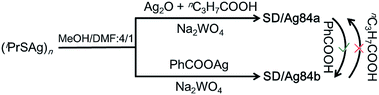Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Carboxylic acid stimulated silver shell isomerism in a triple core–shell Ag84 nanocluster†
Zhi
Wang
a,
Hao-Tian
Sun
a,
Mohamedally
Kurmoo
c,
Qing-Yun
Liu
 d,
Gui-Lin
Zhuang
d,
Gui-Lin
Zhuang
 *b,
Quan-Qin
Zhao
a,
Xing-Po
Wang
a,
Chen-Ho
Tung
*b,
Quan-Qin
Zhao
a,
Xing-Po
Wang
a,
Chen-Ho
Tung
 a and
Di
Sun
a and
Di
Sun
 *ab
*ab
aKey Laboratory of Colloid and Interface Chemistry, Ministry of Education, School of Chemistry and Chemical Engineering, State Key Laboratory of Crystal Materials, Shandong University, Jinan, 250100, People's Republic of China. E-mail: dsun@sdu.edu.cn
bCollege of Chemical Engineering and Materials Science, Zhejiang University of Technology, Hangzhou, 310032, People's Republic of China. E-mail: glzhuang@zjut.edu.cn
cInstitut de Chimie de Strasbourg, Université de Strasbourg, CNRS-UMR 7177, 4 rue Blaise Pascal, 67008 Strasbourg Cedex, France
dCollege of Chemical and Environmental Engineering, Shandong University of Science and Technology, Qingdao, 266590, People's Republic of China
First published on 29th March 2019
Abstract
Isomerization is highly important in all aspects of science, yet it is rarely observed in nanoscience. Here, we synthesized a unique triple core–shell Ag84 nanocluster displaying isomerism, which is controlled by different carboxylic acids and a one-way transformation (SD/Ag84a → SD/Ag84b). The innermost core is a rare Ag10 nanocluster which comprises an Ag6 octahedral unit as seen in face-centred cubic (fcc) silver metal and four capped Ag atoms. It templates two crescent-shaped polyoxometalate (W7O26)10− shells which are then enclosed in a shell of silver shaped as rugby balls. The organic ligands (iPrS−, nPrCOO− and PhCOO−) finally shield the metallic clusters. Due to slight differences in structure at two poles and the steric hindrance of nPrCOO− and PhCOO−, SD/Ag84a and SD/Ag84b adopt the shapes of flat-headed and cuspidal prolate spheres, respectively. Interestingly, PhCOOH is dominant over nPrCOOH whereby crystals of SD/Ag84b were isolated if PhCOOH is added during the synthesis of SD/Ag84a. This demonstrates that PhCOOH not only alters the organic coats but also induces metal shell re-organization. This work reveals carboxylate-controlled skeletal isomerism in silver nanoclusters for the first time, thus deepening the understanding of silver nanocluster assembly, flexibility and reactivity.
Introduction
Isomerization plays a vital role in many fundamental biological processes in nature such as ligand binding, enzymatic catalysis, protein folding and photosynthesis.1 Diverse isomerizations in inorganic, organic, and supramolecular chemistry have also attracted attention since isomers—compounds with the same atoms arranged in different manners—may deliver substantially different physicochemical properties and chemical reactivities without changing their compositions;2 for example, the sweetness of optically active sugars. For polyatomic systems, such as metal nanoclusters, isomerization is not a highly probable event because (i) structural variation usually involves the re-organization of several dozens of metal atoms and motion of several ligands and (ii) unravelling atomic-precision in nanometric structures or even macromolecules is still a major challenge. Thus, it is difficult to experimentally achieve atomic-level isomerization of specific sites/regions in a cluster without destroying the other parts of the main skeleton. An important breakthrough was made in 2015 by Jin and Wu who reported the first genuine gold nanocluster isomers, Au38T and Au38Q.3 Following this work, their group reported ligand-induced core isomerization in two thiolate-protected Au28 nanoclusters.4 Tsukuda also discovered anion-packing induced isomerization between crown- and butterfly-Au9.5 Although there has been sporadic advances in Au nanocluster isomerization recently,6 those for silver are yet to be achieved.The development of silver nanoclusters has expedited the establishment of general synthetic methodologies such as anion-templation and geometric polyhedral principles.7 The gradual accumulation of knowledge about silver nanocluster synthesis is also a reminder for us to revisit the ligand strategy8 based on the classic hard-soft-acid-base (HSAB) theory,9 that is controlling the ‘hardness’ of carboxylic acids in competition with ‘soft’ thiolates, allowing flexibility of the overall silver nanoclusters. The abundant availability of commercial alkyl or aryl carboxylates provides ample options to tune the structure and flexibility of silver nanoclusters through steric hindrance or/and electronic effects. Flexible silver nanoclusters may produce isomers under specific stimuli including acid, base, ligand exchange and so on. Given this situation, using this soft/hard double-ligand strategy in the rational synthesis of isomeric silver nanoclusters is very attractive and challenging.
