Open Access Article
Open Access ArticleMulticolour fluorescent “sulfide–sulfone” diarylethenes with high photo-fatigue resistance†
Kakishi
Uno‡
 a,
Mariano L.
Bossi‡
a,
Mariano L.
Bossi‡
 *b,
Vladimir N.
Belov
*b,
Vladimir N.
Belov
 *a,
Masahiro
Irie
*a,
Masahiro
Irie
 c and
Stefan W.
Hell
c and
Stefan W.
Hell
 a
a
aMax Planck Institute for Biophysical Chemistry (MPI BPC), Am Fassberg 11, 37077 Göttingen, Germany. E-mail: vladimir.belov@mpibpc.mpg.de
bDepartment of Optical Nanoscopy Max Planck Institute for Medical Research, Jahnstrasse 29, 69120 Heidelberg, Germany. E-mail: mariano.bossi@mr.mpg.de
cResearch Center for Smart Molecules, Rikkyo University, Tokyo, Japan. E-mail: iriem@rikkyo.ac.jp
First published on 24th January 2020
Abstract
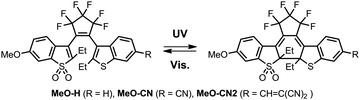
Compact “push–pull” photochromic diaryethenes (DAEs) with unsymmetric oxidation pattern of the benzothiophene core display multicolour fluorescence switching, as a result of dual emission from both “open” and “closed” forms. These DAEs also present an unprecedented photo-fatigue resistance.
Photochromic compounds have isomeric states interconvertible by alternate irradiation with ultraviolet (UV) and visible light via a shared (singlet) excited state.1 They attract great attention in life and materials science.2 The optical properties of two distinct structures are drastically different, and the switching is controllable by changing the irradiation wavelengths. Due to the high sensitivity of fluorescence, as the state-reporting signal, optical systems with fluorescent photochromic units are particularly promising.3 The photoswitching of fluorescence is promising for molecular memories,4 bio-imaging5 and, in particular, as a tool for cutting-edge nanoscopy techniques.6
Due to thermal stability of the two isomers and efficient photo-conversion, diarylethenes (DAEs) have emerged as a versatile platform for constructing fluorescent photochromic compounds.7 Current fluorescent DAEs can be divided into two categories: Turn-Off3,4,8 and Turn-On modes.9 In the former case, DAE is connected with an additional fluorophore (forming a dyad), and the mechanism of fluorescence modulation is based on energy/electron transfer, usually to the closed form. This molecular design is versatile and applicable to various types of fluorophores. The latter case relies on the intrinsic fluorescence of the photogenerated closed-ring isomers9 of the highly fluorescent 1,2-[bis-(2-alkyl-1-benzothiophene-1,1-dioxide-6-aryl-3-yl)]perfluorocyclopentenes.10 Another intriguing category is dual fluorescence systems. Although dual-emissive assemblies combining more than two fluorescent components (e.g. a fluorescent dye, a fluorescent diarylethene, and fluorescent dots) have been reported,11 photochromic compounds that exhibit fluorescence in both isomers are still very rare.12 Due to simple chemistry which is favourable for labelling biomolecules, the compact molecular switches have advantages over complex multicomponent hybrid materials.
Here, we report a series of unique push–pull fluorescent DAEs with unsymmetric oxidation pattern in the benzothiophene core (MeO-H, MeO-CN, MeO-CN2) that not only exhibit reversible blue-to-red multicolour fluorescence switching, but also undergo over 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles (in acetonitrile) without exclusion of air oxygen (Scheme 1).
000 cycles (in acetonitrile) without exclusion of air oxygen (Scheme 1).
