Bifunctional OER/ORR catalytic activity in the tetrahedral YBaCo4O7.3 oxide†
Maria A.
Kirsanova
 *a,
Valery D.
Okatenko
a,
Dmitry A.
Aksyonov
a,
Robin P.
Forslund
b,
J. Tyler
Mefford
c,
Keith J.
Stevenson
*a,
Valery D.
Okatenko
a,
Dmitry A.
Aksyonov
a,
Robin P.
Forslund
b,
J. Tyler
Mefford
c,
Keith J.
Stevenson
 a and
Artem M.
Abakumov
a
a and
Artem M.
Abakumov
a
aCenter for Electrochemical Energy Storage, Skolkovo Institute of Science and Technology, 3 Nobel Street, Moscow 143026, Russia. E-mail: m.kirsanova@skoltech.ru
bDepartment of Chemistry, The University of Texas at Austin, 1 University Station, Austin, Texas 78712, USA
cDepartment of Materials Science & Engineering, Stanford University, 496 Lomita Mall, Stanford, California 94305, USA
First published on 27th November 2018
Abstract
Transition metal oxides, mostly perovskites, have received attention as effective catalysts for the oxygen evolution reaction (OER) and oxygen reduction reaction (ORR) in alkaline media. However, despite the numerous reaction mechanisms and descriptors proposed, the understanding of the nature of their electrochemical activity is still far from complete. Herein, we present YBaCo4O7.3 oxide with tetrahedrally coordinated Co2+/Co3+ as a fundamentally new bifunctional catalyst, which demonstrates OER activity of 1.18 mA cm−2 at an overpotential of 400 mV and ORR activity of 2.76 mA cm−2 at an overpotential of −537 mV, exceeding that of the LaNiO3 benchmark catalyst. The electrochemical activity of YBaCo4O7.3 was rationalized in terms of the Zaanen–Sawatzky–Allen model using DFT-based calculations. According to the results, overstoichiometric oxygen induces octahedral Co positions as active sites in the OER, while large Coulomb on-site repulsion energy for Co in the tetrahedral coordination makes these sites active in the ORR.
Introduction
The practical deployment of low-temperature regenerative alkaline fuel cells is impeded by significant energy loss due to the sluggish kinetics of the oxygen reduction (ORR, O2 + 2H2O + 4e− → 4OH−) and oxygen evolution (OER, 4OH− → O2 + 2H2O + 4e−) reactions.1 Transition metal oxides are considered as promising catalysts for the OER and ORR with fair potential of bifunctionality (i.e. activity for both OER and OER),2–4 resistance to corrosion in alkaline media, moderate cost and low environmental hazard. Precious group metals (PGMs) and their oxides are generally used for benchmarking the catalytic activity in the OER/ORR, but their scarcity and high cost have motivated the extensive search for possible alternatives. Transition metal (TM) oxides are known for their stability in alkaline solutions and ability to catalyze both reactions. The most common non-PGM bifunctional catalysts include the binary oxides MxOy (M = Mn, Co, Ni), oxyhydroxides (M′M′′OOH, particularly with M′, M′′ = Fe, Ni), spinels, conventional perovskites AMO3±δ, and quadruple perovskites (AMn7O12 (A = Ca and La)).5–7Although recent studies on specific perovskites have demonstrated the ability of lattice oxygen to play a role in the OER,8,9 generally the ORR/OER electrocatalytic activity of a material can be rationalized in terms of its surface reaction mechanism, which presents a complex problem requiring knowledge of intermediate steps, associated Gibbs free energies and activation barriers.10–13 However, due to the linear correlation of activation barriers with reaction free energies14–16 and linear relationships between the adsorption energies of intermediates preserved for a wide range of catalyst chemistry, crystal and surface and electronic structures,11,17,18 the qualitative understanding of catalytic activity trends can be achieved in a less sophisticated manner.11,14 For example, Rossmeisl et al. showed that the adsorption energy ΔE(O*(ads)) is a good descriptor of OER activity for rutile-type oxides.16 For perovskites in OER conditions, ΔE(O*(ads)) − ΔE(HO*(ads)) strongly correlates with activity, while for the ORR the ΔE(HO*(ads)) energy is the preferred descriptor.14 The calculation of surface binding energies is complicated by the choice of surface orientation, its termination, and identification of active sites. This type of information is also difficult to obtain both from experimental and computational studies. Hence, to simplify the situation even further, instead of adsorption energies, the electronic structure features of the bulk material are used as effective descriptors of catalytic activity. This fairly crude approach has shown to be useful due to the linear relationships between O*, OO*, HO*, and HOO* adsorption energies, and the relation of the adsorption energies to the surface electronic structure, which in turn inherits the electronic structure of the bulk material. The examples of the discussed electronic structural features are the number of electrons on the d shells19 and on the eg* orbital of the transition metal,20,21 the d-band center of the transition metal,22 the O p-band center,23 and the charge-transfer energy (Δ).24,25
The electronic structure of TM oxide catalysts is generally discussed in terms of the Zaanen–Sawatzky–Allen (ZSA) three-band model,26 in which the TM d-manifold is split due to Coulomb repulsion into two bands (upper and lower Hubbard bands, UHB and LHB, respectively) with the energy difference U, the anionic O2p band is separated from UHB by the charge transfer energy Δ, and the band overlap is controlled by the one-electron bandwidth W. The ZSA model has been demonstrated to be universal, being applicable to oxide materials with various crystal structures and transition metals. Considering ionic bonding, the band positioning with respect to each other and to the Fermi level depends on the ionization energies of the Mn+/M(n+1)+ cations and Madelung site potentials, taking into account the effect of coordination of the M cations with oxygen.27 However, this strongly simplified approach can fail in accounting for the changing coordination geometry of the TM cations (for instance, from octahedral to tetrahedral) in prediction of the true band structure,28 which may be related to the stronger hybridization of the M3d and O2p orbitals in the tetrahedral MO4 unit compared to that in MO6 octahedra.29
The crystal structures of most TM oxide catalysts are based on octahedral MO6 units (with the exception of spinels and some anion-deficient30 and hexagonal perovskites31). Considering that pure tetrahedral structures (i.e. comprised exclusively of MO4 units) have never been considered as potential catalysts for the OER and ORR in alkaline media, the investigation of their activity toward these reactions is of significant fundamental interest and may provide new insight into the relationships between the electronic structure and catalytic activity and verify the applicability of certain descriptors for materials outside the conventional field of research. Thus, herein, we suggest the complex transition metal oxide YBaCo4O7+δ as a potential bifunctional catalyst.