This study is born out of the successes of the recent afore-mentioned synthetic strategy achieving two Ag84 nanoclusters (SD/Ag84a and SD/Ag84b). Their impressive structures comprise a Ag10 nanocluster core, a pair of novel crescent-shaped (W7O26)10− shells and a 74-silver outer shell, thus establishing a novel common rugby-ball shaped three-shell [Ag10@(W7O26)2@Ag74] motif that differs in skeletal organization and ligand coverage at the two poles. Their flat-headed and cuspidal prolate spherical structures, respectively, are likely driven by the different steric hindrances between nPrCOO− and PhCOO−. Although the organic shells are different, these two Ag84 nanoclusters have identical elemental contents, and hence, they belong to pseudo-isomers. What’s more interesting is that we can isolate SD/Ag84b in the mother solution of SD/Ag84a by adding PhCOOH at the second-step reaction, which demonstrates that PhCOOH not only changes the organic coverage but also induces metal shell distortion or re-organization.
Results and discussion
Synthesis of SD/Ag84a and SD/Ag84b
The details of the synthesis of SD/Ag84a and SD/Ag84b are given in the ESI.† Briefly, (iPrSAg)n, silver salts, RCOOH, and Na2WO4 were mixed in MeOH/DMF (v/v = 4/1) and reacted under solvothermal conditions (Scheme 1). Red crystals were collected and characterized by single-crystal and powder X-ray diffraction (SCXRD, PXRD), IR, UV-Vis, elemental analyses, and luminescence.X-ray structures of SD/Ag84a and SD/Ag84b
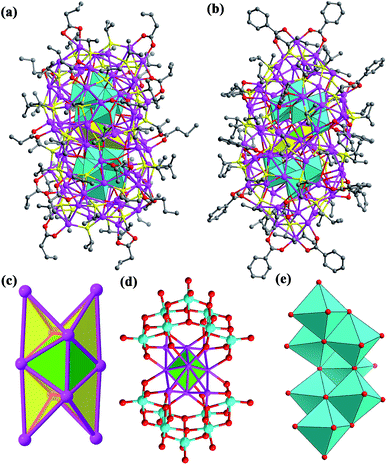
Both SD/Ag84a and SD/Ag84b crystallize in the triclinic P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group with half of the cluster existing as the asymmetric unit. Apart from the carboxylate group they have similar formulae of [Ag10@(W7O26)2@Ag74S2(iPrS)40(nPrCOO)18]·2CH3OH (SD/Ag84a) and [Ag10@(W7O26)2@Ag74S2(iPrS)40(PhCOO)18] (SD/Ag84b). Their overall appearance is a prolate sphere with similar dimensions of about 1.1 × 2.5 nm. Their coverage by organic ligands is also similar to 40 iPrS− and 18 RCOO− ligands (Fig. 1a and b). Both Ag84 clusters consist of an Ag74 outer shell and two crescent-shaped (W7O26)10− inner shells clamping a subnanometer Ag10 kernel. The Ag10 kernel is constructed from an octahedral Ag6 core by adding four Ag caps at the triangular faces (Fig. 1c). Two (W7O26)10− anions symmetrically wrap the Ag10 kernel through Ag–O bonds (Fig. 1d). Each (W7O26)10− anion is formed by seven edge-sharing WO6 octahedra (Fig. 1e) and coordinates to 32 silver atoms in total (Fig. S1†). Such (W7O26)10− anions are not only observed in the POM@Ag cluster family for the first time but also have never been documented in classical POM chemistry. Two in situ generated μ6-S2− ions were inserted into the equatorial waist section, consolidating the linkage between the inner Ag10 kernel and the Ag74 outer shell (Fig. S2†). We listed several important geometry parameters of SD/Ag84a and SD/Ag84b such as Ag⋯Ag distances in the Ag10 kernel and the Ag74 shell, Ag–Sligand, Ag–Ssulfide, Ag–Ocarboxylate, Ag–OPOM, and W–O bond lengths, and coordination modes of ligands and (W7O26)10− for comparison (Table S1†).