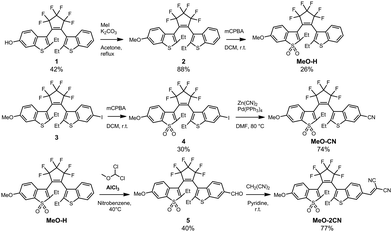
In brief, MeO-H was synthesized via methylation of compound 113 followed by selective oxidation of the π-electron-rich benzothiophene unit bearing methoxy group. MeO-CN was obtained by selective oxidation of compound 314 with mCPBA followed by transformation of aromatic iodide into cyanide using Zn(CN)2/Pd(PPh3)4. Dicyanovinyl substituted diarylethene (MeO-CN2) was prepared by formylation of unoxidized DAE MeO-H followed by condensation with malonodinitrile (Scheme 2).13
To study the photophysical properties, we first measured photoinduced UV-Vis spectral changes of MeO-H, MeO-CN, and MeO-CN2. Upon irradiation with UV light (365 nm) in toluene, the colourless solutions of all compounds turned reddish. The absorption maxima (maxλOF) of the open-ring forms of MeO-H, MeO-CN, and MeO-CN2 were observed at 338 nm, and the corresponding absorption maxima of the closed-ring forms (maxλCF) were 514 nm, 509 nm, and 540 nm respectively (Fig. S8, ESI†). The photocyclization quantum yields (Φ(OF→CF)) of MeO-H and MeO-CN were 0.50 and 0.44; similar to the values reported for analogs.15,16 The value of Φ(OF→CF) found for MeO-CN2 was one order of magnitude smaller. Upon exposure to visible light (505 nm), the coloured solutions (containing mixtures of both forms) were fully converted to colourless open forms. The photo cycloreversion quantum yields (Φ(CF→OF)) in toluene for MeO-H, MeO-CN, and MeO-CN2 were found to be 0.02, 0.18 and 0.06, which are several orders of magnitude higher than the ring-opening efficiencies reported for another red-emissive fluorescent DAEs.17 In all cases, the values of the isomerization quantum yields measured in acetonitrile and toluene were almost identical. Therefore, the photoconversion degree of DAEs with unsymmetric oxidation patterns is scarcely influenced by the solvent polarity.
The photoinduced changes in the emission spectra in toluene and acetonitrile solutions and the main photophysical data are given in Fig. 1 and Table 1. In contrast to “oxidized” fluorescent DAEs9,10,14 with a high ratio of isomerization quantum yields (Φ(OF→CF)/Φ(CF→OF) > 100), now the photostationary states (PSS365nm) contain mixtures of both isomers in comparable amounts. Thus, the optical properties of the UV-irradiated solutions depend on absorption/emission spectra of both isomers, the degree of conversion, and the excitation wavelength.
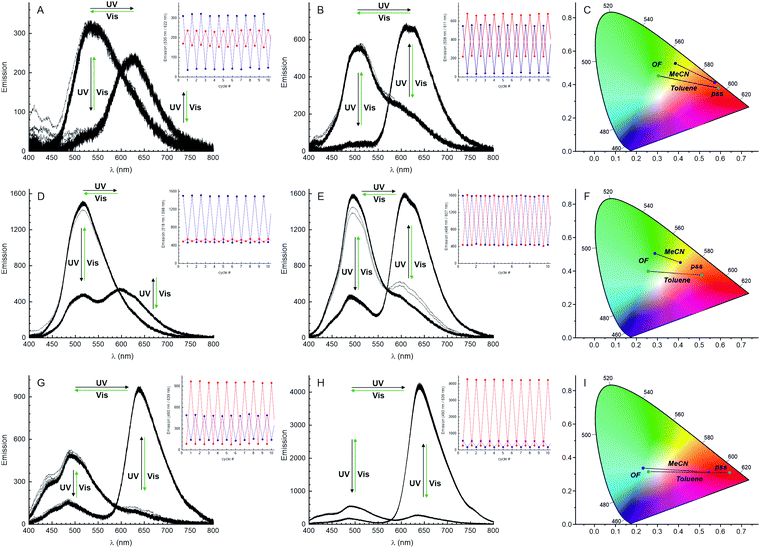 | ||
| Fig. 1 Emission changes observed for DAEs MeO-H (A–C), MeO-CN (D–F), and MeO-CN2 (G–I) for 10 full cycles of irradiation (365 nm/505 nm), in acetonitrile (A, D, G) and toluene solutions (B, E, H). Light of 365 nm ensures excitation of both isomers. The emission changes at the maxima of each isomer are shown in the insets. The emission colours of both states (PSS-365 and PSS-505) are shown in the CIE chromaticity space (400–800 nm range); see also Fig. S9 and S10 (ESI†). | ||
| ab λ max [nm]/ε × 10−3 [M−1cm−1] | fl λ max [nm]/Φfl [%] | ab λ max[nm]/ε × 10−3 [M−1 cm−1] | fl λ max [nm]/Φflb [%] | Φ OF→CF (366 nm) | Φ CF→OF (470 nm) | αPSS (365 nm) | Fatigue resistance [N½] | |
|---|---|---|---|---|---|---|---|---|
| Open form | Closed form | |||||||
| MeO-H | 338/4365 | 535/0.5 | 514/12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 657 657 |
622/0.4 | 0.50 | 0.02 | 0.89 | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
| MeO-CN | 338/4002 | 518/1.9 | 509/12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 018 018 |
598/3.1 | 0.44 | 0.18 | 0.42 | 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
| MeO-CN2 | 338/25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 615 615 |
490/0.1 | 540/19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 844 844 |
639/6.6 | 0.03 | 0.06 | 0.62 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
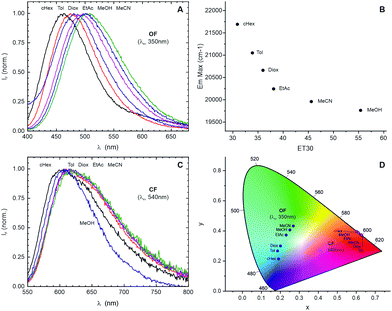
