Preparation of magnetic iron oxide nanoparticles modified with imidazolium-based ionic liquids as a sorbent for the extraction of eight phthalate acid esters in water samples followed by UPLC-MS/MS analysis: an experimental design methodology†
Sayyed Massoud
Bahrololoomi Fard
a,
Seyyed Hamid
Ahmadi
 *b,
Mannan
Hajimahmodi
c,
Reza
Fazaeli
*b,
Mannan
Hajimahmodi
c,
Reza
Fazaeli
 a and
Mohsen
Amini
c
a and
Mohsen
Amini
c
aDepartment of Chemistry, Faculty of Science, South Tehran Branch, Islamic Azad University, Tehran, Iran
bDepartment of Environmental Analytical Chemistry, Chemistry and Chemical Engineering Research Center of Iran, Tehran, 1946813151, Iran. E-mail: Ahmadi@ccerci.ac.ir
cMedicinal Chemistry, Drug and Food Control Department, Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran
First published on 12th November 2019
Abstract
In the present study, different ionic liquid modified magnetic nanoparticles have been prepared and tested as nano-metric adsorbents for the analysis of eight phthalic acid esters (PAEs) from water samples using dispersive micro solid-phase extraction (D-micro-SPE). Determination and identification of the PAEs were performed by ultra performance liquid chromatography-tandem mass spectrometry. Compared with ionic liquid functionalized magnetic nanoparticle adsorbents, benzimidazolium-chloride modified iron oxide nanoparticles (Fe3O4@[Bimi]Cl NPs) show higher extraction efficiency for all PAEs. The Fe3O4@[Bimi]Cl NPs were fully characterized by Fourier-transform infrared spectroscopy, field emission-scanning electron microscopy, energy-dispersive X-ray spectroscopy, transmission electron microscopy, X-ray diffraction, and zeta potential analysis. The D-micro-SPE conditions, including sample pH, adsorbent dosage, extraction time, type of desorption solvent, volume of elution solvent, and desorption time, were optimized using both univariate and multivariate techniques based on response surface methodology. Under optimized conditions, the developed method was validated for PAE analysis in different water samples, including river water, tap water, bottled mineral water, well water, and wastewater. The method features wide linearity and low limits of detection ranging from 0.002–0.012 μg L−1. In addition, recoveries ranging from 83% to 111% with a precision (RSDs) of lower than 8% were obtained. Therefore, the proposed method is an efficient pretreatment procedure and can be utilized for the sensitive determination of PAEs in different water samples.
Introduction
Phthalic acid esters (PAEs) have been widely used as softening agents and plasticizers in the production of plastic materials, beverage bottles, food, and cosmetics containers to increase the durability and flexibility of the product.1–4 Since PAEs form weakly physical bonds (not covalently) with the matrix, they can be gradually released and migrate from the plastic products into the environment and food products.5 PAEs can enter the human body through eating, drinking, and skin contact and disturb the endocrine system. Regarding their carcinogenic properties, they can result in serious health issues.6 Several PAEs have been included in the priority list of pollutants by the US Environmental Protection Agency (EPA) and European Union (EU) due to their potential human health risks.7,8 Hence, reliable, fast, and sensitive analytical methods for the detection of PAEs at low concentration levels are critically required. The direct determination of PAEs in real samples is difficult due to the complexity of matrix interferents. In this regard, the application of an efficient sample preparation method for extraction and enrichment of phthalate analytes from real samples is of high priority. Researchers have developed several sample preparation methods for the extraction of PAEs from water samples, among which liquid–liquid extraction (LLE), liquid-phase microextraction (LPME), stir-bar sorptive extraction (SBSE) and solid-phase extraction (SPE) can be mentioned.1Dispersive SPE (DSPE) is an efficient, convenient method with low solvent consumption, which can be used for pre-treatment and pre-concentration of complex matrices.9 Regarding the dispersion phenomenon, the analytes can equally and quickly interact with all the adsorbent particles; therefore, the extraction time with DSPE is shorter than that with SPE.10–12
Recently, dispersive solid-phase micro-extraction (D-micro-SPE) has been widely developed to modify the DSPE method. The D-micro-SPE process relies on dispersing small quantities of a nano or micro sorbent in a sample solution. Owing to their inherent magnetic features, facile preparation, and low toxicity, ferric oxide magnetic nanoparticles (NPs) have been extensively utilized as sorbents in the D-micro-SPE procedure.13 Regarding the high chemical reactivity of bare magnetic NPs (particularly Fe3O4), they can be easily oxidized in the air leading to loss of dispersibility and adsorption capability.14 Thus, it is of crucial importance to properly cover their surface and employ some effective protection strategies to protect the stability of magnetic NPs.15,16
Ionic liquids (ILs) refer to a class of organic salts composed of various inorganic or organic anions and organic cations.17–19 ILs have attracted increasing interest in the field of analytical chemistry due to their high tendency to interact with another compound via electrostatic interactions, hydrogen bonding, π–π stacking, and hydrophilic/hydrophobic interactions.20,21 Thus, modification of Fe3O4 NPs with ILs can result in synergic benefits of magnetic NPs and ILs which can enhance the extraction efficiency of some analytes. Nevertheless, their reusability is reduced by the unstable IL coatings.22,23 The stability of ILs can be considerably enhanced by their covalent bonding to magnetic NPs. This treatment will also prevent their loss in the over-extraction procedure.24 Imidazole-based ILs have been widely used for the modification of magnetic NPs.25–29
The obvious advantages of magnetic NP adsorbents which appeared to be suitable carriers for ILs in D-micro-SPE are large surface area and short diffusion route, providing improved extraction dynamics, therefore high extraction efficiency, and ease of surface functionalization/modification.30 The possibility of modifying magnetic NPs with ILs resulted in numerous applications of IL-based D-micro-SPE techniques.31–33 Compared with previous reports, the magnetic NP modified IL-based D-micro-SPE method featured a facile, rapid, and efficient sample preparation process, which enabled the treatment of a large volume of samples in a short time.
Considering the combined impacts of these materials, the present work addresses the synthesis and characterization of imidazole-based IL-modified Fe3O4 NPs and evaluates their uses in D-micro-SPE. To assess the viability of the developed method, the appropriateness of the IL-modified Fe3O4 NPs was evaluated as an adsorbent for D-micro-SPE of eight PAEs from various water samples. The factors affecting the efficiency of the extraction were optimized by the multi-response surface methodology (RSM) and the single-factor technique. Furthermore, ultra-performance liquid chromatography coupled with tandem mass spectrometry (UPLC-MS/MS) was employed to detect and separate PAEs. The proposed method was used to analyze PAEs in real water samples.
Materials and methods
Chemicals and reagents
Dimethyl phthalate (DMP 99%), diisononyl phthalate (DINP 99%), dipropyl phthalate (DPP 98%), benzyl butyl phthalate (BBP 98%), bis(4-methylpentyl)phthalate (DIPP 95%), bis(2-ethylhexyl) phthalate (DEEP 99.5%), dicyclohexyl phthalate (DCHP 99%) and diisobutyl phthalate (DIBP 99%) were provided by Sigma-Aldrich company (USA). Dibutyl phthalate deuterated (DBP-d4) was obtained from Accu Standard Co. (USA). The chemical structures of PAEs are shown in Table S1.† (3-Chloropropyl)trimethoxysilane (CTMS, ≥98%) and tetraethyl orthosilicate (TEOS, 99%) were supplied by Merck (Germany). LC-MS grade acetonitrile (MeCN) and methanol (MeOH), 1-benzylimidazole (≥99%), 1-octylimidazole (≥98%), and 1-butylimidazole (≥98%) were obtained from Sigma-Aldrich (USA). Other substances were bought from Chem-Lab company (Belgium). The experiments were performed using ultrapure-water (UPW, provided by ELGA Purelab Ultra Water Purifier, Ultra Genetic, UK) with resistivity higher than 18 MΩ.Sample preparation
To ensure the absence of PAE residues in the laboratory's containers, non-volumetric glass containers were calcined for 4 h at 550 °C. Nochromix® solution (Godox Laboratories, Maryland, USA) was also prepared according to the instruction of the manufacturing factory and used for cleaning the volumetric glass containers. Furthermore, phthalate-free gloves and pipette tips were applied in this study. The individual stock standard solutions (500 μg mL−1) for each analyte and IS were prepared from appropriate amounts of PAEs in MeCN and were stored at −18 °C. The mixture of PAE working solutions was fabricated by proper daily dilution of the standard stock solutions with the initial mobile phase (MeCN with 0.1% formic acid). Britton–Robinson buffer was also used to adjust the pH of the solution. Corning® syringe filters (nylon membrane, pore size 0.2 μm) were employed for the filtration of all solutions before their injection into UPLC-MS/MS.Water sample selection and pretreatment
To validate the developed method, five different water samples (river, tap, mineral plastic bottled, well water and wastewater) were chosen. The tap water sample was directly collected from a laboratory in Tehran (Iran), while the groundwater sample was obtained from a semi-deep well in a private farm in the north of Karaj (Iran). Mineral plastic bottled water was also purchased from a local supermarket in Tehran (Iran). River water was collected from the Kan River in the north of Tehran (Iran).Furthermore, the wastewater sample was collected from a wastewater treatment plant in Tehran (Iran). Using a Microlab-scientific® PVDF membrane filter (0.45 μm), all the water samples were first filtered to remove the suspended solids or particulate substance. The conductivities of river water, mineral water, and wastewater samples were measured to be 895, 218, and 1643 μS cm−1 at 25 °C, respectively.
