Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Highly efficient fullerene and non-fullerene based ternary organic solar cells incorporating a new tetrathiocin-cored semiconductor†
Lethy
Krishnan Jagadamma
 a,
Rupert G. D.
Taylor
a,
Rupert G. D.
Taylor
 b,
Alexander L.
Kanibolotsky
cd,
Muhammad Tariq
Sajjad
b,
Alexander L.
Kanibolotsky
cd,
Muhammad Tariq
Sajjad
 a,
Iain A.
Wright‡
a,
Iain A.
Wright‡
 b,
Peter N.
Horton
b,
Peter N.
Horton
 e,
Simon J.
Coles
e,
Simon J.
Coles
 e,
Ifor D. W.
Samuel
e,
Ifor D. W.
Samuel
 *a and
Peter J.
Skabara
*a and
Peter J.
Skabara
 *c
*c
aOrganic Semiconductor Centre, SUPA, School of Physics and Astronomy, University of St. Andrews, St. Andrews, Fife, KY16 9SS, UK. E-mail: idws@st-andrews.ac.uk
bWestCHEM, Department of Pure and Applied Chemistry, University of Strathclyde, 295 Cathedral Street, Glasgow, G1 1XL, UK
cWestCHEM, School of Chemistry, University of Glasgow, Glasgow, G12 8QQ, UK. E-mail: peter.skabara@glasgow.ac.uk
dInstitute of Physical-Organic Chemistry and Coal Chemistry, Kyiv 02160, Ukraine
eSchool of Chemistry, University of Southampton, Highfield, Southampton, SO17 1BJ, UK
First published on 18th June 2019
Abstract
A new dual-chain oligothiophene-based organic semiconductor, EH-5T-TTC, is presented. The molecule contains two conjugated chains linked by a fused tetrathiocin core. X-ray crystallography reveals a boat conformation within the 8-membered sulfur heterocycle core and extensive π–π and intermolecular sulfur–sulfur interactions in the bulk, leading to a 2-dimensional structure. This unusual molecule has been studied as a ternary component in organic solar cell blends containing the electron donor PTB7-Th and both fullerene (PC71BM) and non-fullerene acceptors ITIC and EH-IDTBR. By incorporating EH-5T-TTC as a ternary component, the power conversion efficiency of the binary blends containing non-fullerene acceptor increases by 17% (from 7.8% to 9.2%) and by 85% for the binary blend with fullerene acceptor (from 3.3% to 6.3%). Detailed characterisation of the ternary blend systems implies that the ternary small molecule EH-5T-TTC functions differently in polymer:fullerene and polymer:non-fullerene blends and has dual functions of morphology modification and complementary spectral absorption.
Introduction
Organic photovoltaic (OPV) devices based on nanocomposites of π-conjugated semiconductors are a prospective solar cell technology1 and have attracted considerable attention due to unprecedented attributes such as printability, foldability, portability, wearability, semi-transparency and amenability to cost-effective large area fabrication.2 Extensive and focussed research to enhance the power conversion efficiency (PCE) of organic solar cells have led to the development of highly efficient bulk heterojunction (BHJ) single and multijunction organic solar cells with PCEs > 15%.3–8 This has been achieved through the development of new photoactive materials, interfacial modification and device architecture design. The power conversion efficiency of solar cells is determined mainly by three performance parameters of open circuit voltage (VOC), fill factor (FF) and short circuit current density (JSC). Even though these parameters are complexly interrelated, each of them is directly influenced by some specific properties of the donor/acceptor (D/A) components constituting the active layer. VOC is primarily determined by the energy gap between the donor HOMO and acceptor LUMO, FF is affected by the D/A morphology and carrier mobility and the JSC by the light absorption properties of the D/A blend active layer. One simple approach to enhance the efficiency of OPVs is to extend the spectral range of light absorption by the photosensitive active layer.Despite the high absorption coefficient of π-conjugated organic semiconductors (105–106 cm−1), due to their bonding properties, the narrow absorption bandwidth of the organic semiconductors limit the spectral overlap of the D/A blend with the solar spectrum, thus adversely restricting the JSC.9 Furthermore, the low exciton diffusion length (5–15 nm) and low charge carrier mobility (∼10−4 cm2 V−1 s−1) of organic semiconductors restrict the active layer thickness of the BHJ solar cells ∼80–150 nm, which also results in incomplete absorption of the incident solar spectrum. To circumvent this, homo and heterojunction tandem solar cell device architectures can be introduced to broaden the light harvesting spectral region and to reduce any thermalisation losses.10 Although the technology is successful in terms of achieving its purpose, the improvement in PCE comes with a high cost and complexity. The design of charge recombination layers, stringent current matching conditions, necessity of solvents with orthogonal solubility properties, complex fabrication steps and low yield, all make the tandem architecture too complicated for a printing process and hence less appealing for adoption by industry.2 Another very promising and simple strategy is to keep the single junction architecture, but combine multiple donor/acceptor molecules to extend the spectral overlap of the organic solar cells with the incident solar spectrum, resulting in so-called ternary blend organic solar cells.11 Here, in this work, this latter approach has been used to obtain highly efficient fullerene and non-fullerene based ternary organic solar cells; the light absorbing active layer is composed of two donor materials and one fullerene/non-fullerene acceptor molecule and such ternary blend systems have been shown to provide significant improvements in power conversion efficiencies when compared to parent binary blends.11,12
The main donor material considered is the polymer poly[4,8-bis(5-(2-ethylhexyl)thiophen-2-yl)benzo[1,2-b;4,5-b′]dithiophene-2,6-diyl-alt-(4-(2-ethylhexyl)-3-fluorothieno[3,4-b]thiophene)-2-(carboxylate-2-6-diyl)] (PTB7-Th), chosen due to its high absorption properties and compatibility with both fullerene and non-fullerene acceptors.3,5 The ternary donor molecule considered is a novel small molecule: EH-5T-TTC, [1,3,6,8-tetrakis(3′-(2-ethylhexyl)-5′-methyl-[2,2′-bithiophen]-5-yl)dithieno[3,4-c:3′,4′-g][1,2,5,6]tetrathiocin]. In a ternary solar cell, the third component enhances the efficiency of the binary host system either by energy transfer or by charge transfer leading to an increase in short circuit current density by extended absorption and increased exciton/charge generation.13,14 A ternary component can also modify the morphology of the binary host blend by increasing the D/A interfacial area for exciton dissociation and forming extended, interpenetrating networks of donor/acceptor domains to facilitate charge transport. However, there are challenges in obtaining efficient ternary blend OPVs. Choosing a compatible ternary component that can be loaded in a sufficient amount into the binary host system, so as to increase the absorption in the complementary spectral range without disrupting the host blend morphology, is a challenging issue. Previously, Yang et al.15 and Thompson et al.16,17 reported that similarity in properties such as surface energy, chemical structure, molecular orientation, crystallite and domain size are necessary for a compatible ternary component. However, recently Mai et al.18 relaxed the condition of molecular structural similarity and demonstrated that morphology compatibility in terms of molecular packing and phase separation is the key to the ternary component selection.
