Open Access Article
Open Access ArticleSelf-assembly of two robust 3D supramolecular organic frameworks from a geometrically non-planar molecule for high gas selectivity performance†
Yue
Zhou
a,
Liang
Kan
a,
Jarrod F.
Eubank
b,
Guanghua
Li
a,
Lirong
Zhang
a and
Yunling
Liu
 *a
*a
aState Key Laboratory of Inorganic Synthesis and Preparative Chemistry, College of Chemistry, Jilin University, Changchun 130012, P. R. China. E-mail: yunling@jlu.edu.cn; Fax: +86-431-85168624; Tel: +86-431-85168614
bFlorida Southern College, 111 Lake Hollingsworth Dr, Lakeland, FL, USA 33801
First published on 29th May 2019
Abstract
The synthesis of highly porous frameworks has received continuous research interest, but achieving the ability to target stable and selective materials remains challenging. Herein, by utilizing a ‘direction-oriented’ strategy and modulating reaction conditions, two novel 3D porous supramolecular organic framework (SOF) materials (JLU-SOF2 and JLU-SOF3, as isomers) are assembled from a non-planar building block (TMBTI = 2,4,6-trimethyl benzene-1,3,5-triyl-isophthalic acid) and they display permanent porosity, high thermal stability, and good recyclability. It is worth mentioning that the CO2 uptake values of JLU-SOF2 and JLU-SOF3 rank among the highest values for SOF-based materials under ambient conditions. Furthermore, these two materials exhibit preferential adsorption of CO2 over N2 and CH4, and can effectively separate the mixtures of light hydrocarbons. These studies indicate the possible application of JLU-SOF2 and JLU-SOF3 in trapping greenhouse gases and upgrading natural gas. In addition, this synthetic strategy introduces an effective method for developing remarkable 3D SOFs among other framework materials.
Introduction
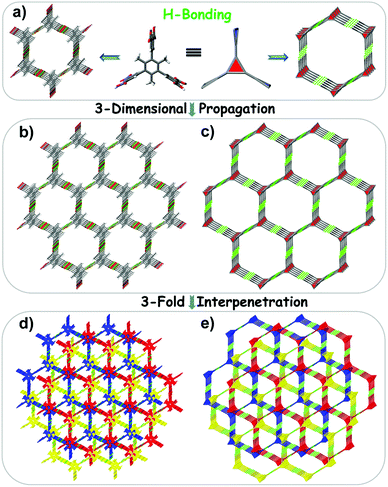
Alleviating the escalation of concentration of atmospheric CO2 is an ongoing research topic.1–3 To solve this issue, adsorption-based separation technology will likely be a feasible method until more effective technologies can be developed, which can realize the post-combustion carbon capture and purification of natural gas.3–5 For gas adsorbents, permanent porosity, sufficient void-space, high selectivity and good recyclability are crucial prerequisites for their use in practical applications. Over the past few decades, some novel porous framework materials, such as metal–organic frameworks (MOFs),6,7 covalent organic frameworks (COFs),8,9 and other crystalline porous materials,10–12 have been explored as potential adsorbents.Supramolecular organic frameworks (SOFs, also called HOFs, abbreviation of hydrogen-bonded organic frameworks) constructed by hydrogen-bonding and π–π interactions are attractive gas adsorbents thanks mainly to their low framework density and regeneration by simple recrystallization.10,13–19 However, the stability of SOFs is still a challenge although a number of SOFs with permanent porosity have been reported.20–35 Considering their highly predictable hydrogen-bonding motif, some (carboxylic acid)-containing organic units were selected to construct SOF materials by the groups of Zentner, Moorthy, Hisaki and others.24,36–41 The vast majority of these SOFs are 2D materials due to the limited propagation ability of planar blocks. Thus, synthesis of new building blocks with a non-planar configuration, that is, utilizing a ‘direction-oriented’ strategy, may be an effective method to construct 3D SOF materials. Li et al. reported the first solution-phase 3D periodic SOF in water, and they pointed out that by utilizing self-assembly of tetrahedral building blocks and cucurbit[8]uril ordered 3D frameworks could be achieved.42 Pillarene-based 3D SOF materials were constructed by Yang and co-workers, and their construction is mainly attributed to the spatial configuration and abundant electron donor–acceptor components of macrocyclic molecules.43 For organic small-molecules, modifying the central component of the planar organic entity to make it rotate or replacing it with a flexible group38 is a feasible way to break the planarity of building blocks. On the basis of the above considerations, a non-planar organic building block, 2,4,6-trimethyl benzene-1,3,5-triyl-isophthalic acid (TMBTI), is synthesized by modifying the central phenyl ring of 3,3′,3′′,5,5′,5′′-benzene-1,3,5-triyl-hexabenzoic acid (H6BHB)44 with methyl groups (Scheme 1). In terms of the ‘direction-oriented’ strategy, desired 3D frameworks would be assembled from TMBTI because it inherently exhibits a high propensity to form multiple hydrogen bonds in different directions (i.e., beyond two dimensions).