UPLC-MS/MS conditions
PAEs were analyzed using a Waters Acquity™ UPLC system (Waters Company, USA). The detection was carried out with a Waters Xevo™ TQD tandem quadrupole mass spectrometer that was equipped with an electrospray ionization (ESI) interface. Masslynx™ software from Waters Chromatography was applied to control the MS parameters and the acquisition and processing of MS spectral data. According to a review of the preceding publications and studies, mass spectrometry was selected for detection and separation of PAEs, UPLC-MS/MS conditions were optimized, and the following results were obtained.PAE separation was carried out in an Acquity™ BEH Shield C18-column (particle size of 1.7 μm, 50 mm × 2.1 mm) utilizing an Acquity™ BEH Shield C18 pre-column (1.7 μm, 5 mm × 2.1 mm), both from Waters Corporation. The oven column's temperature was set at 30 °C. The PAE analysis mobile phase contained MeCN with 0.1% formic acid (solvent A) and UPW containing 0.1% formic acid (solvent B); both solvents were filtered via a Whatman™ polyamide membrane circle (0.2 μm pore size). The initial composition of the mobile phase included 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 (v/v) solvent A
50 (v/v) solvent A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B and was pumped at a flow rate of 0.3 mL min−1. The gradient composition was altered to 70
B and was pumped at a flow rate of 0.3 mL min−1. The gradient composition was altered to 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 (v/v) solvent A
30 (v/v) solvent A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B within 4.5 min. Ultimately, the gradient planned for the column re-equilibrium within 2 min was transformed into 100% solvent A. The injection amount was 5 μL at 10 °C and the overall run time was 6.5 min.
B within 4.5 min. Ultimately, the gradient planned for the column re-equilibrium within 2 min was transformed into 100% solvent A. The injection amount was 5 μL at 10 °C and the overall run time was 6.5 min.
The ESI interface was run in positive mode. The MS system was operated in multiple-reaction monitoring (MRM) mode using one precursor and two product ions (with the highest frequency for each target analyte); the retention time was the identification points giving rise to a maximum tolerance of ±20% for the relative ion intensities of the precursor and product ions.34 The parameters of the mass spectrometer were optimized to achieve the best quantification for each analyte. The MS/MS experiments were carried out by fragmenting the protonated molecule ([M + H]+), which was chosen as the precursor ion. The optimal values of the ionizing source resulting in the highest sensitivity of the precursor ions involved a capillary voltage of 3.5 kV, desolvation gas flow rate of 900 L h−1, source temperature of 150 °C, desolvation gas temperature of 550 °C, and collision cell pressure of 5 mbar. Argon (99.99%) and nitrogen (99.9%) were respectively utilized as the collision gas and the desolvation gas. The transitions of precursor-product ions were monitored for each analyte. For quantification, the product ions with the highest intensity were selected; however, for confirming, the less sensitive transition was utilized. The other MRM parameters were previously optimized for each analyte, and the results are listed in Table S2 (ESI†) as well.
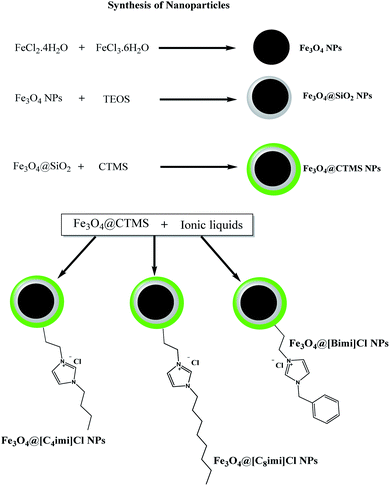
Synthesis of NPs
This work addresses the synthesis of 3 various imidazolium-based IL-functionalized iron oxide NPs (Fe3O4@butylimidazolium chloride (Fe3O4@[Bimi]Cl NPs), Fe3O4@octylimidazolium chloride (Fe3O4@[C8imi]Cl NPs), and Fe3O4@benzylimidazolium chloride (Fe3O4@[C4imi]Cl NPs)). The synthesis procedure was conducted in multiple stages (Fig. 1).Based on the literature, Fe3O4 magnetic NPs could be provided through the chemical co-precipitation technique.35 Previously synthesized magnetite NPs were covered with a silica layer through the modified Stöber technique (Fe3O4@SiO2 NPs).35,36 Chloro-functionalized Fe3O4 NPs (Fe3O4@CTMS NPs) were also prepared according to a previously reported process.37
Three different imidazolium-based IL-functionalized Fe3O4 NPs were synthesized as follows: 0.4 g of Fe3O4@CTMS NPs was dispersed in 50 mL of dry toluene through ultrasonication; then, 2 mmol of the desired imidazole derivative (0.316 g of 1-benzimidazole, 0.360 g of 1-octylimidazole or 0.248 g of 1-butylimidazole) was introduced into the suspension, followed by 15 minutes of sonication. The mixture was then transferred to a 200 mL Synthware™ single-neck round-bottom flask equipped with a reflux condenser and an overhead agitator. The suspension was heated under reflux and mechanical agitation for 24 h. Then, the attained magnetic particles (Fe3O4@[Bimi]Cl NPs, Fe3O4@[C8imi]Cl NPs, and Fe3O4@[C4imi]Cl NPs) were isolated utilizing a magnet and other supernatant substances were removed gradually by decanting. The NPs were then rinsed with dry toluene. Eventually, the particles were dried under vacuum for 48 h at 60 °C.
Batch extraction process
50 mL of the water sample (with a pre-adjusted pH of 6.0 using Britton–Robinson buffer) was introduced into a PYREX® glass beaker containing 47.3 mg of Fe3O4@[Bimi]Cl NPs. The mixture was then sonicated for 3.1 min. After extraction, to settle the NPs, a NdFeB magnet was put at the bottom of the glass beaker. The supernatant solution was discarded, and the sorbent with the retained analytes was dried under a N2 flow. The analytes were then desorbed by introducing 500 μL desorption solvent (MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeCN 75
MeCN 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 (v/v)), and the suspension was sonicated for 2.4 min. Finally, the magnetic particles were isolated from the solvent using a permanent magnet. The eluent was evaporated under a N2 stream. The residues were reconstructed with 500 μL primary mobile phase, vortexed at maximum speed for 1 min, filtered using a syringe filter (Whatman® GD/XP, 0.2 μm), and then gathered into a UPLC vial. For further analysis, 5 μL of the solution was injected into the UPLC-MS/MS system.
25 (v/v)), and the suspension was sonicated for 2.4 min. Finally, the magnetic particles were isolated from the solvent using a permanent magnet. The eluent was evaporated under a N2 stream. The residues were reconstructed with 500 μL primary mobile phase, vortexed at maximum speed for 1 min, filtered using a syringe filter (Whatman® GD/XP, 0.2 μm), and then gathered into a UPLC vial. For further analysis, 5 μL of the solution was injected into the UPLC-MS/MS system.