In the present study, the binary blend systems investigated to enhance the power conversion efficiency are PTB7-Th:ITIC [3,9-bis(2-methylene-(3-(1,1-dicyanomethylene)-indanone))-5,5,11,11-tetrakis(4-hexylphenyl)-dithieno[2,3-d:2′,3′-d′]-s-indaceno[1,2-b:5,6-b′]dithiophene], PTB7-Th:EH-IDTBR and PTB7-Th:PC71BM. Molecular structures of these molecules are shown in Scheme 1. The selected donor materials EH-5T-TTC, EH-5T-Ge [4,4′,6,6′-tetrakis(3′-(2-ethylhexyl)-5′-methyl-[2,2′-bithiophen]-5-yl)-2,2′-spirobi[thieno[3,4-d][1,3,2]dithiagermole]] and P3HT [poly(3-hexylthiophene-2,5-diyl)] are presented initially as potential candidates for ternary devices, with complementary absorption properties with respect to the binary systems considered. The materials P3HT and EH-5T-Ge were chosen simply to compare the main subject of this work (EH-5T-TTC), with a well-known donor polymer and a close structural analogue, respectively. By introducing the ternary donor component EH-5T-TTC into BHJ OPV devices, the PCE of the binary blend systems increased by ∼7% (7.2 to 7.7%) for PTB7-Th:ITIC, ∼85% for PTB7-Th:PC71BM (3.3% to 6.3%) and ∼17% (7.8 to 9.2%) for PTB7-Th:EH-IDTBR. The good generality of the ternary component EH-5T-TTC to both fullerene and non-fullerene based organic solar cells is significant and such wide applicability has seldom been reported in ternary blend organic solar cells. Detailed characterisation of the ternary blend systems implies that the ternary small molecule EH-5T-TTC functions differently in polymer:fullerene and polymer:non-fullerene blends and has dual function in terms of morphology modification and complementary spectral absorption. The improvement in PCE of the ternary blend systems due to the incorporation of EH-5T-TTC is a synergistic effect of enhanced light absorption, improved nanoscale and percolative morphology, reduced recombination losses and increased exciton generation and dissociation probability.
 | ||
| Scheme 1 (a) Molecular structures of the binary blends and (b) P3HT and the ternary components considered in this work. | ||
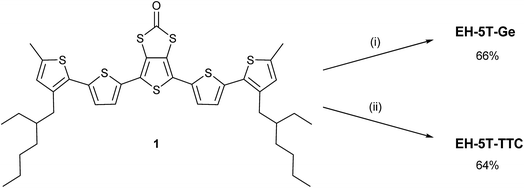
Synthesis of EH-5T-TTC and X-ray crystallographic studies
The syntheses of compounds EH-5T-TTC and EH-5T-Ge are accomplished from the 1,3-dithiole-2-one precursor (1 in Scheme 2), which in turn is prepared from a protocol reported in our previous work on a hexyl analogue of EH-5T-Ge19 and utilises aryl aldehydes for the construction of conjugated oligomers with a fused 1,3-dithiole-2-one heterocycle.20 The full experimental details and characterisation of all new compounds are provided in the ESI section.† The treatment of compound 1 in THF with sodium methoxide removes the carbonyl functionality to give the corresponding dithiolate; this intermediate can then be reacted with GeBr4 or iodine to give compounds EH-5T-Ge and EH-5T-TTC, respectively. In the case of EH-5T-TTC, this is the first time that a tetrathiocin core unit has been introduced into a conjugated oligomer structure and the fascinating structural and packing features resulting from this dual-chain molecule are discussed below.The molecular structure of EH-5T-TTC, determined by single crystal diffractions studies, is shown in Fig. 1. The asymmetric unit is represented by the half-unit formed by the scission drawn in the figure (dashed line). The complete molecule is therefore generated by an inversion centre positioned in the middle of the 8-membered tetrathiocin ring. This heterocycle forms a boat conformer with a dihedral angle of 74.60(12)° (measured between planes of C–S–S–C and S–S–S–S) and C–S–S angles of 102.38(14)° and 103.03(13)°. The thiophenes in the oligomer chains retain good planarity across 5 units, with torsion angles (S–C–C–S) in the range 174.3(2)–179.6(2)°. However, one terminal thiophene is twisted with respect to the remainder of the chain, adopting a syn conformer with the neighbouring ring with a torsion angle of 32.7(4)°. Such a deviation in planarity by a terminal thiophene has been observed in our previous work on related septithiophene21 and quinquithiophene19 dual-chain structures. The stability of the disulfide bridges is inferred by the S–S bond lengths in the structure. The covalent radius of S is stated to be typically in the range of 1.03 Å to 1.05 Å and in ring systems the S–S bond length is quoted as up to 2.10 Å.22 In EH-5T-TTC, the corresponding bond length is 2.0851(15) Å and therefore indicates a stable disulfide bond.
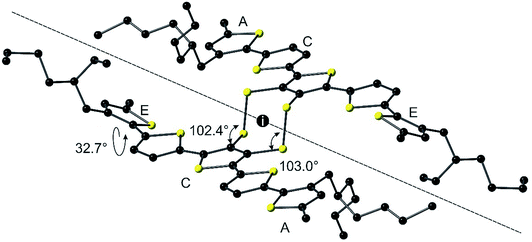 | ||
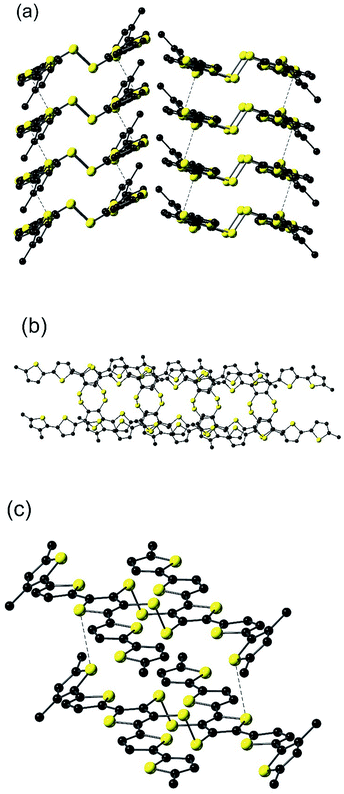
| Fig. 1 Molecular structure of EH-5T-TTC with hydrogens omitted for clarity. The inversion centre of the asymmetric unit is marked with the letter i. | ||
The molecular packing observed in EH-5T-TTC consists of π–π interactions within both sets of quinquithiophene chains in a slip-stacked arrangement (Fig. 2(a) and (b)), such that the coplanar terminal thiophene (E in Fig. 1), resides over the central thiophene ring (C) in the molecule above (and below) it with an inter-ring distance of 3.451(3) Å. Each of the stacks feature close intermolecular S⋯S contacts (Fig. 2(a) and (c)). These interactions are 3.4815(17) Å apart (sum of the van der Waals radii for two sulfurs is 3.6 Å), and involve the sulfur atom of the central thiophene ring and the sulfur atom of the twisted terminal thiophene (ring A in Fig. 1). Because of the nature of the slip-stacked structure, these S⋯S contacts perpetuate through the crystal. In combination with the π–π interactions, these contacts give rise to a structure featuring extensive 2-dimensional orbital overlap which is important for good charge transport properties in the bulk.23 Note that the sheets depicted in Fig. 2(a) are insulated by the alkyl chains, which impedes the material from achieving a 3-dimensional network of close contacts.