Due to the tremendous freedom of hydrogen bonds, the self-assembly of the same constituent could also allow for achievement of different SOFs based on diverse hydrogen-bonding modes.24,45–49 To the best of our knowledge, though some SOF materials have been reported to have hydrogen-bonded isomers, most of these polymorphs were formed simultaneously under the same conditions.24,47 Therefore, design strategies, including precise modulation of reaction conditions, are vitally important to yield unique or desired structures as phase-pure materials.
Herein, as an experimental proof-of-concept, two 3D SOF isomers, JLU-SOF2 and JLU-SOF3, with high thermal stability and permanent microporous nature are assembled by subtly modulating reaction conditions. It is notable that some MOFs have been synthesized using the TMBTI ligand, but all of them are isomorphous.50–52JLU-SOF2 and JLU-SOF3 exhibit excellent CO2 capture and separation ability, which surpasses that of the majority of SOF materials. Besides, these two materials show salient performance for purification of natural gas under ambient conditions.
Results and discussion
Structure descriptions
Single-crystal X-ray diffraction (SCXRD) analysis reveals that JLU-SOF2 and JLU-SOF3 crystallize in the trigonal P![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) 1c and hexagonal P
1c and hexagonal P![[6 with combining macron]](https://www.rsc.org/images/entities/char_0036_0304.gif) 2c space group, respectively. As illustrated in Fig. 1a and b, each TMBTI block existing in both of them is connected with six other organic units through six pairs of intermolecular –COOH⋯HOOC– hydrogen bonds. As expected, these two SOFs exhibit 3D hydrogen-bonded structures, which are mainly attributed to the propagation ability of the geometrically non-planar block in different directions. JLU-SOF3 is assembled by the classic carboxylic acid dimer motif exclusively, and the O–H⋯O distances are 2.605(2), 2.615(7), 2.630(6), and 2.628(3) Å (Table S3†). Meanwhile, two types of hydrogen-bonding motifs are present in JLU-SOF2: one is the typical carboxylic acid dimer formed between two carboxyl groups and the distance of O(4)–H(4)⋯O(3)#4 is 2.642(2) Å; but the other is an uncommon motif formed among three carboxyl groups and the distance of O(2)–H(2)⋯O(1)#3 is 2.6606(19) Å (Table S4†). The different hydrogen-bonding interactions and corresponding packing modes of TMBTI in JLU-SOF2 and JLU-SOF3 were caused by the differences in reaction systems in terms of solvent polarity, solubility and temperature. Nevertheless, these two SOFs display similar pore sizes. JLU-SOF2 contains homogeneous open channels with window sizes of about 5.6 Å × 3.8 Å along the x-axis and y-axis, respectively, considering the van der Waals radius (Fig. 1c and S3†). For JLU-SOF3, two types of triangular cavities (5.8 Å and 3.2 Å in diameter) co-exist in the framework along the z-axis (Fig. 1d and S5†). Interestingly, the whole structure of JLU-SOF3 contains three equivalent interwoven frameworks which are linked by C–H⋯π interactions, as supported by the measured distance of 3.39 Å between the H atoms and the centroids of phenyl rings of the isophthalic acid groups (Fig. 3d, S6 and S7†). There exist typical honeycomb channels in a single net with an internal dimension of about 19.3 Å along the z-axis, considering the van der Waals radius (Fig. 3b and S8†). However, it is expected that the stability of JLU-SOF3 will be significantly increased after three-fold interpenetration.
2c space group, respectively. As illustrated in Fig. 1a and b, each TMBTI block existing in both of them is connected with six other organic units through six pairs of intermolecular –COOH⋯HOOC– hydrogen bonds. As expected, these two SOFs exhibit 3D hydrogen-bonded structures, which are mainly attributed to the propagation ability of the geometrically non-planar block in different directions. JLU-SOF3 is assembled by the classic carboxylic acid dimer motif exclusively, and the O–H⋯O distances are 2.605(2), 2.615(7), 2.630(6), and 2.628(3) Å (Table S3†). Meanwhile, two types of hydrogen-bonding motifs are present in JLU-SOF2: one is the typical carboxylic acid dimer formed between two carboxyl groups and the distance of O(4)–H(4)⋯O(3)#4 is 2.642(2) Å; but the other is an uncommon motif formed among three carboxyl groups and the distance of O(2)–H(2)⋯O(1)#3 is 2.6606(19) Å (Table S4†). The different hydrogen-bonding interactions and corresponding packing modes of TMBTI in JLU-SOF2 and JLU-SOF3 were caused by the differences in reaction systems in terms of solvent polarity, solubility and temperature. Nevertheless, these two SOFs display similar pore sizes. JLU-SOF2 contains homogeneous open channels with window sizes of about 5.6 Å × 3.8 Å along the x-axis and y-axis, respectively, considering the van der Waals radius (Fig. 1c and S3†). For JLU-SOF3, two types of triangular cavities (5.8 Å and 3.2 Å in diameter) co-exist in the framework along the z-axis (Fig. 1d and S5†). Interestingly, the whole structure of JLU-SOF3 contains three equivalent interwoven frameworks which are linked by C–H⋯π interactions, as supported by the measured distance of 3.39 Å between the H atoms and the centroids of phenyl rings of the isophthalic acid groups (Fig. 3d, S6 and S7†). There exist typical honeycomb channels in a single net with an internal dimension of about 19.3 Å along the z-axis, considering the van der Waals radius (Fig. 3b and S8†). However, it is expected that the stability of JLU-SOF3 will be significantly increased after three-fold interpenetration.
