Ultrafine bimetallic Pt–Ni nanoparticles immobilized on 3-dimensional N-doped graphene networks: a highly efficient catalyst for dehydrogenation of hydrous hydrazine†
Amit
Kumar
a,
Xinchun
Yang
 a and
Qiang
Xu
a and
Qiang
Xu
 *ab
*ab
aResearch Institute of Electrochemical Energy, National Institute of Advanced Industrial Science and Technology (AIST), 1-8-31 Midorigaoka, Ikeda, Osaka 563-8577, Japan. E-mail: q.xu@aist.go.jp
bAIST-Kyoto University Chemical Energy Materials Open Innovation Laboratory (ChEM-OIL), National Institute of Advanced Industrial Science and Technology (AIST), Yoshida, Sakyo-Ku, Kyoto 606-8501, Japan
First published on 23rd November 2018
Abstract
Ultrafine and uniformly dispersed bimetallic Pt–Ni nanoparticles (NPs) have been immobilized on novel 3-dimensional N-doped graphene networks (NGNs) by a facile wet chemical reduction method. NGNs were obtained by 3-dimensional assembly of graphene layers with simultaneous nitrogen doping via crosslinking of graphene oxide (GO) with melamine formaldehyde resin (MFR) under hydrothermal conditions followed by carbonization. Surprisingly, NGN-supported Pt0.5Ni0.5 NPs exhibit extremely high catalytic activity for the dehydrogenation of hydrazine hydrate, achieving 100% H2 selectivity with the highest turnover frequency (TOF) of 943 h−1 at 303 K reported thus far. The small size and synergistic effects are responsible for the superior catalytic activity.
Owing to the ever-increasing demand for sustainable energy, there has been enormous research on liquid chemical hydrogen carriers.1 Hydrazine monohydrate (H2NNH2·H2O), a liquid having high hydrogen content (8.0 wt%), high stability and easy recharging ability, has been considered as a promising carrier for hydrogen storage and transportation.2 On the basis of catalysts, the catalytic decomposition of hydrazine occurs through two pathways: complete decomposition N2H4 (l) → N2 (g) + 2H2 (g) and incomplete decomposition N2H4 (l) → 1/3N2 (g) + 4/3NH3 (g).2a In the former case, the produced nitrogen is environmentally friendly without further treatment. However, in the latter, the produced NH3 impurities should be avoided, as they can pollute the environment, reduce the hydrogen production efficiency, and complicate the separation process of fuel cells.3
Although various metal catalysts have been studied for catalytic hydrogen generation from hydrazine (Table S1, ESI†), high-performance catalysts still need to be developed. In this respect, bimetallic Pt–Ni nanoparticles (NPs) have proved their significance for hydrazine dehydrogenation at ambient conditions.2,3 However, severe aggregation and phase separation of the primary bimetallic nanoparticles (NPs) during the synthesis often result in low catalytic activity, selectivity and stability. An ideal strategy is to anchor bimetallic Pt–Ni NPs on an appropriate support. Graphene, which consists of chemically converted single-layer carbon atoms, has emerged as one of the most promising supports for metal NPs owing to its good hydrophilicity and large specific surface area.4 However, owing to the weak interactions between the surface of graphene and metal species, it is still a great challenge to obtain ultrafine and highly dispersed bimetallic NPs on graphene.5 An efficient approach is to rationally modify the graphene surface with electron-rich functional groups/sites, which may improve the binding ability of supports to metal precursors, and thus facilitate the control of NP nucleation and growth. Unfortunately, the lack of controllable surface modification of graphene remains the main downside.
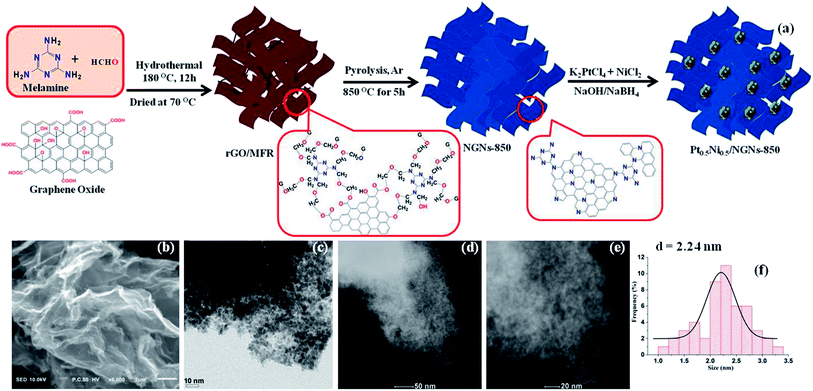
Herein, for the first time, novel porous 3-dimensional (3D) N-doped graphene networks (NGNs) have been synthesized and employed to immobilize ultrafine and highly dispersed bimetallic Pt–Ni NPs (Fig. 1a), which, surprisingly, exhibit exceptionally high catalytic activity toward hydrazine hydrate dehydrogenation with a record-high TOF value of 943 h−1 at 303 K.