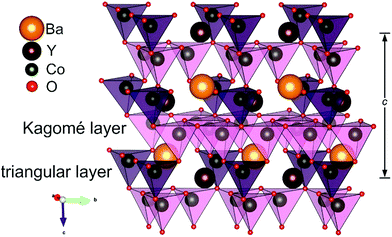
The crystal structure of YBaCo4O7+δ consists of two types of corner-sharing CoO4 tetrahedra in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio linked into a framework by corner sharing (Fig. 1). The tetrahedra are distinguished by different bond lengths and are located in alternating Kagomé and triangular layers. YBaCo4O7+δ can adopt a wide range of oxygen nonstoichiometries with δ varying from 0 to 1.5,32,33 which corresponds to a change in the cobalt average oxidation state from +2.25 to +3. YBaCo4O7+δ demonstrates the ability to reversibly intercalate impressive amounts of oxygen at temperatures ranging from 270–350 °C,32 making it a promising oxygen storage material as well. The oxygen-storage capacity (OSC) of YBaCo4O7+δ (OSC ≈ 2450 μmol-O g−1) substantially exceeds that reported for conventional oxygen-storage materials, e.g. CeO2–ZrO2 (OSC ≈ 1500 μmol-O g−1).34,35 The oxidation capacity of YBaCo4O7+δ has been intensively studied in many works34,36 by thermogravimetric measurements under an O2 flow and under anodic and cathodic polarization in alkaline aqueous solution.37 YBaCo4O7+δ possesses mixed ionic and electronic conductivity with a dominant contribution from p-type electronic conduction, demonstrating conductivity of ∼2.5 S cm−1 at 50 °C.38,39
3 ratio linked into a framework by corner sharing (Fig. 1). The tetrahedra are distinguished by different bond lengths and are located in alternating Kagomé and triangular layers. YBaCo4O7+δ can adopt a wide range of oxygen nonstoichiometries with δ varying from 0 to 1.5,32,33 which corresponds to a change in the cobalt average oxidation state from +2.25 to +3. YBaCo4O7+δ demonstrates the ability to reversibly intercalate impressive amounts of oxygen at temperatures ranging from 270–350 °C,32 making it a promising oxygen storage material as well. The oxygen-storage capacity (OSC) of YBaCo4O7+δ (OSC ≈ 2450 μmol-O g−1) substantially exceeds that reported for conventional oxygen-storage materials, e.g. CeO2–ZrO2 (OSC ≈ 1500 μmol-O g−1).34,35 The oxidation capacity of YBaCo4O7+δ has been intensively studied in many works34,36 by thermogravimetric measurements under an O2 flow and under anodic and cathodic polarization in alkaline aqueous solution.37 YBaCo4O7+δ possesses mixed ionic and electronic conductivity with a dominant contribution from p-type electronic conduction, demonstrating conductivity of ∼2.5 S cm−1 at 50 °C.38,39
Herein, we report the investigation of the activity of YBaCo4O7+δ toward the OER and ORR in alkaline media and attempt to trace the origin of its activity using the intrinsic features of its electronic structure.
Experimental
Synthesis
YBaCo4O7+δ samples were synthesized via an ethylenediaminetetraacetic acid (EDTA)-complexing method followed by high temperature annealing. Stoichiometric amounts of Y(NO3)3·6H2O (Sigma-Aldrich), Ba(NO3)2 (Ruskhim) and Co(CH3COO)2·4H2O (Sigma-Aldrich) were mixed with EDTA (Sigma-Aldrich) and dissolved in a minimum volume of distilled water (∼5 mL per 1 g of metal salts) under vigorous stirring for 10–15 min. The molar ratio between the total amount of metal ions and EDTA was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5, and 20 wt% ammonia was used to adjust the pH value between 9 and 10. The solution was heated over a burner to form a very viscous mass. After evaporation of water, the product ignited spontaneously to turn into a raw ash, which was placed in an alumina crucible and calcined in air at 650 °C for 5 h. The obtained black-colored precursor was reground in an agate mortar, pressed into a pellet, heated at 1100 °C in air for 4–8 h and then quenched to ambient temperature in air.
1.5, and 20 wt% ammonia was used to adjust the pH value between 9 and 10. The solution was heated over a burner to form a very viscous mass. After evaporation of water, the product ignited spontaneously to turn into a raw ash, which was placed in an alumina crucible and calcined in air at 650 °C for 5 h. The obtained black-colored precursor was reground in an agate mortar, pressed into a pellet, heated at 1100 °C in air for 4–8 h and then quenched to ambient temperature in air.
Powder X-ray diffraction
The phase purity and the lattice parameters were determined by powder X-ray diffraction (PXRD) using a Huber G670 Guinier camera (transmission geometry, curved primary Ge (111) monochromator, CoKα1 radiation (λ = 1.78896 Å), image plate detector). The Rietveld refinement was performed using the JANA2006 software.40Oxygen content analysis
The oxygen content was measured via a standard iodometric titration. About 15 mg of sample was placed into a glass flask and 20 mL of 20 wt% KI solution was added. Thereafter, several drops of concentrated HCl were added to dissolve the powder. The flask was tightly closed and kept in the dark for 10 minutes followed by the addition of 50 mL of distilled water. The solution was titrated with 0.0022 M sodium thiosulfate and several drops of starch were added at the very end of the titration to form a transparent violet solution. The procedure was continued until a straw-coloured solution was formed. The calculation of the Co3+ fraction was performed according to the equations:| 2Co3+(aq) + 3I−(aq) = 2Co2+(aq) + I3−(aq) |
| I3−(aq) + 2S2O32−(aq) = 3I−(aq) + S4O62−(aq) |
Electron microscopy
The morphology of the YBaCo4O7+δ crystallites was characterized via scanning electron microscopy (SEM) with a JEOL JSM-6490LV microscope at 30 kV. Electron diffraction (ED) patterns, high-angle annular dark field scanning transmission electron microscopy (HAADF-STEM) images, annular bright-field STEM (ABF-STEM) images and energy dispersive X-ray (EDX) compositional maps were obtained with an aberration-corrected Titan G3 transmission electron microscope operated at 200 kV and equipped with a Super-X EDX system. The sample for the TEM study was prepared by crushing the material under anhydrous ethanol in an agate mortar and depositing a few drops of the suspension onto a holey carbon grid mounted on a Cu support.Surface area analysis
Nitrogen sorption analysis was performed with a Quantachrome Instruments NOVA 2000 high-speed surface area analyzer at a temperature of 77 K. Prior to the measurements, the samples were degassed in vacuum for at least 12 h at room temperature. The specific surface area was calculated using the Brunauer–Emmett–Teller (BET) method from the nitrogen adsorption data in the relative pressure range (P/P0) of 0.05 to 0.30.Electrochemical characterization
YBaCo4O7+δ samples were tested for the OER and ORR and oxygen diffusion rates within the bulk material. LaNiO3 and IrO2 were used as benchmarks. For the OER and ORR comparison, LaNiO3 was tested on Vulcan carbon (VC) at 30 wt%, while IrO2 supported on VC at 20 wt% was tested for the OER only. For OER and ORR testing, the YBaCo4O7.3 sample was supported with 30 wt% catalyst on XC-72 VC via ball milling for 3 min using a Wig-L-Bug ball mill. Catalyst inks were prepared by dispersing 2 mg of the supported catalyst in 2 mL of ethanol containing 0.05 wt% Na-substituted Nafion, followed by bath sonication for 2 h. The catalyst inks were dropcast onto 5 mm glassy carbon electrodes (GCE) with 51 μg cm−2 catalyst/carbon composite, yielding 15.3 μgoxide cmgeo−2. Prior to dropcasting, the electrodes were cleaned via sonication in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 deionized (DI) water
1 deionized (DI) water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol solution followed by polishing using 0.05 μm alumina powder. The powder was removed by sonication in DI water
ethanol solution followed by polishing using 0.05 μm alumina powder. The powder was removed by sonication in DI water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol solution and dried in air. Oxygen diffusion rate tests were performed on 5 mm GCEs prepared in the same manner described above but with the catalyst supported at 85 wt% on VC.
ethanol solution and dried in air. Oxygen diffusion rate tests were performed on 5 mm GCEs prepared in the same manner described above but with the catalyst supported at 85 wt% on VC.
Electrochemical testing was performed on a Metrohm Autolab PGSTAT302N potentiostat equipped with high speed rotators from Pine Instruments. All OER and ORR measurements were performed in 0.1 M KOH at room temperature. Current interrupt and positive feedback methods were used to determine the electrolyte resistance, which was 46 Ω in 0.1 M KOH and 6 Ω in 1 M KOH. All data for the OER and ORR was iR compensated after testing. Testing was performed in a standard three electrode cell using a CH Instruments Hg/HgO (1 M KOH) reference electrode, Au wire counter electrode, and film of catalyst ink on glassy carbon as the working electrode. All potentials are reported versus the Hg/HgO (1 M KOH) reference electrode or versus the regular hydrogen electrode (RHE), which were determined experimentally to be +0.8976 V vs. Hg/HgO (1 M KOH). Cyclic voltammetry (CV) for both OER and ORR testing was performed in O2 saturated 0.1 M KOH at a rotation rate of 1600 rpm. For OER testing, the scan rate was 10 mV s−1, while 5 mV s−1 was used for ORR testing. Activities were measured at 1.63 V vs. RHE-iR for the OER and 0.693 V for the ORR. Oxygen diffusion rates were measured following an adapted method reported previously using chronoamperometry at a potential of 50 mV positive of the Co2+/Co3+ redox wave, while being rotated at 1600 rpm to ensure the experiment was performed at a high enough potential that the measured current was due to a diffusion-limited process.41–43 All OER and ORR stability experiments were performed under the same conditions and in the same experimental setup described above.