In the crystal structure of JLU-SOF2, the central phenyl ring could be described as a 3-connected node, and the isophthalic acid group could be simplified as a 4-connected node (by three hydrogen bonds) (Fig. 2 and S4†). Consequently, the structure of JLU-SOF2 can be rationalized as a new (3,4)-connected network with a point symbol of {63} {65 × 10}3, which possesses four types of tiles [65], [103], [62 × 102] and [63 × 103]. Similarly, the single net of JLU-SOF3 can be reduced into a (3,3)-connected etc net topology with an {83} point symbol, and the total framework consists of three-fold interpenetrated networks (Fig. 3 and S9†). The potential solvent-accessible volume of JLU-SOF2 and JLU-SOF3 is 43.7% and 51.3%, respectively, estimated using PLATON after removal of disordered 1,4-dioxane (DOA) solvent.
 | ||
| Fig. 2 Schematic representation of the (3,4)-connected net of JLU-SOF2 showing the simplified organic building block and hydrogen bonds along the x-axis and z-axis. | ||
Stability studies
Thermogravimetric analyses (TGA) reveal that all solvent is removed at about 230 °C with a weight loss of 23% for JLU-SOF2, followed by a relatively steady plateau until 420 °C, after that the material begins to decompose (Fig. S10†). JLU-SOF3 exhibits a weight loss of 24% before 210 °C, which corresponds to the loss of guest molecules, and then the material begins to decompose at 430 °C. The crystalline phase purity of as-synthesized JLU-SOF2 and JLU-SOF3 was identified from the consistency between the measured powder X-ray diffraction (PXRD) pattern and the simulated one from the SCXRD data (Fig. 4 and S12a†). To investigate their permanent porosity, N2 sorption isotherms of these two compounds were studied. The isotherms exhibit a sharp rise in uptake at the low P/P0 region corresponding to the natural behavior of microporous materials (Fig. S13†). Brunauer–Emmett–Teller surface areas of JLU-SOF2 and JLU-SOF3 are calculated to be 937 and 1141 m2 g−1, respectively, which are higher than those of most SOF-based materials16,53–55 (Tables S5 and S6†). The experimental pore volumes of JLU-SOF2 and JLU-SOF3 (0.47 and 0.63 cm3 g−1) are consistent with the theoretical values (0.47 and 0.63 cm3 g−1, the detailed calculation method is provided in the ESI†) estimated from the crystal structures, demonstrating that the cavities of the frameworks have been activated adequately.In view of practical applications, the reusability and stability of these materials were explored. The N2 sorption was tested for the used materials that were placed in air for one week after adsorption (named JLU-SOF2R and JLU-SOF3R). The BET surface areas are 884 and 1005 m2 g−1 for JLU-SOF2R and JLU-SOF3R, which are slightly lower than those of the pristine samples (Fig. S13 and Table S7†). It indicates that the reused materials remain intact basically. Variable-temperature PXRD and corresponding N2 sorption were performed to test their thermal stability. As shown in Fig. 4 and S11,† the primary peaks are consistent with those of as-synthesized JLU-SOF2, and some changes can be observed, especially for the 350 °C treated sample. The N2 uptake of JLU-SOF2 did not decreased significantly below 250 °C (Fig. S14 and Table S8†). Increasing the temperature above 300 °C lowered the BET surface area and a noticeable up-tail emerged at high P/P0. Yet, the N2 isotherms preserved their type-I shape. These observations may be caused by the cleaving of hydrogen bonds and stacking of the samples.35 These results indicate that the crystallinity and porosity of JLU-SOF2 remain stable up to 250 °C, and are preserved partially up to 350 °C. Similar are made for JLU-SOF3 (Fig. S12, S15 and Table S9†). The up-tail phenomenon was analysed in detail based on the pore volumes and scanning electron microscopy (SEM) images of these materials (Fig. S16, Tables S8 and S9,† the detailed analysis is provided in the ESI†). The 1H NMR spectra and solubility of these materials indicate that the TMBTI blocks remained intact the samples treated at 350 °C (Fig. S17 and S18,† detailed information is presented in the ESI†). The high thermal stability of these two SOFs can be attributed to the strong and multiple hydrogen-bonding interactions. In addition, the CH3CN-exchanged and regenerated samples still exhibit good crystallinity.
