Application of ratiometric fluorescence sensor-based microwave-assisted synthesized CdTe quantum dots and mesoporous structured epitope-imprinted polymers for highly efficient determination of tyrosine phosphopeptide†
Nasibeh
Saeedzadeh Amiri
and
Mohammad-Reza
Milani Hosseini
 *
*
Research Laboratory of Real Samples Analysis, Department of Analytical Chemistry, Faculty of Chemistry, Iran University of Science and Technology, Tehran, Iran. E-mail: drmilani@iust.ac.ir; Fax: +982177491204; Tel: +982177491208
First published on 4th December 2019
Abstract
Molecularly imprinted polymer-coated quantum dots (MIP@QDs) as fluorescent sensors modified with ratiometric fluorescence techniques and mesoporous structured epitope-imprinted silica materials (QDs@SiO2@EMSiO2) were fabricated and applied for the detection of tyrosine phosphopeptide (pTyr) for the first time. The probe exhibited dual-emission peaks at 520 and 625 nm under the single excitation wavelength of 400 nm. The pTyr molecules could selectively quench the QDs, which had red emission, but the intensity of the QDs with green emission did not change; thus, it was considered as an internal reference. These phenomena caused a discernible change in the fluorescence color from orange to green under a UV lamp. In this work, phenylphosphonic acid and 1-tetradecyl-3-methylimidazolium chloride were utilized as the template molecule and template for mesoporous silica, respectively. 1-Propyltrimethoxysilane-3-methylimidazolium chloride was applied as the functional monomer and 3-aminopropyltriethoxysilane was employed as the cross-linker. Meanwhile, the conventional heating technique, namely, hydrothermal synthesis was substituted with the microwave heating method, resulting in fast, uniform and controllable nucleation and growth of the nanoparticles. The suggested epitope-imprinted polymer-coated mesoporous structured fluorescence composite compared with the non-mesoporous structured composite and single-emission MIP biosensor demonstrated excellent efficiency. Under the optimum conditions, the linear range and the detection limit obtained were 0.07–230 μM and 34 nM, respectively. Besides, the suggested nano-biosensor depicted highly reproducible results in the probing of real samples, with recoveries between 95.85% and 103.23%.
Introduction
The need for the recognition of a particular protein as a biomarker of diseases, health conditions, environmental investigation, control of fermentation, and food quality monitoring indicates that the fabrication of highly efficient biosensors in order to fulfil these aims will become progressively more significant.1 Reversible tyrosine phosphorylation has a significant role in several cellular procedures such as growth, differentiation, and migration. The balanced action of protein-tyrosine kinases and protein-tyrosine phosphatases firmly controls phosphotyrosine signalling.2 Hyper-phosphorylation at tyrosine is generally found in tumor proteomes and particular phosphopeptides or phosphoproteins can be considered as biomarkers beneficial for the diagnosis of cancer and in therapeutics.3 Thus, the recognition of tyrosine phosphorylated proteins and the analysis of their participation in signaling pathways are significant. Several techniques for the determination of phosphopeptides such as the use of specific antibodies,4 metal oxide affinity chromatography (MOAC),5,6 strong cation-exchange chromatography,7,8 immobilized metal affinity chromatography (IMAC),9,10 and electrostatic repulsion hydrophilic interaction chromatography11,12 have been employed. However, the immune-affinity recognition relying on antibodies and chemical qualification suffers from inevitable difficulties such as expensive antibodies, protein degradation, and confined applicability. Although some techniques such as MOAC and IMAC have unique characteristics including universality, cost-effectiveness, and insignificant effects on the structural integrity, the binding of non-specific proteins or peptides has restricted their extensive applications.13 Molecularly imprinted polymers (MIPs) have attracted much consideration among the strategies utilized for obtaining specific detection characteristics. Molecular imprinting is an excellent technique for building synthetic and tailor-made diagnosis cavities with the desired selectivity for different molecules. MIPs display intrinsic advantages in comparison with enzymes, antibodies, or biological molecules: (i) the synthesis of MIPs is comparatively easy and cost-effective; (ii) good physical and chemical stability is obtained by MIPs; (iii) MIPs can be employed in harsh chemical media without the loss of their powerful characteristics.14 Generally, in the imprinting procedure, the copolymerization of the functional monomers and cross-linkers helps create molecularly imprinted polymers (MIPs) in the presence of a target molecule that acts as the template. Complementary binding cavities are created in the polymer matrices via the removal of the template, permitting selective rebinding of the template.15 While preparing MIPs for small molecules has been developed and modified in terms of selectivity, the imprinting of large and complex molecules needs to be extended. A feasible approach for imprinting unstable larger molecules relies on the utilization of stable templates, corresponding to the substructures or epitopes of the target. Short sequences of amino acids are utilized as the templates in an epitope imprinting technique in order to bind a bigger analyte molecule including the epitope series as its terminal section.16 This strategy has been eventually incorporated with surface imprinting methods to generate MIPs representing cross-reactivities with peptides17 or more efficiently with proteins18 that rely on only the terminal series of a short peptide, which is complementary. However, specific recognition in biosensing needs the probing of the interaction between a molecule and its receptor. Biosensors can transduce specific molecular detection events into electrical, optical, or mechanical signals by these interactions.19 In recent years, semiconductor quantum dots (QDs) have attracted much consideration for utilization in electrical biosensors because of their nanoscopic and electrical characteristics.20–22 QDs have a very high degree of brightness, size-controllable fluorescence emission, large absorption coefficients, narrow spectral line widths, and distinguished stability against photobleaching in comparison with organic fluorophores. These unparalleled characteristics have attracted great interests in the application of QDs in biosensors and bioassays.23 In this article, an epitope-imprinted polymer incorporated with a quantum dot biosensor was suggested for the highly sensitive and selective recognition of tyrosine phosphopeptide (pTyr). During probe preparation, the selectivity was supplied by the molecular imprinting methods attained by the epitope approach. Meanwhile, in order to improve the sensitivity of the probe, three strategies were applied: (i) employing a ratiometric fluorescence technique,24–27 which uses two different fluorescence emissions for self-calibration based on the core–shell structured composite. (ii) The second strategy involved utilizing periodic mesoporous silica particle imprinting, resulting in large pore volume and nanosized pore wall thickness in silica matrices in order to provide more chances for pTyr to