Photophysical and photovoltaic studies of blends
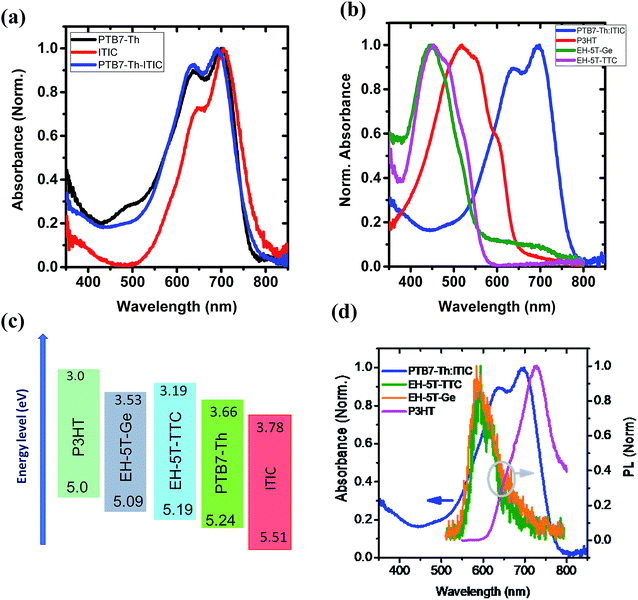
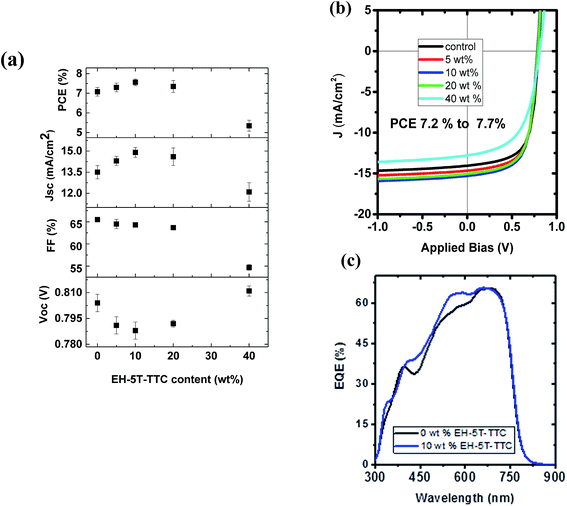
The first series of blends to be studied incorporated PTB7-Th as the donor and ITIC as the acceptor component. The UV-vis absorption spectra of the donor, acceptor and the corresponding binary blend (PTB7-Th:ITIC) films are shown in Fig. 3(a) (see also Fig. S1† for energy level diagrams of the individual components in the binary and each of the ternary configurations). The absorption of the PTB7-Th:ITIC blend is mainly concentrated in the 550–750 nm spectral range, with poor absorption below the wavelength of 550 nm. Fig. 3(b) shows the absorption spectra of the three selected ternary component molecules of P3HT, EH-5T-Ge and EH-5T-TTC and for these molecules the absorption is mainly concentrated in the 400–600 nm wavelength range, thus giving complementary absorption to the PTB7-Th donor molecule. The energy level diagrams of the PTB7-Th:ITIC blend alongside each ternary component, P3HT, EH-5T-Ge and EH-5T-TTC, are shown in Fig. 3(c). The diagram shows that the energy level alignment should support energy/charge transfer across the series of potential ternary components. Further, the photoluminescence (PL) spectra of the ternary components have good overlap with the binary blend absorption spectra (Fig. 3(d)), which is also a criterion for favourable energy transfer to occur.Since the conditions of complementary absorption and energy transfer are satisfied for the three selected ternary donor molecules, solar cells were fabricated to investigate their influence on power conversion efficiency. The OPV device architecture considered was the inverted configuration, consisting of ITO/ZnO/active layer blend/MoO3/Ag. Among the three ternary components tested, only the ternary blend system consisting of EH-5T-TTC has shown an improvement in PCE (Fig. S2†). For the ternary blend system consisting of P3HT and EH-5T-Ge a drastic drop in PCE is observed. This could be due to the surface energy differences among the components leading to their poor miscibility and/or incompatible crystalline structure or orientation. The PCE comparison of the ternary blends with the binary blends and the corresponding J–V characteristics are shown in Fig. S2.† Since the ternary blend system consisting of EH-5T-TTC showed an enhancement in PCE, the optimum content of EH-5T-TTC was investigated. Fig. S3† shows the development of the absorption spectrum of the ternary blend as the loading of EH-5T-TTC increases. As the amount of the ternary component EH-5T-TTC increases in the PTB7-Th:ITIC blend, the absorption in the 400–600 nm wavelength range also increases. The corresponding effect on the photovoltaic performance parameters are shown in Fig. 4. The figure shows that a 10% composition of EH-5T-TTC achieves the highest PCE of the ternary blends studied (0–40 wt% composition), with an improvement to 7.7% PCE, compared to 7.2% for the binary blend which does not contain EH-5T-TTC. This increase is achieved through the improvement of the short circuit current density, despite losses in the values of the open circuit voltage and fill factor. The increase in JSC could be explained by the improved light harvesting properties of the ternary blend and this is supported by the improved EQE in the 10% ternary blend compared with the binary blend (Fig. 4(c)). Moreover, the improved EQE is mainly in the 400–600 nm wavelength range, where the absorption due to the ternary component has shown an improvement in Fig. S3.†
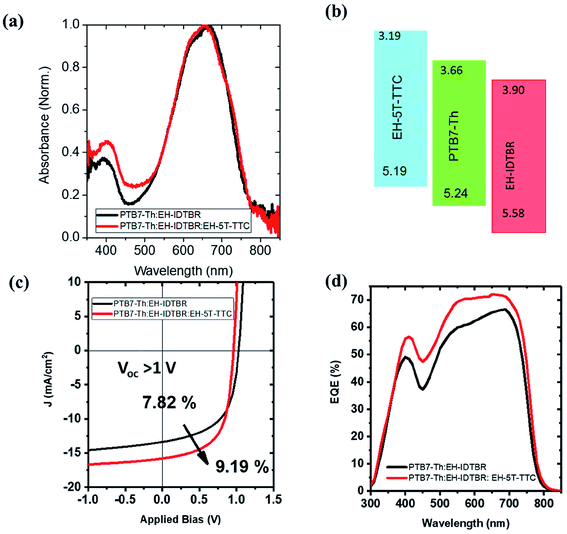
With the promising PCE enhancement observed for the PTB7-Th:ITIC blend with the incorporation of EH-5T-TTC, another ternary blend OPV system was fabricated by replacing ITIC with EH-IDTBR. The UV-vis absorption spectrum of the PTB7-Th:EH-IDTBR blend is shown in Fig. 5(a) and the absorption is mainly concentrated in the 500–750 nm wavelength range with poor absorption for wavelengths below 500 nm. With the incorporation of 10 wt% EH-5T-TTC the absorption of the binary blend PTB7-Th:EH-IDTBR for the spectral range below 500 nm is enhanced. The energy level diagram is shown in Fig. 5(b) and the band positions are favourable for the charge transfer and/or exciton transfer to occur. The comparison of the J–V characteristics corresponding to the binary PTB7-Th:EH-IDTBR and the ternary EH-5T-TTC:PTB7-Th:EH-IDTBR blend systems are shown in Fig. 5(c). The corresponding photovoltaic performance parameters are listed in Table S1.† It was found that the addition of 10 wt% EH-5T-TTC to the PTB7-Th:EH-IDTBR blend increased the PCE by 17%, from 7.82% to 9.19%. This improvement in PCE is mainly due to the increase in the short circuit current density and the fill factor of the ternary blend solar cells. The EQE spectra shown in (Fig. 5(d)), for the ternary blend compared to the binary PTB7-Th:EH-IDTBR imply that the ternary component is not only enhancing the absorption in the wavelength region below 500 nm, but also playing other functions to improve the solar cell device performance which will be further discussed in a later section.