We next studied the multicolour fluorescence properties of MeO-H, MeO-CN, and MeO-CN2. Irradiation of the solutions of MeO-H and MeO-CN in toluene with UV light (365 nm) rapidly changed the initial blue emission to red (Table 1 and TOC graph). The emission maximum (flλmax) and the fluorescence quantum yield (Φfl) of the open form MeO-H were found to be 535 nm and 0.5%, and flλmax and Φfl of the closed form – 622 nm and 0.4%, respectively. To our surprise, the presence of an electron withdrawing cyano group in compound MeO-CN increased the values of Φfl of both open and closed forms to 2% and 3%, respectively. The dyes presented here have very large Stokes shifts (especially open forms). Remarkably, the introduction of an electron acceptor dicyanovinyl group (MeO-CN2) further increased the Φfl of the closed ring form to 6.6%, though it decreased the Φfl of the open form to 0.1%. To evaluate the solvent polarity effect on fluorescent properties, we determined Φfl values and recorded the fluorescence spectra of open- and closed-ring isomers in acetonitrile. Although the emission in acetonitrile was weaker than the emission in toluene, the multicolour fluorescence response was clearly observed. The plots in the CIE chromaticity space show reversible multicolour fluorescence changes of the three compounds in toluene and acetonitrile (Fig. 1C, F and I). The reversible transitions were induced by irradiation with UV and visible light. Furthermore, a distinct difference in the colour transition path in the CIE diagram was observed when solvents with different polarity were used (acetonitrile and toluene).
To explore the effect of solvent polarity on fluorescence properties, we selected MeO-CN for further studies, due to its Φfl values comparable in the open and closed forms. The emission band of the open-ring isomer exhibited red-shift with increasing solvent polarity, which indicates the push–pull electronic effects in this isomer (Fig. 2A and B).18 The emission band of the closed-ring form underwent only a slight red-shift, even in highly polar solvents (methanol and acetonitrile; Fig. 2C). Thus, the overall push–pull interactions in the closed form are weaker than in the open form.
Based on the recorded fluorescence spectra of MeO-CN in various solvents, we plotted CIE diagram describing the colour perception of a human eye (Fig. 2D). The solvatochromism of the open form of MeO-CN combined with its photoinduced multicolour fluorescence switching enabled the control of emission colour in a wide chromaticity range (Fig. 2D).
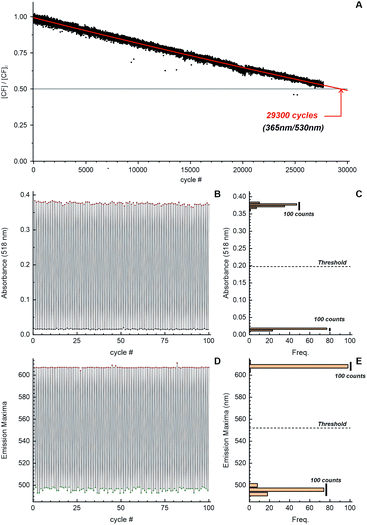
Using the automated optical measurement system,14 we evaluated the photo-fatigue resistance (“cycling number” N½) of the compounds in acetonitrile (Fig. 3A and Table 1). For cyclization reactions, irradiation with UV (365 nm) light was continued until the photostationary state (PSS) was reached, which was monitored by an increase in absorption at 518 nm. Then the samples were irradiated with visible light (505 nm), until cycloreversion reactions were fully complete (see Fig. S11–S13 in ESI†). In addition to the standard observation of the colour changes by monitoring the absorption of the CFs (Fig. 3B and C), we also tracked the fluorescence changes (Fig. 3D and E) with UV excitation of both isomers in acetonitrile (Fig. S14–S19, ESI†) and toluene solutions (Fig. S20–S22, ESI†). After one of the photostationary states of the system was reached (i.e. under irradiation with UV or visible light), the emission maximum (wavelength at the maximum of the detected signal) was automatically searched. A plot of such calculated emission maxima flλmaxvs. the number of cycles, along with the histograms of the total obtained values, demonstrates the value of this parameter as an efficient state assignment of the system. An assignment of the system state to the closed or open form was based on absorption (Fig. 3C) or emission maxima (Fig. 3D). For the first 100 cycles, it showed no errors; the threshold was arbitrarily set at the middle point between the two local maximum bins of the corresponding histograms (Fig. 3C and E). Remarkably, the cycling number of MeO-CN exceeded 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (50% bleaching, relative to the initial absorbance). The fatigue resistance of MeO-H is on the same order (>23
000 (50% bleaching, relative to the initial absorbance). The fatigue resistance of MeO-H is on the same order (>23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles), while compound MeO-CN2 survived over 14
000 cycles), while compound MeO-CN2 survived over 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 switching cycles. To our knowledge, this is the first report on photochromic fluorescent compounds that endure more than 10
000 switching cycles. To our knowledge, this is the first report on photochromic fluorescent compounds that endure more than 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 switching cycles.
