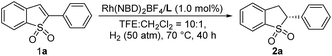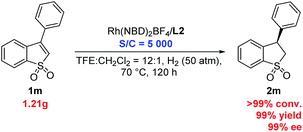Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Efficient synthesis of chiral 2,3-dihydro-benzo[b]thiophene 1,1-dioxides via Rh-catalyzed hydrogenation†
Gongyi
Liu
a,
Heng
Zhang
 a,
Yi
Huang
a,
Zhengyu
Han
a,
Gang
Liu
a,
Yuanhua
Liu
a,
Xiu-Qin
Dong
a,
Yi
Huang
a,
Zhengyu
Han
a,
Gang
Liu
a,
Yuanhua
Liu
a,
Xiu-Qin
Dong
 *a and
Xumu
Zhang
*a and
Xumu
Zhang
 *ab
*ab
aKey Laboratory of Biomedical Polymers, Engineering Research Center of Organosilicon Compounds & Materials, Ministry of Education, College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei 430072, P. R. China. E-mail: xiuqindong@whu.edu.cn
bDepartment of Chemistry, Shenzhen Grubbs Institute, Southern University of Science and Technology, Shenzhen, Guangdong 518055, P. R. China. E-mail: zhangxm@sustc.edu.cn
First published on 15th January 2019
Abstract
Rh-catalyzed asymmetric hydrogenation of prochiral substituted benzo[b]thiophene 1,1-dioxides was successfully developed, affording various chiral 2,3-dihydrobenzo[b]thiophene 1,1-dioxides with high yields and excellent enantioselectivities (up to 99% yield and >99% ee). In particular, for challenging substrates, such as aryl substituted substrates with sterically hindered groups and alkyl substituted substrates, the reaction proceeded smoothly in our catalytic system with excellent results. The gram-scale asymmetric hydrogenation proceeded well with 99% yield and 99% ee in the presence of 0.02 mol% (S/C = 5000) catalyst loading. The possible hydrogen-bonding interaction between the substrate and the ligand may play an important role in achieving high reactivity and excellent enantioselectivity.
Introduction
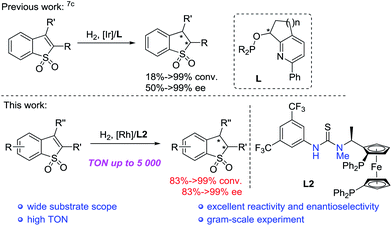
The 2,3-dihydro-benzo[b]thiophene 1,1-dioxides and derivative motifs are widely distributed with significant applications in many biologically active compounds,1–6 such as the inhibitor of tumour necrosis factor-α converting enzyme (TACE),2 antidiabetics3 and HIF-2a inhibitors.4 Other examples include benzothiophene scaffolds, such as 2,3-dihydroraloxifene as raloxifene's analogue with selective estrogen receptor modulator activity5 and a potential HIV-1 reverse transcriptase inhibitor (NSC-380292).6 In addition, they are important synthetic intermediates in the field of organic synthesis.Although chiral 2,3-dihydro-benzo[b]thiophene 1,1-dioxides and their derivatives showed great potential, the development of highly efficient asymmetric synthetic methodologies to construct these compounds still remains very challenging.7,8 In 2017, Pfaltz and co-workers developed the asymmetric hydrogenation of prochiral benzo[b]thiophene 1,1-dioxides by using the Ir/pyridyl phosphinite ligand complex with moderate to excellent enantioselectivities, whereas for some aryl substituted substrates with slightly sterically hindered groups and alkyl substituted substrates it remained difficult to achieve both high reactivity and excellent enantioselectivity (Scheme 1).7c Although some progress was achieved, it is extremely necessary to develop highly efficient asymmetric catalytic systems to prepare chiral 2,3-dihydro-benzo[b]thiophene 1,1-dioxides and their derivatives. Transition metal-catalyzed asymmetric hydrogenation of prochiral unsaturated heterocyclic compounds is a powerful and important method to synthesize chiral heterocyclic compounds.9,10 Meanwhile, chiral ferrocenyl phosphine ligands have emerged as a class of important and privileged ligands, which exhibited excellent performance in asymmetric catalytic reactions.11 Recently, our group successfully developed a series of bifunctional ferrocenyl bisphosphine-thiourea ligands, which were applied in some Rh-catalyzed asymmetric hydrogenation of unsaturated functionalized substrates.12 We envisaged that the asymmetric hydrogenation of prochiral substituted benzo[b]thiophene 1,1-dioxides could proceed well with high reactivity and excellent enantioselective control with the aid of the possible hydrogen-bonding interaction between the sulfonyl group of the substrate and the thiourea motif of the ligand. Herein, we realized Rh-catalyzed asymmetric hydrogenation of prochiral benzo[b]ene 1,1-dioxides with N-methylated bisphosphine-thiourea ZhaoPhos L2 as the ligand, affording various chiral 2,3-dihydro-benzo[b]thiophene 1,1-dioxides with up to >99% conversion, >99% ee and 5000 TON (Scheme 1). Challenging substrates, such as aryl substituted substrates with sterically hindered groups and alkyl substituted substrates, also performed well in our catalytic system with excellent results.