space group with half of the cluster existing as the asymmetric unit. Apart from the carboxylate group they have similar formulae of [Ag10@(W7O26)2@Ag74S2(iPrS)40(nPrCOO)18]·2CH3OH (SD/Ag84a) and [Ag10@(W7O26)2@Ag74S2(iPrS)40(PhCOO)18] (SD/Ag84b). Their overall appearance is a prolate sphere with similar dimensions of about 1.1 × 2.5 nm. Their coverage by organic ligands is also similar to 40 iPrS− and 18 RCOO− ligands (Fig. 1a and b). Both Ag84 clusters consist of an Ag74 outer shell and two crescent-shaped (W7O26)10− inner shells clamping a subnanometer Ag10 kernel. The Ag10 kernel is constructed from an octahedral Ag6 core by adding four Ag caps at the triangular faces (Fig. 1c). Two (W7O26)10− anions symmetrically wrap the Ag10 kernel through Ag–O bonds (Fig. 1d). Each (W7O26)10− anion is formed by seven edge-sharing WO6 octahedra (Fig. 1e) and coordinates to 32 silver atoms in total (Fig. S1†). Such (W7O26)10− anions are not only observed in the POM@Ag cluster family for the first time but also have never been documented in classical POM chemistry. Two in situ generated μ6-S2− ions were inserted into the equatorial waist section, consolidating the linkage between the inner Ag10 kernel and the Ag74 outer shell (Fig. S2†). We listed several important geometry parameters of SD/Ag84a and SD/Ag84b such as Ag⋯Ag distances in the Ag10 kernel and the Ag74 shell, Ag–Sligand, Ag–Ssulfide, Ag–Ocarboxylate, Ag–OPOM, and W–O bond lengths, and coordination modes of ligands and (W7O26)10− for comparison (Table S1†).
Notably, such an Ag10 kernel is observed for the first time, although its innermost subvalent Ag64+ octahedron has been reported in silver nanoclusters10 and some inorganic compounds such as Ag6Ge10P12, Ag5GeO4, Ag5SiO4 and Ag6O2.11 The formation of such subvalent Ag nanoclusters is related to the reductive effect of DMF, which has been recognized as a key factor in the controlled synthesis of multiple-twin silver nanocrystals by reducing Ag+ → Ag0.12 Based on the digested 13C NMR spectra of reaction mother solutions of SD/Ag84a and SD/Ag84b (Fig. S3 and S4†), we observed typical Ccarboxyl resonances at δ = 163.16 and 163.04 ppm corresponding to the oxidization product of DMF, Me2NCOOH.13 In the same region, the expected Caldehyde of DMF and Ccarboxyl of nPrCOOH and PhCOOH were also observed at δ = 164.39, 176.47, and 168.95 ppm, respectively. These results provided experimental evidence that the redox reaction happened in such a complicated self-assembly process. As the smallest unit in fcc bulk silver metal, the subnanometer Ag6 octahedron with eight exposed [111] facets can be seen as an embryonic state of any other bigger silver nanocrystals and thus is of particular interest in the field of nanoparticles.14 The subnanometer Ag10 kernel trapped in SD/Ag84a and SD/Ag84b can be seen as the Ag6 “nuclei” grown by adding four tetrahedra on its four [111] facets, manifesting the atomic-level silver nanocrystal growth route, that is, stepwise growth of tetrahedral caps on specific facets. Such a growth route is quite similar to that proposed for larger decahedral and icosahedral silver nanocrystals by the Tsuji group.15 This result thus sheds light on the atomic details of the growth of silver nanocrystals in the embryonic stage.