With the success of EH-5T-TTC as a ternary component in two non-fullerene based acceptor systems, the influence of EH-5T-TTC as a ternary component in PTB7-Th:PC71BM blend was investigated. The absorption spectra of the PTB7-Th:PC71BM and the EH-5T-TTC:PTB7-Th:PC71BM blend systems are shown in Fig. S4(a).† The addition of 10 wt% of EH-5T-TTC to the binary blend of PTB7-Th:PC71BM mainly enhances the absorption in the spectral range below 550 nm. Photovoltaic performance studies were conducted with and without 1,8-diiodooctane (DIO) in the blend systems, which is often used as an additive in OPVs and acts as a co-solvent and plasticiser to give varying morphologies in blends.24 The corresponding device performance parameters for the various blends are listed in Table S2† and the effect of EH-5T-TTC in the presence of DIO on the J–V characteristics is shown in Fig. S5.† When DIO is present, the incorporation of EH-5T-TTC to the PTB7-Th:PC71BM blend does not result in an increase in PCE compared to the binary blend. However without the presence of DIO in the binary blend of PTB7-Th:PC71BM, the incorporation of 10 wt% EH-5T-TTC increased the PCE by 85% (from 3.3% to 6.3%). The corresponding J–V characteristics are shown in Fig. S4(c)† and the photovoltaic performance parameters are summarised in Table S2.† The main photovoltaic performance factors that are improved by the incorporation of 10 wt% EH-5T-TTC are short circuit current density and FF. This increase in JSC is in line with the increase in EQE as shown in Fig. S4(d).† Also as seen from Fig. S4(d) and (a),† the absorbance of the ternary blend and the external quantum efficiency (EQE) spectra show enhancement in the same spectral region, implying enhanced light harvesting by the ternary blend compared to the binary blend. Table 1 summarises how the incorporation of EH-5T-TTC enhances the photovoltaic properties of different binary blends. So far the role of ternary component EH-5T-TTC in the UV-vis absorption properties and the photovoltaic properties of the PTB7-Th:fullerene/non-fullerene blend with and without EH-5T-TTC were discussed. Now, in the following section the reasons for the improved photovoltaic properties of the ternary blend are discussed.
| Blend composition | Binary/ternary | J SC (mA cm−2) | V OC (V) | FF (%) | PCE (%) avg. | PCE best (%) |
|---|---|---|---|---|---|---|
| PTB7-Th:ITIC | Binary | 13.5 ± 0.5 | 0.804 ± 0.005 | 65.5 ± 0.3 | 7.08 ± 0.20 | 7.26 |
| Ternary | 14.9 ± 0.4 | 0.788 ± 0.005 | 64.3 ± 0.3 | 7.56 ± 0.17 | 7.74 | |
| PTB7-Th:EH-IDTBR | Binary | 12.81 ± 0.3 | 1.02 ± 0.01 | 56.7 ± 0.5 | 7.46 ± 0.17 | 7.82 |
| Ternary | 15.35 ± 0.4 | 0.965 ± 0.005 | 59.7 ± 0.8 | 8.84 ± 0.27 | 9.19 | |
| PTB7-Th:PC71BM | Binary | 11.4 ± 0.3 | 0.770 ± 0.009 | 36.5 ± 0.4 | 3.2 ± 0.1 | 3.36 |
| Ternary | 15.0 ± 0.2 | 0.778 ± 0.004 | 52.0 ± 0.6 | 6.09 ± 0.24 | 6.31 |
Discussion
To understand why EH-5T-TTC enhances the power conversion efficiency of PTB7-Th:fullerene/non-fullerene based blends, various microstructural and optical characterisations were performed. The recombination dynamics of the charge carriers at the open circuit and short circuit current density conditions were investigated by studying the photovoltaic performance as a function of the intensity of incident light. The short circuit current density JSC depends upon the light intensity I by a power law relation of| JSC ∝ Iα |
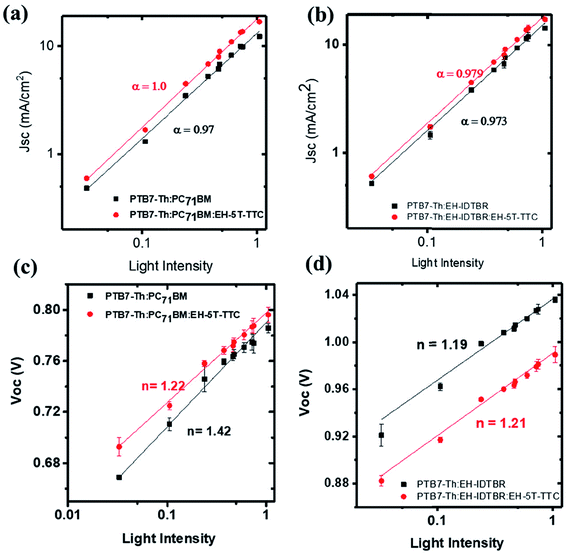 | ||
| Fig. 6 Variation of JSC and VOC as a function of light intensity for binary and ternary blends of PTB7-Th:PC71BM [(a) and (c)] and PTB7-Th:EH-IDTBR [(b) and (d)]. | ||
To gain further information about recombination mechanisms, the variation of VOC as a function of light intensity was studied. VOC is related to the incident light intensity through the relation: 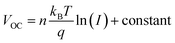 , where kB is the Boltzmann constant, n is the ideality factor, q is the charge and T is the temperature. When the slope of VOCversus the logarithm of the light intensity is