Results and discussion
Characterization of NPs
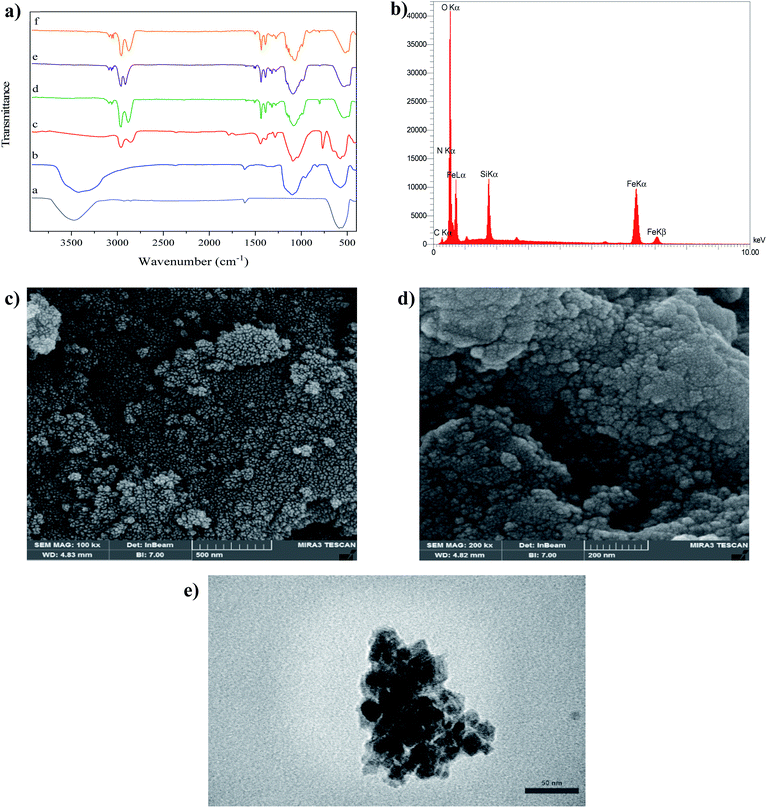
Fourier-transform infrared spectroscopy (FTIR) spectra of Fe3O4 NPs, Fe3O4@SiO2 NPs, Fe3O4@CPTS NPs, Fe3O4@[Bimi]Cl NPs, Fe3O4@[C8imi]Cl NPs and Fe3O4@[C4imi]Cl NPs are shown in Fig. 2a. The absorbance band at about 570 cm−1 is assigned to Fe–O bond vibrations.38 The absorption bands at 1050, 987, and 815 cm−1 for all silica core–shell magnetic NPs are associated with the asymmetric stretching vibrations of Si–O–Si and Si–O, and the symmetric Si–O–Si stretching, respectively.39The characteristic bands of the organic group modified with CPTS on the Fe3O4@SiO2 framework are represented in the FTIR spectrum. The C–H stretching of methylene groups is observed in the range of 2990–2800 cm−1. Furthermore, further evidence for grafting CPTS onto the magnetic Fe3O4@SiO2 NPs is provided by asymmetric Si–CH2 and Fe–O–Si stretching vibration peaks appearing at 1286 and 1095 cm−1, respectively. The C–Cl stretching vibration peaks were detected in the FTIR spectrum of Fe3O4@CPTS NPs appearing at 750 cm−1 in comparison with Fe3O4@SiO2, and the band of C–Cl disappeared for the imidazolium-based IL modified NPs, due to the nucleophilic substitution reaction between chlorine atoms and nitrogen atoms, leading to the emergence of nitrogen element in the spectrum.
In the case of butyl- and octyl-imidazolium-based IL-modified NPs, new bands emerged at 1560 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N vibrations and C
N vibrations and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C vibrations of the imidazole ring), 2937, 2848 and 1447 cm−1 (alkyl chain stretching and deformation vibrations).40 In the spectrum of Fe3O4@[Bimi]Cl NPs, the bands at 3031–3099 cm−1, 2915 cm−1, 1560 cm−1, 1506 cm−1, and 736 cm−1 can be assigned to the C–H stretching vibration in the aromatic group, C–H stretching vibration mode of alkyl moieties, C
C vibrations of the imidazole ring), 2937, 2848 and 1447 cm−1 (alkyl chain stretching and deformation vibrations).40 In the spectrum of Fe3O4@[Bimi]Cl NPs, the bands at 3031–3099 cm−1, 2915 cm−1, 1560 cm−1, 1506 cm−1, and 736 cm−1 can be assigned to the C–H stretching vibration in the aromatic group, C–H stretching vibration mode of alkyl moieties, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N vibration of the imidazole ring, C–C stretching in the aromatic ring and out of plane C–H bending, respectively.41 Notably, the new band at around 1430 cm−1 can be assigned to the stretching vibration of the C–N+ group, which was observed in IL-modified magnetic NPs, implying the successful IL modification of the Fe3O4@SiO2 NP surface.42
N vibration of the imidazole ring, C–C stretching in the aromatic ring and out of plane C–H bending, respectively.41 Notably, the new band at around 1430 cm−1 can be assigned to the stretching vibration of the C–N+ group, which was observed in IL-modified magnetic NPs, implying the successful IL modification of the Fe3O4@SiO2 NP surface.42
The energy dispersive X-ray spectrometry (EDX) pattern confirms the formation of the core–shell Fe3O4 NPs (Fig. 2b) as a result of the presence of Fe. The C and N peaks together with the Si, O, and Fe peaks validate the successful functionalization of Fe3O4@SiO2 core–shell NPs.
Using field emission-scanning electron microscopy (FESEM), the morphology, structure and size of Fe3O4 and Fe3O4@[Bimi]Cl NPs were characterized. Fig. 2c shows the spherical Fe3O4 NPs with an approximate particle size distribution of 15 nm. Furthermore, the FESEM image in Fig. 2d indicates that the surface of Fe3O4@[Bimi]Cl NPs became rougher and more irregular as compared to that of the pure Fe3O4 NPs. The average Fe3O4@[Bimi]Cl NP diameter was measured to be 35 nm.
The transmission electron microscopy (TEM) image of Fe3O4@[Bimi]Cl NPs is presented in Fig. 2e. In this image, solid black spheres are Fe3O4 NPs, which endow the magnetic D-micro-SPE sorbent with an excellent magnetic response; the light shell is the IL layer, which can protect the inner magnetic core from oxidation and corrosion and provide strong affinity to the target analytes.
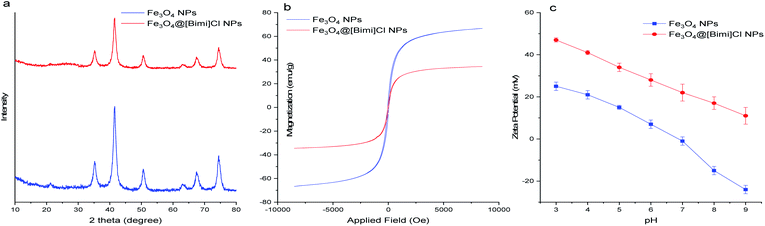
Fig. 3a presents the X-ray diffraction (XRD) patterns of bare Fe3O4 NPs and Fe3O4@[Bimi]Cl NPs. The diffraction peaks were observed at 2θ = 35.3°, 41.5°, 50.5°, 63.4°, 67.5°, and 74.5° which can be allocated to the (220), (311), (400), (422), (511) and (440) planes, respectively, indicating an inverse cubic spinel phase of Fe3O4 NPs according to the standard data in the literature for magnetite structure (JCPDS card no. 85-1436).43,44 Amorphous layers can also be observed in the range of 2θ = 15–25°, proving the existence of a silica layer on the modified Fe3O4 NP surface.19,45
The chemical states of NPs can be confirmed by the use of X-ray photoelectron spectroscopy (XPS) analysis. The XPS spectrum of Fe3O4@[Bimi]Cl NPs is shown in Fig. S1a.† As this figure suggests, Fe3O4@[Bimi]Cl NPs have photoelectron peaks at 711.3 and 724.9 eV, which can be assigned to Fe 2p3/2 and Fe 2p1/2 characteristics of iron oxide core-level conforming with the iron oxidation state in Fe3O4, respectively.46,47
The high-resolution XPS patterns prove the successful surface modification of the silica-coated Fe3O4 NPs with benzylimidazolium chloride IL (Fig. S1b–e†). Fig. S1b† shows the C1s spectrum with an asymmetric peak structure due to the presence of variable carbon bonds. The C1s peak can be deconvoluted into four chemically shifted peak components at 284.8, 285.8, 286.3, and 287.1 eV, allocated to C–C/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C/C–H, C–N, C–O, and N–C–N, respectively.48 The high-resolution N1s XPS spectrum represents a peak at 402.2 eV, which can be attributed to the positively charged nitrogen (Fig. S3c†).49 The O1s and Si2p peaks also emerged at 531.9 eV and 102.6 eV, respectively, confirming the presence of Si–O linkage besides the oxygen functionalities in the silica surface coated on Fe3O4 NPs.