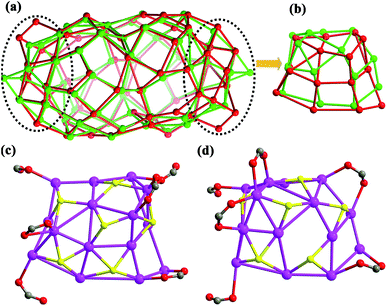
Upon further carefully checking and comparing the structural features such as ligand distributions and skeletons of these two silver nanoclusters, we surprisingly found that the distinct differences of silver skeletons between SD/Ag84a and SD/Ag84b are in the polar sections and have nothing to do with the inner Ag10 kernel and (W7O26)10−, although the silver polygons on the other regions of surfaces are almost identical with slight dislocations and distortions. The Ag74 shells in SD/Ag84a and SD/Ag84b can be described as flat-headed (red skeleton in Fig. 2a) and cuspidal (green skeleton in Fig. 2a) prolate spheres, respectively. The superposed Ag74 shells, especially in the polar regions (Ag15 caps, Fig. 2b), showed silver polygon migrations and severe distortions, which are caused by the different distributions of iPrS− and RCOO− ligands on this region. There are in total six iPrS− and six RCOO− ligands on the polar regions of SD/Ag84a and SD/Ag84b. On one pole of SD/Ag84a, six nPrCOO− ligands are equally distributed at the two sides (Fig. 2c), whereas four PhCOO− ligands are located at one side and the other two at another side of one pole of SD/Ag84b (Fig. 2d). Moreover, two μ2-κ1:κ1 PhCOO− ligands are adjacent and simultaneously coordinate to the same Ag atom (Ag32), creating a single peak (Ag32) on the pole of SD/Ag84b. Although the organic coats are different in SD/Ag84a and SD/Ag84b, the same silver atom counts suggested that they are cage isomers. The driving force of the isomeric silver nanocluster should be most probably generated from the steric hindrance of different carboxylate groups. Thus, we successfully filled in the blanks in isomeric silver nanoclusters using a soft/hard double-ligand strategy.
Inspired by the isomeric silver skeletons of SD/Ag84a and SD/Ag84b, we also tried to explore the possibility of conversion between them under the carboxylic acid stimulus. The isomerization experiments were performed in respective reaction mother liquors without removing crystals and by adding a portion of another kind of carboxylic acid. Interestingly, we found that SD/Ag84b can be isolated after the synthesis of SD/Ag84a by adding bulkier PhCOOH for the second-step reaction; however, the transformation from SD/Ag84b to SD/Ag84a failed by adding smaller nPrCOOH into the system for synthesizing SD/Ag84b, which indicated that the bulkier the carboxylic acid, the stronger the inducing effect.
Considering the subvalent characteristics of the Ag10 kernel, we deduced the charge of it to be +4 based on the formula determined by high-quality SCXRD data, which was also further confirmed by the DFT calculations (see details in ESI†). The electronic structure of SD/Ag84a (Fig. 3a) demonstrates that both 5s states of the outer Ag74 shell and 3p states of S play a pivotal role in the valence band maximum (VBM), while only the 5s states of Ag (especially from the inner Ag10 kernel) are dominant in the conduction band minimum (CBM). The resultant band gap of 1.12 eV is comparable with the observed value (Eg = 1.24 eV) from solid UV-Vis measurement results discussed below. Thus, the corresponding absorption peak can be attributed to electronic transition from the outer Ag74 shell to the inner Ag10 kernel. Moreover, frontier molecular orbitals (Fig. 3b–g) also indicate that the three highest occupied orbitals (HOMO, HOMO−1 and HOMO−2) are concentrated in the 5s orbitals and 4d orbitals of the outer Ag74 shell, while the two lowest unoccupied orbitals (LUMO and LUMO+1) mainly consist of 5s orbitals (derived from octahedron-like Ag6) and 4d orbitals (involving two wing-like Ag2 units) of the inner Ag10 kernel. Therefore, it is concluded that the inner Ag10 core features an electron-deficient state, consistent with the +4 valence estimated crystallographically.