DFT calculations
Total energies and optimized geometries were calculated with the Vienna Ab Initio Simulation Package (VASP), which implemented the widely established density-functional theory (DFT).44–46 The Perdew–Burke–Ernzerhof (PBE) flavor of the generalized gradient approximation (GGA) and projected augmented wave (PAW) method were employed.47,48 The standard library potentials “Y_sv”, “Ba_sv”, “Co”, and “O”, were used. To consider the strongly correlated character of the d-electrons of cobalt, a Hubbard-like correction was added within the Dudarev scheme and U–J value of 4.2 eV.49,50 All calculations were performed with spin polarization with the antiferromagnetic ground state (AFM) and tetrahedron method with Blöchl corrections for partial occupancies. The initial crystal structures for YBaCo4O7 with P63mc (26 atoms) and YBaCo4O8 with Pbc21 (112 atoms) space groups were taken from the ICSD database.51,52 The ground state AFM configuration for YBaCo4O7 was found by checking all possible configurations (70 in total). For YBaCo4O8, the AFM configuration with the lowest energy was chosen from 10 random configurations. The convergence study showed that the energy cut-off of 400 eV and k-point grids of 4 × 4 × 3 and 2 × 2 × 3 were sufficient for the reliable description of lattice constants and formation energies. The relaxation was performed until the forces acting on atoms were smaller than 0.05 eV Å−1. The (001) surface and adsorption energies were calculated using the slab model within the periodic boundary conditions and vacuum thickness of 11 Å. For the slab calculations, only one k-point and dipole correction in the [001] direction, as well as relaxation of all atomic position were chosen. The convergence of adsorption energies was checked with respect to the increase in slab thickness in the [001] direction, increase in surface area, fixing of bottom atomic layers, and increase in k-point density and energy cut-off. The resulting precision for the calculated adsorption energies was in the order of ∼0.3 eV improvement in energy differences. The projected density of states (PDOS) was calculated using the increased k-point density of 8 × 8 × 5 and 4 × 4 × 5 for YBaCo4O7 and YBaCo4O8, respectively. To make the correct t2 and e separation of d-orbitals on Co atoms, we rotated the simulation cells in such a way that the oxygen tetrahedra coincided with the tetrahedral crystal field splitting. An effective O p-band center was determined by taking the centroid of the projected density of states of the O 2p states relative to the Fermi level.Results and discussion
Compositional and crystallographic characterization
The EDX compositional maps of the YBaCo4O7+δ crystallites showed a homogeneous distribution of Y, Ba and Co (Fig. S1 of ESI†). Iodometric titration revealed the oxygen non-stoichiometry index δ = 0.3. The PXRD pattern of YBaCo4O7.3 was indexed on a hexagonal lattice with the unit cell parameters a = 6.30038(3) Å and c = 10.24280(6) Å and refined by the Rietveld method (Fig. 2, Tables S1 and S2 in ESI†). The unit cell parameters and the c/a ratio of 1.626 correspond well to the δ = 1/3 material prepared by annealing at the partial oxygen pressure P(O2) = 0.2 bar.53 The PXRD pattern could be fitted with the highest P63mc symmetry, but the electron diffraction patterns of YBaCo4O7.3 (Fig. 3) demonstrated the presence of satellite reflections corresponding to the modulation vector q = 1/3(a* + b*) + c*. This modulation vector reduces the crystal symmetry down to trigonal, corresponding to the superspace group P31c(1/3,1/3,γ)000. Indeed, a distortion from P63mc to P31c has been reported for the room temperature structure of slightly overstoichiometric YBaCo4O7+δ.53 However, the refinement of the complete modulated structure requires synchrotron PXRD data as the satellites are too weak to be detected in the laboratory PXRD pattern. The [010] and [1![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0] high resolution HAADF-STEM images confirm that the cation arrangement well-fits the basic P31c structure (Fig. S2 in ESI†). The [1
0] high resolution HAADF-STEM images confirm that the cation arrangement well-fits the basic P31c structure (Fig. S2 in ESI†). The [1![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0] ABF-STEM image (Fig. S3†) provides a clue on the origin of the superstructure. The ABF intensity profile along the Ba/Y/Co1 triangular layers demonstrates a tripled periodicity along the {110} direction. This tripled periodicity is due to the systematic increase in ABF intensity at the positions between two neighboring Ba/Y/Co1 columns, where two out of three positions show a decrease in scattering density compared to the third (marked with arrowheads in Fig. S3,† bottom). This may be associated with the insertion of overstoichiometric oxygens into the Ba/Y/Co1 triangular layers in an ordered manner. The Kagomé layers stay intact at the given oxygen overstoichiometry index δ = 0.3.
0] ABF-STEM image (Fig. S3†) provides a clue on the origin of the superstructure. The ABF intensity profile along the Ba/Y/Co1 triangular layers demonstrates a tripled periodicity along the {110} direction. This tripled periodicity is due to the systematic increase in ABF intensity at the positions between two neighboring Ba/Y/Co1 columns, where two out of three positions show a decrease in scattering density compared to the third (marked with arrowheads in Fig. S3,† bottom). This may be associated with the insertion of overstoichiometric oxygens into the Ba/Y/Co1 triangular layers in an ordered manner. The Kagomé layers stay intact at the given oxygen overstoichiometry index δ = 0.3.