quench the QDs. In order to construct the proposed probe, the green-emitting QDs were embedded in silica nanoparticles to perform as blank (core) and the red ones were deposited on the silica-coated green-emitting QDs (shell). This part constructed the fluorescence section of the sensor. Subsequently, the fluorescence part was coated with the mesoporous structured epitope-imprinted polymer. Only the silica shell contained the molecular diagnosis cavities. (iii) The third strategy involved applying microwave irradiation for the synthesis of nanoparticles. The synthesis with microwave irradiation afforded unique nanoparticles with a high quantum yield. Compared with the conventional MIP@QD sensors, they provided more sensitivity and selectivity, which resulted in the highly efficient determination of the target analyte. The suggested strategy was utilized in order to generate a ratiometric mesoporous biosensor based on Mn-doped ZnS@Cu-doped ZnS QDs as ratiometric fluorescence dyes by the present research group and it was successfully employed for the quantification of cytochrome c in real samples.28 This probe was successfully utilized for the highly efficient detection of pTyr in real samples, whereas sample preparation was not necessary. The suggested sensor can be successfully developed for the sensitive detection of the ultra-trace amounts of wide groups of proteins and other macromolecules.Experimental
Reagents and chemicals
All solution preparation was performed using water with conductivity less than 0.1 μS cm−1, purified via the Milli-Q system. These solutions were made of analytical grade chemicals produced without any treatment or subsequent purification. Cadmium chloride (CdCl2, 99.99%), sodium tellurite (Na2TeO3, 99.0%), sodium citrate tribasic dihydrate (C6H5Na3O7·2H2O, 99.0%), sodium borohydride (NaBH4, 96.0%), 4-mercaptobutyric acid (C4H8O2S, MBA, 99.0%), tetraethoxysilane (TEOS, 98%), ammonia solution (25–28%), 3-aminopropyltriethoxysilane (APTES, ≥98%), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC, ≥98%) and N-hydroxysuccinimide (NHS, 98%), urea (98%), glutamic acid, ascorbic acid, glucose, fructose, acetone, ethanol, and all other reagents were purchased from Sigma-Aldrich (USA). 1-Chlorooctadecane (CH3(CH2)16CH2Cl, 96%), sodium carbonate, and sodium hydrogen carbonate were purchased from Merck (Darmstadt, Germany). 1-Propyltrimethoxysilane-3-methylimidazolium chloride (PTESMIC) was purchased from Cheng Jie and 1-methylimidazole was obtained from Aladdin company (China). The standard pTyr peptide (TRG[pY]GTSTRK), pTyr1 peptide (TRDI[pY]ETDYYRK), pThr peptide (GP[pT]LTYGAR), and pSer peptide (TVDME[pS]TEVF) were purchased from ChinaPeptides Co., Ltd. Cytochrome c (Cyt c, MW = 12.4 kDa), hemoglobin (Hb, MW = 65 kDa), and myoglobin (Mb, MW = 44 kDa) were supplied by Solarbio (China).Apparatus
Fourier transform infrared (FT-IR) spectroscopic measurements were performed on a Shimadzu FTIR-4800S. The synthesis of CdTe QDs was performed by utilizing a single mode CEM Discover SP® microwave synthesis platform. The device was operated by utilizing Synergy™ software (Matthews, NC, US) to adjust the stirring and also to control the irradiation power (0–300 W), reaction temperature (30–300 °C), and pressure power (0–200 psi). The reactions were carried out in 35 mL borosilicate glass vessels. An integrated infrared (IR) sensor was used for continuously monitoring the temperature. An automated pressure sensing and controlling system (ActiVentTM), and an active cooling system (PowerMAXTM) equipped within the microwave was utilized to carry out the synthesis under controlled pressure and to guarantee a quick decrease in the reaction vessel temperature at the end of the synthesis. Absolute and photoluminescence quantum yield spectrometer (C11347-11; Hamamatsu, Japan) supplied with an integration sphere was employed to determine the quantum yield amounts of the synthesized quantum dots nanoparticles.The fluorescence emission spectra of the QDs were obtained by using a RF-6000 spectrofluorophotometer (Shimadzu Co.). Transmission electron microscopic (TEM) images were obtained using a Philips CM30 TEM. The Brunauer–Emmett–Teller (BET), Barrett–Joyner–Halenda (BJH) techniques, and nitrogen adsorption porosimetry carried out on a model ASAP 2020 instrument (Micromeritics Instrument Corporation) were utilized to calculate the surface areas and to obtain the pore size distribution data. The X-ray diffraction (XRD) spectra were obtained by employing an X-ray diffractometer (Shimadzu XRD-6100).
Synthesis of tailor-made microwave-assisted CdTe QDs with two different sizes
The synthesis of MBA–CdTe QDs was carried out according to the reported route described by Ribeiro et al.29 Typically, 2.5 × 10−4 mol CdCl2 was mixed with an equi-molar quantity of MBA in 25 mL Milli-Q water. Then, in another beaker, 5.0 × 10−4 mol Na2TeO3, 8.0 × 10−4 mol NaBH4, and 2.5 × 10−4 mol citrate were placed separately, the mixture of CdCl2 and MBA was injected to the proposed beaker, and the pH of the obtained solution was adjusted to 11 with NaOH solution (1.0 M mol L−1) under magnetic stirring. The molar ratio of Cd2+/Te2−/MBA was adjusted to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The CdTe precursor solution was transferred into a 35 mL reaction vessel. This mixture was utilized for the synthesis of both red-emissive and green-emissive QDs. These different-sized QDs were subsequently obtained by applying different amounts of time and temperature to the prepared mixture in the microwave device. Under microwave irradiation, the synthesis of MBA–CdTe QDs was performed at 84 °C for 53 min to obtain the core quantum dots with the emission peak at 540 nm and at 128 °C for 65 min to obtain the shell quantum dots with emission peak at 630 nm. The reaction vessel was cooled rapidly to 50 °C by utilizing a high-pressure air stream (C25 psi) connected to the synthesizer at the end of the synthesis. The obtained mixture was precipitated with ethanol and the product was collected by centrifugation at 15
1. The CdTe precursor solution was transferred into a 35 mL reaction vessel. This mixture was utilized for the synthesis of both red-emissive and green-emissive QDs. These different-sized QDs were subsequently obtained by applying different amounts of time and temperature to the prepared mixture in the microwave device. Under microwave irradiation, the synthesis of MBA–CdTe QDs was performed at 84 °C for 53 min to obtain the core quantum dots with the emission peak at 540 nm and at 128 °C for 65 min to obtain the shell quantum dots with emission peak at 630 nm. The reaction vessel was cooled rapidly to 50 °C by utilizing a high-pressure air stream (C25 psi) connected to the synthesizer at the end of the synthesis. The obtained mixture was precipitated with ethanol and the product was collected by centrifugation at 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 15 min. Finally, the MBA-capped CdTe QD nanoparticles were dried under vacuum and stored in a desiccator for subsequent utilization. The fluorimetric measurements at the end of the experiments represented the synthesis of two kinds of quantum dots with the emission peaks at 520 and 625 nm for the core and shell part of the composite, respectively, which represented a difference between the predicted and experimental values. The QY of the 520CdTe QDs and the 625CdTe QDs was 56% and 72%, respectively.