Gas adsorption and separation
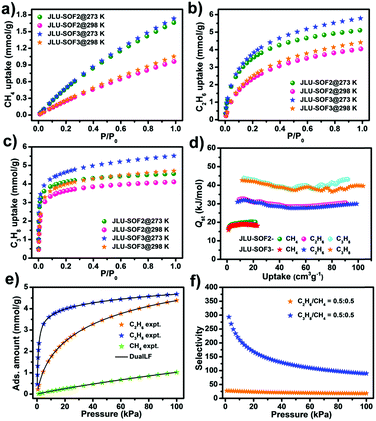
The high pore volume and permanent porosity of these two materials inspired us to probe into their potential for application as gas adsorbents. Both JLU-SOF2 and JLU-SOF3 exhibit stepwise CO2 adsorption at 195 K with a slight hysteresis loop, and the uptake values are 13.75 and 15.18 mmol g−1, respectively (Fig. 5a). Furthermore, JLU-SOF2 and JLU-SOF3 also display notable CO2 adsorption amounts at higher temperatures with 4.25 and 4.49 mmol g−1 at 273 K, and 1.98 and 2.34 mmol g−1 at 298 K, respectively (Fig. 5b). It is worth mentioning that the CO2 uptake values of JLU-SOF3 rank among the values of activated carbon,56 ZIF-100,57 JLU-MOF50 (ref. 58) and the highest values for SOFs except for HOF-5a15 and IISERP-HOF1 (ref. 38) (Tables S10 and S11†). In contrast to the high CO2 uptake values, much less N2 is adsorbed, 0.24 and 0.36 mmol g−1 at 273 K, and these values are decreased to 0.11 and 0.14 mmol g−1 at 298 K, respectively (Fig. S20†). These observed differential adsorption behaviors between CO2 and N2 for JLU-SOF2 and JLU-SOF3 imply that these materials are probably potential separators for capture of CO2 from flue-gas.To predict their separation ability for CO2/N2, ideal adsorbed solution theory (IAST) was used to calculate the selectivity for the binary mixtures based on single-component adsorption data fitting using the dual-site Langmuir-Freundlich (DSLF) equation at 298 K. As expected, the selectivity of JLU-SOF2 was calculated to be 30.0 and 29.1, respectively, for the binary mixtures of CO2/N2 (v/v: 15/85 and 10/90) at 298 K and 1 bar and the values are 22.8 and 23.6 for JLU-SOF3 under the same conditions (Fig. 5c–f). Encouragingly, these values are higher than those of considerable SOF materials, and exceed those of MOF materials such as PCN-88,3 UiO-67,59 and NU-1000 (ref. 60) (Table S12†). The highly selective adsorption of CO2 over N2 may be explained by the following aspects: (1) the kinetic diameter of CO2 (3.3 Å) is smaller than that of N2 (3.64 Å); (2) the attractive and unique interactions formed by the quadrupole moment of CO2 will increase the adsorption ability; and (3) the host cavities containing carboxyl groups exhibit stronger affinity behavior for CO2 than for N2, which can be identified using the isosteric adsorption enthalpy (Qst). As shown in Fig. S21 and S22,† the Qst of CO2 and N2 are 24.5 and 12.1 kJ mol−1 for JLU-SOF2, and 21.8 and 17.0 kJ mol−1 for JLU-SOF3 at zero coverage, respectively, as calculated from the adsorption data at 273 and 298 K.
Another particularly salient feature of JLU-SOF2 and JLU-SOF3 is the adsorption and separation ability for light hydrocarbons under ambient conditions. Single-component gas sorption isotherms measured at 298 K under 1 bar show that the adsorption amounts of CH4 for JLU-SOF2 and JLU-SOF3 are 0.96 and 1.05 mmol g−1, those for C2H6 are 4.04 and 4.41 mmol g−1, and those for C3H8 are 4.11 and 4.70 mmol g−1, respectively (Fig. 6a–c). Following a temperature decrease to 273 K, the maximum adsorption amounts are increased to 1.67 and 1.73 mmol g−1 for CH4, 5.10 and 5.79 mmol g−1 for C2H6, and 4.55 and 5.51 mmol g−1 for C3H8. The used materials exhibit a similar adsorption capacity for these gases to the pristine samples (Fig. S23 and Table S7†). The selectivity of JLU-SOF2 for equimolar binary mixtures of C2H6/CH4 and C3H8/CH4 is 16.3 and 48.1 at 298 K and 1 bar, respectively (Fig. S24†). There are two primary reasons for the different adsorption ability and high selectivity: (1) the strength of mutual gas interaction decreases as the molecular size decreases;61 and (2) the hydrocarbon-building block dispersion interaction is higher for larger and more polarizable molecules,62,63 which can be proved from the gas affinity towards JLU-SOF2 in the order of C3H8 > C2H6 > CH4 according to the Qst of 43.8, 32.3 and 16.6 kJ mol−1 at zero coverage, respectively (Fig. 6d). With respect to JLU-SOF3, the selectivity for C2H6/CH4 and C3H8/CH4 is 17.8 and 89.2, respectively (Fig. 6e and f). Similarly, the gas affinity of JLU-SOF3 shows a consistent trend for C3H8, C2H6, and CH4 and the Qst is 42.7, 31.0, and 15.8, respectively. These results imply that the suitable pore sizes of these two SOFs induce stronger host–guest interactions with larger hydrocarbons than with the smaller ones. It should be mentioned that only one SOF material, HOF-TCPB, has been reported to separate light hydrocarbons significantly, and the article mainly focuses on the separation of CH4 from C4 hydrocarbons.64 In addition to this separation, the selectivity of our materials for CO2/CH4 mixtures (v/v: 50/50 and 5/95) was explored, and the values of which are 2.2 and 2.1 for JLU-SOF2 and 2.3 and 2.2 for JLU-SOF3 at 298 K and 1 bar, respectively (Fig. S25†). The above studies indicate the high potential for application of these two materials in selective capturing of light hydrocarbons and upgrading of natural gas.