Results and discussion
The initial investigation of the Rh-catalyzed asymmetric hydrogenation of 2-phenylbenzo[b]thiophene 1,1-dioxide (1a)13 as a model substrate was conducted with different metal sources using ligand ZhaoPhos L1 (S/C = 100) under 50 atm H2 in CH2Cl2 at 50 °C for 40 h (Table 1, entries 1–4). The Rh(NBD)2BF4 afforded the best result with high conversion and excellent enantioselectivity (94% conversion, 93% ee, Table 1, entry 1). The conversion can be improved to 99%, when the reaction temperature is increased from 50 °C to 70 °C (Table 1, entry 5). In order to achieve good solubility of the Rh(NBD)2BF4/ZhaoPhos L1 catalytic system, the catalyst was generated in stiu by mixing Rh(NBD)2BF4/ZhaoPhos L1 in CH2Cl2. The solvent effect of this asymmetric hydrogenation was investigated in various solvents. Excellent enantioselectivities can be obtained in mixed solvents of dichloroethane, CHCl3, MeOH, EtOH, iPrOH or tetrahydrofuran in CH2Cl2 with the volume ratio of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, but the reactivities were very poor (15–51% conversion, 90–99% ee, Table 1, entries 6–7, 9–11, and 14). Good to excellent reactivities and enantioselectivities were observed in the mixed solvents of CF3CH2OH, ethyl acetate or toluene in CH2Cl2 (87–>99% conversion, 87–>99% ee, Table 1, entries 8 and 12–13). And the mixed solvent CF3CH2OH/CH2Cl2 (10
1, but the reactivities were very poor (15–51% conversion, 90–99% ee, Table 1, entries 6–7, 9–11, and 14). Good to excellent reactivities and enantioselectivities were observed in the mixed solvents of CF3CH2OH, ethyl acetate or toluene in CH2Cl2 (87–>99% conversion, 87–>99% ee, Table 1, entries 8 and 12–13). And the mixed solvent CF3CH2OH/CH2Cl2 (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was chosen as the best reaction solvent with full conversion and >99% ee (Table 1, entry 8).
1) was chosen as the best reaction solvent with full conversion and >99% ee (Table 1, entry 8).
| Entry | Metal source | Solvent | Conv.b [%] | eec [%] |
|---|---|---|---|---|
a Unless otherwise noted, all reactions were carried out with a [Rh]/ligand L1/substrate 1a (0.1 mmol) ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 at 70 °C in 1.0 mL solvent under 50 atm H2 for 40 h, and the catalyst was pre-complexed in CH2Cl2 (0.1 mL for each reaction vial).
b Determined by 1H NMR analysis.
c Determined by HPLC on a chiral phase.
d Reaction temperature is 50 °C. NR = no reaction, NA = not available. DCE is dichloroethane. TFE is CF3CH2OH. EA is ethyl acetate. THF is tetrahydrofuran. 100 at 70 °C in 1.0 mL solvent under 50 atm H2 for 40 h, and the catalyst was pre-complexed in CH2Cl2 (0.1 mL for each reaction vial).