, where kB is the Boltzmann constant, n is the ideality factor, q is the charge and T is the temperature. When the slope of VOCversus the logarithm of the light intensity is 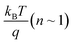 , the recombination mechanism that dominates is bimolecular recombination. A stronger dependence of VOC on light intensity corresponding to (n > 1) indicates the presence of Shockley–Read–Hall (SRH) or trap assisted recombination competing with bimolecular recombination. In the case of binary and ternary blends with non-fullerene acceptors, the n values are similar and close to 1 implying that incorporation of EH-5T-TTC is not contributing towards the reduction of trap-assisted recombination losses [Fig. 6(c), (d) and S6(b)†]. However, for the blends with the fullerene acceptor as shown in Fig. 6(c), the variation of VOC with incident light intensity implies that under open circuit conditions, in the presence of EH-5T-TTC, the n value has come down from 1.4 to 1.2 indicating that trap-assisted recombination losses are reduced for the fullerene-based ternary blend.
, the recombination mechanism that dominates is bimolecular recombination. A stronger dependence of VOC on light intensity corresponding to (n > 1) indicates the presence of Shockley–Read–Hall (SRH) or trap assisted recombination competing with bimolecular recombination. In the case of binary and ternary blends with non-fullerene acceptors, the n values are similar and close to 1 implying that incorporation of EH-5T-TTC is not contributing towards the reduction of trap-assisted recombination losses [Fig. 6(c), (d) and S6(b)†]. However, for the blends with the fullerene acceptor as shown in Fig. 6(c), the variation of VOC with incident light intensity implies that under open circuit conditions, in the presence of EH-5T-TTC, the n value has come down from 1.4 to 1.2 indicating that trap-assisted recombination losses are reduced for the fullerene-based ternary blend.
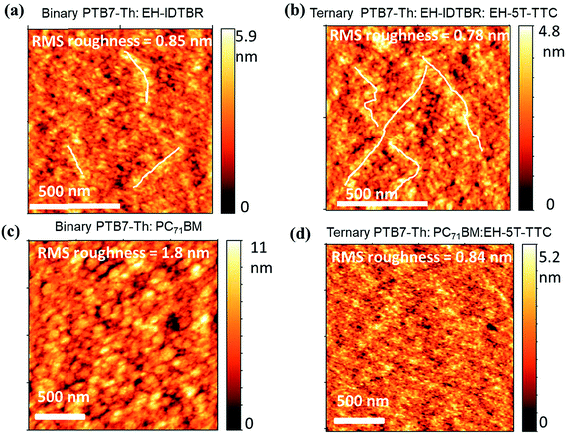
To understand the differences in recombination dynamics of the blends containing EH-5T-TTC and fullerene/non-fullerene acceptors, detailed morphological characterisation was performed using atomic force microscopy (AFM). AFM height images of the binary blends (PTB7-Th:EH-IDTBR and PTB7-Th:PC71BM) and their ternary blends with EH-5T-TTC are shown in Fig. 7(a)–(d). The corresponding AFM height images for PTB7-Th:ITIC blends are shown in Fig. S7.† In the case of blends based on the non-fullerene acceptors, incorporation of EH-5T-TTC did not make any change in the domain size, but the appearance of self-assembled percolated structures could be observed. This type of percolated pathway, which enhances the charge transport properties of the material, is more obvious for PTB7-Th:EH-IDTBR (Fig. 7(a) and (b)) compared to PTB7-Th:ITIC (Fig. S7†). This could be the reason for the improved fill factor for the ternary blend EH-5T-TTC:PTB7-Th:EH-IDTBR based solar cells, compared to the binary PTB7-Th:EH-IDTBR blend as shown in Table S1.† Also the root mean square (RMS) roughness values of the binary blends with non-fullerene acceptors are not changed much due to the incorporation of EH-5T-TTC. In contrast to this, in the case of fullerene-based blends of PTB7-Th:PC71BM, the incorporation of EH-5T-TTC brings a dramatic drop in the domain size and reduces the RMS roughness from 1.8 nm to 0.84 nm, similar to the effect of DIO as additive [Fig. 7(c) and (d)]. This finer morphology of the PTB7-Th:PC71BM:EH-5T-TTC ternary blend compared to PTB7-Th:PC71BM can explain the reduced recombination losses of the ternary compared to the binary blend. The difference in morphology observed between fullerene and non-fullerene based blends upon the incorporation of EH-5T-TTC can account for the variation in recombination dynamics detailed above.
To understand the role of surface energy as the driving force for the difference in morphology for fullerene and non-fullerene based ternary blends containing EH-5T-TTC, contact angle measurements were performed. Previous studies have shown that the surface energy of different components in a blend determines their segregation and location in the blend system.27,28 The contact angles of polymer PTB7-Th, fullerene acceptor PC71BM, non-fullerene acceptors EH-IDTBR and ITIC, and the ternary component EH-5T-TTC were measured to evaluate the surface energy (Fig. S8† and Table 2). The contact angles were measured with water, ethylene glycol and diiodomethane. The surface energy is calculated using the Owens and Wendt equation (eqn (1)), corresponding to three different solvents: deionised water, diiodomethane and ethylene glycol.27,29–31 The estimated surface energy values are shown in Table 2.