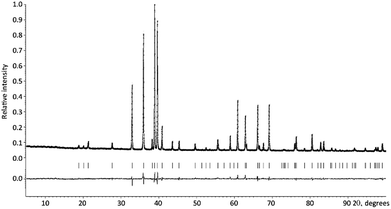 | ||
| Fig. 2 Experimental, calculated and difference PXRD patterns of YBaCo4O7.3 after Rietveld refinement. The Bragg positions are indicated with the vertical lines. | ||
 | ||
Fig. 3 Electron diffraction patterns of YBaCo4O7.3. Weak satellite reflections are visible in the [001] and [1![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0] ED patterns. 0] ED patterns. | ||
Microstructural characterization
Since YBaCo4O7.3 was prepared via high-temperature calcination, the obtained material consisted of relatively large crystallites with a size of 2–5 μm, as visualized by scanning electron microscopy (ESI, Fig. S4a†). Thus, to decrease the particle size and increase the surface area, the sample was milled in a SPEX 8000M high-energy ball mill for 20 min in ethanol. Ball milling reduced the average particle size by a factor of two (ESI, Fig. S4b†). The BET surface area measured through N2 adsorption in the milled sample was 3.3 m2 g−1 (ESI, Fig. S5†). The ABF-STEM images demonstrate that the surface of the YBaCo4O7.3 crystallites retained a high degree of crystallinity (ESI, Fig. S6†).Electrochemical characterization
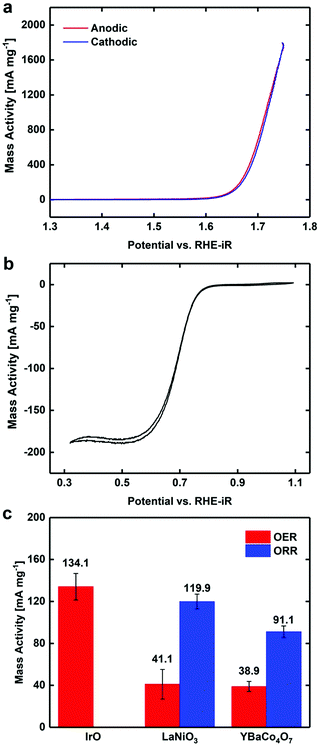
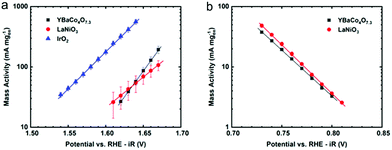
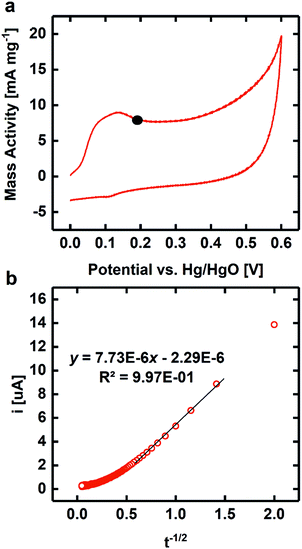
CV was used to quantify the bifunctional capabilities of YBaCo4O7.3 in the OER and ORR. The representative CVs for both OER and ORR in O2-saturated 0.1 M KOH are shown in Fig. 4. The catalyst was supported at 30 wt% on VC because without the carbon support, the catalyst itself is much less conductive than other metallic systems and only contacts the GCE through point contacts. Mixing the catalyst material with VC increased the electrical contact between adjacent catalyst particles and between the catalyst and the electrode, leading to higher conductivity. The activity of YBaCo4O7.3 towards the OER at 1.63 V vs. RHE-iR was 39 mA mg−1, which is similar to that of the benchmark catalysts in this field, such as IrO2 and LaNiO3 perovskite (see ESI Fig. S7†). Furthermore, its activity towards the ORR was measured to be −91 mA mg−1 at 0.693 V, which also compares well with the abovementioned benchmark catalysts. Although the mass activity of YBaCo4O7.3 seems to be moderate, its low surface area of 3.3 m2 g−1 has to be considered due to sintering of the metal oxide precursor particles during calcination at high temperature. The specific activities of YBaCo4O7.3 calculated with the given surface area scale were determined to be 1.18 mA cm−2 (OER) and 2.76 mA cm−2 (ORR). As the surface of the LaNiO3 sample used here for benchmarking is almost 3 times higher (11.1 m2 g−1), the specific activity of YBaCo4O7.3 in both the OER and ORR significantly exceeds that of LaNiO3 (0.37 mA cm−2 and 1.08 mA cm−2, respectively). The bifunctional window or the potential difference between the voltage at which 10 mA cmox−2 is generated for the OER and −3 mA cmox−2 for the ORR is 1.00 V. The OER Tafel slope for YBaCo4O7.3 is 58 mV dec−1 (Fig. 5), which is significantly lower than the 82 mV dec−1 measured for the benchmark precious metal catalyst IrO2. The ORR Tafel slope for YBaCo4O7.3 is 66 mV dec−1, which is similar to the Tafel slope measured for Pt. The comparisons to other well-studied TM oxides studied by our group in the same experimental setup are shown in the ESI, Table S3.†Previous studies have demonstrated that YBaCo4O7+δ can absorb and store significant amounts of oxygen upon heated in a relatively low temperature range of 270–350 °C in an O2-containing atmosphere,32,33 making it interesting as a possible pseudocapacitor material in basic electrolyte. CV was performed in Ar-saturated 1 M KOH to determine the Co redox features concomitant with oxygen intercalation. By consulting the Pourbaix diagram for cobalt, as shown in Fig. 6, Co2+ is oxidized to Co3+ at 0.2 V on the anodic scan, which is in agreement with the results reported by Dan et al.37 against an Ag/AgCl reference electrode (−0.1 V). To measure the oxygen diffusion rate within YBaCo4O7.3, chronoamperometry was performed at a potential 50 mV positive of the peak potential to ensure a diffusion-limited process and the current was plotted as a function of t−1/2. The oxygen diffusion rate was calculated by extrapolating the linear portion of the data to find the intercept with the t−1/2 axis (Fig. 6b) and then using a bounded diffusion model to calculate the diffusion rate coefficient. More details on the method used can be found elsewhere.41–43 Using an average particle size of 1 μm, as calculated from the SEM images in Fig. S4,† the oxygen diffusion rate within YBaCo4O7.3 was measured to be 2.74 × 10−10 cm2 s−1, which is faster than that reported for LaMnO3−δ in our previous work.54
To investigate the durability of YBaCo4O7.3 for the OER and ORR, both chronoamperometry and constant current stability experiments were performed under the same experimental conditions as the CV experiments described above. The chronoamperometry experiments were performed for hour periods at three potentials for both the OER and ORR. The potentials were chosen by consulting the CVs in Fig. 4 to ensure that the constant potentials were located in the OER or ORR regions. As can be seen in Fig. S8a,† YBaCo4O7.3 was able to maintain a steady potential while generating up to 180 mA mg−1 for the ORR and 58 mA mg−1 for the OER, although when a potential of 1.74 V vs. RHE was applied, there was a significant decrease in the amount of current generated over the hour-long period. This may be because the carbon support is not stable at these high potentials and may undergo significant oxidation, as has been previously shown.12 Thus, to further examine the catalyst stability, both the OER and ORR were performed under the same conditions described above and the current generated was held constant at 10 mA mg−1 while the potential necessary to generate that current was measured. As can be seen in Fig. S8b,† YBaCo4O7.3 was able to maintain this current for 12 h for both oxygen evolution and reduction, and although there was a slight increase in the potential for the OER over this time frame, we again attribute this to oxidation of the VC support.
DFT calculations of oxygen adsorption energies
To rationalize the observed bifunctional activity of the YBaCo4O7.3 material in terms of the surface reaction mechanism, we estimated the adsorption energies of the intermediates along the (001) surface, which provide transition metal-O termination. This type of termination was repeatedly studied for perovskites and proved its usefulness for rationalizing catalytic activity trends.14,21 The calculations for the stoichiometric YBaCo4O7 (001) surface were performed using a 10.5 Å thick slab with a 1 × 1 surface area and vacuum region of 11 Å. Due to the lack of appropriate symmetry of the slab center, it had two non-equivalent surfaces. In total, seven different terminations were considered for the (001) surface. By calculating the energies, the most stable combination of two surfaces with an average surface energy (cleavage energy) of 0.8 J m−2 was identified.55 This relatively low value of energy for free surfaces proves that the found surface terminations were thermodynamically stable or close to stable. The two surfaces had Co–O and Y–Ba–Co–O terminations, respectively. The coordination of atoms at both surfaces conserved their bulk topology; however the surface O and Co atoms were shifted in opposite ways along the [001] direction, reducing the polarity of the surfaces. Further, we focused only on the first Co–O surface, which had more transition metal sites per unit area. The top-view of the optimized structure of Co–O surface is shown in Fig. 7.To find the most favorable adsorption sites for the intermediates, the surface was probed by placing an O atom with a 1 Å step along the surface plane, optimizing the distance between O and the surface, and calculating adsorption energy. The adsorption energies for O* and HO* were calculated as follows:
| ΔE(O*(ads)) = E(O*(ads)) − E(*) − (EH2O(g) − EH2(g)), |
| ΔE(HO*(ads)) = E(HO*(ads)) − E(*) − (EH2O(g) − 1/2EH2(g)), |
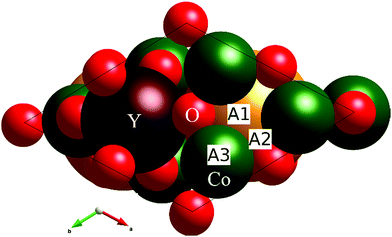
The most favorable adsorption sites for the oxygen atom are shown in Fig. 7 with labels A1, A2, and A3. The A1 position is located above the Co triangle with the Co–O distances of 2.1, 2.1, and 1.8 Å. The oxygen atom at this position forms a nearly linear O–Co–O fragment with one of the remaining lattice O atoms of the tetrahedral oxygen coordination of Co. The A2 position is located between two Co atoms, and the Co–O distances are 2 and 1.7 Å. The third position A3 is located just above the Co atom at a distance of 1.7 Å, completing its coordination back to tetrahedron. The corresponding adsorption energies ΔE(O*) of O for the A1, A2, and A3 positions are 1.8, 2.0, and 2.8 eV, respectively. The most favorable position for O adsorption is located near the center of the triangles formed by the Co atoms on the surface. This is different to that in perovskites, where their surface structures do not contain transition metal triangles, and oxygen prefers to be either above the metal site or between two metal sites. The local coordination of the most energetically favorable A1 position for the adsorbates is reminiscent to that of the oxygen position in the octahedral layers of LiCoO2, i.e. due to the formation of the linear O–Co–O fragment, the A1 position looks more “octahedral” rather than “tetrahedral”. Surprisingly, the “tetrahedral” A3 position inherited from the regular YBaCo4O7 structure is the least energetically favorable.