b Determined by 1H NMR analysis.
c Determined by HPLC on a chiral phase.
d Reaction temperature is 50 °C. NR = no reaction, NA = not available. DCE is dichloroethane. TFE is CF3CH2OH. EA is ethyl acetate. THF is tetrahydrofuran.
|
||||
| 1d | Rh(NBD)2BF4 | CH2Cl2 | 94 | 93 |
| 2d | Rh(COD)2BF4 | CH2Cl2 | 92 | 87 |
| 3d | [Rh(COD)Cl]2 | CH2Cl2 | 44 | 95 |
| 4d | Rh(COD)2CF3SO3 | CH2Cl2 | NR | NA |
| 5 | Rh(NBD)2BF4 | CH2Cl2 | 99 | 93 |
| 6 | Rh(NBD)2BF4 | DCE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH2Cl2 = 10 CH2Cl2 = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
23 | 95 |
| 7 | Rh(NBD)2BF4 | CHCl3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH2Cl2 = 10 CH2Cl2 = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
15 | 95 |
| 8 | Rh(NBD)2BF4 | TFE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH2Cl2 = 10 CH2Cl2 = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
>99 | >99 |
| 9 | Rh(NBD)2BF4 | MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH2Cl2 = 10 CH2Cl2 = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
34 | 95 |
| 10 | Rh(NBD)2BF4 | EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH2Cl2 = 10 CH2Cl2 = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
44 | 90 |
| 11 | Rh(NBD)2BF4 |
iPrOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH2Cl2 = 10 CH2Cl2 = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
39 | 98 |
| 12 | Rh(NBD)2BF4 | EA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH2Cl2 = 10 CH2Cl2 = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
87 | 87 |
| 13 | Rh(NBD)2BF4 | Toluene![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH2Cl2 = 10 CH2Cl2 = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
99 | 93 |
| 14 | Rh(NBD)2BF4 | THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH2Cl2 = 10 CH2Cl2 = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
51 | 99 |
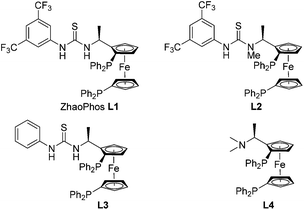
A series of bisphosphine-thiourea ligands were then investigated in this Rh-catalyzed asymmetric hydrogenation (Fig. 1). As shown in Table 2, ZhaoPhos ligand L1 and N-methylated ZhaoPhos ligand L2 provided the same result with >99% conversion and >99% ee (Table 2, entries 1 and 2), which indicates that one hydrogen bond is sufficient to obtain high reactivity and excellent enantioselectivity in this asymmetric transformation. The ligand L3 without the CF3 group on the phenyl ring provided poor results (73% conversion, 56% ee, Table 2, entry 3). In addition, no reaction was observed using ligand L4 without the thiourea group, which showed that the possible hydrogen bonding interaction between the ligand and the sulfonyl group of the substrate was essential to achieve high reactivity and excellent enantioselectivity. In order to obtain the optimal ligand, this Rh-catalyzed asymmetric hydrogenation was conducted in the presence of ZhaoPhos ligand L1 and N-methylated ZhaoPhos ligand L2 with a lower catalyst loading (0.5 mol%). We found that ligand L2 provided better results than ligand L1 (95% conversion, 98% ee, Table 2, entry 6).
| Entry | Ligand | Conv.b [%] | eec [%] |
|---|---|---|---|
a Unless otherwise mentioned, all reactions were carried out with a [Rh(NBD)2]BF4/ligand/substrate 1a (0.1 mmol) ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 in 1.0 mL CF3CH2OH under 50 atm H2 at 70 °C for 40 h, and the catalyst was pre-complexed in CH2Cl2 (0.1 mL for each reaction vial).
b Determined by 1H NMR analysis.
c The ee value was determined by HPLC on a chiral phase.