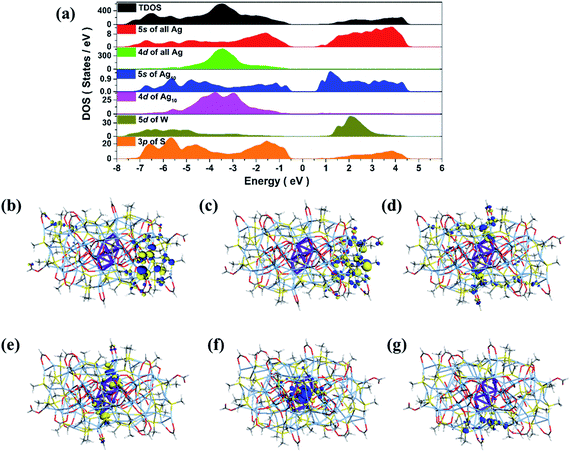 | ||
| Fig. 3 Total DOS and partial DOS of SD/Ag84a (a). Frontier molecular orbitals: HOMO−2 (b), HOMO−1 (c), HOMO (d) and LUMO (e), LUMO+1 (f) and LUMO+2 (g). | ||
The optical properties of SD/Ag84a
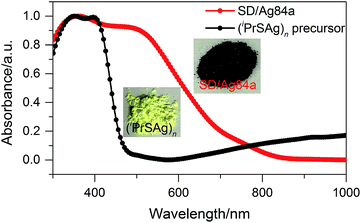
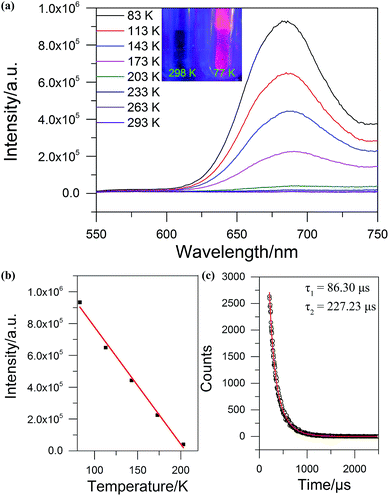
The solid state UV-Vis absorption spectrum of SD/Ag84a was recorded at room temperature in the wavelength range from 300 to 1000 nm. As depicted in Fig. 4, there is one intense absorption band centered at 361 nm and a shoulder peak at 500 nm, respectively. The high energy absorption centered at 361 nm originates from the n → π* transition of iPrS−, as similarly observed in the absorption spectrum of the (iPrSAg)n precursor. The low energy absorption centered at 500 nm can be attributed to the charge transfer transition from the S 3p to Ag 5s orbitals. Using a transformed Kubelka–Munk plot (Fig. S5†), the HOMO–LUMO gap of SD/Ag84a was found to be 1.24 eV, indicating that SD/Ag84a is a potential narrow-band-gap semiconductor. By contrast, the optical gap of the (iPrSAg)n precursor was calculated to be 2.52 eV, which matches with the color of the samples: SD/Ag84a is red, whereas the (iPrSAg)n precursor is yellow (see the insets of Fig. 4).SD/Ag84a does not emit at room temperature in the solid state under a 365 nm hand-held UV light; however, it emits bright-red light at 77 K, which is detectable by the naked eye (see the insets of Fig. 5). The temperature dependent fluorescence spectra were recorded under 468 nm excitation in the temperature range of 293–83 K. From Fig. 5a, we can see that SD/Ag84a is almost non-emissive from 293 to 203 K. Upon cooling from 203 to 83 K, SD/Ag84a starts emitting with the maximum emission band centered at 689 nm, which is gradually blue-shifted to 685 nm at 83 K along with the increase of luminous intensity. This low-temperature emission should originate from the ligand-to-metal charge transfer (LMCT, charge transfer from S 3p to Ag 5s) perturbed by Ag⋯Ag interactions.16 The temperature dependence behavior should be relevant with variable molecular rigidity and Ag⋯Ag interactions at different temperatures. The emission intensity was found to be sensitive to temperature and showed good linearity with the corresponding temperature ranging from 83 to 203 K (Fig. 5b), with the linear equation Imax = 1511090 − 7364.75T. The linear equation correlation coefficient is 0.992, which is suitable for temperature detection in the temperature range of 83–203 K. The fluorescence lifetime of SD/Ag84a was measured at 83 K (Fig. 5c), with the lifetime value falling on the scale of microseconds (τ1 = 86.30 μs and τ2 = 227.23 μs), suggesting a triplet state emission.