000 rpm for 15 min. Finally, the MBA-capped CdTe QD nanoparticles were dried under vacuum and stored in a desiccator for subsequent utilization. The fluorimetric measurements at the end of the experiments represented the synthesis of two kinds of quantum dots with the emission peaks at 520 and 625 nm for the core and shell part of the composite, respectively, which represented a difference between the predicted and experimental values. The QY of the 520CdTe QDs and the 625CdTe QDs was 56% and 72%, respectively.
Synthesis of amphiphilic ionic liquid 1-tetradecyl-3-methylimidazolium chloride
1-Tetradecyl-3-methylimidazolium chloride (C14MIMCl) synthesis was carried out according to the synthetic process.30 Typically, 0.1 mol 1-methylimidazole was mixed with equi-molar quantity of 1-chlorotetradecane. The obtained mixture was injected into a 250 mL flask and refluxed at 90 °C overnight. A solid with white color was obtained while the solution was cooled to room temperature. After that, the product was subsequently purified by recrystallization in THF. The product was washed with THF several times and after that, it was collected and dried under vacuum at room temperature.Synthesis of mesoporous structured epitope imprinted polymer capped ratiometric CdTe QDs
The green emissive QDs (520QDs, λem = 520 nm) embedded silica nanoparticles were obtained according to the reported route explained by Yao et al.31 Briefly, 40 mL ethanol, 15 mL Milli-Q water, and 2 mL 520QD solution (containing 4 mg 520QDs) were blended in a one-necked flask (volume = 100 mL) and stirred for 10 min at room temperature. The flask was packed with aluminium foil and then, 4 mL EDC/NHS solution ([EDS] = [NHS] = 10 g L−1) and 30 μL APTES were added, followed by stirring for 12 h. Followed by the addition of 130 μL TEOS dropwise into the solution, 0.5 mL ammonium hydroxide was introduced into the solution and the mixture was stirred for another 12 h in order to modify the silica surface with amino groups. The resultant mixture was centrifuged after 12 h of reaction and the precipitate was washed several times with the mixture of ethanol and acetic acid. Finally, the resulting nanoparticles were re-dispersed in 10 mL Milli-Q water for subsequent utilization.Then, the 520QDs@SiO2 were capped with 625CdTe QDs and the mesoporous structured epitope imprinted polymer capped ratiometric CdTe QDs were provided on the surface of the 520QDs@SiO2@625QDs by dummy molecular imprinting method. Firstly, 1.6 × 10−4 mol C14MIMCl and 4.3 × 10−3 mol NaOH were dissolved in 288 mL Milli-Q water immediately. Afterwards, the mixture was transferred into a three-necked flask (volume = 500 mL) and stirred at 80 °C for 15 min. Afterwards, 10 mL 520QD@SiO2 solution (containing 10 mg) was introduced into the mixture of C18MIMCl solution and NaOH. Meanwhile, 4 mL EDC/NHS solution ([EDS] = [NHS] = 10 g L−1) was added into 10 mL 625CdTe QD solution (containing 10 mg of 625CdTe QDs) in another beaker and after the carboxyl groups of the 625CdTe QDs were activated, the solution was added drop by drop into the mixture of 520QD@SiO2 and C14MIMCl, and the mixture was stirred for 30 min. Subsequently, 100 μL TEOS, 35 μL APTES, 0.4 g PTESMIC, and 25 mg N-terminal nonapeptide were added. The mixture was stirred in darkness for 24 h and the obtained product was centrifuged. The templates for molecular recognition and mesoporous structure were rinsed with a binary solvent of methanol/HCl (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) by 4 submersions for 4 h.
1) by 4 submersions for 4 h.
Also, the non-imprinted mesoporous composite was prepared in the absence of the nonapeptide template by the same process.
Measurement procedure
In all the experiments, the fluorescence (FL) measurements were carried out under the same conditions: 400 nm was chosen as the excitation wavelength, both slit widths of the excitation and emission were 5 nm, and the photomultiplier tube voltage was adopted as 800 V. Into the centrifuge tube (volume = 5 mL), 1.5 mL stock solution of the composite (100 mg L−1) was added, then different amounts of pTyr with the final concentration of 0.07–230 μM were introduced one by one in the mixture and the mixture was diluted to 3.0 mL with phosphate buffer solution (PBS, pH = 7.0, 10 mM). After mixing completely, the amount of pTyr in the mixture was measured by the spectrofluorometer.Sample preparation
In order to evaluate the practical operation of the suggested biosensor for the detection of pTyr, the tryptic digest of β-casein was employed as the representative real protein sample. In order to provide the tryptic digested β-casein, 1.0 mg β-casein was dissolved in 1.0 mL ammonium carbonate solution (50 mM) and digested for 14 h at 38 °C with protein/trypsin in the ratio of 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w). Different concentrations of pTyr (10.0, 20.0, 50.0, 100.0, 150.0, and 200.0 μM) were spiked into the tryptic digest of β-casein and then the amount of pTyr was measured according to the above procedure.
1 (w/w). Different concentrations of pTyr (10.0, 20.0, 50.0, 100.0, 150.0, and 200.0 μM) were spiked into the tryptic digest of β-casein and then the amount of pTyr was measured according to the above procedure.