To synthesize 3D NGNs, melamine and formaldehyde were chosen as the cross-linking and N agents. Briefly, a homogeneous aqueous suspension of graphene oxide (GO) (10 mg ml−1) was mixed with formaldehyde and melamine at room temperature, followed by hydrothermal treatment at 180 °C for 12 h. GO was cross-linked and reduced simultaneously by melamine formaldehyde resin (MFR) in this process, resulting in a graphene/MFR composite (Scheme S1, ESI†),6 which was established by Fourier transform infrared (FTIR) and powder X-ray diffraction (PXRD) analyses (Fig. S1 and S2, ESI†). Finally, the as-prepared graphene/MFR composite was calcined at different temperatures under an argon atmosphere to yield 3D NGNs-T (T, the calcination temperature). In contrast, graphene networks (GNs) were also prepared without using melamine and formaldehyde (see the ESI†).
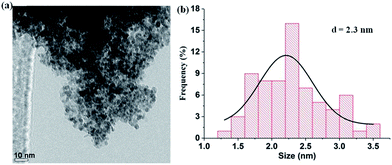
Pt–Ni NPs were loaded on the NGNs by using a facile wet chemical reduction method. Briefly, the as-prepared NGNs-T was dispersed in water by ultra-sonication and mixed with an aqueous solution of K2PtCl4 and NiCl2·6H2O with the designated Pt/Ni molar ratio at room temperature. Finally, NGNs-T supported bimetallic Pt–Ni NPs, denoted as PtxNi1−x/NGNs-T (x, the molar ratio of Pt in the Pt–Ni system), were obtained by addition of an aqueous solution of NaBH4/NaOH. In addition, control samples of Pt0.5Ni0.5 NPs supported on GNs and graphene were also prepared under similar conditions. Clearly, the scanning electron microscopy (SEM) images of NGNs-850 and Pt0.5Ni0.5/NGNs-850 exhibit porous 3D frameworks constructed from graphene layers with random open pores (Fig. 1b and S3, ESI†). The transmission electron microscopy (TEM) and high-angle annular dark-field scanning TEM (HAADF-STEM) images of Pt0.5Ni0.5/NGNs-850 indicate the uniform dispersion of Pt0.5Ni0.5 NPs on NGNs-850 with an average particle size of ∼2.24 nm (Fig. 1c–f and S4, ESI†). Energy-dispersive X-ray spectroscopy (EDX) analysis shows the presence of C, N, Pt and Ni (Fig. S4c, ESI†). In contrast, the TEM images of Pt0.5Ni0.5 NPs on GNs reveal the random distribution and severe aggregation with large particle size (∼4.27 nm) (Fig. S5, ESI†).
The powder X-ray diffraction (PXRD) patterns of the as-synthesized PtxNi1−x/NGNs-850 show that the characteristic peaks of the Pt–Ni NPs are located between the characteristic peaks of individual Pt (JCPDS File no. 65-2868) and Ni (JCPDS File no. 65-0380) (Fig. S6, ESI†), indicating the formation of a Pt–Ni alloy.4a,7 X-ray photoelectron spectroscopy (XPS) analyses (Fig. S7 and S8, ESI†) reveal the doping of N sites in graphene with the signals of the N 1s level at 398.1, 400.6 and 402.4 eV, corresponding to pyridinic, pyrrolic and graphitic N, respectively.6,8 The signals at 71.2 and 74.3 eV correspond to the Pt 4f7/2 and 4f5/2 levels of Pt0, while the signals at 855.7 and 874.8 eV are associated with the Ni 2p3/2 and 2p1/2 levels of Ni0.4a Elemental analysis further confirms the successful doping of N in the graphene surface (Table S2, ESI†), while inductively coupled plasma optical emission spectroscopy (ICP-OES) analysis reveals that Pt0.5Ni0.5/NGNs-850 contains 38 wt% of Pt and 13 wt% of Ni (Table S3, ESI†), which are similar to the designated mass ratio.