Conclusions
In conclusion, we successfully achieved the assembly of isomeric Ag84 nanoclusters for the first time by the combination of anion template and soft/hard double-ligand strategies. The comparative structural analysis indicated that the isomerism mainly occurred on the polar regions of the prolate spheres. The differences are found in both the silver skeleton and iPrS−/RCOO− ligand distributions. The driving force for such an isomerism is dominated by the steric hindrance of carboxylates. What’s more interesting is that the Ag10 kernel as a grown nanocrystal from the smallest octahedral Ag6 unit in face-centred cubic (fcc) silver metal was first identified in the innermost region of the Ag84 nanocluster. The results obtained in this study are not only the pioneering results for isomeric silver nanoclusters but also enrich the library of multi-shell silver nanoclusters containing both novel POM templates and ultrasmall reductive silver nanocrystals.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant No. 21822107, 21571115, 21671172), the Natural Science Foundation of Shandong Province (No. JQ201803 and ZR2017MB061), the Taishan Scholar Project of Shandong Province of China, the Qilu Youth Scholar Funding of Shandong University and the Fundamental Research Funds of Shandong University (104.205.2.5). Dr Zhuang was financially supported by the Zhejiang Provincial Natural Science Foundation (No. LR19B010001).References
-
(a) D. B. Ritchie and M. T. Woodside, Curr. Opin. Struct. Biol., 2015, 34, 43–51 CrossRef CAS PubMed
; (b) S. W. Englander and L. Mayne, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, 8253–8258 CrossRef CAS PubMed
; (c) P. Leippe and J. A. Frank, Curr. Opin. Struct. Biol., 2019, 57, 23–30 CrossRef CAS PubMed
; (d) N. Ankenbruck, T. Courtney, Y. Naro and A. Deiters, Angew. Chem., Int. Ed., 2018, 57, 2768–2798 CrossRef CAS PubMed
; (e) L. A. Acevedo, J. Kwon and L. K. Nicholson, Proc. Natl. Acad. Sci. U. S. A., 2019, 116, 2589–2594 CrossRef CAS PubMed
.