The relation between electronic structure and catalytic activity
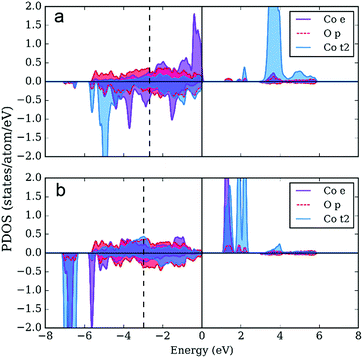
The distinct feature of the electronic structure of YBaCo4O7+δ is the charge disproportionation with the Co atoms in the Co2+ and Co3+ oxidation states. Thus, to gain more insight into the character of the bonding between Co and O, we investigated the partial density of electronic states (PDOS), such as t2 and e orbitals in the tetrahedral field splitting for Co2+ and Co3+, as well as for the p orbitals on the O atoms. As expected, the local orbital projected PDOS for Co2+ and Co3+ are different (Fig. 8). First, the Co3+ atom is characterized by a lower density on the e orbitals, which serve as a source of electrons during oxidation. Second, the low-lying d peak is located 2 eV lower on Co3+ than that on Co2+. The local electronic structure of both atoms has a band gap, which is smaller in the case of Co3+. On the one hand, the DOS on Co2+ locally resembles the Mott–Hubbard insulator scenario (with U < Δ, where U is the difference between the centers of the occupied and unoccupied d-bands and Δ is the difference between the center of the valence p-band and unoccupied d band56). On the other hand, the local electronic structure of Co3+ resembles that of a typical charge-transfer insulator, with U > Δ. The centers of the valence p-bands, shown with dashed vertical lines on the PDOS plots, usually correlate with the adsorption energies and consequently with catalytic activity.14 Since the observed adsorption can be considered too strong (using adsorption energies on perovskite surfaces as a reference14), the upshift of the p-band center should be accompanied with an increase in catalytic activity.As the next step, we calculated the PDOS for partially oxidized YBaCo4O8, where a fraction of the tetrahedrally coordinated Co acquires octahedral oxygen coordination.51 Oxidation results in the upshifting of the p-band center by 0.1 eV compared to the p-band center in YBaCo4O7 located 2.7 eV below the Fermi level, which indicates that the increase in oxygen concentration could be responsible for the weaker adsorption of the intermediates and corresponding increase in catalytic activity. Besides the upshift of the p-band center, the PDOSs for the tetrahedral Co2+ and Co3+ atoms in YBaCo4O8 (not shown) resemble that for YBaCo4O7. However, in YBaCo4O8, 1/4 of the Co atoms adopt a 6-fold oxygen octahedral coordination. The PDOS for the octahedrally coordinated Co (Fig. 9) has an additional peak above the Fermi level with significant contribution from both the Co3d and O2p states, which represent the σ* band characteristic of TM cations in the octahedral oxygen coordination. The appearance of the σ* band results in a very small band gap and increased p-density below the Fermi level (in agreement with the drastic conductivity increase in YBaCo4O7+δ upon increasing the oxygen content32,39). The oxidation state is +3, locally resembling a charge-transfer insulator with a small Δ. According to Hong et al., the charge-transfer energy serves as a utility descriptor of the OER catalytic activity.24,25 Moreover, in contrast to other descriptors that usually obey the volcano shape of activity, Δ has a simpler inverse linear relationship with activity. Thus, the octahedrally-coordinated atoms in YBaCo4O8 should serve as active catalytic centers for the OER reaction.
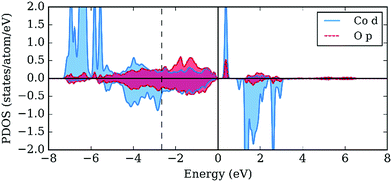
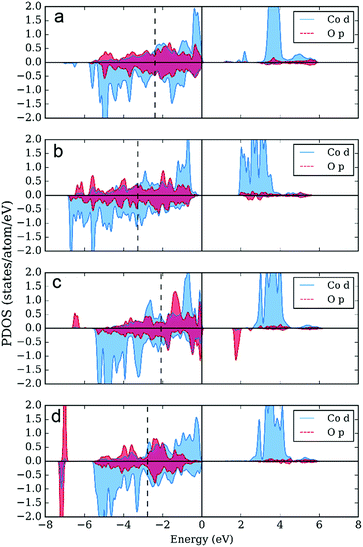
The drastic increase in the Co3d contribution to the PDOS near the Fermi level is not the sole effect of the oxygen insertion into the YBaCo4O7+δ crystal structure. The adsorbates at the “octahedral” A1 position provide a similar effect on the local PDOS at the surface Co atoms. A comparison of the local PDOS on the bulk Co and O atoms, bare surface Co and O atoms, and surface Co and adsorbed O atoms for O* and HO* is provided in Fig. 10. The d-PDOS on the Co atom at the surface resembles that obtained for the bulk Co, although more density is acquired in the −6 to −7 eV region in the case of Co at the surface (Fig. 10b). For both the bulk and surface Co atoms, there is an e-peak under the Fermi-level (Fig. 10a and b). The adsorption of O causes oxidation of the Co atom, removing the electrons from the e-orbital and forming a strongly hybridized p–d bond in a wide range of energies (Fig. 10c). The adsorption of O is accompanied by the formation of p–d hybridized σ* anti-bonding states right below the Fermi level. The p-band center of adsorbed oxygen surprisingly coincides with that of the bulk oxygen. In the case of HO*, oxygen forms s–p hybridized bonds with hydrogen, which are manifested around −7 eV as sharp peaks in Fig. 10d. The p–d hybridized band is also present in the case of HO*, but is shifted downwards from the Fermi level. Thus, the qualitative changes in the local band structure caused by oxygen insertion and surface adsorption are very similar, suggesting that the undercoordinated octahedral sites on the surfaces should be more active and responsible for the increase in OER catalytic activity.