000 switching cycles.
In summary, we introduced three photochromic diaryethenes with unsymmetrically oxidized cores, exhibiting photoswitchable emission signal, with a blue-to-red multicolour fluorescence modulation. Diarylethenes with a distinctive dual fluorescence switching capacities were recently reported by Yokoyama et al.12 Compound MeO-CN presented here is advantageous in terms of its improved fluorescence quantum yield in both open/closed ring isomers, efficient photocyclization/cycloreversion quantum yields (faster switches), and the compact structure. In addition, it exhibited an outstanding photofatigue resistance, which is essential for all practical applications. While several fluorescent diarylethenes are reported to endure several thousands of photocycles, such highly photostable fluorescent DAEs, as MeO-H, MeO-CN and MeO-CN2 are still rare.14,19 We believe that our work will contribute to the design and facile synthesis of diarylethenes with unsymmetric oxidation patterns and unusual photophysical properties interesting for the optical superresolution techniques.
The authors are grateful to J. Bienert (Facility for Synthetic chemistry, MPI BPC) for measuring NMR and mass-spectra. Open Access funding provided by the Max Planck Society.
Conflicts of interest
There are no conflicts to declare.Notes and references
- New Frontiers in Photochromism, ed. M. Irie, Y. Yokoyama and T. Seki, Springer, Tokyo, 2013 Search PubMed.
- (a) A. A. Beharry and G. A. Woolley, Chem. Soc. Rev., 2011, 40, 4422–4437 RSC; (b) C. Falenczyk, M. Schiedel, B. Karaman, T. Rumpf, N. Kuzmanovic, M. Grøtli, W. Sippl, M. Jung and B. König, Chem. Sci., 2014, 5, 4794–4799 RSC; (c) L. Wang and Q. Li, Chem. Soc. Rev., 2018, 47, 1044–1097 RSC; (d) T. Fukaminato, S. Ishida and R. Métivier, NPG Asia Mater., 2018, 10, 859–881 CrossRef CAS; (e) M. Li, L.-J. Chen, Y. Cai, Q. Luo, W. Li, H.-B. Yang, H. Tian and W.-H. Zhu, Chem, 2019, 5, 634–648 CrossRef CAS; (f) H. Xi, Z. Zhang, W. Zhang, M. Li, C. Lian, Q. Luo, H. Tian and W.-H. Zhu, J. Am. Chem. Soc., 2019, 141, 18467–18474 CrossRef CAS PubMed.
- (a) L. Giordano, T. M. Jovin, M. Irie and E. A. Jares-Erijman, J. Am. Chem. Soc., 2002, 124, 7481–7489 CrossRef CAS PubMed; (b) M. Irie, T. Fukaminato, T. Sasaki, N. Tamai and T. Kawai, Nature, 2002, 420, 759–760 CrossRef CAS PubMed; (c) H. Al Sabea, L. Norel, O. Galangau, H. Hijazi, R. Métivier, T. Roisnel, O. Maury, C. Bucher, F. Riobé and S. Rigaut, J. Am. Chem. Soc., 2019, 141, 20026–20030 CrossRef CAS PubMed.
- T. Fukaminato, T. Doi, N. Tamaoki, K. Okuno, Y. Ishibashi, H. Miyasaka and M. Irie, J. Am. Chem. Soc., 2011, 133, 4984–4990 CrossRef CAS PubMed.
- K. Liu, Y. Wen, T. Shi, Y. Li, F. Li, Y.-l. Zhao, C. Huang and T. Yi, Chem. Commun., 2014, 50, 9141–9144 RSC.