Furthermore, the Brunauer–Emmett–Teller (BET) surface areas and pore volumes of NGNs-750, NGNs-800, NGNs-850, NGNs-900 and Pt0.5Ni0.5/NGNs-850 were evaluated using N2 sorption measurements (Fig. 2, S9 and Table S4, ESI†). The results reveal that these NGNs possess high surface areas and large pore volumes, which may benefit the diffusion of reactants to metal NPs during the catalysis. Notably, the BET surface area and pore volume of Pt0.5Ni0.5/NGNs-850 were measured and found to be 365 m2 g−1 and 0.672 cm3 g−1, respectively, which are appreciably lower than those of NGNs-850 (859 m2 g−1 and 3.349 cm3 g−1), indicating that bimetallic Pt–Ni NPs are immobilized inside the pores of NGNs-850 (Fig. 2).
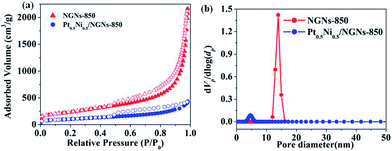 | ||
| Fig. 2 (a) N2 sorption isotherms and (b) pore size distribution of NGNs-850 and Pt0.5Ni0.5/NGNs-850 at 77 K. | ||
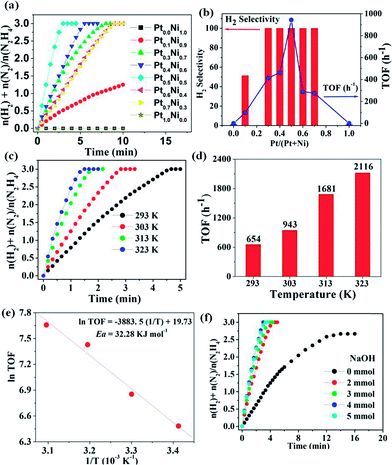
The catalytic activity of PtxNi1−x/NGNs (0 ≤ x ≤ 1.0) for the hydrogen release of hydrazine hydrate was evaluated at 303 K with a constant molar ratio of Pt–Ni/N2H4·H2O = 0.05. Obviously, the catalytic activity and selectivity are highly dependent on the Pt/Ni molar ratio (Fig. 3a and b). The monometallic Pt/NGNs-850 and Ni/NGNs-850 catalysts show no activity for hydrazine dehydrogenation, while the incorporation of Pt with Ni together results in a significant enhancement in catalytic performance, attaining complete dehydrogenation of hydrazine with 100% H2 selectivity. From our evaluations, it is found that Pt0.5Ni0.5/NGNs-850 shows extremely high catalytic activity toward the catalytic hydrogen generation from hydrazine hydrate, with the highest TOF of 943 h−1 at 303 K reported thus far. As expected, Pt0.5Ni0.5/GNs and Pt0.5Ni0.5/graphene show poor activity for hydrazine dehydrogenation under analogous conditions (Fig. S10, ESI†).
Moreover, the catalytic dehydrogenation of hydrazine over Pt0.5Ni0.5/NGNs-850 at different temperatures was performed (Fig. 3c). High TOFs of 654, 943, 1681 and 2116 h−1 were obtained at 293, 303, 313 and 323 K, respectively (Fig. 3d). The Arrhenius plot fitted on ln(TOF) versus 1/T yields an activation energy (Ea) of 32.29 kJ mol−1, which is much lower than most of the previously reported values (Fig. 3e and Table S1, ESI†). Such remarkably high activity of Pt–Ni NPs supported on NGNs is mainly attributed to the ultra-small size effect, the strong synergistic effect between Pt and Ni atoms, and the excellent porous structures of the 3D networks, which are enabled by enhancing the interactions between the graphene surface and metal species through N-doping and cross-linking of graphene layers.