d Catalyst loading is 0.5 mol%, 12 h. NR = no reaction, NA = not available. 100 in 1.0 mL CF3CH2OH under 50 atm H2 at 70 °C for 40 h, and the catalyst was pre-complexed in CH2Cl2 (0.1 mL for each reaction vial).
b Determined by 1H NMR analysis.
c The ee value was determined by HPLC on a chiral phase.
d Catalyst loading is 0.5 mol%, 12 h. NR = no reaction, NA = not available.
|
|||
| 1 | ZhaoPhos L1 | >99 | >99 |
| 2 | L2 | >99 | >99 |
| 3 | L3 | 73 | 56 |
| 4 | L4 | NR | NA |
| 5d | ZhaoPhos L1 | 81 | 96 |
| 6d | L2 | 95 | 98 |
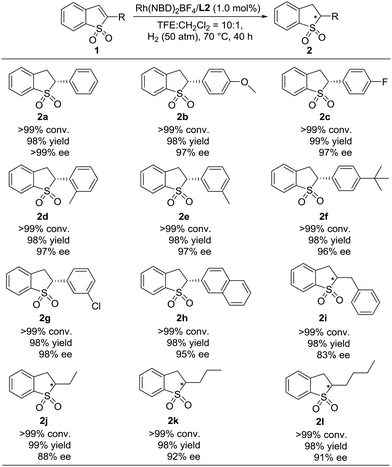
Under the optimized reaction conditions, the substrate scope of Rh-catalyzed asymmetric hydrogenation of prochiral substituted benzo[b]thiophene 1,1-dioxides was explored, and the results are summarized in Table 3. A wide range of 2-substituted benzo[b]thiophene 1,1-dioxides were hydrogenated smoothly catalyzed by Rh(NBD)2BF4/L2. When the 2-substituted benzo[b]thiophene 1,1-dioxides bearing the electron-donating group (1b and 1d–1f) or electron-withdrawing group on the phenyl ring (1c and 1g) were used, the corresponding hydrogenation products chiral 2-substituted 2,3-dihydro-benzo[b]thiophene 1,1-dioxides (2b–2g) were obtained with full conversions, high yields and excellent enantioselectivities (>99% conversion, 98–99% yields, 96–>99% ee). And the position of the substituent on the phenyl ring had little effect on the reactivity and enantioselectivity. To our delight, 2-substituted benzo[b]thiophene 1,1-dioxides with an ortho- (1d) or meta- (1e and 1g) substituted group on the phenyl ring with steric hindrance were hydrogenated smoothly with excellent results (>99% conversion, 98% yield and 97–98% ee). The asymmetric hydrogenation of the substrate with a bulky 2-naphthyl group also proceeded efficiently to afford the product (2h) with >99% conversion, 98% yield and 95% ee. Noticeably, the alkyl substituted benzo[b]thiophene 1,1-dioxides (1i–1l) were also hydrogenated well in our catalytic system, providing the desired products (2i–2l) with >99% conversion, 98–99% yields and 83–92% ee.