-
(a) B. Moulton and M. J. Zaworotko, Chem. Rev., 2001, 101, 1629–1658 CrossRef CAS PubMed
; (b) J. A. DeVine, M. L. Weichman, B. Laws, J. Chang, M. C. Babin, G. Balerdi, C. J. Xie, C. L. Malbon, W. C. Lineberger, D. R. Yarkony, R. W. Field, S. T. Gibson, J. Y. Ma, H. Guo and D. M. Neumark, Science, 2017, 358, 336–339 CrossRef CAS PubMed
; (c) Y.-Z. Tan, Z.-J. Liao, Z.-Z. Qian, R.-T. Chen, X. Wu, H. Liang, X. Han, F. Zhu, S.-J. Zhou, Z. Zheng, X. Lu, S.-Y. Xie, R.-B. Huang and L.-S. Zheng, Nat. Mater., 2008, 7, 790–794 CrossRef CAS PubMed
; (d) L.-Y. Yao and V. W.-W. Yam, J. Am. Chem. Soc., 2015, 137, 3506–3509 CrossRef CAS PubMed
; (e) J.-P. Zhang, X.-C. Huang and X.-M. Chen, Chem. Soc. Rev., 2009, 38, 2385–2396 RSC
; (f) C. B. Williamson, D. R. Nevers, A. Nelson, I. Hadar, U. Banin, T. Hanrath and R. D. Robinson, Science, 2019, 363, 731–735 CrossRef CAS PubMed
; (g) B. Zhang, T. Zhu, M. Ou, N. Rowell, H. Fan, J. Han, L. Tan, M. T. Dove, Y. Ren, X. Zuo, S. Han, J. Zeng and K. Yu, Nat. Commun., 2018, 9, 2499 CrossRef PubMed
.
- S. Tian, Y.-Z. Li, M.-B. Li, J. Yuan, J. Yang, Z. Wu and R. Jin, Nat. Commun., 2015, 6, 8667 CrossRef CAS PubMed
.
- Y. Chen, C. Liu, Q. Tang, C. Zeng, T. Higaki, A. Das, D.-e. Jiang, N. L. Rosi and R. Jin, J. Am. Chem. Soc., 2016, 138, 1482–1485 CrossRef CAS PubMed
.
- S. Yamazoe, S. Matsuo, S. Muramatsu, S. Takano, K. Nitta and T. Tsukuda, Inorg. Chem., 2017, 56, 8319–8325 CrossRef CAS PubMed
.
-
(a) X.-Y. Liu, Y. Yang, Z. Lei, Z.-J. Guan and Q.-M. Wang, Chem. Commun., 2016, 52, 8022–8025 RSC
; (b) L.-Q. Mo, J.-H. Jia, L.-j. Sun and Q.-M. Wang, Chem. Commun., 2012, 48, 8691–8693 RSC
; (c) S. Zhuang, L. Liao, J. Yuan, N. Xia, Y. Zhao, C. Wang, Z. Gan, N. Yan, L. He, J. Li, H. Deng, Z. Guan, J. Yang and Z. Wu, Angew. Chem., Int. Ed., 2019, 58, 4510–4514 CrossRef CAS PubMed
.
-
(a) G.-G. Gao, P.-S. Cheng and T. C. W. Mak, J. Am. Chem. Soc., 2009, 131, 18257–18259 CrossRef CAS PubMed
; (b) C. W. Liu, H.-W. Chang, C.-S. Fang, B. Sarkar and J.-C. Wang, Chem. Commun., 2010, 46, 4571–4573 RSC
; (c) Y.-P. Xie and T. C. W. Mak, Angew. Chem., Int. Ed., 2012, 51, 8783–8786 CrossRef CAS PubMed
; (d) Y.-P. Xie, J.-L. Jin, G.-X. Duan, X. Lu and T. C. W. Mak, Coord. Chem. Rev., 2017, 331, 54–72 CrossRef CAS
; (e) Y.-P. Xie and T. C. W. Mak, J. Am. Chem. Soc., 2011, 133, 3760–3763 CrossRef CAS PubMed
; (f) H. Liu, C.-Y. Song, R.-W. Huang, Y. Zhang, H. Xu, M.-J. Li, S.-Q. Zang and G.-G. Gao, Angew. Chem., Int. Ed., 2016, 55, 3699–3703 CrossRef CAS PubMed
; (g) R.-W. Huang, Q.-Q. Xu, H.-L. Lu, X.-K. Guo, S.-Q. Zang, G.-G. Gao, M.-S. Tang and T. C. W. Mak, Nanoscale, 2015, 7, 7151–7154 RSC
; (h) R.-W. Huang, Y.-S. Wei, X.-Y. Dong, X.-H. Wu, C.-X. Du, S.-Q. Zang and T. C. W. Mak, Nat. Chem., 2017, 9, 689–697 CrossRef CAS PubMed
; (i) C.-Y. Gao, X. He, L. Zhao and M.-X. Wang, Chem. Commun., 2012, 48, 8368–8370 RSC
; (j) Q.-M. Wang, Y.-M. Lin and K.-G. Liu, Acc. Chem. Res., 2015, 48, 1570–1579 CrossRef CAS PubMed
.