Notably, the use of NaOH plays a key role in promoting the catalytic performance. To gain insights into the effects of the amount of NaOH on the catalytic activity, hydrazine dehydrogenation reaction over the Pt0.5Ni0.5/NGNs-850 catalyst was performed in the presence of different amounts of NaOH (0.0–5.0 mmol). Fig. 3f indicates that the catalytic activity of Pt0.5Ni0.5/NGNs-850 increases rapidly until the amount of NaOH increases to 4.0 mmol. The further increase has no actual effect on the dehydrogenation of hydrazine. The presence of NaOH in hydrazine solution could promote the rate determining deprotonation step (N2H4 → N2H3* + H*) while limiting the formation of undesirable N2H5+ ions (N2H5+ + OH− → N2H4 + H2O), which is crucial and beneficial for the elimination of N–H bonds to produce N2 and H2.4a,9
The durability and recyclability of catalysts are crucial for practical applications. To test the durability and recyclability of Pt0.5Ni0.5/NGNs-850, firstly, hydrazine monohydrate (2.0 mmol) was injected into a reactor (only 4.0 mmol of NaOH was added in the first cycle). A slight change in catalytic activity was observed owing to the dilution of NaOH in the reaction mixture (Fig. S11, ESI†). Furthermore, a durability test was performed through recovering the Pt0.5Ni0.5/NGNs-850 catalyst by centrifugation and washing with water, as well as adding a fixed concentration of substrates (2.0 mmol of hydrazine and 4.0 mmol of NaOH) in each cycle. No change in catalytic activity was observed over 5 cycles (Fig. S12, ESI†). PXRD, SEM and TEM analyses of Pt0.5Ni0.5/NGNs-850 after catalytic reactions show no significant changes in size, distribution and structure, suggesting that the Pt0.5Ni0.5/NGNs-850 catalyst possesses high durability and stability under the current conditions (Fig. 4 and S13–S14, ESI†).
 | ||
| Fig. 4 (a) STEM images and (b) the corresponding size histogram of Pt–Ni NPs in Pt0.5Ni0.5/NGNs-850 after 5 cycles. | ||
Conclusions
In summary, ultrafine (∼2.24 nm) and uniformly dispersed bimetallic Pt–Ni NPs were immobilized on NGNs by a facile wet chemical reduction method. The novel porous 3D NGNs were prepared via crosslinking of GO and MFR under hydrothermal conditions followed by carbonization. Surprisingly, the as-prepared Pt0.5Ni0.5/NGNs-850 catalyst exhibits extremely high catalytic activity for hydrogen generation from hydrazine hydrate with a record-high TOF value of 943 h−1 at 303 K. The exceptionally high catalytic activity of Pt0.5Ni0.5/NGNs-850 is mainly attributed to the ultra-small size effect, the strong synergistic effect between Pt and Ni atoms, and the excellent porous structures of the 3D networks, which are enabled by enhancing the interactions between the graphene surface and metal species through N-doping and cross-linking of graphene layers.Conflicts of interest
The authors declare no competing interests.Acknowledgements
The authors acknowledge the Japan Society for the Promotion of Science (JSPS) for financial support. A. K. thanks JSPS for the postdoctoral fellowship.Notes and references
- (a) K. Sordakis, C. Tang, L. K. Vogt, H. Junge, P. J. Dyson, M. Beller and G. Laurenczy, Chem. Rev., 2018, 118, 372 CrossRef CAS PubMed; (b) X. Yang, J.-K. Sun, M. Kitta, H. Pang and Q. Xu, Nat. Catal., 2018, 1, 214 CrossRef; (c) J. Yao, J. Chen, K. Shen and Y. Li, J. Mater. Chem. A, 2018, 6, 3571 RSC; (d) Q. Yao, Z.-H. Lu, R. Zhang, S. Zhang, X. Chen and H.-L. Jiang, J. Mater. Chem. A, 2018, 6, 4386 RSC; (e) Y. He, F. Chen, B. Lin, G. Qian, W. Zhou and B. Chen, Coord. Chem. Rev., 2017, 373, 163 Search PubMed; (f) D. Mellmann, P. Sponholz, H. Junge and M. Beller, Chem. Soc. Rev., 2016, 45, 3954 RSC; (g) X. Yang and Q. Xu, Chin. J. Catal., 2016, 37, 1594 CrossRef CAS; (h) T. He, P. Pachfule, H. Wu, Q. Xu and P. Cheng, Nat. Rev. Mater., 2016, 1, 16059 CrossRef CAS.