Experimental design
In order to synthesize CdTe QDs with desired size, optimization of the experimental conditions is very important. Statistical optimization methods can discuss the interactions of variables in producing the process responses versus the conventional methods. One of the most powerful statistical techniques for examining multiple variables is Response Surface Methodology (RSM), which requires fewer experimental trials compared with the “one-factor-at-a-time” method.32 RSM is a mathematical method for optimizing the complex processes, which can produce an empirical model for the assessment of relationship among a series of controlled experimental factors and the obtained results. This technique is employed extensively to evaluate the effect of independent variables and to optimize the responses of processes using the adequate values of the factors in different chemical, bio-chemical, and nanochemical processes.33–35Results and discussion
The synthesis and characterization of QDs@SiO2@EMSiO2
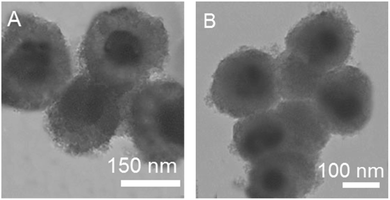
The QDs@SiO2@EMSiO2 composite was synthesized as shown in Fig. 1. Firstly, in order to get the 520QDs-embedded silica nanoparticles, the green emissive QDs, 520QDs (emission peak at 520 nm), were modified with APTES and then totally wrapped by a silica shell. The coated silica shell of the green 520QDs has exclusive benefit. According to the article published by Wu et al.,36 it modifies their optical and chemical stabilities and also inhibits the direct contact of 520QDs with the template, therefore producing a valid reference signal for the ratiometric recognition of pTyr. In the following, the amino groups of 3-aminopropyltriethoxysilane was reacted with the carboxylic groups of the 625QD surface, whose conjugation was performed by EDC and NHS; so, the surface of the 625QD nanoparticles was functionalized with APTES. In the presence of NHS, succinimidyl ester (–COOSuc)-terminal groups were prepared. The COOSuc is obtained by reacting carboxyl end groups with NHS in the presence of a water-soluble carbodiimide (here, it is EDC).37 Then, the 625QDs were attached chemically on the surface of the silica nanoparticles (520QDs@SiO2) by a condensation process to get the ratiometric fluorescence probe with dual emissions at 520 and 625 nm. The mesoporous structured epitope imprinted part (EIMs) was prepared by combining the epitope imprinting technique and the sol–gel method with mesoporous approach. The amphiphilic IL with an imidazole ring (C14MIMCl) was employed as the surfactant. Amphiphilic ionic liquids (ILs), including an imidazolium cation and a hydrophobic alkyl chain accompanied by a kosmotropic anion could produce the mesophase configuration in the synthetic medium and generate well-ordered mesoporous materials. The mesopore size can be tuned by varying the length of the alkyl chain and the anion. In addition, ILs with an imidazolium cation as the polar group could provide multiple interactions in immobilizing the template molecules, which guarantees the adsorption capacity of the MIPs.30 So, via several interactions produced by surfactant–template complexes such as ion–ion electrostatic interaction, π–π stacking, and hydrogen bonding, the nonapeptide templates were attached on the surface of the C14MIMCl micelle rods. After elimination of the surfactant–template complexes, all of the imprinted sites were created on the surface of the mesoporous channels. The prepared EIMs could be the desired sorbent for the selective recognition of pTyr. The quenching of fluorescence intensity, which happened between the CdTe QDs and the pTyr, represented the concentration of polypeptide in the samples. In addition, non-imprinted QDs@SiO2@EMSiO2 were prepared under the same conditions but without adding the template molecule.Fig. S2† shows the fluorescence intensities of the NIP coated QDs (A) and MIP-coated QDs after (B) and before (C) removal of the template. The structure and optical properties of the obtained mesoporous structured epitope imprinted polymer capped ratiometric fluorescence probe were studied in detail. First, the morphology of the fluorescence probe was characterized by TEM. QDs@SiO2@EMSiO2 (Fig. 2A) and non-imprinted QDs@SiO2@EMSiO2 (Fig. 2B) show the same diameter (approximately 180 nm).
The TEM images of 520QDs (A), 625QDs (B), and 520QDs@SiO2 (C) are also illustrated in Fig. S1.† The average size of these nanoparticles was also calculated (2.6 nm for 520QDs, 3.5 nm for 625QDs, and 100 nm for 520QDs@SiO2).
The fluorescence spectra of the green emissive QDs (a), the ratiometric probe (b), and red emissive QD solutions (c) are shown in Fig. 2S and 3S.† The green QD-embedded silica nanoparticles displayed fluorescence maximum at 520 nm and show strong intense fluorescence excited by UV light at 400 nm. When the red emissive QD embedded silica nanoparticles were coated on the surface of the green QD-embedded silica nanoparticles, the ratiometric fluorescence probe represented well-resolved dual emission bands under excitation wavelength of 400 nm and showed yellow-orange fluorescence color. These outcomes all demonstrate that the core–shell structured ratiometric fluorescence probe was prosperously manufactured. A slight decrease in the fluorescence intensities of the composite in comparison with the 520QDs and 625QDs was because of the surface modification of these QDs.
The N2 adsorption–desorption isotherm (Fig. 4S(A)†) and pore size distribution of the QDs@ SiO2@EMSiO2 composite (Fig. 4S(B)†) were investigated. As can be seen from the figure, the pore diameter of the composite was determined to be 3.74 nm and the specific surface area was calculated to be 826 m2 g−1.