C/C–H, C–N, C–O, and N–C–N, respectively.48 The high-resolution N1s XPS spectrum represents a peak at 402.2 eV, which can be attributed to the positively charged nitrogen (Fig. S3c†).49 The O1s and Si2p peaks also emerged at 531.9 eV and 102.6 eV, respectively, confirming the presence of Si–O linkage besides the oxygen functionalities in the silica surface coated on Fe3O4 NPs.
The magnetic properties of nanoparticles were assessed using a vibrating sample magnetometer (VSM) at room temperature. No hysteresis can be observed in Fig. 3b indicating the superparamagnetic behavior of the NPs at room temperature. Indeed, no magnetism remained in the NPs after removing the magnetic field.50 According to Fig. 3b, the magnetization saturation of pure Fe3O4 NPs was 67 emu g−1, which decreased to 34 emu g−1 after the coating procedure.
The zeta potential at various pH values was measured to determine the pH of the isoelectric point of the NPs; as shown in Fig. 3c, the zeta potential of Fe3O4@[Bimi]Cl NPs revealed a positive charge for most of the pH values. The presence of quaternary ammonium moieties is responsible for this positively charged surface leading to the creation of positive forms at most of the values of pH.
Optimization of magnetic D-micro-SPE conditions
To optimize influential factors such as type of adsorbent, sample pH, and type and quantity of the elution solvent, the single factor method was utilized. The other factors were optimized using the multivariate optimization technique. All the optimization tests were carried out in triplicate, utilizing 50.0 mL of spiked UPW containing a concentration of 10.0 μg L−1 of each analyte.Extraction recovery (ER) was utilized to assess the optimum conditions. ER is defined as the percentage of the total amount of analyte (n0) which is extracted into the sedimented phase (nsed). The ER was calculated correctly as an analytical response as follows:
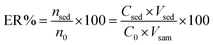 | (1) |
Nanosorbent selection
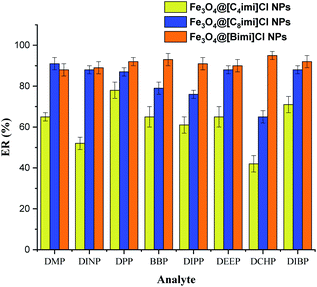
In order to compare the magnetic nano-sorbents to achieve efficient extraction of PAEs, the extraction capability of Fe3O4@[Bimi]Cl, Fe3O4@[C8imi]Cl and Fe3O4@[C4imi]Cl NPs was investigated. As depicted in Fig. 4, Fe3O4@[C4imi]Cl NPs exhibited limited extraction performance towards PAEs, while the highest extraction efficiency was achieved by Fe3O4@[Bimi]Cl. The presence of benzyl groups on the surfaces of Fe3O4@[Bimi]Cl NPs may enhance π–π interactions between the adsorbent and the PAE molecules, as compared to the other adsorbents. Therefore, this study indicated the significant role of benzylimidazolium chloride as a surface modifier in the efficient extraction of PAEs. Therefore, magnetic D-micro-SPE based on Fe3O4@[Bimi]Cl NPs was selected for further experiments.Effect of sample pH
The pH of the sample solution is an essential factor in SPE methods. The pH value was adjusted within 2.0–9.0 to evaluate the effect of the sample pH on extraction efficiency (Fig. S2†). The pH values higher than 9.0 were not considered due to their potentially destructive impact on the silica layer of the adsorbent. The ER of the analytes increased with the increase of sample pH from 2 to 6, but it sharply decreased in alkaline media. This is probably due to the target analytes (esters), which tend to be hydrolyzed to phthalic acid under both alkaline and acidic conditions, but hydrolysis is irreversible when the sample pH exceeded 7.51 Another critical point is the higher stability of the NPs in suspension within the zeta potential range of ±30 mV.52 For pH < 6.0, the zeta potential of the Fe3O4@[Bimi]Cl NPs was higher than +30 mV which can explain the excellent dispersion stability of Fe3O4@[Bimi]Cl NPs in the acidic solutions. Therefore, the pH of the sample solution was set to 6.0 for the following optimization experiments.Optimization of the elution solvent type and volume
In the current study, the desorption capability of five solvents with different polarities was evaluated. The results are presented in Fig. S3† showing that MeOH exhibited a comparatively better elution capability than the other solvents for PAE desorption from the Fe3O4@[Bimi]Cl NPs. Pure methanol induced a peak shape leading to a weak resolution of the acquired chromatographic peaks. As such, the application of a mixture of MeCN and MeOH can significantly affect the desorption procedure. In order to evaluate the role of the mixed elution solvent, different ratios of MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeCN were tested. Based on the results, the most appropriate eluent for PAEs was MeOH
MeCN were tested. Based on the results, the most appropriate eluent for PAEs was MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeCN at a ratio of 75
MeCN at a ratio of 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25.
25.
Furthermore, the effect of desorption solvent volume was studied. This optimal volume of solvent should be sufficient to prompt the highest concentration and complete desorption of analytes from the sorbent.53 For this purpose, different volumes of the desorption solvent (400–4000 μL) were considered. Based on the results, 500 μL of the solvent was enough for the desorption of analytes.
Multivariate optimization
The adsorbent quantity, contact time, and desorption time were optimized via the Box–Behnken design (BBD) method using RSM (refer to the ESI† for complete description). Based on our preliminary experiments, the adsorbent quantity, contact time, and desorption time were ultimately selected and optimized as independent variables which were optimized through the Box–Behnken design (BBD) method using RSM (refer to the ESI† for complete description). The range of each factor was determined according to single-factor experiments. Each factor was coded by a 3-level scale (−1, 0, and +1 corresponding to the low-level, central point, and high-level, respectively), as listed in Table 1.| Independent variable | Symbol | Range and level | ||
|---|---|---|---|---|
| Low-level (−1) | Central point (0) | High-level (+1) | ||
| Adsorbent quantity (mg) | A | 20.0 | 40.0 | 60.0 |
| Contact time (min) | B | 1.0 | 2.5 | 4.0 |
| Desorption time (min) | C | 1.0 | 2.0 | 3.0 |
The response factors (dependent variables, Yi) were the analyte's ER percentage, and three repetitions for these factors were assessed. A series of experiments were carried out for optimization using Design-Expert software (V 10.0.3) as summarized in Table S3.† Typically, for minimizing the systematic error, the experiments were performed in a randomized order (Table S3†).
The adequacy and significance of the established models were assessed via analysis of variance (ANOVA) at a certain confidence interval (α = 0.05). Table S4† presents the ANOVA test results. Least squares regression was also used for fitting the results to a second-order polynomial equation, as presented in Table S5.† In order to compare the model variance or the variables with residual error variances, Fisher's test in ANOVA was utilized where the terms with higher F-values or probability critical levels (p-value) below 0.05 had a significant contribution to the model terms.54
The F-values of the suggested quadratic models varied within the range of 40.8–151.0 (p-value < 0.0001). The adequate reliability and robustness of the fitted models were verified from p-values of lack-of-fit of the models over 0.05 and correlation coefficients more than 0.98.55,56 In the suggested models, all lack-of-fit p-values were higher than 0.05, and all the correlation coefficients were above 0.98. Since the traditional R2 values for all responses were close to the predicted R2 values and adjusted R2 (differences not more than 0.2), unbiasedness of the predictions of the established extraction models is confirmed (Table S4†).56,57 As mentioned above, it can be stated that all the validations were carried out for the BBD models, and an acceptable explanation of the effects of variables studied on the extraction with a high accuracy level can be provided by regression equations.