- (a) L. He, B. Liang, Y. Huang and T. Zhang, Natl. Sci. Rev., 2018, 5, 356 CrossRef; (b) F.-Z. Song, Q.-L. Zhu and Q. Xu, J. Mater. Chem. A, 2015, 3, 23090 RSC; (c) J.-K. Sun and Q. Xu, ChemCatChem, 2015, 7, 526 CrossRef CAS; (d) H. L. Wang, J. M. Yan, S. J. Li, X. W. Zhang and Q. Jiang, J. Mater. Chem. A, 2015, 3, 121 RSC; (e) L. He, B. Liang, L. Li, X. Yang, Y. Huang, A. Wang, X. Wang and T. Zhang, ACS Catal., 2015, 5, 1623 CrossRef CAS; (f) S. Singh and Q. Xu, Catal. Sci. Technol., 2013, 3, 1889 RSC; (g) L. He, Y. Huang, A. Wang, X. Wang, X. Chen, J. J. Delgado and T. Zhang, Angew. Chem., Int. Ed., 2012, 51, 6191 CrossRef CAS PubMed; (h) S. K. Singh, A. K. Singh, K. Aranishi and Q. Xu, J. Am. Chem. Soc., 2011, 133, 19638 CrossRef CAS PubMed; (i) S. K. Singh, X.-B. Zhang and Q. Xu, J. Am. Chem. Soc., 2009, 131, 9894 CrossRef CAS PubMed; (j) S. K. Singh and Q. Xu, J. Am. Chem. Soc., 2009, 131, 18032 CrossRef CAS PubMed.
- (a) Z. Zhang, S. Zhang, S. Zhang, Q. Yao, X. Chen and Z.-H. Lu, Inorg. Chem., 2017, 56, 11938 CrossRef CAS PubMed; (b) Q.-L. Zhu and Q. Xu, Energy Environ. Sci., 2015, 8, 478 RSC; (c) A. K. Singh and Q. Xu, ChemCatChem, 2013, 5, 652 CrossRef CAS.
- (a) F.-Z. Song, Q.-L. Zhu, X. Yang, W. Zhan, P. Pachfule, N. Tsumori and Q. Xu, Adv. Energy Mater., 2018, 8, 1701416 CrossRef; (b) C. Ye, Z. Fan, Z. Zhang, W. Niu, C. Li, N. Yang, B. Chen and H. Zhang, Chem. Rev., 2018, 118, 6409 CrossRef PubMed; (c) A. Kumar and Q. Xu, ChemNanoMat, 2018, 4, 28 CrossRef CAS; (d) X. Yang, P. Pachfule, Y. Chen, N. Tsumori and Q. Xu, Chem. Commun., 2016, 52, 4171 RSC; (e) Z.-L. Wang, J.-M. Yan, Y.-F. Zhang, Y. Ping, H.-L. Wang and Q. Jiang, Nanoscale, 2014, 6, 3073 RSC; (f) C. Tan, X. Huang and H. Zhang, Mater. Today, 2013, 16, 29 CrossRef CAS.
- (a) B. Zhu, C. Qu, S. Gao, Z. Liang, H. Zhang and R. Zou, ChemCatChem, 2018, 10, 1 CrossRef; (b) Y.-P. Zhu, J. Ran and S.-Z. Qiao, ACS Appl. Mater. Interfaces, 2017, 9, 41980 CrossRef CAS PubMed; (c) Y. Chen, X. Yang, M. Kitta and Q. Xu, Nano Res., 2017, 11, 3811 CrossRef; (d) T. Hassina, W. Guo, W. Meng, A. Mahmood, R. Zhao, Q. Wang and R. Zou, Adv. Energy Mater., 2017, 7, 1601671 CrossRef; (e) K. Parvez, S. Yang, Y. Hernandez, A. Winter, A. Turchanin, X. Feng and K. Müllen, ACS Nano, 2012, 6, 9541 CrossRef CAS PubMed.
- Y. Qin, J. Yuan, J. Li, D. Chen, Y. Kong, F. Chu, Y. Tao and M. Liu, Adv. Mater., 2015, 27, 5171 CrossRef CAS PubMed.
- (a) J. Mathiyarasu, A. M. Remona, A. Mani, K. L. N. Phani and V. Yegnaraman, J. Solid State Electrochem., 2004, 8, 968 CrossRef CAS; (b) T. C. Deivaraj, W. Chen and J. Y. Lee, J. Mater. Chem., 2003, 13, 2555 RSC.
- Z. Li, X. Yang, N. Tsumori, Z. Liu, Y. Himeda, T. Autrey and Q. Xu, ACS Catal., 2017, 7, 2720 CrossRef CAS.
- (a) V. Rosca, M. Duca, M. T. Groot and M. T. M. Koper, Chem. Rev., 2009, 109, 2209 CrossRef CAS PubMed; (b) Q.-L. Zhu, D.-C. Zhong, U. B. Demirci and Q. Xu, ACS Catal., 2014, 4, 4261 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: The experimental details, scheme for synthesis, structural characterization and catalytic activity of the catalysts, Fig. S1–S14, and Tables S1–S4. See DOI: 10.1039/c8ta09003c |
| This journal is © The Royal Society of Chemistry 2019 |

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TOF
TOF