Origin of bifunctional behavior in the frame of the ZSA model
If the OER activity of YBaCo4O7+δ can still be rationalized in the frame of the ZSA model, understanding the ORR activity in the same framework is much more challenging. This is due to the lack of systematic correlations between the ORR activity and the parameters of the ZSA band structure unequivocally established thus far. Thus, based on the knowledge on the perovskite OER and ORR activities from our own experiments and that extrapolated from the published data,19–21,57 we attempted to establish the correlations between them and the U and Δ values for perovskites in the ZSA model determined within the configuration interaction method from the XPS spectra.58–60 The correlations of the overpotentials (taken as a measure of the catalytic activity at the current density of 10 mA cm−2 and 3 mA cm−2 for the OER and ORR, respectively) with U and Δ are shown in Fig. 11.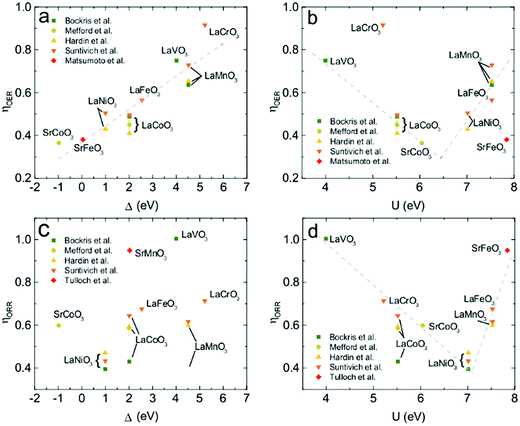 | ||
| Fig. 11 Relationships of the OER (a and b) and ORR (c and d) overpotentials for perovskite oxides with the Coulomb on-site repulsion energy, U, and charge transfer energy, Δ. Dash lines shown on the plots are guides for the eye. Part of the data points are extrapolated from the published Tafel curves;12,21,57,64–67 thus they have no error bars. | ||
The linear decrease in the OER overpotential with Δ (Fig. 11a) is strikingly similar to the latest observations by Hong et al. on a monotonic increase in the OER kinetic current with a decrease in Δ.25 The volcano-like plot of the OER overpotential as a function of U with a minimum on SrCoO3−δ (Fig. 11b), the most active OER catalyst reported to date,12 suggests that U can be considered as a secondary descriptor for OER catalytic activity. Surprisingly, the ORR overpotential does not show any noticeable correlation with the charge transfer energy (Fig. 11c). However, the ORR overpotential demonstrates a strong volcano-type dependence on U with a minimum at 7.0 eV for LaNiO3 (Fig. 11d). Thus, in terms of the ZSA model, the Coulomb on-site repulsion U may be considered as a primary descriptor governing the ORR activity of perovskites in alkaline solutions.
Different primary descriptors for the OER and ORR stems from the different rate determining steps (RDS) on the surface for two regimes. In the case of the OER, the RDS step is associated with electron-transfer energy, explaining its correlation with Δ. In the case of YBaCo4O7+δ, oxidation leads to the formation of octahedral Co with a small Δ, thus rendering this Co site more electrochemically active. In the case of the ORR, the RDS step is associated with the adsorption energies of the intermediates, making the on-site repulsion more important. Qualitatively, this can be understood from the correlation between U and the adsorption strength of the intermediates. On the one hand, the adsorption of surface intermediates weakens from early to late transition metals.61 On the other hand, the on-site Coulomb repulsion energy, which is a measure of d-orbital localization, increases from early to late transition metals since the spatial extent of the d orbital shrinks with atomic number.62 As a result, the higher U values correspond to weaker adsorption. The direct weakening of adsorption due to the increase in U value was shown using DFT+U calculations.63 Comparing the Co atoms in the tetrahedral and octahedral coordination in YBaCo4O8, the former is characterized with more compact d orbitals and, therefore, a higher U value. This was confirmed from the calculated local PDOS on the tetrahedral and octahedral atoms (the d-peak separations are ∼7 and ∼5.5 eV, respectively). The larger value of U in the tetrahedral case weakens the adsorption strength towards the optimal value, corresponding to an increase in catalytic activity for the ORR and renders the tetrahedrally coordinated Co a catalytically active center.
Conclusions
The tetrahedral oxide YBaCo4O7.3 demonstrated bifunctional catalytic activity of 1.18 mA cm−2 and 2.76 mA cm−2 in the OER and ORR, respectively, similar to that of the benchmark LaNiO3 perovskite. At the same time, this compound was chosen as an interesting object to study the correlations between the OER and ORR catalytic activity and the peculiarities of the bulk band structure. The bifunctional activity of YBaCo4O7.3 was rationalized in terms of the ZSA model with the parameters U (Coulomb on-site repulsion energy) and Δ (charge transfer energy). The Co2+ and Co3+ cations in the stoichiometric mixed-valent YBaCo4O7 are charge-ordered, with the local DOS on Co2+ being strongly reminiscent of a Mott–Hubbard insulator (U < Δ); whereas, the local electronic structure at Co3+ is typical for a charge transfer insulator (U > Δ). However, prior to the OER, YBaCo4O7.3 absorbs overstoichiometric oxygen, inducing octahedrally coordinated Co and giving rise to a narrow d–p σ* band near the Fermi level. This drastically reduces Δ and renders the octahedral Co positions active sites in the OER according to the established correlation between the OER catalytic activity and charge transfer energy. The analysis of the possible positions for the adsorbates on the model (001) Co–O terminated surface reflects that the most energetically favorable position is along the nearly linear O–Co–O bridges, introducing similar a d–p σ* band near the Fermi level and making the surface Co act more like an “octahedral” redox center rather than a “tetrahedral” one. In the ORR, the Coulomb on-site repulsion energy may serve as a descriptor of the catalytic activity scaling inversely with the binding energies of the adsorbates. Apparently, the comparative qualitative analysis of the U-overpotential trends in the OER and ORR demonstrated that the highest catalytic activity in the ORR requires a lower binding energy of the adsorbates compared to the OER, as reflected by ∼1 eV larger U. In YBaCo4O7, a larger U was realized for Co in the tetrahedral coordination due to its more compact coordination environment, thus making these sites active in the ORR.Author contributions
M. A. K. and V. D. O. performed the synthesis, XRPD and iodometric titration. M. A. K. performed microstructural characterization. D. A. A. performed DFT modelling. R. P. F. performed electrochemical characterization. J. T. M. performed calculations for perovskite overpotentials in frame of ZSA model. A. M. A. performed the SAED, HAAFD-STEM, ABF-STEM and EDX measurements. K. J. S. planned the electrochemical measurements and analyzed the data. A. M. A. and K. J. S. conceived and guided the work. All authors contributed to the writing of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
M. A. K. thanks Victoria Nikitina for fruitful discussion of electrochemical results.References
- W. T. Hong, M. Risch, K. A. Stoerzinger, A. Grimaud, J. Suntivich and Y. Shao-Horn, Toward the Rational Design of Non-Precious Transition Metal Oxides for Oxygen Electrocatalysis, Energy Environ. Sci., 2015, 8, 1404–1427 RSC.
- C. C. L. McCrory, S. Jung, J. C. Peters and T. F. Jaramillo, Benchmarking Heterogeneous Electrocatalysts for the Oxygen Evolution Reaction, J. Am. Chem. Soc., 2013, 135, 16977–16987 CrossRef CAS PubMed.
- Y. Gorlin and T. F. Jaramillo, A Bifunctional Nonprecious Metal Catalyst for Oxygen Reduction and Water Oxidation, J. Am. Chem. Soc., 2010, 132, 13612–13614 CrossRef CAS PubMed.
- S. M. Jasem and A. C. C. Tseung, A Potentiostatic Pulse Study of Oxygen Evolution on Teflon-Bonded Nickel-Cobalt Oxide Electrodes, J. Electrochem. Soc., 1979, 126, 1353–1360 CrossRef CAS.
- M. P. Browne, C. Domínguez and P. E. Colavita, Emerging Trends in Metal Oxide Electrocatalysis: Bifunctional Oxygen Catalysis, Synergies and New Insights from in situ Studies, Curr. Opin. Electrochem., 2018, 7, 208–215 CrossRef CAS.
- H. Osgood, S. V. Devaguptapu, H. Xu, J. Cho and G. Wu, Transition Metal (Fe, Co, Ni, and Mn) Oxides for Oxygen Reduction And evolution Bifunctional Catalysts in Alkaline Media, Nano Today, 2016, 11, 601–625 CrossRef CAS.