3D response surface plots were constructed from the data obtained using the BBD depending on the model equations.
As an example, the effects of three factors on the ER of DMP are presented in Fig. 5. The response surface plot for the interaction effect of the adsorbent quantity and contact time at constant desorption time revealed that at any fixed contact time, the ER of DMP increases with increasing sorbent amount (Fig. 5a). As suggested by the results, the relative response was enhanced by increasing the extraction time, and the maximum ER was achieved at a contact time of 3.1 min.
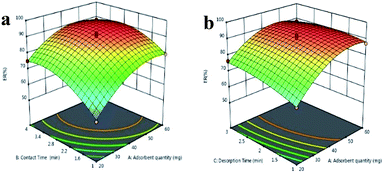 | ||
| Fig. 5 3D response surface plots for the ER of DMP versus (a) adsorbent quantity and extraction time, and (b) adsorbent quantity and desorption time. | ||
Study of the simultaneous effect of sorbent dosage and desorption time (Fig. 5b) leads us to the conclusion that they can significantly affect the ER. In the sorbent range of 20–50 mg, the ER increased with a gradual slope upon the enhancement of desorption time for all adsorbent amounts. Increase of desorption time, however, did not positively affect the ER in the sorbent range of 50–60 mg.
In order to determine the specific point maximizing the global desirability function (DF), the numerical optimization technique of the software was chosen. Based on the response optimizer data, optimal responses are achievable under the following conditions: adsorbent quantity = 47.3 mg, contact time = 3.1 min and desorption time = 2.4 min. The obtained DF was 0.999 representing an entirely desirable and ideal response value of these conditions.58 The predicted ER values varied from 90.2% to 95.8%. Verification experiments were performed under similar conditions. The actual experimental values of ER% ranged from 89.8–94.7% showing a close correlation with the predicted values, proving the reliability of RSM optimization.
Method validation
The matrix effect, linearity range, LOQ, LOD, precision, and trueness of the developed method were evaluated under optimum conditions.To guarantee the reliability of methodical data attained from the validating procedure and the analysis of the real samples, laboratory blanks were studied in a periodical manner to evaluate the effect of the background signal. Furthermore, due to the accessibility of this type of compounds in the water samples, the blank water samples (unspiked) were also evaluated to examine the presence of PAE residues in all the matrices. Consequently, the peak areas of these compounds were subtracted to attain a sufficient validation process for all the target analytes.
Matrix effect study
The matrix effect of the proposed method was assessed at two concentrations based on the Matuszewski method.59 Moreover, regarding the different natures of the studied water samples, this study was established for each water sample to individually evaluate their influence on the extraction procedure. The data (Table S6†) showed negligible ion suppression or enhancement in tap water, river water, well water, and mineral bottled plastic water samples under the considered circumstances.60 Nonetheless, the complexity in the matrix of the wastewater sample resulted in significant matrix effects for DINP (122%) and BBP (118%). Isotopically labeled IS is a consistent tool for compensating for the unwanted matrix effect.61 Deuterated DBP (DBP-d4) was utilized in this work, as IS for PEN analysis. It was added at the beginning of the extraction procedure to correct the possible errors that occurred during the sample preparation and improve the reproducibility of the methodology.62Linearity and analytical performance characteristics of the developed method
Regarding the matrix effect assessment results, matrix-matched calibration curves were plotted for the matrices to evaluate the linearity of the method within the intended concentration range. Table 2 lists the findings of the linearity investigation.| Analyte | Type of water | Concentration studied (μg L−1) | Slope | Intercept | R 2 | LOQ (μg L−1) |
|---|---|---|---|---|---|---|
| DMP | River water | 0.02–250 | 6.45 × 10−2 ± 1.98 × 10−4 | 1.66 × 10−3 ± 1.23 × 10−4 | 0.9993 | 0.017 |
| Tap water | 0.01–250 | 6.23 × 10−2 ± 1.54 × 10−4 | −1.98 × 10−3 ± 1.47 × 10−4 | 0.9992 | 0.010 | |
| Mineral bottled plastic water | 0.02–250 | 6.79 × 10−2 ± 1.39 × 10−4 | 1.53 × 10−3 ± 1.11 × 10−4 | 0.9991 | 0.011 | |
| well water | 0.03–250 | 6.64 × 10−2 ± 1.92 × 10−4 | 1.88 × 10−4 ± 1.23 × 10−3 | 0.9988 | 0.029 | |
| Wastewater | 0.04–250 | 6.55 × 10−2 ± 4.27 × 10−4 | −2.71 × 10−3 ± 1.78 × 10−3 | 0.9981 | 0.035 | |
| DINP | River water | 0.03–250 | 5.11 × 10−2 ± 3.28 × 10−5 | 2.27 × 10−3 ± 1.34 × 10−3 | 0.9989 | 0.026 |
| Tap water | 0.03–250 | 5.34 × 10−2 ± 4.05 × 10−5 | 2.34 × 10−3 ± 2.06 × 10−3 | 0.9992 | 0.024 | |
| Mineral bottled plastic water | 0.03–250 | 4.98 × 10−2 ± 5.39 × 10−5 | −2.29 × 10−3 ± 1.55 × 10−3 | 0.9990 | 0.028 | |
| well water | 0.03–250 | 5.09 × 10−2 ± 2.95 × 10−4 | 3.52 × 10−3 ± 2.78 × 10−3 | 0.9991 | 0.029 | |
| Wastewater | 0.04–250 | 4.85 × 10−2 ± 6.22 × 10−4 | 4.47 × 10−3 ± 2.54 × 10−3 | 0.9987 | 0.034 | |
| DPP | River water | 0.01–250 | 5.28 × 10−2 ± 2.78 × 10−4 | 1.34 × 10−3 ± 1.29 × 10−3 | 0.9993 | 0.006 |
| Tap water | 0.01–250 | 5.48 × 10−2 ± 2.34 × 10−4 | 1.44 × 10−3 ± 1.31 × 10−3 | 0.9992 | 0.007 | |
| Mineral bottled plastic water | 0.01–250 | 4.99 × 10−2 ± 2.89 × 10−4 | 2.08 × 10−3 ± 2.76 × 10−3 | 0.9995 | 0.008 | |
| well water | 0.01–250 | 4.84 × 10−2 ± 1.51 × 10−4 | −2.31 × 10−3 ± 1.55 × 10−3 | 0.9992 | 0.008 | |
| Wastewater | 0.01–250 | 5.12 × 10−2 ± 3.57 × 10−4 | 1.98 × 10−3 ± 1.76 × 10−3 | 0.9991 | 0.010 | |
| BBP | River water | 0.01–250 | 1.08 × 10−2 ± 4.21 × 10−5 | 5.21 × 10−4 ± 6.78 × 10−3 | 0.9992 | 0.010 |
| Tap water | 0.01–250 | 1.11 × 10−2 ± 5.87 × 10−6 | 1.15 × 10−3 ± 1.71 × 10−4 | 0.9991 | 0.007 | |
| Mineral bottled plastic water | 0.02–250 | 9.78 × 10−3 ± 3.54 × 10−5 | −1.92 × 10−4 ± 3.23 × 10−3 | 0.9992 | 0.005 | |
| well water | 0.01–250 | 1.05 × 10−2 ± 3.81 × 10−5 | 3.68 × 10−4 ± 4.86 × 10−3 | 0.9992 | 0.008 | |
| Wastewater | 0.01–250 | 8.89 × 10−3 ± 4.53 × 10−5 | −1.73 × 10−3 ± 4.27 × 10−4 | 0.9992 | 0.013 | |
| DIPP | River water | 0.01–250 | 3.17 × 10−2 ± 5.48 × 10−5 | −2.58 × 10−3 ± 2.35 × 10−3 | 0.9993 | 0.008 |
| Tap water | 0.01–250 | 3.03 × 10−2 ± 5.23 × 10−5 | 2.63 × 10−3 ± 3.43 × 10−3 | 0.9994 | 0.009 | |
| Mineral bottled plastic water | 0.01–250 | 3.11 × 10−2 ± 3.72 × 10−5 | 2.46 10−3 ± 1.82 × 10−3 | 0.9992 | 0.008 | |
| well water | 0.01–250 | 2.93 × 10−2 ± 1.38 × 10−5 | −2.18 × 10−3 ± 3.03 × 10−3 | 0.9992 | 0.008 | |
| Wastewater | 0.01–250 | 1.75 × 10−2 ± 4.18 × 10−5 | −2.89 × 10−3 ± 4.29 × 10−3 | 0.9989 | 0.013 | |
| DEEP | River water | 0.02–250 | 8.23 × 10−2 ± 2 78 × 10−4 | −1.23 × 10−3 ± 1.11 × 10−2 | 0.9992 | 0.015 |
| Tap water | 0.02–250 | 8.18 × 10−2 ± 3.25 × 10−4 | 2.44 × 10−3 ± 1.75 × 10−3 | 0.9993 | 0.013 | |
| Mineral bottled plastic water | 0.02–250 | 7.92 × 10−2 ± 3.49 × 10−4 | −2.36 × 10−3 ± 3.63 × 10−3 | 0.9994 | 0.012 | |
| well water | 0.02–250 | 7.86 × 10−2 ± 4.57 × 10−4 | 3.72 × 10−3 ± 2.59 × 10−3 | 0.9991 | 0.016 | |