- (a) B. Roubinet, M. L. Bossi, P. Alt, M. Leutenegger, H. Shojaei, S. Schnorrenberg, S. Nizamov, M. Irie, V. N. Belov and S. W. Hell, Angew. Chem., Int. Ed., 2016, 55, 15429–15433 CrossRef CAS PubMed; (b) B. Roubinet, M. Weber, H. Shojaei, M. Bates, M. L. Bossi, V. N. Belov, M. Irie and S. W. Hell, J. Am. Chem. Soc., 2017, 139, 6611–6620 CrossRef CAS PubMed; (c) O. Nevsky, D. Sysoiev, A. Oppermann, T. Huhn and D. Wöll, Angew. Chem., Int. Ed., 2016, 55, 12698–12702 CrossRef PubMed; (d) O. Nevskyi, D. Sysoiev, A. Oppermann, T. Huhn and D. Wöll, Angew. Chem., Int. Ed., 2016, 55, 12698–12702 CrossRef CAS PubMed.
- (a) M. Irie, Chem. Rev., 2000, 100, 1685–1716 CrossRef CAS PubMed; (b) M. Irie, T. Fukaminato, K. Matsuda and S. Kobatake, Chem. Rev., 2014, 114, 12174–12277 CrossRef CAS PubMed.
- (a) G. M. Tsivgoulis and J.-M. Lehn, Angew. Chem., Int. Ed. Engl., 1995, 34, 1119–1122 CrossRef CAS; (b) M.-S. Kim, T. Kawai and M. Irie, Chem. Lett., 2001, 702–703 CrossRef CAS.
- (a) Y.-C. Jeong, S. I. Yang, K.-H. Ahn and E. Kim, Chem. Commun., 2005, 2503–2505 RSC; (b) M. Taguchi, T. Nakagawa, T. Nakashima and T. Kawai, J. Mater. Chem., 2011, 21, 17425–17432 RSC; (c) S.-C. Pang, H. Hyun, S. Lee, D. Jang, M. J. Lee, S.-H. Kang and K. H. Ahn, Chem. Commun., 2012, 48, 3745–3747 RSC.
- K. Uno, H. Niikura, M. Morimoto, Y. Ishibashi, H. Miyasaka and M. Irie, J. Am. Chem. Soc., 2011, 133, 13558–13564 CrossRef CAS PubMed.
- (a) Q. Ai and K.-H. Ahn, RSC Adv., 2016, 6, 43000–43006 RSC; (b) C.-W. Hsu, C. Sauvée, H. Sundén and J. Andréasson, Chem. Sci., 2018, 9, 8019–8023 RSC; (c) D. Kim, K. Jeong, J. E. Kwon, H. Park, S. Lee, S. Kim and S. Y. Park, Nat. Commun., 2019, 10, 3089 CrossRef PubMed; (d) G. Naren, C.-W. Hsu, S. Li, M. Morimoto, S. Tang, J. Hernando, G. Guirado, M. Irie, F. M. Raymo, H. Sundén and J. Andréasson, Nat. Commun., 2019, 10, 3996 CrossRef CAS PubMed.
- T. Nakagawa, Y. Miyasaka and Y. Yokoyama, Chem. Commun., 2018, 54, 3207–3210 RSC.
- See ESI†.
- K. Uno, M. L. Bossi, T. Konen, V. N. Belov, M. Irie and S. W. Hell, Adv. Opt. Mater., 2019, 7, 1801746 CrossRef.
- M. Hanazawa, R. Sumiya, Y. Horikawa and M. Irie, J. Chem. Soc., Chem. Commun., 1992, 206–207 RSC.
- (a) Y.-C. Jeong, D. G. Park, E. Kim, K.-H. Ahn and S. I. Yang, Chem. Commun., 2006, 1881–1883 RSC; (b) Y.-C. Jeong, D. G. Park, I. S. Lee, S. I. Yang and K.-H. Ahn, J. Mater. Chem., 2009, 19, 97–103 RSC.
- (a) M. Morimoto, Y. Takagi, K. Hioki, T. Nagasaka, H. Sotome, S. Ito, H. Miyasaka and M. Irie, Dyes Pigm., 2018, 153, 144–149 CrossRef CAS; (b) A. L. Schleper, M. L. Bossi, V. N. Belov and S. W. Hell, Beilstein J. Org. Chem., 2019, 15, 2344–2354 CrossRef CAS PubMed.
- C. Reichardt, Chem. Rev., 1994, 94, 2319–2358 CrossRef CAS.
- K. Uno, M. L. Bossi, M. Irie, V. N. Belov and S. W. Hell, J. Am. Chem. Soc., 2019, 141, 16471–16478 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthesis and photophysical studies. See DOI: 10.1039/c9cc09390g |
| ‡ Equal contributions. |
| This journal is © The Royal Society of Chemistry 2020 |