- I. Yamada, et al., Bifunctional Oxygen Reaction Catalysis of Quadruple Manganese Perovskites, Adv. Mater., 2017, 29, 1603004 CrossRef PubMed.
- W. G. Hardin, D. A. Slanac, X. Wang, S. Dai, K. P. Johnston and K. J. Stevenson, Highly Active, Nonprecious Metal Perovskite Electrocatalysts for Bifunctional Metal–Air Battery Electrodes, J. Phys. Chem. Lett., 2013, 4, 1254–1259 CrossRef CAS PubMed.
- R. P. Forslund, et al., Exceptional Electrocatalytic Oxygen Evolution via Tunable Charge Transfer Interactions in La0.5Sr1.5Ni1−xFexO4±δ Ruddlesden-Popper Oxides, Nat. Commun., 2018, 9, 3150 CrossRef PubMed.
- J. K. Nørskov, et al., Origin of the Overpotential for Oxygen Reduction at a Fuel-Cell Cathode, J. Phys. Chem. B, 2004, 108, 17886–17892 CrossRef.
- I. C. Man, et al., Universality in Oxygen Evolution Electrocatalysis on Oxide Surfaces, ChemCatChem, 2011, 3, 1159–1165 CrossRef CAS.
- J. T. Mefford, et al., Water Electrolysis on La1-xSrxCoO3-δ Perovskite Electrocatalysts, Nat. Commun., 2016, 7, 11053 CrossRef CAS PubMed.
- A. Grimaud, et al., Activating Lattice Oxygen Redox Reactions in Metal Oxides to Catalyse Oxygen Evolution, Nat. Chem., 2017, 9, 457–465 CrossRef CAS PubMed.
- Y.-L. Lee, M. J. Gadre, Y. Shao-Horn and D. Morgan, Ab Initio GGA+U Study of Oxygen Evolution and Oxygen Reduction Electrocatalysis on the (001) Surfaces of Lanthanum Transition Metal Perovskites LaBO3 (B = Cr, Mn, Fe, Co and Ni), Phys. Chem. Chem. Phys., 2015, 17, 21643–21663 RSC.
- J. K. Nørskov, et al., Universality in Heterogeneous Catalysis, J. Catal., 2002, 209, 275–278 CrossRef.
- J. Rossmeisl, Z.-W. Qu, H. Zhu, G.-J. Kroes and J. K. Nørskov, Electrolysis of Water on Oxide Surfaces, J. Electroanal. Chem., 2007, 607, 83–89 CrossRef CAS.
- F. Calle-Vallejo, et al., Number of Outer Electrons as Descriptor for Adsorption Processes on Transition Metals and Their Oxides, Chem. Sci., 2013, 4, 1245–1249 RSC.
- F. Calle-Vallejo, D. Loffreda, M. T. M. Koper and P. Sautet, Introducing Structural Sensitivity into Adsorption–Energy Scaling Relations by Means of Coordination Numbers, Nat. Chem., 2015, 7, 403–410 CrossRef CAS PubMed.
- J. O. Bockris and T. Otagawa, The Electrocatalysis of Oxygen Evolution on Perovskites, J. Electrochem. Soc., 1984, 131, 290–302 CrossRef CAS.
- J. Suntivich, H. A. Gasteiger, N. Yabuuchi, H. Nakanishi, J. B. Goodenough and Y. Shao-Horn, Design Principles for Oxygen-Reduction Activity on Perovskite Oxide Catalysts for Fuel Cells and Metal–Air Batteries, Nat. Chem., 2011, 3, 546–550 CrossRef CAS PubMed.
- J. Suntivich, K. J. May, H. A. Gasteiger, J. B. Goodenough and Y. Shao-Horn, A Perovskite Oxide Optimized for Oxygen Evolution Catalysis from Molecular Orbital Principles, Science, 2011, 334, 1383–1385 CrossRef CAS PubMed.
- V. Stamenkovic, et al., Changing the Activity of Electrocatalysts for Oxygen Reduction by Tuning the Surface Electronic Structure, Angew. Chem., 2006, 118, 2963–2967 CrossRef.
- A. Grimaud, et al., Double Perovskites as a Family of Highly Active Catalysts for Oxygen Evolution in Alkaline Solution, Nat. Commun., 2013, 4, 2439 CrossRef PubMed.
- W. T. Hong, R. E. Welsch and Y. Shao-Horn, Descriptors of Oxygen-Evolution Activity for Oxides: A Statistical Evaluation, J. Phys. Chem. C, 2016, 120, 78–86 CrossRef CAS.
- W. T. Hong, et al., Charge-Transfer-Energy-Dependent Oxygen Evolution Reaction Mechanisms for Perovskite Oxides, Energy Environ. Sci., 2017, 10, 2190–2200 RSC.
- J. Zaanen, G. A. Sawatzky and J. W. Allen, Band Gaps and Electronic Structure of Transition-Metal Compounds, Phys. Rev. Lett., 1985, 55, 418–421 CrossRef CAS PubMed.
- J. B. Torrance, P. Lacorre, C. Asavaroengchai and R. M. Metzger, Why are Some Oxides Metallic, While Most are Insulating?, Physica C, 1991, 182, 351–364 CrossRef CAS.
- A. A. Tsirlin, M. G. Rabie, A. Efimenko, Z. Hu, R. Saez-Puche and L. H. Tjeng, Importance of Tetrahedral Coordination for High-Valent Transition-Metal Oxides: YCrO4 as a Model System, Phys. Rev. Lett., 2014, 90, 085106 Search PubMed.
- L. Qiao, et al., The Impact of Crystal Symmetry on the Electronic Structure and Functional Properties of Complex Lanthanum Chromium Oxides, J. Mater. Chem. C, 2013, 1, 4527–4535 RSC.
- E. Tsuji, et al., Brownmillerite-Type Ca2FeCoO5 as a Practicable Oxygen Evolution Reaction Catalyst, ChemSusChem, 2017, 10, 2864–2868 CrossRef CAS PubMed.
- A. Grimaud, C. E. Carlton, M. Risch, W. T. Hong, K. J. May and Y. Shao-Horn, Oxygen Evolution Activity and Stability of Ba6Mn5O16, Sr4Mn2CoO9, and Sr6Co5O15: The Influence of Transition Metal Coordination, J. Phys. Chem. C, 2013, 117, 25926–25932 CrossRef CAS.
- M. Karppinen, et al., Oxygen Nonstoichiometry in YBaCo4O7+δ: Large Low-Temperature Oxygen Absorption/Desorption Capability, Chem. Mater., 2006, 18, 490–494 CrossRef CAS.
- S. Räsänen, H. Yamauchi and M. Karppinen, Oxygen Absorption Capability of YBaCo4O7+δ, Chem. Lett., 2008, 37, 638–639 CrossRef.
- T. Motohashi, S. Kadota, H. Fjellvåg, M. Karppinen and H. Yamauchi, Uncommon Oxygen Intake/Release Capability of Layered Cobalt Oxides, REBaCo4O7+δ: Novel Oxygen-Storage Materials, Mater. Sci. Eng., B, 2008, 148, 196–198 CrossRef CAS.
- Y. Nagai, et al., X-ray Absorption fine Structure Analysis of Local Structure of CeO2–ZrO2 Mixed Oxides With the Same Composition Ratio (Ce/Zr=1), Catal. Today, 2002, 74, 225–234 CrossRef CAS.
- E. V. Tsipis, D. D. Khalyavin, S. V. Shiryaev, K. S. Redkina and P. Núñez, Electrical and Magnetic Properties of YBaCo4O7+δ, Mater. Chem. Phys., 2005, 92, 33–38 CrossRef CAS.
