed.
- M. S. Tremblay, M. Halim and D. Sames, J. Am. Chem. Soc., 2007, 129, 7570–7577 CrossRef CAS PubMed.
- D. Kovacs, D. Phipps, A. Orthaber and K. E. Borbas, Dalton Trans., 2018, 47, 10702–10714 RSC.
- D. Kovacs, X. Lu, L. S. Mészáros, M. Ott, J. Andres and K. E. Borbas, J. Am. Chem. Soc., 2017, 139, 5756–5767 CrossRef CAS PubMed.
- J. Lehr, P. D. Beer, S. Faulkner and J. J. Davis, Chem. Commun., 2014, 50, 5678–5687 RSC.
- L. Armelao, S. Quici, F. Barigelletti, G. Accorsi, G. Bottaro, M. Cavazzini and E. Tondello, Coord. Chem. Rev., 2010, 254, 487–505 CrossRef CAS.
- J. A. González-Vera, F. Fueyo-González, I. Alkorta, M. Peyressatre, M. C. Morris and R. Herranz, Chem. Commun., 2016, 52, 9652–9655 RSC.
- W. M. F. Fabian, K. S. Niederreiter, G. Uray and W. Stadlbauer, J. Mol. Struct., 1999, 477, 209–220 CrossRef CAS.
- CCDC numbers 1980627–1980630† contains the supplementary crystallographic data for compounds 2, Eu(2)2(H2O)4OTf, 1, and Eu(1)2(H2O)4 respectively.
- G. Stein and E. Wurzberg, J. Chem. Phys., 1975, 62, 208–213 CrossRef CAS.
- J. R. Lakowicz, Principles of Fluorescence Spectroscopy, Springer, New York, 3rd edn, 2006 Search PubMed.
- P. Kumar, R. Kaushik, A. Ghosh and D. A. Jose, Anal. Chem., 2016, 88, 11314–11318 CrossRef CAS PubMed.
- A. Douvali, A. C. Tsipis, S. V. Eliseeva, S. Petoud, G. S. Papaefstathiou, C. D. Malliakas, I. Papadas, G. S. Armatas, I. Margiolaki, M. G. Kanatzidis, T. Lazarides and M. J. Manos, Angew. Chem., Int. Ed., 2015, 54, 1651–1656 CrossRef CAS PubMed.
- B. Li, W. Wang, Z. Hong, E.-S. M. El-Sayed and D. Yuan, Chem. Commun., 2019, 55, 6926–6929 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedures; photophysical and X-ray diffraction methods; and NMR spectra. CCDC 1980627–1980630. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0cc01725f |
| ‡ Current address: Department of Medicine, Translational Transplant Research Center, Immunology Institute, Icahn School of Medicine at Mount Sinai, New York, USA. |
| This journal is © The Royal Society of Chemistry 2020 |