| a 0.1 mmol substrate 1, substrate 1/Rh(NBD)2BF4/L2 = 1/0.01/0.011 at 70 °C under 50 atm H2 in 1.0 mL CF3CH2OH for 40 h, and the catalyst was pre-complexed in CH2Cl2 (0.1 mL for each reaction vial). Conversion was determined by 1H NMR analysis. Yield is isolated yield. The ee value was determined by HPLC on a chiral column. The absolute configuration of 2a was determined as (R) according to previous work.7c |
|---|
|
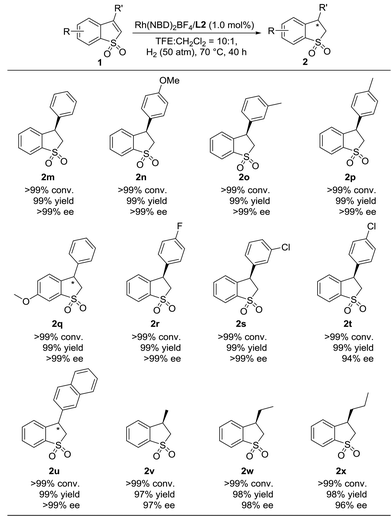
Encouraged by the success in the highly enantioselective hydrogenation of various 2-substituted benzo[b]thiophene 1,1-dioxides catalyzed by Rh(NBD)2BF4/L2, we turned our attention to investigate the substrate generality of 3-substituted benzo[b]thiophene 1,1-dioxides. As shown in Table 4, a variety of 3-substituted benzo[b]thiophene 1,1-dioxides were reduced efficiently, providing the desired hydrogenation products (2m–2x) with excellent results (>99% conversion, 97–99% yields, and 94–>99% ee). We found that the electronic properties and position of the substituted group on the phenyl ring of 3-aromatic substituted benzo[b]thiophene 1,1-dioxides have little influence on the reactivity and enantioselectivity. In addition, the substrate (1u) with a bulky 2-naphthyl group also worked well to afford the product (2u) with >99% conversion, 99% yield and >99% ee. Furthermore, when the 3-substituted aromatic group was changed to an alkyl group, the Rh-catalyzed asymmetric hydrogenation of 3-alkyl substituted benzo[b]thiophene 1,1-dioxides (1v–1x) proceeded smoothly with excellent results (>99% conversion, 97–98% yields, and 96–98% ee).
| a 0.1 mmol substrate 1, substrate 1/Rh(NBD)2BF4/L2 = 1/0.01/0.011 at 70 °C under 50 atm H2 in 1.0 mL CF3CH2OH for 40 h, and the catalyst was pre-complexed in CH2Cl2 (0.1 mL for each reaction vial). Conversion was determined by 1H NMR analysis. Yield is isolated yield. The ee value was determined by HPLC on a chiral column. The absolute configurations of 2n and 2v were determined as (R) according to previous work.7c |
|---|
|
In addition, the gram-scale asymmetric hydrogenation of 3-phenyl benzo[b]thiophene 1,1-dioxide (1m) proceeded efficiently with only 0.02 mol% (S/C = 5000) catalyst, affording the desired product (2m) with >99% conversion, 99% yield and 99% ee (Scheme 2). This result showed that this Rh/ligand L2 catalytic system possessed very high activity in this reaction.
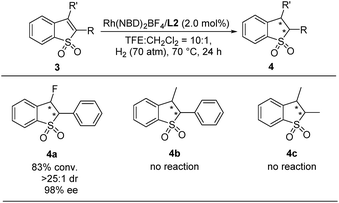
It is very challenging to realize the asymmetric hydrogenation of tetrasubstituted cyclic olefins owing to their unfavorable bulky steric hindrance. The tetrasubstituted cyclic olefins 2,3-disubstituted benzo[b]thiophene 1,1-dioxides were applied in this Rh-catalyzed asymmetric hydrogenation to further investigate the substrate generality. As shown in Scheme 3, the desired product 3-fluoro-2-phenyl-2,3-dihydrobenzo[b]thiophene 1,1-dioxide (4a) can be obtained with good conversion, high diastereoselectivity and excellent enantioselectivity (83% conversion, >25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr, and 98% ee). In addition, no reaction was detected with more challenging substrates, 3-methyl-2-phenylbenzo[b]thiophene 1,1-dioxide (3b) and 2,3-dimethylbenzo[b]thiophene 1,1-dioxide (3c).
1 dr, and 98% ee). In addition, no reaction was detected with more challenging substrates, 3-methyl-2-phenylbenzo[b]thiophene 1,1-dioxide (3b) and 2,3-dimethylbenzo[b]thiophene 1,1-dioxide (3c).
A nonlinear effect suggests that the potential dimerization or high-order aggregation of catalysts should exist in catalytic asymmetric reactions.14 In order to verify the possible catalytic model, the asymmetric hydrogenation of substrate 1m was performed in the presence of ligand L2 with different ee values. And no nonlinear effect was observed in this transformation, which revealed that there should be no catalyst self-aggregation or ligand–substrate agglomeration in this catalytic system. Furthermore, a Job plot was drawn and the curve suggests a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding pattern between ligand L2 and substrate 1m. On the basis of these observations and the reaction results, 3D catalytic models for the asymmetric hydrogenation of substrates 1a and 1m were built through DFT calculations to account for the possible hydrogen bonding interaction between the Rh-catalyst and the substrate (summarized in the ESI†).