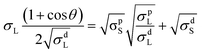 | (1) |
| Material | Surface energy (mJ m−2) |
|---|---|
| PTB7-Th | 19.7 |
| EH-5T-TTC | 20.4 |
| PC71BM | 32.1 |
| ITIC | 19.5 |
| EH-IDTBR | 18.3 |
In eqn (1), θ is the contact angle, σL and σS are the liquid and solid surface tension, respectively. The addition of d and p in the superscript refer to the dispersive and polar components of each solvent. The calculated surface energy values of PTB7-Th, EH-5T-TTC, and the non-fullerene acceptors ITIC and EH-IDTBR are closely matching and in the range of 18–20 mJ m−2. The small difference in surface energy between the blend components results in a well-mixed morphology and the same reason can account for the similar morphology for binary and ternary blends containing non-fullerene acceptors. In the case of the PTB7-Th:PC71BM blend, the surface energy of PC71BM (32.1 mJ m−2) is higher than for PTB7-Th (19.7 mJ m−2) and this large difference in surface energy results in a phase separated morphology with large domains. However, with the incorporation of EH-5T-TTC (20.4 mJ m−2) a well-mixed morphology is observed for the EH-5T-TTC:PTB7-Th:PC71BM blend, implying that the thermodynamic driving force for phase separation of PTB7-Th and PC71BM has been reduced by the ternary component.
After identifying the role of EH-5T-TTC in the morphology of each ternary blend compared to the binary blend containing fullerene and non-fullerene acceptors, the contribution of this altered morphology towards the photogeneration process was studied by the photocurrent analysis method. The role of EH-5T-TTC in the enhanced photocurrent is investigated by considering the charge generation and exciton dissociation processes in the binary and ternary blends by determining the saturation current density (Jsat) and exciton dissociation probabilities P(E,T) of the respective blends. Fig. 8 shows the photocurrent density (Jph) vs. effective voltage (Veff) characteristics for the PTB7-Th:ITIC, PTB7-Th:EH-IDTBR and PTB7-Th:PC71BM blends compared to their respective ternary blend with EH-5T-TTC. Jph is obtained using the following relation:32,33
| Jph = JL − JD |
| Jsat = eGmax × L |
G max values for the binary and ternary blends are listed in Fig. 8(d). With the incorporation of the ternary component EH-5T-TTC, the overall exciton generation rate Gmax of the binary blend has been increased (by 5–10%). This improvement can be attributed to the increased absorption of the active layer blend due to the ternary component EH-5T-TTC. Thus, enhanced exciton generation was observed for EH-5T-TTC-added ternary components.
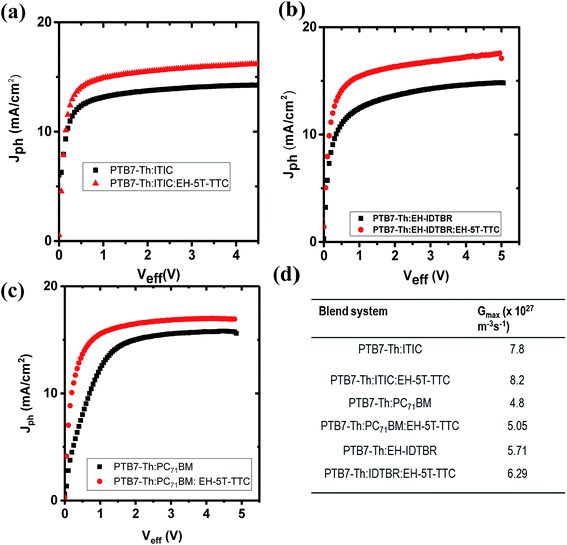 | ||
| Fig. 8 Comparison of photocurrent Jphvs. Veff for binary and ternary blends of (a) PTB7-Th:ITIC, (b) PTB7-Th:EH-IDTBR and (c) PTB7-Th:PC71BM, and (d) Gmax values for all the blends. | ||
Along with increased exciton generation rate, exciton dissociation probability is also important for enhanced photocurrent. The exciton dissociation probability has now been estimated using the relation:
| Jph = eGmaxP(E,T) × L |
Finally, to investigate the possible energy transfer process between the EH-5T-TTC and PTB7-Th, steady state PL spectra were taken (Fig. S10†). The excitation wavelength used is 515 nm. No measurable PL signal could be obtained from EH-5T-TTC. In the case of PTB7-Th, the PL spectra show a broad emission peaking at 760 nm. The excitation wavelength of 515 nm used in this study excited both PTB7-Th and EH-5T-TTC. The change observed in the PL of PTB7-Th with 20 wt% and 40 wt% blend may be either due to reduced self-quenching upon dilution with EH-5T-TTC or energy transfer from EH-5T-TTC to PTB7-Th.38
In order to prove that the beneficial properties of EH-5T-TTC are unique to the core tetrathiocin structure, three types of fullerene-containing organic solar cells were prepared and characterised. The devices, which were made under the same conditions, were not optimised and the experiments were carried out simply to compare a binary device of PTB7-Th:PC71BM with ternary blend devices incorporating the non-tetrathiocin compound 1 (half-unit of EH-5T-TTC, Scheme 2) and EH-5T-TTC. The highest recorded PCE of the binary device was found to be 3.33% (Table S3†). This value increased to 4.26% in PTB7-Th:PC71BM:1 devices, but a much greater increase was observed for the ternary blend containing EH-5T-TTC (5.89%). The corresponding J–V characteristics and the EQE spectra are shown in Fig. S11.† These results clearly indicate that the enhancement in device characteristics is not simply a function of the quinquithiophene chains, but is due to the physical properties of the more complex tetrathiocin structure.
So far we have shown how EH-5T-TTC can help with the important problem of spectral coverage in OPVs and simultaneously achieve morphology modification and energy transfer in forming an efficient ternary component in OPV blends. Now in the final section, the shelf life stability for binary and ternary blend systems has been carried out (Fig. S12†). The encapsulated solar cells were kept in ambient conditions. In the case of the ternary blend incorporating the fullerene acceptor, the shelf life study (for 6 months) has been found to be better with EH-5T-TTC added as a ternary component compared to DIO as the additive. This implies that in addition to the improved absorption of the visible spectral range, the incorporation of EH-5T-TTC can enhance the shelf-life stability compared to the commonly used DIO additive for PTB7-Th:PC71BM blend.