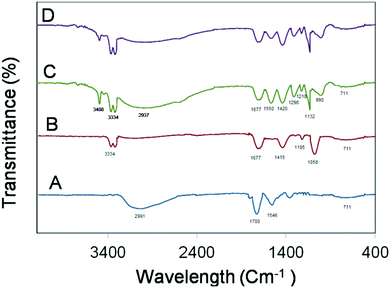
The FT-IR spectra of 520CdTe QDs (A), 520CdTe QDs@SiO2 (B), QDs@SiO2@EMSiO2 before (C) and after (D) washing were recorded and compared in the region of 400–4000 cm−1 in Fig. 3. As it is understood from the FT-IR absorption spectrum of CdTe QDs, the absorption of the stretching vibration of O–H is displayed at 2991 cm−1. Additionally, the bands at about 1700 and 1546 cm−1 belong to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration. All these peaks confirm the presence of carboxyl groups, which are related to the presence of the carboxylic acid group of MBA. In 520CdTe QDs@SiO2, the carboxyl group was modified with amino-group of APTES and the amide group disappeared; instead, the C
O stretching vibration. All these peaks confirm the presence of carboxyl groups, which are related to the presence of the carboxylic acid group of MBA. In 520CdTe QDs@SiO2, the carboxyl group was modified with amino-group of APTES and the amide group disappeared; instead, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration and N–H stretching vibration of amide appeared at 1677 cm−1 and 3334 cm−1, respectively. Meanwhile, a new band at 1415 nm appeared, which belongs to the C–N stretching vibration. Two peaks at about 1195 cm−1 and 1058 cm−1 in the FT-IR absorption spectrum of 520CdTe QDs@SiO2 are attributed to the Si–O–Si and Si–N asymmetric stretching, respectively, which guarantee the attachment of the silica part on the 520CdTe QDs.38–40
O stretching vibration and N–H stretching vibration of amide appeared at 1677 cm−1 and 3334 cm−1, respectively. Meanwhile, a new band at 1415 nm appeared, which belongs to the C–N stretching vibration. Two peaks at about 1195 cm−1 and 1058 cm−1 in the FT-IR absorption spectrum of 520CdTe QDs@SiO2 are attributed to the Si–O–Si and Si–N asymmetric stretching, respectively, which guarantee the attachment of the silica part on the 520CdTe QDs.38–40
 | ||
| Fig. 3 FT-IR spectra of 520CdTe (A), 520CdTe QDs@SiO2 (B), QDs@SiO2@EMSiO2 before (C) and after (D) washing. | ||
The FT-IR spectra of QDs@SiO2@EMSiO2 before and after washing were also recorded. As it is understood from the FT-IR absorption spectra of QDs@SiO2@EMSiO2 before washing, the absorption of stretching vibration of N–H belonging to the amine group of peptide sequence appeared at 3488 cm−1 while the amide N–H vibrations emerged at 3334 cm−1, which is higher than the one in the 520CdTe QDs@SiO2 spectrum, thus illustrating that the amide group on the surface of both 520CdTe QDs@SiO2 and 625CdTe QDs@SiO2 composites was created. The increase in the height of the N–H vibrations peak at 3334 cm−1 confirms that the QDs@SiO2@EMSiO2 composite had more amide groups. The strong and broad peak at about 2937 cm−1 is the characteristic peak for O–H stretching vibration, which belongs to the carboxyl group of the analyte. The peak at 1677 cm−1 belongs to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration of amide. Additionally, the absorption peaks at about 1550 cm−1 and 1420 cm−1 indicate the presence of N–H bending in the amine and C–N stretching vibration of amide in the proposed composite. The C–O stretching band of carboxyl and C–N stretching band of amine emerged at 1298 cm−1 and 1218 cm−1, respectively. The strong 1132 peak at about 1030 cm−1 is indicative of the Si–O–Si asymmetric stretching vibration and the band at about 990 cm−1 illustrates the Si–N vibration, which is related to the sol–gel part. The peak at 711 cm−1 is attributed to the C–H bending vibration. The QDs@SiO2@EMSiO2 spectrum after rinsing is similar to the one before rinsing but the peak intensities, which are related to the presence of the analyte such as the peaks at 3488, 2937, and 1550 cm−1, are decreased, thus proving the elimination of analytes from the composite.41
O stretching vibration of amide. Additionally, the absorption peaks at about 1550 cm−1 and 1420 cm−1 indicate the presence of N–H bending in the amine and C–N stretching vibration of amide in the proposed composite. The C–O stretching band of carboxyl and C–N stretching band of amine emerged at 1298 cm−1 and 1218 cm−1, respectively. The strong 1132 peak at about 1030 cm−1 is indicative of the Si–O–Si asymmetric stretching vibration and the band at about 990 cm−1 illustrates the Si–N vibration, which is related to the sol–gel part. The peak at 711 cm−1 is attributed to the C–H bending vibration. The QDs@SiO2@EMSiO2 spectrum after rinsing is similar to the one before rinsing but the peak intensities, which are related to the presence of the analyte such as the peaks at 3488, 2937, and 1550 cm−1, are decreased, thus proving the elimination of analytes from the composite.41
Fig. 4 shows the X-ray diffraction patterns of 520CdTe QDs (curve A) 520CdTe QDs@SiO2 (curve B), 625CdTe QDs (curve C), and the composite (curve D). From this figure, it was obviously indicated that the samples' crystal structures illustrated cubic zinc blende structure with peaks at (111), (220), and (311). The diffraction peak intensities of 520CdTe QDs@SiO2 and the composite are weaker than that of 520CdTe QDs and the total intensities of the diffraction peaks of 520CdTe QDs and 625CdTe QDs because of the imprinted polymer shell generation on the surface of the 520CdTe QDs and 625CdTe QDs. The identical XRD patterns of the different sized CdTe QDs and their composites reveal that CdTe still retains the crystal lattice of zinc blende in all conditions.42,43
 | ||
| Fig. 4 The X-ray diffraction patterns of 520CdTe QDs (A), 520CdTe QDs@SiO2 (B), 625CdTe QDs (C), and the composite (D). | ||
Effect of time and temperature on the emission wavelength of the quantum dots
Central composite design (CCD) was employed for the verification of the physical conditions for the synthesis of MBA–CdTe QDs. Two important parameters affecting the size of the quantum dots, namely, temperature and time were examined by the model. The independent variables, their symbols and levels are shown in Table 1S.† The design matrix (coded value of the variables) and responses (the maximum fluorescence emission wavelength of the synthesized QDs) are demonstrated in Table 2S.† The ANOVA data are shown in Table 3S.† This model in terms of the coded level factors is shown in eqn (1). The insignificant factors were eliminated from the equation;| R = 589.83 + 18.59A + 47.98B + 0.85B2 | (1) |
According to eqn (1), the positive and negative coefficients of the main effects illustrate how the response is altered with respect to these variables. The absolute quantum of a coefficient demonstrates the effectiveness of the related effect.