| Wastewater | 0.02–250 | 8.32 × 10−3 ± 6.13 × 10−4 | −5.86 × 10−4 ± 3.27 × 10−3 | 0.9989 | 0.020 | |
| DCHP | River water | 0.02–250 | 1.92 × 10−1 ± 3.41 × 10−5 | 4.22 × 10−2 ± 4.21 × 10−3 | 0.9989 | 0.017 |
| Tap water | 0.02–250 | 2.02 × 10−1 ± 2.83 × 10−5 | −1.77 × 10−3 ± 5.22 × 10−4 | 0.9986 | 0.016 | |
| Mineral bottled plastic water | 0.02–250 | 1.81 × 10−1 ± 3.08 × 10−4 | −3.22 × 10−2 ± 4.18 × 10−3 | 0.9989 | 0.020 | |
| well water | 0.02–250 | 1.87 × 10−1 ± 2.95 × 10−4 | 2.54 × 10−2 ± 3.48 × 10−3 | 0.9981 | 0.021 | |
| Wastewater | 0.02–250 | 1.93 × 10−1 ± 4.23 × 10−4 | −1.95 × 10−3 ± 6.22 × 10−3 | 0.9978 | 0.015 | |
| DIBP | River water | 0.02–250 | 1.57 × 10−1 ± 2.86 × 10−4 | 4.78 × 10−2 ± 4.98 × 10−3 | 0.9994 | 0.021 |
| Tap water | 0.02–250 | 1.26 × 10−4 ± 1.18 × 10−3 | −2.26 × 10−3 ± 1.37 × 10−2 | 0.9992 | 0.020 | |
| Mineral bottled plastic water | 0.01–250 | 1.11 × 10−1 ± 8.27 × 10−4 | −2.82 × 10−2 ± 4.51 × 10−3 | 0.9993 | 0.013 | |
| well water | 0.03–250 | 1.19 × 10−1 ± 1.63 × 10−4 | 3.08 × 10−2 ± 2.89 × 10−3 | 0.9989 | 0.027 | |
| Wastewater | 0.04–250 | 1.24 × 10−1 ± 2.88 × 10−3 | 5.65 × 10−1 ± 4.34 × 10−3 | 0.9989 | 0.038 |
The LOQs of the developed method and the lowest concentration of the matrix-matched calibration for each analyte were considered. The signal-to-noise ratio exceeded 10 for the quantification transition for each analyte, in calculation of the recovery of the proposed method.8,19,63 The LOQ values of the method are tabulated in Table 2. In the current work, the LODs were determined from the signal-to-noise criterion equal to three obtained from analyzing several blank samples spiked with a low-level concentration of the compounds.64,65 Table S7† presents the results of this study. The LOD values of the proposed method ranged from 0.002 to 0.12 μg L−1.
Assessing the accuracy of the proposed method
The method's precision and trueness were studied in the recovery investigations. Considering the lack of certified reference materials for these kinds of samples, these investigations were carried out at three spiked levels of concentration at the beginning of the extraction process for all water samples. For instance, Fig. 6 reveals the chromatogram of compounds for the river water sample spiked with 5 μg L−1 for each PAE using the developed method (UPLC-DAD chromatogram of PAEs in the river water sample using the proposed method is shown in Fig. S4†). Acceptable reproducibility and efficiency were found in the data with average recovery values in the range of 82.7–111.2% with relative standard deviation (RSD, %) values of less than 7.2% in all samples (Table S7†).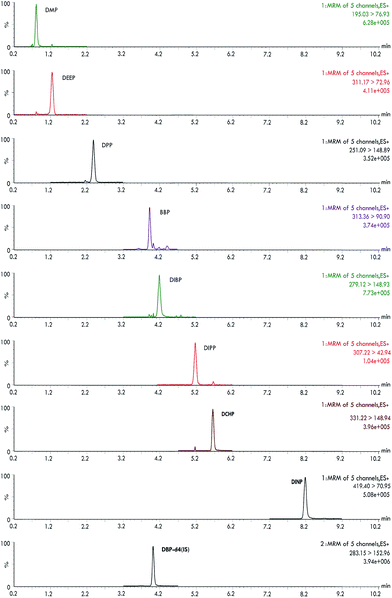 | ||
| Fig. 6 UPLC-MS/MS chromatograms of PAEs of a spiked river water sample after the D-μ-SPE procedure (analyte and IS concentration: 5.0 μg L−1). | ||
Application of the developed method
The applicability of the developed method was assessed by analyzing PAEs in water samples. For this purpose, different types of water samples (including bottled mineral water, plastic container water, and wastewater) were used. The concentration of PAEs was calculated from the calibration curve. According to Table 3, four of the chosen PAEs (DPP, DMP, DIBP, and BBP) were obtained in some analyzed water samples. The presence of BBP and DIBP was particularly notable since both compounds were involved by both the EPA and the EU as significant materials needing monitoring. These results indicated that the established technique might be useful in the successful detection of the ultra-trace levels of PAEs in water samples.| Analyte | PAE concentration (RSD/%)a | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| M1 | M2 | M3 | M4 | M5 | PC1 | PC2 | PC3 | PC4 | W1 | W2 | |
| a Mean of analyte concentration (μg L−1) (n = 5), M: mineral bottled water samples, PC: plastic container water samples, W: wastewater samples. | |||||||||||
| DMP | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | 0.120 (2.8) | <LOQ | 0.084 (3.9) | <LOQ | <LOQ | <LOQ |
| DPP | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | 0.093 (3.2) | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| BBP | 0.139 (3.4) | 0.28 (3.3) | 0.082 (3.6) | <LOQ | <LOQ | 0.089 (3.5) | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| DIBP | <LOQ | 0.621(3.7) | 0.247 (2.9) | 0.524 (3.1) | <LOQ | 1.972 (3.1) | <LOQ | 0.935 (2.8) | <LOQ | 1.272 (5.9) | 0.852 (6.2) |
Comparison of the proposed D-micro-SPE technique with previous methods
The common SPE method is an exhaustive extraction procedure with time-consuming and multistep operation.66 In magnetic SPE, the extraction procedure was enhanced and simplified by introducing magnetic NPs using an external magnetic field. Compared to traditional SPE, magnetic D-micro-SPE has the main advantages, including the opportunity of using small volumes of organic solvents and employing a dispersive extraction approach. Moreover, no column-packing is required by the proposed D-micro-SPE method; hence, the process is simplified. Considerable enhancement in the extraction efficiency of PAEs was obtained by the provided D-micro-SPE together with the UPLC-MS/MS method.Furthermore, compared to the methods reported previously, the current procedure is similar regarding linearity, LOD, accuracy, sorbent dosage and solvent used. Table 4 presents a succinct evaluation for comparison of the present technique with some techniques utilized formerly in extracting PAEs from water samples. As is evident, the developed method showed the advantages of wide linearity range, extreme sensitivity, and desirable accuracy. Moreover, it is noteworthy that the proposed method included lower LODs compared to the other previously reported methods, indicating outstanding extraction efficiency of the proposed D-micro-SPE method. Compared to other studies, the established method had RSD values considerably or marginally lower. In the Khedr method, the RSD values are less than 3.9%.67 Although this method achieves higher precision, it has lower sensitivity and lower linear range compared to the proposed method. In this study, for effective PAE extraction, only a small quantity of core–shell Fe3O4@[Bimi]Cl NPs was needed, probably due to their excellent adsorption capability. Besides, as a result of the fast magnetic separation, the magnetic adsorbent could be simply isolated from sample after extraction and elution. In addition, the adsorbents can be reused several times. Moreover, in the present study, only limited organic solvent was utilized (no use of water-immiscible hazardous solvents), making it an environmentally friendly method. Optimizing the parameters influencing the PAE extraction using a multivariate technique in terms of the RSM is another difference in the present work. This technique was used to assess the effects of individual factors and their interactions on the extraction efficiency; consequently, the true optimum level was recognized with the minimum cost, time, chemicals, and labor effort. These outcomes indicate that the high sensitivity and efficiency provided by the proposed method contributes to the determination of PAEs at trace levels in complex matrices with a simple, low-cost and environmentally friendly mode.