Conclusions
In summary, by utilizing a ‘direction-oriented’ strategy and selectively modulating reaction conditions, two 3D SOF materials were synthesized based on different hydrogen-bonding modes from a singular pre-designed non-planar building block. JLU-SOF2 and JLU-SOF3 exhibit permanent porosity, high thermal stability, and good recyclability. It is worth mentioning that these two SOF materials display favourable CO2 adsorption capacity at ambient temperature. More importantly, these two materials show preferential adsorption of CO2 over N2, as well as CO2, C2H6 and C3H8 over CH4. These substantial results indicate that JLU-SOF2 and JLU-SOF3 could serve as effective reservoirs and separators for greenhouse gas trapping and natural gas upgrading. Notably, the successful crystallization of these two materials offers a feasible method to fabricate 3D SOFs, and in principle, this strategy is not limited to the construction of SOF materials, but suitable for other porous framework materials also.Experimental
Materials and methods
The chemicals were purchased from commercial sources and used without further purification. The Fourier transform infrared (FT-IR) spectrum was recorded on a Bruker IFS-66v/S FT-IR spectrometer from 400 to 4000 cm−1 using KBr pellets. TGA analyses were performed on a TGA Q500 thermogravimetric analyzer with a heating rate of 10 °C min−1 under an air atmosphere. Elemental analyses were completed on a vario MICRO elemental analyzer. PXRD patterns were collected on a Rigaku D/max-2550 diffractometer (Cu-Kα radiation, λ = 1.5418 Å). Nuclear magnetic resonance (NMR) spectra were obtained using a Varian 300 MHz NMR analyzer. The SEM images were measured using a HITACHI SU-8010.Synthesis of TMBTI
The TMBTI building block was synthesized by Suzuki–Miyaura reaction according to the literature with some adjustments,50 and the detailed information is given in the ESI.†Preparation of JLU-SOF2
TMBTI (6 mg) dissolved in DOA (1 mL) was sealed in a vial, and then heated at 85 °C for 2 days. Colorless hexagonal prism-shaped crystals, JLU-SOF2, were obtained and dried in air with a yield of 75%. Elemental analysis (%) calcd for JLU-SOF2 (C33H24O12·2C4H8O2, TMBTI·2DOA): C 62.43, H 5.11; found: C 60.13, H 4.99.Preparation of JLU-SOF3
TMBTI (6 mg) was dissolved in DOA (1 mL), and then acetonitrile was slowly diffused into the solution at room temperature for 1 day. The colorless hexagonal rod-like crystals, JLU-SOF3, were collected and air-dried with a yield of 72%. Elemental analysis (%) calcd for JLU-SOF3 (C33H24O12·2C4H8O2, TMBTI·2DOA): C 62.43, H 5.11; found: C 60.11, H 4.88.Regeneration of JLU-SOF2 and JLU-SOF3
The prepared JLU-SOF2 (or JLU-SOF3) was dissolved in ethanol by ultrasonication, and then the solution was evaporated in a rotary evaporator to give the TMBTI building block. The regeneration procedures were the same as the original preparation of JLU-SOF2 (or JLU-SOF3).X-ray crystallography
Crystallographic data of as-synthesized JLU-SOF2 and JLU-SOF3 were acquired on a Bruker Apex II CCD diffractometer using graphite-monochromated Mo-Kα radiation (λ = 0.71073 Å) at 293 K and at 153 K, respectively. The structures of these two SOFs were resolved by a direct method and refined by full-matrix least-squares on F2 using the SHELXTL program.65 The non-hydrogen atoms were refined with anisotropic displacement parameters and the hydrogen atoms were located geometrically with isotropic parameters. In addition, the “SQUEEZE” command was utilized because of the significantly disordered solvent molecules in the pores. Topological analysis was carried out using TOPOS 4.0.66 The final formulae of the structures were derived from the crystallographic data, TGA and elemental analyses data. The summarized crystallographic data and all the hydrogen-bonds data are presented in Tables S2–S4.† The supplementary crystallographic data for JLU-SOF2 and JLU-SOF3 (CCDC numbers: 1842179 and 1861545†) can be achieved free of charge from the Cambridge Crystallographic Data Centre upon request at http://www.ccdc.cam.ac.uk/data_request/cif.Gas adsorption measurements