Factors affecting the enhancement of photoluminescence of the QDs@SiO2@EMSiO2 due to the presence of pTyr
Some parameters that may influence the fluorescence-quenching performance such as the pH, response time, and amount of composite were optimized.The pH value was optimized by utilizing eqn (2).
| (F0 − F/F) | (2) |
The pH value can affect the surface environment of the composite and the charge of the protein; thus, it has a considerable effect on the fluorescence intensity of the QDs. Table 4S† represents the influence of pH on the fluorescence variations of the imprinted and non-imprinted probe, and that of the NIP-coated QDs reduced rapidly as pH was enhanced due to the nucleophilic attack of OH− on the silica shell surface; thus, the shell was ionized at high pH. Therefore, this factor could affect the interaction between the composite and the template protein. The best imprinting efficiency with an imprinting factor of 5.42 was achieved at pH 7.0, so a pH of 7.0 was selected for further trials.
From the determination of fluorescence intensity of the sensor, the stability of the proposed ratiometric probe towards time was studied in the range of 2–20 min and the result is shown in Table 5S.† The signal quenching, which is defined as F0 − F (eqn (3)), was utilized for this optimization process.
| F0 − F | (3) |
The experimental results illustrated that 10 min was acceptable as the response time. Also, the stability of the sensor was studied during a week and as it is clear from Table 6S,† the probe had an acceptable stability for 7 days.
As the amount of composite significantly affects the detection sensitivity, so the amount of composite was optimized by utilizing eqn (3). From Table 7S,† the maximum sensitivity (maximum amount of (F0 − F)) was obtained when 50 mg of the composite was utilized; therefore, the concentration of the composite was fixed at 50 mg L−1 in subsequent experiments.
Validation of the synthesis optimization model
After the physical optimization process, the appropriate times and temperatures should be chosen in order to synthesis the quantum dots of both core and shell part separately. Under the optimum conditions, five different syntheses of MBA–CdTe QDs for each of the core and shell QDs were performed in order to prove that the obtained desired fluorescence emission wavelength values were comparable with those predicted by the experimental model, supporting that the applied CCD method was applicable. The results are demonstrated in Table 8S.†Analysis of real samples
To demonstrate the feasibility of the proposed method, pTyr concentration in the spiked real samples was determined. The tryptic digest of β-casein solution (100 μg mL−1) was utilized as the representative real sample to characterize the ability of the biosensor. As the sample did not contain pTyr, the sample solution was spiked with different concentrations of pTyr. The spiking recoveries of pTyr and their relative standard deviation (RSD) are summarized in Table 1. The results showed that the spiking recoveries of pTyr ranged from 95.85% and 103.23% with the RSD from 1.84–4.20%, which indicated that the probe had high ability for assaying pTyr in complex matrices.Selectivity study
The selectivity of the suggested probe was evaluated by observing the changes in the fluorescence intensities of QDs@SiO2@EMSiO2 in the presence of various interferents. For this purpose, several solutions containing constant quantities of pTyr (100 μM) and different quantities of the interferents were prepared and analyzed according to the suggested procedure. The main possible interferences that can be found in the biological samples, such as some metal ions, anions, uric acid, ascorbic acid, glucose, fructose, some proteins such as Hb, Mb, and Cyt c, and substances with similar chemical structures such as peptides like pTyr1 peptide, pSer peptide, and pThr peptide were selected in this study. An ion or substance was considered to interfere when its existence caused a variation of more than 5% in the composite fluorescence intensities. As can be understood from Table 2, the common metal ions and anions had no significant effect on the fluorescence responses of the composite (fluorescence quenching or enhancement) compared to pTyr. The result also confirmed that the suggested probe had a high specificity towards pTyr peptide, illustrating that many specific imprinted sites with the memory of the size, shape, and functional groups of PPA were produced during the synthesis of the composite, and as PPA functioned as the “epitope” of the pTyr peptide, the imprinted sites should selectively capture the pTyr peptide molecules; thus, similar chemical structures with different epitopes had no considerable effect on the fluorescence responses of the composite. For the proteins Cyt c, Hb, and Mb, it could not interact with the imprinted sites specifically as it had a larger molecular volume than that of pTyr.| Potential interferences | Added amount (molar ratio) | Relative error (%) |
|---|---|---|
| Na+, Li+, K+ | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
3.7 |
| Mg2+, Ba2+, Mn2+, Ca2+ | 1000 | 1.9 |
| Al3+, Ni2+, Cr2+, Cu2+, Fe3+ | 500 | 2.7 |
| Ag+, Pb2+ | 1000 | 3.8 |
| Cl−, CO32−, HCO3−, SO42−, C2O42−, NO3− | 1000 | 3.1 |
| Glutamic acid | 500 | 1.8 |
| Urea | 500 | 2.8 |
| Glucose, ascorbic acid, fructose | 1000 | 2.7 |
| Hb | 50 | 3.1 |
| Mb | 50 | 2.4 |
| Cyt c | 50 | 3.2 |
| pSer | 50 | 1.5 |
| pThr | 50 | 2.3 |
| pTyr1 | 50 | 1.7 |
Reusability of QDs@SiO2@EMSiO2 for the detection of pTyr
The reusability of the composite for the detection of pTyr molecules is significant because the synthesis of the material is difficult and time consuming. The reusability of the suggested probe was evaluated by utilizing it for pTyr detection while monitoring their fluorescence intensity and quenching efficiency 8 times. Fig. 5S† demonstrates that the probe could retain its fluorescence intensity and detection sensitivity without a significant drop during those 8 cycles (RSD = 4.7%).Analytical figures of merit
In order to examine the binding capacity and the fluorescent features of QD@SiO2@EMSiO2 towards pTyr, the fluorescent assays were performed and investigated by the following Stern–Volmer equation:| F0/F = 1 + Ksv[Q] | (4) |
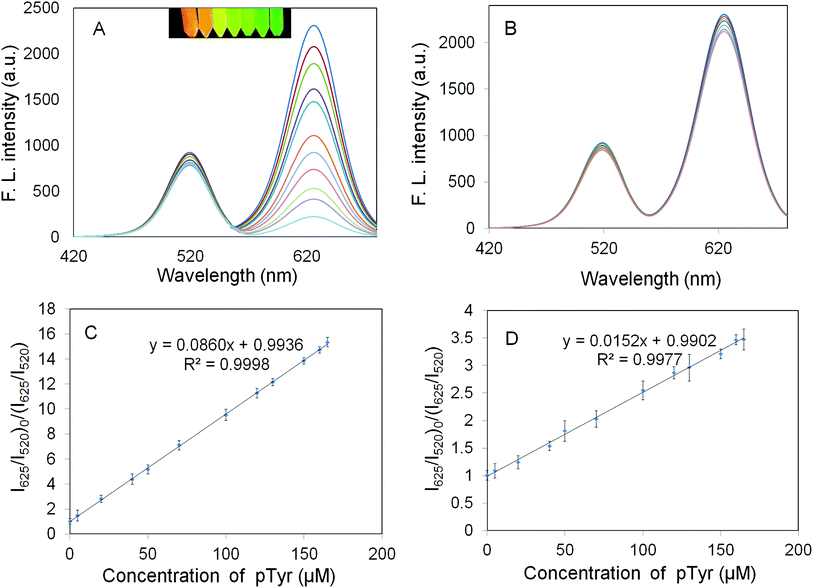
According to the Fig. 5A, in the absence of pTyr, the suggested sensor emitted two well-resolved emission peaks focused at 520 and 625 nm. After pTyr addition, the fluorescence intensity at 625 nm was constantly quenched while the one at 520 nm did not change. The fluorescence intensity ratio was closely associated with the pTyr concentration in the range of 0.07–230.0 μM, which can be employed for the identification and determination of pTyr with the detection limit of 34 nM (3S/N).