-
(a) J.-W. Liu, H.-F. Su, Z. Wang, Y.-A. Li, Q.-Q. Zhao, X.-P. Wang, C.-H. Tung, D. Sun and L.-S. Zheng, Chem. Commun., 2018, 54, 4461–4464 RSC
; (b) Y.-M. Su, W. Liu, Z. Wang, S.-A. Wang, Y.-A. Li, F. Yu, Q.-Q. Zhao, X.-P. Wang, C.-H. Tung and D. Sun, Chem.–Eur. J., 2018, 24, 4967–4972 CrossRef CAS PubMed
.
- R. G. Pearson, J. Am. Chem. Soc., 1963, 85, 3533–3539 CrossRef CAS
.
-
(a) D. Sun, G.-G. Luo, N. Zhang, R.-B. Huang and L.-S. Zheng, Chem. Commun., 2011, 47, 1461–1463 RSC
; (b) Y. Kikukawa, Y. Kuroda, K. Suzuki, M. Hibino, K. Yamaguchi and N. Mizuno, Chem. Commun., 2013, 49, 376–378 RSC
; (c) H. Yang, J. Lei, B. Wu, Y. Wang, M. Zhou, A. Xia, L. Zheng and N. Zheng, Chem. Commun., 2013, 49, 300–302 RSC
.
-
(a) H. G. V. Schnering and K. G. Hausler, Rev. Chim. Miner., 1976, 13, 71–81 Search PubMed
; (b) W. Beesk, P. G. Jones, H. Rumpel, E. Schwarzmann and G. M. Sheldrick, J. Chem. Soc., Chem. Commun., 1981, 664–665 RSC
; (c) M. Jansen and C. Linke, Angew. Chem., Int. Ed., 1992, 31, 653–654 CrossRef
; (d) C. Linke and M. Jansen, Inorg. Chem., 1994, 33, 2614–2616 CrossRef CAS
.
- I. Pastoriza-Santos and L. M. Liz-Marzan, Nano Lett., 2002, 2, 903–905 CrossRef CAS
.
- I. Pastoriza-Santos and L. M. Liz-Marzan, Pure Appl. Chem., 2000, 72, 83–90 CAS
.
- A. Tao, P. Sinsermsuksakul and P. Yang, Angew. Chem., Int. Ed., 2006, 45, 4597–4601 CrossRef CAS PubMed
.
-
(a) M. Tsuji, Y. Maeda, S. Hikino, H. Kumagae, M. Matsunaga, X.-L. Tang, R. Matsuo, M. Ogino and P. Jiang, Cryst. Growth Des., 2009, 9, 4700–4705 CrossRef CAS
; (b) M. Tsuji, M. Ogino, R. Matsuo, H. Kumagae, S. Hikino, T. Kim and S.-H. Yoon, Cryst. Growth Des., 2010, 10, 296–301 CrossRef CAS
; (c) M. Tsuji, X. Tang, M. Matsunaga, Y. Maeda and M. Watanabe, Cryst. Growth Des., 2010, 10, 5238–5243 CrossRef CAS
.
-
(a) V. W.-W. Yam, V. K.-M. Au and S. Y.-L. Leung, Chem. Rev., 2015, 115, 7589–7728 CrossRef CAS PubMed
; (b) A. Barbieri, G. Accorsi and N. Armaroli, Chem. Commun., 2008, 2185–2193 RSC
.
Footnote |
| † Electronic supplementary information (ESI) available: IR, 13C NMR, UV-Vis, EDS and PXRD data, and details of the data collection and structure refinements, and crystal data. CCDC 1878911 and 1878912 for SD/Ag84a and SD/Ag84b. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c8sc05666h |
| This journal is © The Royal Society of Chemistry 2019 |