Before gas adsorption measurements, JLU-SOF2 and JLU-SOF3 were exchanged with acetonitrile 8 times for 2 days to fully remove the guest molecules, DOA, which can be identified by TGA analyses. Then, the exchanged samples were activated by using the ‘outgas’ function of the surface area analyzer at 100 °C for 10 h. Surface areas were calculated using N2 adsorption isotherms at 77 K obtained using a Micromeritics ASAP 2040 instrument. CO2 adsorption isotherms measured at 195 K were acquired on a Micromeritics ASAP 2020 instrument. CO2, CH4, C2H6, C3H8, and N2 adsorption isotherms were collected on a Micromeritics 3-Flex and a Micromeritics ASAP 2020 PLUS HD88 instrument at 273 and 298 K.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge the financial support of the National Natural Science Foundation of China (No. 21771078, 21671074, and 21621001), the 111 Project (B17020), the National Key Research and Development Program of China (2016YFB0701100). We thank Dr Libo Sun, Mr Xiaodong Sun, Mr Jiantang Li and Mr Jiaming Gu from Jilin University for their assistance in the synthesis of TMBTI and helpful discussions. We also thank Dr Kui-Zhan Shao from Northeast Normal University for his kind technical support relating to the crystallographic data.Notes and references
- D. W. Keith, Science, 2009, 325, 1654 CrossRef CAS PubMed.
- D. M. D'Alessandro, B. Smit and J. R. Long, Angew. Chem., Int. Ed., 2010, 49, 6058 CrossRef.
- J.-R. Li, J. Yu, W. Lu, L.-B. Sun, J. Sculley, P. B. Balbuena and H.-C. Zhou, Nat. Commun., 2013, 4, 1538 CrossRef.
- K. Sumida, D. L. Rogow, J. A. Mason, T. M. McDonald, E. D. Bloch, Z. R. Herm, T.-H. Bae and J. R. Long, Chem. Rev., 2012, 112, 724 CrossRef CAS PubMed.
- J.-R. Li, R. J. Kuppler and H.-C. Zhou, Chem. Soc. Rev., 2009, 38, 1477 RSC.
- K. Adil, Y. Belmabkhout, R. S. Pillai, A. Cadiau, P. M. Bhatt, A. H. Assen, G. Maurinb and M. Eddaoudi, Chem. Soc. Rev., 2017, 46, 3402 RSC.
- T. L. Easun, F. Moreau, Y. Yan, S. Yang and M. Schröder, Chem. Soc. Rev., 2017, 46, 239 RSC.
- A. P. Côte, A. I. Benin, N. W. Ockwig, M. O'Keeffe, A. J. Matzger and O. M. Yaghi, Science, 2005, 310, 1166 CrossRef PubMed.
- S. Das, P. Heasman, T. Ben and S. Qiu, Chem. Rev., 2017, 117, 1515 CrossRef CAS PubMed.
- J. Lü and R. Cao, Angew. Chem., Int. Ed., 2016, 55, 9474 CrossRef PubMed.
- B. Wang, A. P. Côté, H. Furukawa, M. O'Keeffe and O. M. Yaghi, Nature, 2008, 453, 207 CrossRef CAS PubMed.
- Y. Zhu, H. Long and W. Zhang, Chem. Mater., 2013, 25, 1630 CrossRef CAS.
- Z.-T. Li, Beilstein J. Org. Chem., 2015, 11, 2057 CrossRef CAS PubMed.
- Y.-F. Han, Y.-X. Yuan and H.-B. Wang, Molecules, 2017, 22, 266 CrossRef PubMed.
- H. Wang, B. Li, H. Wu, T.-L. Hu, Z. Yao, W. Zhou, S. Xiang and B. Chen, J. Am. Chem. Soc., 2015, 137, 9963 CrossRef CAS.
- J. Lü, C. Perez-Krap, M. Suyetin, N. H. Alsmail, Y. Yan, S. Yang, W. Lewis, E. Bichoutskaia, C. C. Tang, A. J. Blake, R. Cao and M. Schröder, J. Am. Chem. Soc., 2014, 136, 12828 CrossRef.
- Y. Zhou, B. Liu, X. Sun, J. Li, G. Li, Q. Huo and Y. Liu, Cryst. Growth Des., 2017, 17, 6653 CrossRef CAS.
- A. Chaix, G. Mouchaham, A. Shkurenko, P. Hoang, B. Moosa, P. M. Bhatt, K. Adil, K. N. Salama, M. Eddaoudi and N. M. Khashab, J. Am. Chem. Soc., 2018, 140, 14571 CrossRef CAS PubMed.
- Y.-H. Luo, X.-T. He, D.-L. Hong, C. Chen, F.-H. Chen, J. Jiao, L.-H. Zhai, L.-H. Guo and B.-W. Sun, Adv. Funct. Mater., 2018, 28, 1804822 CrossRef.