In addition, the quantity response of the NIP-coated QDs towards pTyr has been evaluated. From Fig. 5B, it can be understood that the fluorescence intensity of the NIP-coated QDs can also be quenched by pTyr.
Fig. 5C and D represent the Ksv of QDs@SiO2@EMSiO2 and the Ksv of the non-imprinted QDs@SiO2@MSiO2 corresponding to the slopes of calibration curves, respectively. The IF of 5.66 was attained, which was measured as the ratio of Ksv of QDs@SiO2@EMSiO2 to the Ksv of the non-imprinted QDs@SiO2@MSiO2 and was applied to evaluate the imprinting influence of the fluorescent probe to the protein molecules.
Comparison with the non-mesoporous and single fluorescence biosensors
The mesoporous structured ratiometric fluorescence sensor advantages were investigated by the comparison of the proposed probe with the non-mesoporous and single fluorescence one reported before.44 According to the published work, the non-mesoporous and single fluorescence sensor had a linear range of 0.50–35.00 μM with the detection limit of 0.37 μM. The comparison illustrated that the reported biosensor, compared with the proposed probe, had a very narrow linear range and higher LOD. The results illustrate that the mesoporous structured ratiometric fluorescence sensor probe possess significantly higher sensitivity and selectivity for the pTyr molecules than a non-mesoporous structured single fluorescence quenching probe.Conclusion
The mesoporous structured CdTe quantum dots capped epitope imprinted polymer biosensor has been prepared using phenylphosphonic acid as the characteristic template, which acts as the “epitope” of tyrosine phosphopeptides. In this work, the preparation of water-soluble MBA-capped CdTe QDs was figured out using the advantages of the microwave dielectric heating, thus permitting fast, identical and controlled nucleation and growth of the nanocrystals. Compared with the single-color fluorescence sensors, the ratiometric fluorescence sensors supply more accurate determination and can achieve rapid visual detection of the target protein. On the other hand, the multiple interactions between C14MIMCl and nonapeptide sequences in the mesoporous structure could guarantee the arrangement of the nonapeptide sequences at the micelle interface, which was an essential initial level for the production of imprinted sites at the mesoporous channels' surfaces. The obtained imprinted mesoporous substances supplied an appropriate pore diameter, which enhanced the mass transfer rate of pTyr. The large specific surface areas of the proposed composite ensured enough determination cavities. Meanwhile, the method proposed herein is for pTyr monitoring and it can be a universally applicable technique for other molecules' analysis.Note added in proof: After acceptance of this manuscript, it was noted that there were repeated patterns in the XRD spectrum in Fig. 4. This experiment was subsequently repeated by the authors and the corrected XRD spectrum was included prior to first publication.
Conflicts of interest
The authors declare that they have no conflict of interest.Acknowledgements
Support for this investigation by The Research Council of Iran University of Science and Technology through grant is gratefully acknowledged.References
- M. J. Whitcombe, I. Chianella, L. Larcombe, S. A. Piletsky, J. Noble, R. Porter and H. Adrian, Chem. Soc. Rev., 2011, 40, 1547–1571 RSC.
- P. J. Boersema, L. Y. Foong, V. M. Ding, S. Lemeer, B. van Breukelen, R. Philp, J. Boekhorst, B. Snel, J. den Hertog, A. B. Choo and A. J. Heck, Mol. Cell. Proteomics, 2010, 1, 84–99 CrossRef PubMed.
- D. Y. Li, Y. P. Qin, H. Y. Li, X. W. He, W. Y. Li and Y. K. Zhang, Biosens. Bioelectron., 2015, 66, 224–230 CrossRef CAS PubMed.
- P. Mallick and B. Kuster, Nat. Biotechnol., 2010, 2821, 695–709 CrossRef PubMed.
- S. Piovesana, A. L. Capriotti, C. Cavaliere, F. Ferraris, D. Iglesias, S. Marchesan and A. Laganà, Anal. Chem., 2016, 88, 12043–12050 CrossRef CAS PubMed.
- A. Leitner, M. Sakeye, C. E. Zimmerli and J. H. Smått, Analyst, 2017, 142, 1993–2003 RSC.
- S. A. Beausoleil, M. Jedrychowski, D. Schwartz, J. E. Elias, J. Villen, J. X. Li, M. A. Cohn, L. C. Cantley and S. P. Gygi, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 12130–12135 CrossRef CAS PubMed.
- H. Buncherd, W. Roseboom, D. Chokchaichamnankit, P. Sawangareetrakul, A. Phongdara, C. Srisomsap, L. de Jong and J. Svasti, Rapid Commun. Mass Spectrom., 2016, 30, 1695–1704 CrossRef CAS PubMed.
- J. Lu, Y. Li and C. H. Deng, Nanoscale, 2011, 3, 1225–1233 RSC.