1 binding pattern between ligand L2 and substrate 1m. On the basis of these observations and the reaction results, 3D catalytic models for the asymmetric hydrogenation of substrates 1a and 1m were built through DFT calculations to account for the possible hydrogen bonding interaction between the Rh-catalyst and the substrate (summarized in the ESI†).
Conclusions
In summary, a highly efficient synthetic methodology for the construction of various chiral 2,3-dihydro-benzo[b]thiophene 1,1-dioxides was successfully developed through Rh/N-methylated ZhaoPhos ligand L2-catalyzed asymmetric hydrogenation. Our catalytic system possessed wide tolerance of substrate scope, both aromatic and alkyl substituted groups at the 2-position or the 3-position of prochiral benzo[b]thiophene 1,1-dioxides worked well in this asymmetric hydrogenation to provide the desired products with high yields and excellent enantioselectivities (up to 99% yield and >99% ee). In addition, our catalytic system showed very high activity, and the gram-scale asymmetric hydrogenation of 3-phenyl benzo[b]thiophene 1,1-dioxide proceeded well catalyzed by only 0.02 mol% (S/C = 5000) Rh/ligand L2 catalyst loading with >99% conversion, 99% yield and 99% ee. The possible hydrogen-bonding interaction between the substrate and the thiourea motif of the ligand may make an important contribution to achieving high reactivity and excellent enantioselectivity in this reaction. Further investigations toward a catalytic asymmetric variant of this reaction process are under way.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful for financial support from the National Natural Science Foundation of China (Grant No. 21432007, 21502145), Wuhan Morning Light Plan of Youth Science and Technology (Grant No. 2017050304010307), Shenzhen Nobel Prize Scientists Laboratory Project (Grant No. C17783101) and Fundamental Research Funds for and the Central Universities (Grant No. 2042018kf0202). The Program of Introducing Talents of Discipline to Universities of China (111 Project) is also appreciated. The numerical calculations in this paper have been carried out on the supercomputing system in the Supercomputing Center of Wuhan University.Notes and references
-
(a)
M. Saitou, H. Sekiguchi and S. Ogawa, WO 2000069853, 2000
; (b) M. Saitou, H. Sekiguchi and S. Ogawa, WO 2000020408, 2000
; (c) R. G. Hall, O. Loiseleur, J. Pabba, S. Pal, A. Jeanguenat, A. Edmunds and A. Stoller, WO 2009010260, 2009
; (d) A. Edmunds, M. Mghlebach, A. Stoller, O. Loiseleur, A. Buchholz, O. F. Hueter, A. Bigot, R. G. Hall, D. Emery, P. Jung, L. Lu, Y. Wu and R. Chen, WO 2015000715, 2015
.
-
L. Aigars, L. Gundars, K. Ivars, B. Daniel, F. Paul and K. Nagma, WO 2008142376, 2008
.
-
F. Wendelin, G. Heiner, T. Stefan and E. Ralf, WO 2011107494, 2011
.
-
(a)
D. Dixon, J. Grina, J. A. Josey, J. P. Rizzi, S. T. Schlachter, E. M. Wallace, B. Wang, P. Wehn, R. Xu and H. Yang, WO 2015095048, 2015
; (b) P. Wehn and P. Yang, US 20160362390, 2016
.
- A. L. Glasebrook, J. W. Misner, G. A. Stephenson and C. R. Schmid, Bioorg. Med. Chem. Lett., 1999, 9, 1137–1140 CrossRef
.
- K. Krajewski, Y. J. Zhang, D. Parrish, J. Deschamps, P. Roller and V. K. Pathak, Bioorg. Med. Chem. Lett., 2006, 16, 3034–3038 CrossRef CAS PubMed
.