- W. Yang, A. Greenaway, X. Lin, R. Matsuda, A. J. Blake, C. Wilson, W. Lewis, P. Hubberstey, S. Kitagawa, N. R. Champness and M. Schröder, J. Am. Chem. Soc., 2010, 132, 14457 CrossRef CAS PubMed.
- T.-H. Chen, I. Popov, W. Kaveevivitchai, Y.-C. Chuang, Y.-S. Chen, O. Daugulis, A. J. Jacobson and O. Š. Miljanić, Nat. Commun., 2014, 5, 5131 CrossRef CAS PubMed.
- X.-Z. Luo, X.-J. Jia, J.-H. Deng, J.-L. Zhong, H.-J. Liu, K.-J. Wang and D.-C. Zhong, J. Am. Chem. Soc., 2013, 135, 11684 CrossRef CAS PubMed.
- M. Mastalerz and I. M. Oppel, Angew. Chem., Int. Ed., 2012, 51, 5252 CrossRef CAS.
- C. A. Zentner, H. W. H. Lai, J. T. Greenfield, R. A. Wiscons, M. Zeller, C. F. Campana, O. Talu, S. A. FitzGerald and J. L. C. Rowsell, Chem. Commun., 2015, 51, 11642 RSC.
- P. Li, Y. He, Y. Zhao, L. Weng, H. Wang, R. Krishna, H. Wu, W. Zhou, M. O'Keeffe, Y. Han and B. Chen, Angew. Chem., Int. Ed., 2015, 54, 574 CAS.
- R. S. Patil, D. Banerjee, C. Zhang, P. K. Thallapally and J. L. Atwood, Angew. Chem., Int. Ed., 2016, 55, 4523 CrossRef CAS PubMed.
- W. Yan, X. Yu, T. Yan, D. Wu, E. Ning, Y. Qi, Y. F. Han and Q. Li, Chem. Commun., 2017, 53, 3677 RSC.
- T. Yu, D. Ou, Z. Yang, Q. Huang, Z. Mao, J. Chen, Y. Zhang, S. Liu, J. Xu, M. R. Bryce and Z. Chi, Chem. Sci., 2017, 8, 1163 RSC.
- D.-D. Zhou, Y.-T. Xu, R.-B. Lin, Z.-W. Mo, W.-X. Zhang and J.-P. Zhang, Chem. Commun., 2016, 52, 4991 RSC.
- M. Morshedi, M. Thomas, A. Tarzia, C. J. Doonan and N. G. White, Chem. Sci., 2017, 8, 3019 RSC.
- A. Karmakar, R. Illathvalappil, B. Anothumakkool, A. Sen, P. Samanta, A. V. Desai, S. Kurungot and S. K. Ghosh, Angew. Chem., Int. Ed., 2016, 55, 10667 CrossRef CAS.
- J. Lü, C. Perez-Krap, F. Trousselet, Y. Yan, N. H. Alsmail, B. Karadeniz, N. M. Jacques, W. Lewis, A. J. Blake, F.-X. Coudert, R. Cao and M. Schröder, Cryst. Growth Des., 2018, 18, 2555 CrossRef PubMed.
- D. F. Sava, V. Ch. Kravtsov, J. Eckert, J. F. Eubank, F. Nouar and M. Eddaoudi, J. Am. Chem. Soc., 2009, 131, 10394 CrossRef CAS PubMed.
- I. Hisaki, Y. Suzuki, E. Gomez, B. Cohen, N. Tohnai and A. Douhal, Angew. Chem., Int. Ed., 2018, 57, 12650 CrossRef CAS PubMed.
- Q. Yin, P. Zhao, R.-J. Sa, G.-C. Chen, J. Lü, T.-F. Liu and R. Cao, Angew. Chem., Int. Ed., 2018, 57, 7691 CrossRef CAS PubMed.
- A. Bajpai, P. Venugopalan and J. N. Moorthy, Cryst. Growth Des., 2013, 13, 4721 CrossRef CAS.
- H. W. H. Lai, R. A. Wiscons, C. A. Zentner, M. Zeller and J. L. C. Rowsell, Cryst. Growth Des., 2016, 16, 821 CrossRef CAS.
- S. Nandi, D. Chakraborty and R. Vaidhyanathan, Chem. Commun., 2016, 52, 7249 RSC.
- I. Hisaki, S. Nakagawa, N. Ikenaka, Y. Imamura, M. Katouda, M. Tashiro, H. Tsuchida, T. Ogoshi, H. Sato, N. Tohnai and M. Miyata, J. Am. Chem. Soc., 2016, 138, 6617 CrossRef CAS PubMed.
- I. Hisaki, S. Nakagawa, N. Tohnai and M. Miyata, Angew. Chem., Int. Ed., 2015, 54, 3008 CrossRef CAS.
- I. Hisaki, H. Toda, H. Sato, N. Tohnai and H. Sakurai, Angew. Chem., Int. Ed., 2017, 56, 15294 CrossRef CAS.