Conclusions
We have presented a new, soluble ‘double-chain’ oligothiophene compound (EH-5T-TTC), based on two quinquithiophene units bridged by a fused 8-membered tetrathiocin core. This non-conventional structure provides intermolecular contacts (π–π and S⋯S) in two dimensions. The synthetic strategy towards EH-5T-TTC is versatile and one can envisage a wide range of new fascinating conjugated structures incorporating the tetrathiocin core. We have demonstrated EH-5T-TTC as an efficient ternary component for both fullerene and non-fullerene based OPVs containing the efficient narrow band gap donor polymer PTB7-Th. The improved efficiency is mainly due to the combined effect of enhanced absorption and beneficial modification of blend morphology enhancing the photogenerated exciton generation and dissociation. A comparison of the shelf life stability studies shows that ternary blend OPVs with EH-5T-TTC are as stable as its binary components and in the case of the DIO-containing binary blend, the shelf life has been found to be better with EH-5T-TTC added as a ternary component compared to DIO as the additive.Experimental details
Fabrication of organic solar cells
Inverted organic solar cells were fabricated on pre-patterned ITO-coated glass. The ITO-coated glass substrates were cleaned in detergent (sodium dodecyl sulfate-SDS), successively ultrasonicated in deionised water, acetone, and isopropyl alcohol, and exposed to Plasma Asher for 3 minutes. The PTB7-Th:PC71BM blend solutions were prepared by dissolving the components in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 (by weight), with a total concentration of 25 mg mL−1 in chlorobenzene, with and without 3 vol% DIO. The solution was kept stirring at 60 °C for ∼8 h before spin-coating.
1.5 (by weight), with a total concentration of 25 mg mL−1 in chlorobenzene, with and without 3 vol% DIO. The solution was kept stirring at 60 °C for ∼8 h before spin-coating.
In the case of PTB7-Th:ITIC blend solution was prepared by mixing 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3 (wt%) donor to acceptor with a total concentration of 25 mg mL−1 in chlorobenzene solvent. For PTB7-Th:EH-IDTBR blend, the donor and acceptor weight ratio was 1
1.3 (wt%) donor to acceptor with a total concentration of 25 mg mL−1 in chlorobenzene solvent. For PTB7-Th:EH-IDTBR blend, the donor and acceptor weight ratio was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with a total concentration of 20 mg mL−1 in o-dichlorobenzene (oDCB) solvent. The blend solutions were kept stirring at 60 °C for ∼6 hours before spin coating. In all the inverted organic solar cells fabricated, the electron transporting layer was amorphous ZnO (a-ZnO) thin film having thickness ∼25 nm and was prepared according to a previous report.39 The active layer was deposited by spin-coating (1000 rpm, 60 s) on glass/ITO/a-ZnO substrates inside a nitrogen filled glove box. The samples were then transferred to a vacuum thermal evaporator (1 × 10−6 mbar base pressure) and kept under vacuum overnight before thermally evaporating the hole transporting layer of MoOx (7 nm) and anode Ag (100 nm) using a shadow mask. The active area of the device was 0.07 cm2. All the processing related to the active layer was performed inside a nitrogen-filled glove box.
1 with a total concentration of 20 mg mL−1 in o-dichlorobenzene (oDCB) solvent. The blend solutions were kept stirring at 60 °C for ∼6 hours before spin coating. In all the inverted organic solar cells fabricated, the electron transporting layer was amorphous ZnO (a-ZnO) thin film having thickness ∼25 nm and was prepared according to a previous report.39 The active layer was deposited by spin-coating (1000 rpm, 60 s) on glass/ITO/a-ZnO substrates inside a nitrogen filled glove box. The samples were then transferred to a vacuum thermal evaporator (1 × 10−6 mbar base pressure) and kept under vacuum overnight before thermally evaporating the hole transporting layer of MoOx (7 nm) and anode Ag (100 nm) using a shadow mask. The active area of the device was 0.07 cm2. All the processing related to the active layer was performed inside a nitrogen-filled glove box.
After the electrode deposition, the devices were encapsulated with a UV optical adhesive and a glass coverslip. The current–voltage characteristics were determined under an illumination intensity of 100 mW cm−2 in air using an air mass 1.5 global (AM 1.5G) Sciencetech solar simulator and a Keithley 2400 source-measure unit. The illumination intensity was verified with a calibrated monosilicon detector and a KG-5 filter. The external quantum efficiency (EQE) measurements were performed at zero bias by illuminating the device with monochromatic light supplied from a Xenon arc lamp in combination with a dual-grating monochromator. The number of photons incident on the sample was calculated for each wavelength by using a silicon photodiode calibrated by the National Physical Laboratory (NPL).
Characterisation of the binary and ternary active layer blend
The surface morphology of the PTB7-Th:PC71BM films was characterised using atomic force microscopy (AFM). AFM images were obtained with a Bruker MultiMode 8 instrument in the tapping mode. NANOSENSORS™ PPP-NCSTR Si cantilever tips with force constant of 6–7 Nm−1 were used as AFM probes. Steady state absorption spectra of the blend films were recorded using a Cary 300 spectrometer for the wavelength range of 300–800 nm. For this, the active layer blend was deposited under identical conditions to that used for OPV devices on a quartz disc. Steady state PL spectra were measured with a Hamamatsu streak camera C10910-05 with S-20ER photocathode and 650 nm as excitation wavelength from an optical parametric amplifier pumped by Pharos laser (from Light Conversion). To measure the contact angle, a goniometer was used with angles estimated by eye using the built-in protractor.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the EPSRC for funding under grants EP/L012200/1 and EP/L012294/1.References
- Nanostructured Materials for Type III Photovoltaics, ed. P. J. Skabara and M. A. Malik, RSC Publishing, Cambridge, 2017 Search PubMed.
- K. Hongkyu, K. Geunjin, K. Junghwan, K. Sooncheol, K. Heejoo and L. Kwanghee, Adv. Mater., 2016, 28, 7821–7861 CrossRef PubMed.
- D. Baran, R. S. Ashraf, D. A. Hanifi, M. Abdelsamie, N. Gasparini, J. A. Röhr, S. Holliday, A. Wadsworth, S. Lockett, M. Neophytou, C. J. M. Emmott, J. Nelson, C. J. Brabec, A. Amassian, A. Salleo, T. Kirchartz, J. R. Durrant and I. McCulloch, Nat. Mater., 2016, 16, 363–369 CrossRef PubMed.
- S. Chen, Y. Liu, L. Zhang, P. C. Y. Chow, Z. Wang, G. Zhang, W. Ma and H. Yan, J. Am. Chem. Soc., 2017, 139, 6298–6301 CrossRef CAS PubMed.
- J. L. Krishnan, M. Al-Senani, A. El-Labban, I. Gereige, G. O. Ngongang_Ndjawa, J. C. D. Faria, T. Kim, K. Zhao, F. Cruciani, D. H. Anjum, M. A. McLachlan, P. M. Beaujuge and A. Amassian, Adv. Energy Mater., 2015, 5, 1500204 CrossRef.
- H.-C. Liao, C.-C. Ho, C.-Y. Chang, M.-H. Jao, S. B. Darling and W.-F. Su, Mater. Today, 2013, 16, 326–336 CrossRef CAS.