-
(a) S. Urban, B. Beiring, N. Ortega, D. Paul and F. Glorius, J. Am. Chem. Soc., 2012, 134, 15241–15244 CrossRef CAS PubMed
; (b) D. Janssen-Müller, M. Fleige, D. Schlüns, M. Wollenburg, C. G. Daniliuc, J. Neugebauer and F. Glorius, ACS Catal., 2016, 6, 5735–5739 CrossRef
; (c) P. Tosatti and A. Pfaltz, Angew. Chem., Int. Ed., 2017, 56, 4579–4582 CrossRef CAS PubMed
; (d) H. Deng, F.-S. He, C.-S. Li, W.-L. Yang and W.-P. Deng, Org. Chem. Front., 2017, 4, 2343–2347 RSC
.
- F. Toda, H. Miyamoto and S. Kikuchi, J. Am. Chem. Soc., 1996, 118, 11315–11316 CrossRef CAS
.
- Selected recent reviews:
(a) D.-S. Wang, Q.-A. Chen, S.-M. Lu and Y.-G. Zhou, Chem. Rev., 2012, 112, 2557–2590 CrossRef CAS PubMed
; (b) Z. Yu, W. Jin and Q. Jiang, Angew. Chem., Int. Ed., 2012, 51, 6060–6072 CrossRef CAS PubMed
; (c) Y.-M. He, F.-T. Song and Q.-H. Fan, Top. Curr. Chem., 2014, 343, 145–190 CrossRef CAS PubMed
; (d) Z.-P. Chen and Y.-G. Zhou, Synthesis, 2016, 1769–1781 CAS
; (e) D. Zhao, L. Candish, D. Paul and F. Glorius, ACS Catal., 2016, 6, 5978–5988 CrossRef CAS
.
- Selected examples, see:
(a) A. Baeza and A. Pfaltz, Chem.–Eur. J., 2010, 16, 2036–2039 CrossRef CAS PubMed
; (b) D.-S. Wang, Q.-A. Chen, W. Li, C.-B. Yu, Y.-G. Zhou and X. Zhang, J. Am. Chem. Soc., 2010, 132, 8909–8911 CrossRef CAS PubMed
; (c) D.-S. Wang, J. Tang, Y.-G. Zhou, M.-W. Chen, C.-B. Yu, Y. Duan and G.-F. Jiang, Chem. Sci., 2011, 2, 803–806 RSC
; (d) N. Ortega, S. Urban, B. Beiring and F. Glorius, Angew. Chem., Int. Ed., 2012, 51, 1710–1713 CrossRef CAS PubMed
; (e) C. Li, J. Chen, G. Fu, D. Liu, Y. Liu and W. Zhang, Tetrahedron, 2013, 69, 6839–6844 CrossRef CAS
; (f) Y. Duan, L. Li, M.-W. Chen, C.-B. Yu, H.-J. Fan and Y.-G. Zhou, J. Am. Chem. Soc., 2014, 136, 7688–7700 CrossRef CAS PubMed
; (g) L. Pauli, R. Tannert, R. Scheil and A. Pfaltz, Chem.–Eur. J., 2015, 21, 1482–1487 CrossRef CAS PubMed
; (h) T. Touge and T. Arai, J. Am. Chem. Soc., 2016, 138, 11299–11305 CrossRef CAS PubMed
; (i) Z. Yang, F. Chen, Y. He, N. Yang and Q.-H. Fan, Angew. Chem., Int. Ed., 2016, 55, 13863–13866 CrossRef CAS PubMed
.