- J. Tian, T.-Y. Zhou, S.-C. Zhang, S. Aloni, M. V. Altoe, S.-H. Xie, H. Wang, D.-W. Zhang, X. Zhao, Y. Liu and Z.-T. Li, Nat. Commun., 2014, 5, 5574 CrossRef CAS.
- L.-L. Tan, H. Li, Y. Tao, S. X.-A. Zhang, B. Wang and Y.-W. Yang, Adv. Mater., 2014, 26, 7027 CrossRef CAS.
- Z. Guo, H. Wu, G. Srinivas, Y. Zhou, S. Xiang, Z. Chen, Y. Yang, W. Zhou, M. O'Keeffe and B. Chen, Angew. Chem., Int. Ed., 2011, 50, 3178 CrossRef CAS PubMed.
- H. Yamagishi, H. Sato, A. Hori, Y. Sato, R. Matsuda, K. Kato and T. Aida, Science, 2018, 361, 1242 CrossRef CAS.
- Y. Li, M. Handke, Y.-S. Chen, A. G. Shtukenberg, C. T. Hu and M. D. Ward, J. Am. Chem. Soc., 2018, 140, 12915 CrossRef CAS.
- I. Hisaki, N. Ikenaka, S. Tsuzuki and N. Tohnai, Mater. Chem. Front., 2018, 2, 338 RSC.
- P. Li, O. Alduhaish, H. D. Arman, H. Wang, K. Alfooty and B. Chen, Cryst. Growth Des., 2014, 14, 3634 CrossRef CAS.
- H. Wang, Z. Bao, H. Wu, R.-B. Lin, W. Zhou, T.-L. Hu, B. Li, J. C.-G. Zhao and B. Chen, Chem. Commun., 2017, 53, 11150 RSC.
- X. Zhao, X. Wang, S. Wang, J. Dou, P. Cui, Z. Chen, D. Sun, X. Wang and D. Sun, Cryst. Growth Des., 2012, 12, 2736 CrossRef CAS.
- L. Li, S. Tang, X. Lv, M. Jiang, C. Wang and X. Zhao, New J. Chem., 2013, 37, 3662 RSC.
- J. Yang, X. Wang, F. Dai, L. Zhang, R. Wang and D. Sun, Inorg. Chem., 2014, 53, 10649 CrossRef CAS PubMed.
- Y. He, S. Xiang and B. Chen, J. Am. Chem. Soc., 2011, 133, 14570 CrossRef CAS PubMed.
- W. Yang, B. Li, H. Wang, O. Alduhaish, K. Alfooty, M. A. Zayed, P. Li, H. D. Arman and B. Chen, Cryst. Growth Des., 2015, 15, 2000 CrossRef CAS.
- H. Wang, H. Wu, J. Kan, G. Chang, Z. Yao, B. Li, W. Zhou, S. Xiang, J. C.-G. Zhao and B. Chen, J. Mater. Chem. A, 2017, 5, 8292 RSC.
- D. P. Bezerra, R. S. Oliveira, R. S. Vieira, C. L. Cavalcante and D. C. Azevedo, Adsorption, 2011, 17, 235 CrossRef CAS.
- B. Wang, A. P. Côté, H. Furukawa, M. O'Keeffe and O. M. Yaghi, Nature, 2008, 453, 207 CrossRef CAS PubMed.
- X. Sun, S. Yao, C. Yu, G. Li, C. Liu, Q. Huo and Y. Liu, J. Mater. Chem. A, 2018, 6, 6363 RSC.
- B. Wang, H. Huang, X.-L. Lv, Y. Xie, M. Li and J.-R. Li, Inorg. Chem., 2014, 53, 9254 CrossRef CAS PubMed.
- P. Deria, S. Li, H. Zhang, R. Q. Snurr, J. T. Hupp and O. K. Farha, Chem. Commun., 2015, 51, 12478 RSC.
- H. Kim, J. Park and Y. Jung, Phys. Chem. Chem. Phys., 2013, 15, 19644 RSC.
- J. Li, X. Luo, N. Zhao, L. Zhang, Q. Huo and Y. Liu, Inorg. Chem., 2017, 56, 4141 CrossRef CAS PubMed.
- Y. He, W. Zhou, R. Krishna and B. Chen, Chem. Commun., 2012, 48, 11813 RSC.
- F. Hu, C. Liu, M. Wu, J. Pang, F. Jiang, D. Yuan and M. Hong, Angew. Chem., Int. Ed., 2017, 56, 2101 CrossRef CAS PubMed.
- G. M. Sheldrick, SHELXTL-NT, version 5.1, Bruker AXS Inc., Madison, WI, 1997 Search PubMed.
- V. A. Blatov, A. P. Shevchenko and D. M. Proserpio, Cryst. Growth Des., 2014, 14, 3576 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis procedures of TMBTI, structure descriptions, materials characterization, and crystal data for JLU-SOF2 and JLU-SOF3. CCDC 1842179 and 1861545. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9sc00290a |
| This journal is © The Royal Society of Chemistry 2019 |