- A. Wadsworth, R. S. Ashraf, M. Abdelsamie, S. Pont, M. Little, M. Moser, Z. Hamid, M. Neophytou, W. Zhang, A. Amassian, J. R. Durrant, D. Baran and I. McCulloch, ACS Energy Lett., 2017, 2, 1494–1500 CrossRef CAS.
- Z. Wenchao, L. Sunsun, Z. Shaoqing, L. Xiaoyu and H. Jianhui, Adv. Mater., 2017, 29, 1604059 CrossRef PubMed.
- C. Winder and N. S. Sariciftci, J. Mater. Chem., 2004, 14, 1077–1086 RSC.
- B. Minnaert and P. Veelaert, Materials, 2012, 5, 1933–1953 CrossRef.
- X. Liu, Y. Yan, Y. Yao and Z. Liang, Adv. Funct. Mater., 2018, 28, 1802004 CrossRef.
- N. Gasparini, A. Salleo, I. McCulloch and D. Baran, Nat. Rev. Mater., 2019, 4, 229–242 Search PubMed.
- Q. Jiang, L. Zhang, H. Wang, X. Yang, J. Meng, H. Liu, Z. Yin, J. Wu, X. Zhang and J. You, Nat. Energy, 2016, 2, 16177 CrossRef.
- L. Lu, M. A. Kelly, W. You and L. Yu, Nat. Photonics, 2015, 9, 491–500 CrossRef CAS.
- Y. Yang, W. Chen, L. Dou, W.-H. Chang, H.-S. Duan, B. Bob, G. Li and Y. Yang, Nat. Photonics, 2015, 9, 190–198 CrossRef CAS.
- P. P. Khlyabich, A. E. Rudenko, R. A. Street and B. C. Thompson, ACS Appl. Mater. Interfaces, 2014, 6, 9913–9919 CrossRef CAS PubMed.
- P. P. Khlaybich, A. E. Rudenko, B. C. Thompson and L. Yueh-Lin, Adv. Funct. Mater., 2015, 25, 5557–5563 CrossRef.
- J. Mai, T.-K. Lau, J. Li, S.-H. Peng, C.-S. Hsu, U. S. Jeng, J. Zeng, N. Zhao, X. Xiao and X. Lu, Chem. Mater., 2016, 28, 6186–6195 CrossRef CAS.
- I. A. Wright, A. L. Kanibolotsky, J. Cameron, T. Tuttle, P. J. Skabara, S. J. Coles, C. T. Howells, S. A. J. Thomson, S. Gambino and I. D. W. Samuel, Angew. Chem., Int. Ed., 2012, 51, 4562–4567 CrossRef CAS PubMed.
- P. J. Skabara, I. M. Serebryakov, D. M. Roberts, I. F. Perepichka, S. J. Coles and M. B. Hursthouse, J. Org. Chem., 1999, 64, 6418–6424 CrossRef CAS.
- I. A. Wright, P. J. Skabara, J. C. Forgie, A. L. Kanibolotsky, B. Gonzalez, S. J. Coles, S. Gambino and I. D. W. Samuel, J. Mater. Chem., 2011, 21, 1462–1469 RSC.
- S. Ralf, Angew. Chem., Int. Ed. Engl., 1975, 14, 655–664 CrossRef.
- P. J. Skabara, J. B. Arlin and Y. H. Geerts, Adv. Mater., 2013, 25, 1948–1954 CrossRef CAS PubMed.
- Y. Zhang, A. J. Parnell, F. Pontecchiani, J. F. K. Cooper, R. L. Thompson, R. A. L. Jones, S. M. King, D. G. Lidzey and G. Bernardo, Sci. Rep., 2017, 7, 44269 CrossRef PubMed.
- S. R. Cowan, A. Roy and A. J. Heeger, Phys. Rev. B: Condens. Matter Mater. Phys., 2010, 82, 245207 CrossRef.
- L. J. A. Koster, V. D. Mihailetchi, R. Ramaker and P. W. M. Blom, Appl. Phys. Lett., 2005, 86, 3 CrossRef.
- J.-H. Huang, Y.-S. Hsiao, E. Richard, C.-C. Chen, P. Chen, G. Li, C.-W. Chu and Y. Yang, Appl. Phys. Lett., 2013, 103, 043304 CrossRef.
- S. Honda, H. Ohkita, H. Benten and S. Ito, Adv. Energy Mater., 2011, 1, 588–598 CrossRef CAS.
- R. L. Bendure, J. Colloid Interface Sci., 1973, 42, 137–144 CrossRef CAS.
- S. Siboni, C. Della Volpe, D. Maniglio and M. Brugnara, J. Colloid Interface Sci., 2004, 271, 454–472 CrossRef CAS PubMed.
- D. Y. Kwok and A. W. Neumann, Adv. Colloid Interface Sci., 1999, 81, 167–249 CrossRef CAS.
- P. W. M. Blom, V. D. Mihailetchi, L. J. A. Koster and D. E. Markov, Adv. Mater., 2007, 19, 1551–1566 CrossRef CAS.
- L. Yang, J. R. Tumbleston, H. Zhou, H. Ade and W. You, Energy Environ. Sci., 2013, 6, 316–326 RSC.
- V. Shrotriya, Y. Yao, G. Li and Y. Yang, Appl. Phys. Lett., 2006, 89, 063505 CrossRef.
- P. Gautam, R. Sharma, R. Misra, M. L. Keshtov, S. A. Kuklin and G. D. Sharma, Chem. Sci., 2017, 8, 2017–2024 RSC.
- V. D. Mihailetchi, L. J. A. Koster, J. C. Hummelen and P. W. M. Blom, Phys. Rev. Lett., 2004, 93, 216601 CrossRef CAS PubMed.
- N. Bauer, Q. Zhang, J. Zhao, L. Ye, J.-H. Kim, I. Constantinou, L. Yan, F. So, H. Ade, H. Yan and W. You, J. Mater. Chem. A, 2017, 5, 4886–4893 RSC.
- S. M. Menke, W. A. Luhman and R. J. Holmes, Nat. Mater., 2012, 12, 152 CrossRef PubMed.
- L. K. Jagadamma, M. Abdelsamie, A. El Labban, E. Aresu, G. O. Ngongang Ndjawa, D. H. Anjum, D. Cha, P. M. Beaujuge and A. Amassian, J. Mater. Chem. A, 2014, 2, 13321–13331 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: General experimental and synthetic and crystallography details; AFM images, spectroscopic data and device data. CCDC 1856815. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9se00343f. Additional supporting data are accessible from http://dx.doi.org/10.5525/gla.researchdata.833 |
| ‡ Present address: Department of Chemistry, Loughborough University, Loughborough LE11 3TU, UK. |
| This journal is © The Royal Society of Chemistry 2019 |