- H. L. Yang, C. H. Deng and X. M. Zhang, Talanta, 2016, 153, 285–294 CrossRef CAS PubMed.
- A. J. Alpert, O. Hudecz and K. Mechtler, Anal. Chem., 2015, 87, 4704–4711 CrossRef CAS PubMed.
- S. Loroch, R. P. Zahedi and A. Sickmann, Anal. Chem., 2015, 87, 1596–1604 CrossRef CAS.
- Z. G. Wang, N. Lv, W. Z. Bi, J. L. Zhang and J. Z. Ni, ACS Appl. Mater. Interfaces, 2015, 7, 8377–8392 CrossRef CAS PubMed.
- R. Schirhagl, Anal. Chem., 2013, 86, 250–261 CrossRef PubMed.
- L. Wu, Z. Z. Lin, H. P. Zhong, A. H. Peng, X. M. Chen and Z. Y. Huang, Food Chem., 2017, 229, 847–853 CrossRef CAS PubMed.
- B. Sellergren and A. J. Hall, Molecularly Imprinted Polymers, John Wiley & Sons, Ltd, 2012 Search PubMed.
- M. M. Titirici, A. J. Hall and B. Sellergren, Chem. Mater., 2003, 15(4), 822–824 CrossRef CAS.
- H. Nishino, C. S. Huang and K. J. Shea, Angew. Chem., Int. Ed., 2006, 45(15), 2392–2396 CrossRef CAS.
- M. D. Baaske, M. R. Foreman and F. Vollmer, Nat. Nanotechnol., 2014, 9(11), 933 CrossRef CAS.
- I. L. Medintz, A. R. Clapp, H. Mattoussi, E. R. Goldman, B. Fisher and J. M. Mauro, Nat. Mater., 2003, 2, 630 CrossRef CAS.
- W. R. Algar, A. J. Tavares and U. J. Krull, Anal. Chim. Acta, 2010, 673, 1–25 CrossRef CAS PubMed.
- X. Ji, J. Zheng, J. Xu, V. K. Rastogi, T. C. Cheng, J. J. DeFrank and R. M. Leblanc, J. Phys. Chem. B, 2005, 109, 3793–3799 CrossRef CAS.
- Z. Li, Y. Wang, J. Wang, Z. Tang, J. G. Pounds and Y. Lin, Anal. Chem., 2010, 82, 7008–7014 CrossRef CAS.
- S. Xu and H. Lu, Biosens. Bioelectron., 2015, 73, 160–166 CrossRef CAS.
- J. Yao, K. Zhang, H. Zhu, F. Ma, M. Sun, H. Yu, J. Sun and S. Wang, Anal. Chem., 2013, 85, 6461–6468 CrossRef CAS.
- X. Yan, H. Li, X. Han and X. Su, Biosens. Bioelectron., 2015, 74, 277–283 CrossRef CAS.
- K. Zhang, H. Zhou, Q. Mei, S. Wang, G. Guan, R. Liu, J. Zhang and Z. Zhang, J. Am. Chem. Soc., 2011, 133, 8424–8427 CrossRef CAS.
- M. R. Milani Hosseini and N. Saeedzadeh Amiri, Anal. Methods, 2019 10.1039/c8ay02773k.
- D. S. M. Ribeiro, G. C. S. de Souza, A. Melo, J. X. Soares, S. S. M. Rodrigues, A. N. Araújo, M. C. B. S. M. Montenegro and J. L. M. Santos, J. Mater. Sci., 2017, 52, 3208–3224 CrossRef CAS.
- Z. Li, P. Guan, X. Hu, S. Ding, Y. Tian, Y. Xu and L. Qian, Polymers, 2018, 10, 298–311 CrossRef.
- J. Yao, K. Zhang, H. Zhu, F. Ma, M. Sun, H. Yu, J. Sun and S. Wang, Anal. Chem., 2013, 85(13), 6461–6468 CrossRef CAS.
- A. Nazari, M. Mirjalili, N. Nasirizadeh and S. Torabian, J. Ind. Eng. Chem., 2015, 21, 1068–1076 CrossRef CAS.
- B. Qi, X. Chen, F. Shen, Y. Su and Y. Wan, Ind. Eng. Chem. Res., 2009, 48, 7346–7353 CrossRef CAS.
- R. Tatineni, K. K. Doddapaneni, R. C. Potumarthi and L. N. Mangamoori, Appl. Biochem. Biotechnol., 2007, 141, 187–201 CrossRef CAS PubMed.
- Z. Kui and Q. Wang, Carbohydr. Polym., 2010, 80, 19–25 CrossRef.
- L. Wu, Q. S. Guo, Y. Q. Liu and Q. J. Sun, Anal. Chem., 2015, 87, 5318–5323 CrossRef CAS.
- S. Sam, L. Touahir, J. S. Andresa, P. Allongue, J. N. Chazalviel, A. C. Gouget-Laemmel and C. H. de Villeneuve, et al. , Langmuir, 2009, 26, 809–814 CrossRef.
- Y. Zhou, Z. B. Qu, Y. Zeng, T. Zhou and G. Shi, Biosens. Bioelectron., 2014, 52, 317–323 CrossRef CAS.
- M. Koneswaran and R. Narayanaswamy, Sens. Actuators, B, 2009, 139, 104–109 CrossRef CAS.
- J. Hou, H. Li, L. Wang, P. Zhang, T. Zhou, H. Ding and L. Ding, Talanta, 2016, 146, 34–40 CrossRef CAS.
- D. L. Pavia, G. M. Lampman, G. S. Kriz and J. A. Vyvyan, Introduction to Spectroscopy, Cengage Learning, 2008 Search PubMed.
- X. Wei, Z. Zhou, J. Dai, T. Hao, H. Li, Y. Xu, L. Gao, J. Pan, C. Li and Y. Yan, J. Lumin., 2014, 298–304 CrossRef CAS.
- M. Molaei, H. Hasheminejad and M. Karimipour, Electron. Mater. Lett., 2015, 11, 7–12 CrossRef CAS.
- Y. P. Qin, D. Y. Li, X. W. He, W. Y. Li and Y. K. Zhang, ACS Appl. Mater. Interfaces, 2016, 8, 10155–10163 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ay00276f |
| This journal is © The Royal Society of Chemistry 2020 |