- Selected examples:
(a) T. Hayashi, T. Mise, M. Fukushima, M. Kagotani, N. Nagashima, Y. Hamada, A. Matsumoto, S. Kawakami, M. Konishi, K. Yamamoto and M. Kumada, Bull. Chem. Soc. Jpn., 1980, 53, 1138–1151 CrossRef CAS
; (b) T. Hayashi, N. Kawamura and Y. Ito, J. Am. Chem. Soc., 1987, 109, 7876–7878 CrossRef CAS
; (c) T. Hayashi, K. Kanehira, T. Hagihara and M. Kumada, J. Org. Chem., 1988, 53, 113–120 CrossRef CAS
; (d) T. Hayashi and A. Yamazaki, J. Organomet. Chem., 1991, 413, 295–302 CrossRef CAS
; (e) M. Sawamura and Y. Ito, Chem. Rev., 1992, 92, 857–871 CrossRef CAS
; (f) J. W. Han and T. Hayashi, Tetrahedron: Asymmetry, 2010, 21, 2193–2197 CrossRef CAS
; (g) T. J. Colacot, Chem. Rev., 2003, 103, 3101–3118 CrossRef CAS PubMed
; (h) P. Barbaro, C. Bianchini, G. Giambastiani and S. L. Parisel, Coord. Chem. Rev., 2004, 248, 2131–2150 CrossRef CAS
; (i) A. Yanagisawa and T. Arai, Chem. Commun., 2008, 1165–1172 RSC
; (j) W. Zeng, G.-Y. Chen, Y.-G. Zhou and Y.-X. Li, J. Am. Chem. Soc., 2007, 129, 750–751 CrossRef CAS PubMed
; (k) R. G. Arrayás, J. Adrio and J. C. Carretero, Angew. Chem., Int. Ed., 2006, 45, 7674–7715 CrossRef PubMed
; (l) R. C. J. Atkinson, V. C. Gibson and N. J. Long, Chem. Soc. Rev., 2004, 33, 313–328 RSC
; (m) H. B. Kagan, P. Diter, A. Gref, D. Guillaneux, A. Masson-Szymczak, F. Rebière, O. Riant, O. Samuel and S. Taudien, Pure Appl. Chem., 1996, 68, 29–36 CAS
.
-
(a) Q. Zhao, S. Li, K. Huang, R. Wang and X. Zhang, Org. Lett., 2013, 15, 4014–4017 CrossRef CAS PubMed
; (b) Q. Zhao, J. Wen, R. Tan, K. Huang, P. Metola, R. Wang, E. V. Anslyn and X. Zhang, Angew. Chem., Int. Ed., 2014, 53, 8467–8470 CrossRef CAS PubMed
; (c) P. Li, M. Zhou, Q. Zhao, W. Wu, X. Hu, X.-Q. Dong and X. Zhang, Org. Lett., 2016, 18, 40–43 CrossRef CAS PubMed
; (d) Z. Han, P. Li, Z. Zhang, C. Chen, Q. Wang, X.-Q. Dong and X. Zhang, ACS Catal., 2016, 6, 6214–6218 CrossRef CAS
; (e) P. Li, X. Hu, X.-Q. Dong and X. Zhang, Chem. Commun., 2016, 52, 11677–11680 RSC
; (f) J. Wen, R. Tan, S. Liu, Q. Zhao and X. Zhang, Chem. Sci., 2016, 7, 3047–3051 RSC
; (g) P. Li, Y. Huang, X. Hu, X.-Q. Dong and X. Zhang, Org. Lett., 2017, 19, 3855–3858 CrossRef CAS PubMed
; (h) Z. Han, R. Wang, G. Gu, X.-Q. Dong and X. Zhang, Chem. Commun., 2017, 53, 4226–4229 RSC
; (i) J. Wen, J. Jiang and X. Zhang, Org. Lett., 2016, 18, 4451–4453 CrossRef CAS PubMed
; (j) X. Li, C. You, Y. Yang, Y. Yang, P. Li, G. Gu, L. Wang, H. Lv and X. Zhang, Chem. Sci., 2018, 9, 1919–1924 RSC
.
- D. Antonow, T. Marrafa, I. Dawood, T. Ahmed, M. R. Haque, D. E. Thurston and G. Zinzalla, Chem. Commun., 2010, 46, 2289–2291 RSC
.
- D. Guillaneux, S.-H. Zhao, O. Samuel, D. Rainford and H. B. Kagan, J. Am. Chem. Soc., 1994, 116, 9430–9439 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8sc05397a |
| This journal is © The Royal Society of Chemistry 2019 |