| Method | Instrument | Linearity (μg L−1) | LOD (μg L−1) | Recovery (%) | Precision (RSD%) | Sorbent dosage (mg) | Solvent used | References |
|---|---|---|---|---|---|---|---|---|
| a Molecularly imprinted solid-phase extraction. | ||||||||
| SPE | 100–1000 | 0.013 | 97–109 | ≤3.9 | 2000 | 50 mL of MeOH containing 10% NH3 | 67 | |
| MIP-SPEa | HPLC-MS/MS | 10–500 | 0.16–17.6 | 74–97 | ≤4.9 | 200 | 4 mL of MeOH | 68 |
| DSPE | HPLC-MS/MS | 35–500 | 0.051–0.428 | 71–117 | ≤18 | 80 | 25 mL of MeCN | 69 |
| SPE | UHPLC-MS/MS | — | — | 98–106 | ≤10 | 500 | 9 mL of 0.1% formic acid in MeCN | 70 |
| D-μ-SPE | UPLC-MS/MS | 0.5–250 | 0.002–0.135 | 79–120 | ≤15 | 60 | 4 mL of dichloromethane | 8 |
| D-μ-SPE | UPLC-MS/MS | 10–300 | 0.007–0.02 | 70–117 | ≤20 | 120 | 30 mL of MeCN | 71 |
| D-μ-SPE | UPLC-MS/MS | 0.01–250 | 0.002–0.012 | 82–115 | ≤8 | 48 | 0.5 mL of MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeCN 75 MeCN 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 (v/v) 25 (v/v) |
This research |
Conclusion
The present work provided an effective and versatile adsorbent composed of Fe3O4 NPs functionalized with benzylimidazolium chloride. Moreover, it was applied in D-micro-SPE for extraction and preconcentration of PAEs from different water samples before their analysis by liquid chromatography-mass spectrometry. The validation experiment results showed acceptable precisions in the linear range. The synthesized nano-sorbent can be considered as an attractive and innovative candidate to substitute traditional DSPE adsorbents utilized for extracting such compounds from a complex matrix. Therefore, lower quantities of the adsorbent can be employed to attain satisfactory extraction effectiveness. Consequently, it is possible to use a small volume of organic solvents, which is highly favorable in green chemistry.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work supported by the South Tehran Branch of Islamic Azad University.References
- S. Net, A. Delmont, R. Sempéré, A. Paluselli and B. Ouddane, Sci. Total Environ., 2015, 515–516, 162–180 CrossRef CAS.
- Y. Yan, Y. Lu, B. Wang, Y. Gao, J. Ge, H. Liang and D. Wu, Anal. Methods, 2018, 10, 2924–2930 RSC.
- T. Karunasekara, S. N. Atapattu and C. F. Poole, Chromatographia, 2012, 75, 1135–1146 CrossRef CAS.
- M. L. Oca, L. Rubio, L. A. Sarabia and M. C. Ortiz, J. Chromatogr. A, 2016, 1464, 124–140 CrossRef CAS.
- D.-W. Gao and Z.-D. Wen, Sci. Total Environ., 2016, 541, 986–1001 CrossRef CAS.
- Q. Zhou, Z. Zheng, J. Xiao, H. Fan and X. Yan, Anal. Bioanal. Chem., 2016, 408, 5211–5220 CrossRef CAS.
- S. R. Bandforuzi and M. R. Hadjmohammadi, J. Chromatogr. A, 2018, 1561, 39–47 CrossRef CAS.
- Á. Santana-Mayor, B. Socas-Rodríguez, M. d. M. Afonso, J. A. Palenzuela-López and M. Á. Rodríguez-Delgado, J. Chromatogr. A, 2018, 1565, 36–47 CrossRef.
- Z. Lv, C. Yang, Y. Pang, W. Xie and X. Shen, Anal. Methods, 2019, 11, 3467–3473 RSC.
- H. Shirkhanloo, A. Khaligh, H. Z. Mousavi and A. Rashidi, Microchem. J., 2017, 130, 245–254 CrossRef CAS.
- F. Abujaber, M. Zougagh, S. Jodeh, Á. Ríos, F. J. Guzmán Bernardo and R. C. Rodríguez Martín-Doimeadios, Microchem. J., 2018, 137, 490–495 CrossRef CAS.
- N. Yahaya, T. Mitome, N. Nishiyama, M. M. Sanagi, W. A. Wan Ibrahim and H. Nur, J. Pharm. Innov., 2013, 8, 240–246 CrossRef.
- A. Asfaram, M. Ghaedi, A. Goudarzi, M. Soylak and S. Mehdizadeh Langroodi, New J. Chem., 2015, 39, 9813–9823 RSC.
- N. Ma, L. Zhang, R. Li, Y. Zhou, Z. Cai, C. Dong and S. Shuang, Anal. Methods, 2014, 6, 6736–6744 RSC.
- S. Naghibi and H. Sahebi, Biomed. Chromatogr., 2018, 32, e4082 CrossRef.
- E. Konoz, A. H. M. Sarrafi and H. Sahebi, Can. J. Chem., 2015, 94, 9–14 CrossRef.
- C. Liu, Y. Liao and X. Huang, Talanta, 2017, 172, 23–30 CrossRef CAS PubMed.
- M. M. Seitkalieva, V. V. Kachala, K. S. Egorova and V. P. Ananikov, ACS Sustainable Chem. Eng., 2015, 3, 357–364 CrossRef CAS.
- H. Sahebi, E. Konoz and A. Ezabadi, New J. Chem., 2019, 43, 13554–13570 RSC.
- H. Wang, H. Zhang, S. Wei and Q. Jia, J. Chromatogr. A, 2018, 1566, 23–31 CrossRef CAS.
- A. K. Tripathi, Y. L. Verma and R. K. Singh, J. Mater. Chem. A, 2015, 3, 23809–23820 RSC.
- H. Yan, M. Gao, C. Yang and M. Qiu, Anal. Bioanal. Chem., 2014, 406, 2669–2677 CrossRef CAS.
- G. Liu, P. Su, L. Yang and Y. Yang, J. Sep. Sci., 2015, 38, 3936–3944 CrossRef CAS.
- S. Chen, X. Qin, W. Gu and X. Zhu, Talanta, 2016, 161, 325–332 CrossRef CAS.
- Y. Liu, Y. Li and Y. Wei, J. Sep. Sci., 2014, 37, 3745–3752 CrossRef CAS PubMed.
- Y. Wang, M. Deng and L. Jia, Microchim. Acta, 2014, 181, 1275–1283 CrossRef CAS.
- Y. Wei, Y. Li, A. Tian, Y. Fan and X. Wang, J. Mater. Chem. B, 2013, 1, 2066–2071 RSC.