- M. Dan, V. Pralong, N. Vaszilcsin, A. Kellenberger and N. Duteanu, Electrochemical Behaviour of YBaCo4O7 in Alkaline Aqueous Solution, J. Solid State Electrochem., 2011, 15, 1227–1233 CrossRef CAS.
- E. V. Tsipis, V. V. Kharton and J. R. Frade, Transport Properties and Electrochemical Activity of YBa(Co,Fe)4O7 Cathodes, Solid State Ionics, 2006, 177, 1823–1826 CrossRef CAS.
- H. Hao, Q. He, Y. Cheng and L. Zhao, Oxygen Adsorption and Electronic Transport Properties of Fe-Substituted YBaCo4O7 Compounds, Mater. Res. Bull., 2014, 53, 84–88 CrossRef CAS.
- V. Petricek, M. Dusek and L. Palatinus, Crystallographic Computing System JANA2006: General features, Z. Kristallogr., 2014, 229, 345–352 CAS.
- F. R. van Buren, G. H. J. Broers, A. J. Bouman and C. Boesveld, An Electrochemical Method for the Determination–xSrxCoO3–y Compounds: Theoretical Aspects of Oxygen Ion Diffusion Coefficients in La1, J. Electroanal. Chem. Interfacial Electrochem., 1978, 87, 389–394 CrossRef CAS.
- F. R. van Buren, G. H. J. Broers, A. J. Bouman and C. Boesveld, The Electrochemical Determination of Oxygen Ion Diffusion Coefficients in La0.50Sr0.50CoO3–y: Experimental Results and Related Properties, J. Electroanal. Chem. Interfacial Electrochem., 1978, 88, 353–361 CrossRef CAS.
- A. G. C. Kobussen, F. R. van Buren and G. H. J. Broers, The Influence of the Particle Size Distribution on the Measurement of Oxygen Ion Diffusion Coefficients in La0.50Sr0.50CoO3–y, J. Electroanal. Chem. Interfacial Electrochem., 1978, 91, 211–217 CrossRef CAS.
- G. Kresse and J. Furthmüller, Efficiency of Ab-Initio Total Energy Calculations for Metals and Semiconductors Using a Plane-Wave Basis Set, Comput. Mater. Sci., 1996, 6, 15–50 CrossRef CAS.
- P. Hohenberg and W. Kohn, Inhomogeneous Electron Gas, Phys. Rev. B, 1964, 136, B864–B871 CrossRef.
- W. Kohn and L. J. Sham, Self-Consistent Equations Including Exchange and Correlation Effects, Phys. Rev. A, 1965, 140, A1133–A1138 CrossRef.
- J. Perdew, K. Burke and M. Ernzerhof, Generalized Gradient Approximation Made Simple, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS PubMed.
- P. E. Blöchl, Projector Augmented-Wave Method, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 50(24), 17953 CrossRef.
- S. L. Dudarev, G. A. Botton, S. Y. Savrasov, C. J. Humphreys and A. P. Sutton, Electron-Energy-Loss Spectra and the Structural Stability of Nickel Oxide: An LSDA+U Study, Phys. Rev. B: Condens. Matter Mater. Phys., 1998, 57, 1505–1509 CrossRef CAS.
- S. Chatterjee and T. Saha-Dasgupta, Electronic and Magnetic Structure of the Mixed-Valence Cobaltite CaBaCo4O7, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 84, 85116 CrossRef.
- N. V Podberezskaya, A. I. Smolentsev, L. P. Kozeeva, M. Y. Kameneva and A. N. Lavrov, Yttrium Barium Heptaoxocobaltate YBaCo4O7+δ: Refinement of the Structure and Determination of the Composition, Crystallogr. Rep., 2013, 58, 682–686 CrossRef.
- O. Chmaissem, H. Zheng, A. Huq, P. W. Stephens and J. F. Mitchell, Formation of Co3+ Octahedra and Tetrahedra in YBaCo4O8.1, J. Solid State Chem., 2008, 181, 664–672 CrossRef CAS.
- S. Avci, O. Chmaissem, H. Zheng, A. Huq, P. Manuel and J. F. Mitchell, J. F. Oxygen Stoichiometry in the Geometrically Frustrated Kagomé System YBaCo4O7+δ: Impact on Phase Behavior and Magnetism, Chem. Mater., 2013, 25, 4188–4196 CrossRef CAS.
- J. T. Mefford, W. G. Hardin, S. Dai, K. P. Johnston and K. J. Stevenson, Anion Charge Storage Through Oxygen Intercalation in LaMnO3 Perovskite Pseudocapacitor Electrodes, Nat. Mater., 2014, 13, 726–732 CrossRef CAS PubMed.
- W. Sun and G. Ceder, Efficient Creation and Convergence of Surface Slabs, Surf. Sci., 2013, 617, 53–59 CrossRef CAS.
- C. Yang and A. Grimaud, Factors Controlling the Redox Activity of Oxygen in Perovskites: from Theory to Application for Catalytic Reactions, Catalysts, 2017, 7, 149 CrossRef.
- J. Tulloch and S. W. Donne, Activity of Perovskite La1−xSrxMnO3 Catalysts Towards Oxygen Reduction in Alkaline Electrolytes, J. Power Sources, 2009, 188, 359–366 CrossRef CAS.
- A. E. Bocquet, T. Mizokawa, T. Saitoh, H. Namatame and A. Fujimori, Electronic Structure of 3d-Transition-Metal Compounds by Analysis of the 2p Core-Level Photoemission Spectra, Phys. Rev. B: Condens. Matter Mater. Phys., 1992, 46, 3771–3784 CrossRef CAS.
- A. E. Bocquet, et al., Electronic Structure of SrFe4+O3 and Related Fe Perovskite Oxides, Phys. Rev. B: Condens. Matter Mater. Phys., 1992, 45, 1561–1570 CrossRef CAS.
- A. E. Bocquet, et al., Electronic Structure of Early 3d-Transition-Metal Oxides by Analysis of the 2p Core-Level Photoemission Spectra, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 53, 1161–1170 CrossRef CAS.
- S. A. Akhade and J. R. Kitchin, Effects of Strain, d-Band Filling, and Oxidation State on the Surface Electronic Structure and Reactivity of 3d Perovskite Surfaces, J. Chem. Phys., 2012, 137, 084703 CrossRef PubMed.
- M. Imada, A. Fujimori and Y. Tokura, Metal-Insulator Transitions, Rev. Mod. Phys., 1998, 70, 1039–1263 CrossRef CAS.
- Y.-L. Lee, J. Kleis, J. Rossmeisl and D. Morgan, Ab Initio Energetics of LaBO3(001) (B = Mn, Fe, Co, and Ni) for Solid Oxide Fuel Cell Cathodes, Phys. Rev. B: Condens. Matter Mater. Phys., 2009, 80, 224101 CrossRef.
- J. O'M Bockris and T. Otagawa, Mechanism of Oxygen Evolution on Perovskites, J. Phys. Chem., 1983, 87, 2960–2971 CrossRef.
- W. G. Hardin, et al., Tuning the Electrocatalytic Activity of Perovskites through Active Site Variation and Support Interactions, Chem. Mater., 2014, 26, 3368–3376 CrossRef CAS.
- Y. Matsumoto, J. Kurimoto and E. Sato, Oxygen Evolution on SrFeO3 Electrode, J. Electroanal. Chem. Interfacial Electrochem., 1979, 102, 77–83 CrossRef CAS.
- J. Suntivich, H. A. Gasteiger, N. Yabuuchi, H. Nakanishi, J. B. Goodenough and Y. Shao-Horn, Design Principles for Oxygen-Reduction Activity on Perovskite Oxide Catalysts for Fuel Cells and Metal–Air Batteries, Nat. Chem., 2011, 3, 546–550 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ta09862j |
| This journal is © The Royal Society of Chemistry 2019 |