- Y. Yamini, S. Seidi and F. Latifeh, Int. J. Environ. Anal. Chem., 2017, 97, 1223–1236 CrossRef CAS.
- L. Qian, J. Sun, C. Hou, J. Yang, Y. Li, D. Lei, M. Yang and S. Zhang, Talanta, 2017, 168, 174–182 CrossRef CAS.
- A. A. Asgharinezhad, H. Ebrahimzadeh, F. Mirbabaei, N. Mollazadeh and N. Shekari, Anal. Chim. Acta, 2014, 844, 80–89 CrossRef CAS PubMed.
- R. Marcinkowska, K. Konieczna, Ł. Marcinkowski, J. Namieśnik and A. Kloskowski, TrAC, Trends Anal. Chem., 2019, 119, 115614 CrossRef CAS.
- Z. Lotfi, H. Z. Mousavi and S. M. Sajjadi, RSC Adv., 2016, 6, 90360–90370 RSC.
- F. Liu, X. Yang, X. Wu, X. Xi, H. Gao, S. Zhang, W. Zhou and R. Lu, Food Chem., 2018, 268, 485–491 CrossRef CAS.
- EU Commission, Commission Decision EC 2002/657 of 12 August 2002 implementing Council Directive 96/23/EC concerning the performance of analytical methods and the interpretation of results, Off. J. Eur. Communities: Legis., 2002, 221 Search PubMed.
- A. L. Capriotti, C. Cavaliere, F. Ferraris, V. Gianotti, M. Laus, S. Piovesana, K. Sparnacci, R. Zenezini Chiozzi and A. Laganà, Talanta, 2018, 178, 274–281 CrossRef CAS.
- W. Stöber, A. Fink and E. Bohn, J. Colloid Interface Sci., 1968, 26, 62–69 CrossRef.
- N. Fattahi, A. Ramazani and V. Kinzhybalo, Silicon, 2019, 11, 1745–1754 CrossRef CAS.
- A. Ghorbani-Choghamarani and M. Norouzi, J. Magn. Magn. Mater., 2016, 401, 832–840 CrossRef CAS.
- M. Farahi, B. Karami, R. Keshavarz and F. Khosravian, RSC Adv., 2017, 7, 46644–46650 RSC.
- M. Davudabadi Farahani and F. Shemirani, Microchim. Acta, 2012, 179, 219–226 CrossRef CAS.
- C. James, C. Ravikumar, V. S. Jayakumar and I. Hubert Joe, J. Raman Spectrosc., 2009, 40, 537–545 CrossRef CAS.
- A. B. Hashkavayi and J. B. Raoof, Biosens. Bioelectron., 2017, 91, 650–657 CrossRef CAS.
- M. Anbarasu, M. Anandan, E. Chinnasamy, V. Gopinath and K. Balamurugan, Spectrochim. Acta, Part A, 2015, 135, 536–539 CrossRef CAS PubMed.
- V. A. J. Silva, P. L. Andrade, M. P. C. Silva, A. Bustamante D, L. De Los Santos Valladares and J. Albino Aguiar, J. Magn. Magn. Mater., 2013, 343, 138–143 CrossRef CAS.
- H. Touir, J. F. Morhange and J. Dixmier, Solid State Commun., 1999, 110, 315–319 CrossRef CAS.
- X. Teng, D. Black, N. J. Watkins, Y. Gao and H. Yang, Nano Lett., 2003, 3, 261–264 CrossRef CAS.
- R. Liu, Y. Zhao, R. Huang, Y. Zhao and H. Zhou, Eur. J. Inorg. Chem., 2010, 2010, 4499–4505 CrossRef.
- L. Bekalé, S. Barazzouk and S. Hotchandani, J. Mater. Chem., 2012, 22, 2943–2951 RSC.
- A. Maetz, L. Delmotte, G. Moussa, J. Dentzer, S. Knopf and C. M. Ghimbeu, Green Chem., 2017, 19, 2266–2274 RSC.
- H. Yan, J. Zhang, C. You, Z. Song, B. Yu and Y. Shen, Mater. Chem. Phys., 2009, 113, 46–52 CrossRef CAS.
- T. Wang, R. Zhang, D. Li, P. Su and Y. Yang, J. Sep. Sci., 2019, 42, 1600–1609 CrossRef CAS PubMed.
- J. M. Berg, A. Romoser, N. Banerjee, R. Zebda and C. M. Sayes, Nanotoxicology, 2009, 3, 276–283 CrossRef CAS.
- H. Sahebi, E. Konoz and A. Ezabadi, New J. Chem., 2019, 43, 13554–13570 RSC.
- L. Vinicius de Faria, T. P. Lisboa, R. Arromba de Sousa, G. S. Gonçalves de Carvalho, M. A. Costa Matos and R. C. Matos, Anal. Methods, 2018, 10, 5327–5334 RSC.
- T. Shojaeimehr, F. Rahimpour, M. A. Khadivi and M. Sadeghi, J. Ind. Eng. Chem., 2014, 20, 870–880 CrossRef CAS.
- S. M. Pourmortazavi, H. Sahebi, H. Zandavar and S. Mirsadeghi, Composites, Part B, 2019, 175, 107130 CrossRef CAS.
- M. H. Essa, N. D. Mu’azu, S. Lukman and A. Bukhari, Soil and Sediment Contamination: An International Journal, 2015, 24, 30–48 CrossRef CAS.
- E. Bazrafshan, T. J. Al-Musawi, M. F. Silva, A. H. Panahi, M. Havangi and F. K. Mostafapur, Microchem. J., 2019, 147, 643–653 CrossRef CAS.
- B. K. Matuszewski, M. L. Constanzer and C. M. Chavez-Eng, Anal. Chem., 2003, 75, 3019–3030 CrossRef CAS PubMed.
- C. Ferrer Amate, H. Unterluggauer, R. J. Fischer, A. R. Fernández-Alba and S. Masselter, Anal. Bioanal. Chem., 2010, 397, 93–107 CrossRef CAS PubMed.
- S. Wang, M. Cyronak and E. Yang, J. Pharm. Biomed. Anal., 2007, 43, 701–707 CrossRef CAS PubMed.
- J. D. Carrillo, M. P. Martínez and M. T. Tena, J. Chromatogr. A, 2008, 1181, 125–130 CrossRef CAS PubMed.
- D. Castilla-Fernández, D. Moreno-González, M. Beneito-Cambra and A. Molina-Díaz, Anal. Bioanal. Chem., 2019, 411, 1433–1442 CrossRef PubMed.
- I. Zabaleta, E. Bizkarguenaga, A. Prieto, M. Ortiz-Zarragoitia, L. A. Fernández and O. Zuloaga, J. Chromatogr. A, 2015, 1387, 13–23 CrossRef CAS PubMed.
- A. L. Heffernan, K. Thompson, G. Eaglesham, S. Vijayasarathy, J. F. Mueller, P. D. Sly and M. J. Gomez, Talanta, 2016, 151, 224–233 CrossRef CAS PubMed.
- H. Sahebi, S. M. Pourmortazavi, H. Zandavar and S. Mirsadeghi, Analyst, 2019 10.1039/C9AN01654F.
- A. Khedr, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2013, 930, 98–103 CrossRef CAS PubMed.
- M. C. Barciela-Alonso, N. Otero-Lavandeira and P. Bermejo-Barrera, Microchem. J., 2017, 132, 233–237 CrossRef CAS.
- J. González-Sálamo, J. Hernández-Borges, M. d. M. Afonso and M. Á. Rodríguez-Delgado, J. Sep. Sci., 2018, 41, 2613–2622 CrossRef PubMed.
- S. Huysman, L. Van Meulebroek, O. Janssens, F. Vanryckeghem, H. Van Langenhove, K. Demeestere and L. Vanhaecke, Anal. Chim. Acta, 2019, 1049, 141–151 CrossRef CAS PubMed.
- J. González-Sálamo, M. Á. González-Curbelo, J. Hernández-Borges and M. Á. Rodríguez-Delgado, Talanta, 2019, 195, 236–244 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ay02073j |
| This journal is © The Royal Society of Chemistry 2020 |